Aromatic compounds found in different beers fermented with non-conventional Saccharomyces yeasts whose concentrations are above their threshold.
Abstract
Beer is a world-famous beverage, second only to tea and coffee, where the yeasts traditionally used are Saccharomyces cerevisiae and Saccharomyces pastorianus for the production of ale and lager beer, respectively. Their production, especially craft beer production, has grown in recent years, as has the development of new products. For this reason, research has focused on the selection of yeasts with good fermentation kinetics, as well as beers with outstanding aromatic profiles. The final flavor and aroma of beer is a combination of hundreds of active aroma compounds produced mostly during fermentation as a result of yeast metabolism (higher alcohols, esters, aldehydes, and vicinal diketones). Likewise, several studies have demonstrated the potential of wild yeasts of the genus Saccharomyces, both in aromatic production and in the production of healthy compounds of interest such as melatonin. This chapter therefore focuses on non-conventional Saccharomyces yeasts as they have the capacity to produce outstanding aroma compounds, as well as compounds that can provide health benefits, under moderate consumption.
Keywords
- non-conventional yeast
- Saccharomyces
- beer
- aromatic compounds
- functional beer
1. Introduction
Nowadays, there is a growing global interest in craft beer, as well as in the production of new beers that meet market needs. For this reason, brewers have focused especially on the use of yeasts, especially non-conventional yeast, to innovate in the brewing sector [1, 2, 3, 4, 5]. Wild yeast strains can provide different aroma and flavor characteristics with which to obtain varieties and styles of beer that are alternative and new to existing ones [6]. However, the use of these domesticated yeasts may show variable fermentative characteristics and affect the consistency and quality of the beers produced [4, 7]. Unlike the current commercial strains of
The present review focuses on the use of non-conventional yeasts of the genus
2. Beer ingredients
Beer is a worldwide-known beverage and the most popular after tea and water. However, people do not focus their attention on how it is brewed, as well as on its ingredients, which are barley as the main cereal, water, hops, and yeast. Other cereals or adjuncts may also be added [18, 19].
The basis of beer production is
In the past, these four ingredients were not well-known, but it was known that yeast was necessary for the production of fermented beverages. It was not until the development of lager beers that the exact composition of yeast was discovered. Although yeasts have been used for centuries to brew beer, they were first identified as responsible for the fermentation of malted barley water in the 19th century. The first principles of yeast function were discovered during the 17th and 18th centuries [26]; however, it was not until the mid-19th century that the French scientist Louis Pasteur was able to demonstrate that yeast is made up of living cells responsible for the fermentation process [27]. In the same vein, sugars were also not known to be another essential ingredient and can be classified according to the type of fermented beverage, whether from sugar cane (sucrose), milk (lactose), fruit or honey (fructose and glucose), or cereals (maltose) [19].
3. Beginnings in the use of yeast for beer production
The beginnings of beer production date back to the Neolithic civilization. Beer is a traditional product and valued for its physicochemical properties that give it quality. Therefore, the history of brewing is not only the history of scientific and technological developments but also the history of people: their government, their culture, and their daily life [28]. Early brewers, winemakers, and bakers realized that by using small portions of finished products that had already been fermented, it was possible to obtain products with faster and more predictable fermentation. Thus, the ability of
The most studied yeast at the industrial level is
4. Brewing potential of wild Saccharomyces
The increase in the consumption of craft beer [42], as well as consumers’ interest in trying new beer styles [43], has encouraged the application of new yeasts in brewing [44, 45]. These yeasts include the wild yeast
The natural biodiversity of microorganisms representing the habitat of a geographic region can be tapped.
These new species can bring added value to beer in terms of organoleptic qualities.
Some yeasts isolated from nature already have the status of Generally Recognized as Safe/Qualified Presumption of Safety.
Current regulations favor the use of unmodified genetic stocks, as they add identity and uniqueness to differentiate the production line.
On an industrial level, not only ethanol and glycerol production but also the utilization of available sugars in the wort, hop tolerance and resistance to low temperatures, and the relative production of aromatic compounds such as esters and higher alcohols, as well as low levels of acetic acid and hydrogen sulfide, are important [2, 45, 49]. Not all species are able to ferment the sugars present in the wort (glucose, fructose, maltose, and maltotriose). In the case of
Another advantage of wild yeasts is that they can be subjected to different fermentation conditions to observe their behavior. In this case, from a technological point of view, the application of aeration during fermentation is an interesting tool for controlling yeast metabolism during fermentation [54]. First, due to Crabtree-negative yeasts, when the oxygen concentration in the medium is saturated, the yeast metabolism begins to be predominantly oxidative, thus reducing the ethanol content and increasing the yeast biomass. On the other hand, aeration may also affect aromatic compounds by reducing or increasing their concentration in beer (the acetaldehyde content may decrease, and the concentration of higher alcohols increases) [55, 56, 57]. Within the non-conventional strains of
5. Aroma production by non-conventional yeast
The beverage industry has focused on the search for fruity and floral aromas, which is why consumers demand beers with fruitier aromatic profiles. The ingredients in beer that can provide such aromas and flavors are hops, but mostly yeast during the fermentation process, as it will provide a fruitier organoleptic profile to beers. Various aroma compounds can be found in beer, although studies mainly focus on the alcohols and esters produced by yeast as they will provide the main aromas found in beer [10, 62, 63].
Meilgaard elaborated the “beer aroma wheel,” where all important beer aromas were included, including all yeast-derived aromas, as well as all other raw materials used during brewing [64].
Main aromatic compounds relevant in beer:
The use of non-conventional yeasts in winemaking has been extensively studied in both
The study carried out by Postigo et al. [103] with 114
| Yeast strain | Species | Source of isolation | Apparent attenuation (%) | Flavor compound | Concentration (mg/L) | Reference |
|---|---|---|---|---|---|---|
| DBVPG 1058 | Baker’s yeast | 71.25 | Ethyl acetate | 32.00 | [105] | |
| Isoamyl acetate | 1.90 | |||||
| Ethyl hexanoate | 0.60 | |||||
| M4 | Craft beer Ale | 69.85 | Acetaldehyde | 164.92 | [106] | |
| Isoamyl alcohol | 161.04 | |||||
| MT-15 | Sourdoughs | 60.29 | Acetaldehyde | 43.14 | [106] | |
| Isoamyl alcohol | 101.88 | |||||
| Granvin2 | Norwegian kveik | n.a. | Ethyl caproate | 0.37 | [107] | |
| Ethyl caprilate | 4.56 | |||||
| Ethyl decanoate | 0.46 | |||||
| Grancin6 | Norwegian kveik | n.a. | Ethyl caproate | 0.37 | [107] | |
| Ethyl caprilate | 5.01 | |||||
| Ethyl decanoate | 0.88 | |||||
| G 520 | Organic cellar | 72.00 | Isoamyl acetate | 2.34 | [103] | |
| Guaiacol | 0.05 | |||||
| CLI 1109 | Vineyard | 71.00 | Isoamyl acetate | 1.90 | [103] | |
| Ethyl hexanoate | 0.60 | |||||
| Guaiacol | 0.03 |
Table 1.
n.a.: not appear.
6. Functional beer
Functional beer is defined as a beer that can provide health benefits under moderate consumption. Functional beers include those that have a low ethanol content, as well as those that provide high concentrations of compounds such as fiber, vitamins, minerals, polyphenols, and probiotics [1].
Recent years have seen an increase in consumption and interest in low-alcohol beers. This is mainly due to health and safety reasons, in addition to an increase in strict social regulations [112]. Low-ethanol beers can have health benefits due to the healthy components they contain, besides a lower energy intake and the total absence of negative effects of alcohol consumption.
Several studies have shown that with the use of non-conventional yeast, functional beers can be obtained, as they not only possess the ability to produce remarkable aromatic compounds, as well as other by-products such as melatonin [6, 113].
Functional beers also include beers that are gluten-free, thus covering consumers suffering from coeliac disease, which is a gluten-sensitive, immune-mediated enteropathy.
Probiotic beers are also included in functional beers. Probiotics include those live microorganisms that are added to food and that under certain doses can be potentially beneficial for human health, especially for the intestinal microbial balance [114]. Therefore, a probiotic beer is one obtained by using probiotic microorganisms during the fermentation process. The best known microorganisms used for their probiotic characteristics are lactic acid bacteria (
6.1 Melatonin production
Melatonin is a mammalian hormone that regulates sleep and has antioxidant properties. It is produced by yeasts during fermentation and can therefore be a source of exogenous melatonin for the body, since as people age, less melatonin is produced in the body. In addition, it provides beer with antioxidant, antiaging, anti-inflammatory, antitumor, and immunomodulatory capacities [122, 123, 124].
Much of the food and beverages we consume on a daily basis contain melatonin. Therefore, their intake helps to increase the melatonin level in the body and its antioxidant status in human serum, which is the reason that this molecule is absorbed in the gastrointestinal tract [123, 125, 126] and readily crosses all morphophysiological barriers and tissue and cell membranes [124, 125, 126, 127, 128, 129]. Likewise, melatonin interacts with toxic reagents, generating other metabolites that are in turn direct free radical scavengers. The combined actions of melatonin and its derivatives greatly enhance the efficacy of melatonin in protecting against free radical damage and reducing the likelihood of human disease [130].
Melatonin is a by-product of yeast that is produced in the final stages of fermentation and is excreted into the medium during the stationary phase of the yeast fermentation [131]. In the studies carried out by Postigo et al. [103, 132] with different strains of wild
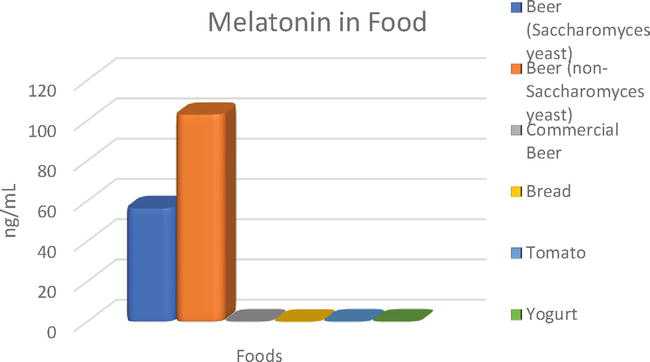
Figure 1.
Melatonin concentrations found in different foods. Beer (Saccharomyces yeast) [
These levels of melatonin that can be found in various foods, as well as in beer, are concentrations below those studied that have supposed positive effects on health (1–10 mg) [137, 138]; however, if ingested together with other foods, they can contribute to increase its concentration in the human serum. Studies carried out by Maldonado et al. [123] determined that the intake of beer with high melatonin content (169.7 pg/ml) contributed to increase the antioxidant properties of human serum. However, although melatonin can act as a strong antioxidant, it can be degraded in the presence of oxygen, light, or free radicals that are present during the aging process [139]. This fact could be observed in the studies of lambic beer carried out by Postigo et al. [140], where the analysis at different maturation times of the lambic beers brewed determined their degradation over time. It should also be taken into account whether the final product is subjected to final heat treatments to prolong the stability of the product (such as pasteurization), since it has been shown that high temperatures can also degrade it and reduce its concentration in food [136].
6.2 Antioxidant capacity
Different compounds such as phenolics can be found in beer [141]. These substances can also be found naturally in fruits, vegetables, nuts, seeds, and beverages [142]. When studying the antioxidant fraction of beer, phenolic compounds are the most studied, which are found in hops (20–30%) as well as in malt (70–80%) [141]. Hops provide the beer with phenolic acids, prenylated chalcones, flavonoids, catechins, and proanthocyanidins [141]. Malt contains an overall phenolic mass of 1.0–1.9 mg g−1 dry matter [143]. Likewise, the yeast used in brewing can also influence the phenolic composition and antioxidant capacity of the final product [106]. Several studies, such as the one carried out by Viana et al. [144], showed that the use of certain yeast strains for the production of Pale Ale beer significantly influences the antioxidant capacity of the beers.
Antioxidant capacity is related to parameters such as total phenolic and flavonoid content; 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity; and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation scavenging activity [145]. In the studies carried out by Postigo et al. [103] with different strains of wild yeast, it was observed that there were no major differences between the yeasts of
| Beer | Concentration | Method | Reference |
|---|---|---|---|
| Ale ( | 9.50 to 13.67 mmol TE/L | TEAC | [103] |
| Ale (sequential fermentation) | 9.63 to 13.70 mmol TE/L | TEAC | [133] |
| Commercial Ale (S-04) | 11.18 mmol TE/L | TEAC | [103] |
| Commercial Ale | 0.76 to 10.51 mmol TE/L | ORAC | [113] |
| Commercial Lager | 0.44 to 7.72 mmol TE/L | ORAC | [113] |
| Commercial Ale | 3.70 to 29.11 mmol TE/L | ORAC | [113] |
| Commercial Lager | 0.58 to 1.02 mmol TE/L | TEAC | [146] |
| Commercial Ale | 0.73 to 1.19 mmol TE/L | TEAC | [146] |
| Commercial Lager | 3.70 to 14.79 mmol TE/L | ORAC | [146] |
| Commercial Ale | 10.65 to 29.11 mmol TE/L | ORAC | [146] |
Table 2.
Antioxidant capacity of different ale and lager beers analyzed by different methods.
ORAC (Oxygen Radical Absorption Capacity), TEAC (Trolox equivalent antioxidant capacity).
7. Conclusions
The use of non-conventional
References
- 1.
Yeo HQ , Liu SQ. An overview of selected specialty beers: Developments, challenges and prospects. International Journal of Food Science and Technology. 2014; 49 (7):1607-1618. DOI: 10.1111/ijfs.12488 - 2.
Steensels J, Verstrepen KJ. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annual Review of Microbiology. 2014; 68 (1):61-80. DOI: 10.1146/annurev-micro-091213-113025 - 3.
James SA, Stratford M. Zygosaccharomyces . In: The Yeasts. London: Elsevier; 2011. pp. 937-947. DOI: 10.1016/B978-0-444-52149-1.00084-7 - 4.
Lentz M, Putzke T, Hessler R, Luman E. Genetic and physiological characterization of yeast isolated from ripe fruit and analysis of fermentation and brewing potential. Journal of the Institute of Brewing. 2014; 120 (4):559-564. DOI: 10.1002/jib.154 - 5.
Petruzzi L, Rosaria Corbo M, Sinigaglia M, Bevilacqua A. Brewer’s yeast in controlled and uncontrolled fermentations, with a focus on novel, nonconventional, and superior strains. Food Review International. 2016; 32 (4):341-363. DOI: 10.1080/87559129.2015.1075211 - 6.
Basso RF, Alcarde AR, Portugal CB. Could non- Saccharomyces yeasts contribute on innovative brewing fermentations? Food Research International. 2016;86 :112-120. DOI: 10.1016/j.foodres.2016.06.002 - 7.
Vanderhaegen B, Neven H, Coghe S, Verstrepen KJ, Derdelinckx G, Verachtert H. Bioflavoring and beer refermentation. Applied Microbiology and Biotechnology. 2003; 62 (2-3):140-150. DOI: 10.1007/s00253-003-1340-5 - 8.
Piskur J, Rozpedowska E, Polakova S, Merico A, Compagno C. How did Saccharomyces evolve to become a good brewer? Trends in Genetics. 2006; 22 (4):183-186. DOI: 10.1016/j.tig.2006.02.002 - 9.
Lodolo EJ, Kock JLF, Axcell BC, Brooks M. The yeast Saccharomyces cerevisiae -the main character in beer brewing. FEMS Yeast Research. 2008;8 (7):1018-1036. DOI: 10.1111/j.1567-1364.2008.00433.x - 10.
Pires EJ, Teixeira JA, Brányik T, Vicente AA. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Applied Microbiology and Biotechnology. 2014; 98 (5):1937-1949. DOI: 10.1007/s00253-013-5470-0 - 11.
Verstrepen KJ, Derdelinckx G, Pierre DJ, Winderickx J, Thevelein JM, Pretorius IS, et al. Flavor-active esters: Adding fruitiness to beer. Journal of Bioscience and Bioengineering. 2003; 96 (2):110-118. DOI: 10.1016/S1389-1723(03)90112-5 - 12.
Procopio S, Qian F, Becker T. Function and regulation of yeast genes involved in higher alcohol and ester metabolism during beverage fermentation. European Food Research and Technology. 2011; 233 (5):721-729. DOI: 10.1007/s00217-011-1567-9 - 13.
Procopio S, Brunner M, Becker T. Differential transcribed yeast genes involved in flavour formation and its associated amino acid metabolism during brewery fermentation. European Food Research and Technology. 2014; 239 (3):421-439. DOI: 10.1007/s00217-014-2236-6 - 14.
Steensels J, Snoek T, Meersman E, Picca Nicolino M, Aslankoohi E, Christiaens JF, et al. Selecting and generating superior yeasts for the brewing industry. Cerevisia. 2012; 37 (2):63-67. DOI: 10.1016/j.cervis.2012.08.001 - 15.
Verstrepen KJ, Derdelinckx G, Delvaux FR. Esters in beer-part 1: The fermentation process: More than ethanol formation. Cerevisia. 2003; 28 (3):41-49 - 16.
Meilgaard MC. Flavor chemistry in beer: Part I: Flavor interaction between principal volatiles. Technical Quarterly—Master Brewers Association. 1975; 12 (2):107-117 - 17.
Kollmannsberger H, Biendl M, Nitz S. Occurrence of glycosidically bound flavour compounds in hops, hop products and beer. Monatsschrift fur Brauwissenschaft. 2006; 5 (6):83-89 - 18.
Bogdan P, Kordialik-Bogacka E. Alternatives to malt in brewing. Trends in Food Science and Technology. 2017; 65 :1-9. DOI: 10.1016/j.tifs.2017.05.001 - 19.
Nelson M. The Barbarian’s Beverage [Internet]. New York: Routledge; 2005. DOI: 10.4324/9780203309124 - 20.
Belitz H, Grosch W. Alcoholic beverages. In: Food Chemistry. Berlin, Heidelberg: Springer Berlin Heidelberg; 2004. pp. 892-937. DOI: 10.1007/978-3-540-69934-7_21 - 21.
Eumann M. Water in brewing. In: Brewing. London: Elsevier; 2006. pp. 183-207. DOI: 10.1533/9781845691738.183 - 22.
Niu C, Han Y, Wang J, Zheng F, Liu C, Li Y, et al. Malt derived proteins: Effect of protein Z on beer foam stability. Food Bioscience. 2018; 25 :21-27. DOI: 10.1016/j.fbio.2018.07.003 - 23.
Parr H, Bolat I, Cook D. Modelling flavour formation in roasted malt substrates under controlled conditions of time and temperature. Food Chemistry. 2021; 337 :127641. DOI: 10.1016/j.foodchem. 2020.127641 - 24.
Kishimoto T, Teramoto S, Fujita A, Yamada O. Evaluation of components contributing to the international bitterness unit of wort and beer. Journal of the American Society of Brewing Chemists. 2022; 80 (1):53-61. DOI: 10.1080/03610470.2021.1878684 - 25.
Astray G, Gullón P, Gullón B, Munekata PES, Lorenzo JM. Humulus lupulus L. as a natural source of functional biomolecules. Applied Sciences. 2020;10 (15):5074. DOI: 10.3390/app10155074 - 26.
Barnett JA. Beginnings of microbiology and biochemistry: The contribution of yeast research. Microbiology. 2003; 149 (3):557-567. DOI: 10.1099/mic.0.26089-0 - 27.
Barnett JA. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850-1880. Yeast. 2000; 16 (8):755-771. DOI: 10.1002/1097-0061 (20000615)16:8<755::AID-YEA587>3.0.CO;2-4 - 28.
Meussdoerffer FG. A comprehensive history of beer brewing. In: Handbook of Brewing. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009. pp. 1-42. DOI: 10.1002/ 9783527623488.ch1 - 29.
Michel RH, McGovern PE, Badler VR. Chemical evidence for ancient beer. Nature. 1992; 360 (6399):24-24 - 30.
Goddard MR, Greig D. Saccharomyces cerevisiae : A nomadic yeast with no niche? FEMS Yeast Research. 2015;15 (3):1-6 - 31.
Warringer J, Zörgö E, Cubillos FA, Zia A, Gjuvsland A, Simpson JT, et al. Trait variation in yeast is defined by population history. PLoS Genetics. 2011; 7 (6):e1002111 - 32.
Driscoll CA, Macdonald DW, O’Brien SJ. An evolutionary view of domestication. PNAS. 2009; 106 :9971-9978 - 33.
Purugganan MD, Fuller DQ. The nature of selection during plant domestication. Nature. 2009; 457 (7231):843-848 - 34.
Marsit S, Mena A, Couloux A, Guy J, Luc LJ, Barrio E, et al. Evolutionary advantage conferred by an eukaryote-to-eukaryote gene transfer event in wine yeasts. Molecular Biology and Evolution. 2015; 32 (7):1695-1707 - 35.
Marsit S, Sanchez I, Galeote V. Horizontally acquired oligopeptide transporters favour adaptation of Saccharomyces cerevisiae wine yeast to oenological environment. Environmental Microbiology. 2016;18 :1148-1161 - 36.
Almeida P, Barbosa R, Zalar P, Imanishi Y, Shimizu K, Turchetti B, et al. A population genomics insight into the Mediterranean origins of wine yeast domestication. Molecular Ecology. 2015; 24 (21):5412-5427 - 37.
Marsit S, Dequin S, Montpellier F, Supagro M, Montpellier F, Montpellier F. Diversity and adaptive evolution of Saccharomyces wine yeast: A review. FEMS Yeast Research. 2015; 15 :fov067 - 38.
Dunn B, Richter C, Kvitek DJ, Pugh T, Sherlock G. Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Research. 2012;22 :908-924 - 39.
Steenwyk J, Rokas A. Extensive copy number variation in fermentation-related genes among Saccharomyces cerevisiae wine strains. G3 (Bethesda). 2017;7 (May):1475-1485 - 40.
Strope PK, Skelly DA, Kozmin SG, Mahadevan G, Stone EA, Magwene PM, et al. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Research. 2015;25 :762-774 - 41.
Almeida P, Barbosa R, Bensasson D, Lisboa UN De. Adaptive divergence in wine yeasts and their wild relatives, Molecular Ecology. 2017;26(7):2167-2182 - 42.
CJS G. Economics of the craft beer revolution: A comparative international perspective. In: Garavaglia C, Swinnen J, editors. Economic Perspectives on Craft Beer. Cham: Springer International Publishing; 2018. pp. 3-54. DOI: 10.1007/978-3-319-58235-1 - 43.
Aquilani B, Laureti T, Poponi S, Secondi L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Quality and Preference. 2015; 41 :214-224. DOI: 10.1016/j.foodqual.2014.12.005 - 44.
Hittinger CT, Steele JL, Ryder DS. Diverse yeasts for diverse fermented beverages and foods. Current Opinion in Biotechnology. 2018; 49 :199-206. DOI: 10.1016/j.copbio.2017.10.004 - 45.
Gibson B, Geertman JMA, Hittinger CT, Krogerus K, Libkind D, Louis EJ, et al. New yeasts-new brews: Modern approaches to brewing yeast design and development. FEMS Yeast Research. 2017; 17 (4):1-13 - 46.
De FG, Sannino C, Sileoni V, Marconi O, Filippucci S, Tasselli G, et al. Mrakia Gelida in brewing process: An innovative production of low alcohol beer using a psychrophilic yeast strain. Journal of Food Microbiology. 2018; 76 (April):354-362. DOI: 10.1016/j.fm.2018.06.018 - 47.
Domizio P, House JF, Joseph CML, Bisson LF, Bamforth CW. Lachancea thermotolerans as an alternative yeast for the production of beer. Journal of the Institute of Brewing. 2016;122 (4):599-604. DOI: 10.1002/jib.362 - 48.
Michel M, Kopecká J, Meier- Dörnberg T, Zarnkow M, Jacob F, Hutzler M. Screening for new brewing yeasts in the non- Saccharomyces sector withTorulaspora delbrueckii as model. Yeast. 2016;33 (4):129-144. DOI: 10.1002/yea.3146 - 49.
Gallone B, Mertens S, Gordon JL, Maere S, Verstrepen KJ, Steensels J. Origins, evolution, domestication and diversity of Saccharomyces beer yeasts. Current Opinion in Biotechnology. 2018;49 :148-155. DOI: 10.1016/j.copbio.2017.08.005 - 50.
Nikulin J, Krogerus K, Gibson B. Alternative Saccharomyces interspecies hybrid combinations and their potential for low-temperature wort fermentation. Yeast. 2018;35 (1):113-127. DOI: 10.1002/yea.3246 - 51.
Coghe S, Benoot K, Delvaux F, Vanderhaegen B, Delvaux FR. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in Saccharomyces cerevisiae . Journal of Agricultural and Food Chemistry. 2004;52 (3):602-608. DOI: 10.1021/jf0346556 - 52.
Gallone B, Steensels J, Prahl T, Soriaga L, Saels V, Herrera-Malaver B, et al. Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell. 2016;166 (6):1397-1410. DOI: 10.1016/j.cell.2016.08.020 - 53.
Cubillos FA, Gibson B, Grijalva- Vallejos N, Krogerus K, Nikulin J. Bioprospecting for brewers: Exploiting natural diversity for naturally diverse beers. Yeast. 2019; 36 (6):383-398. DOI: 10.1002/yea.3380 - 54.
Rodrigues AJ, Raimbourg T, Gonzalez R, Morales P. Environmental factors influencing the efficacy of different yeast strains for alcohol level reduction in wine by respiration. LWT. 2016; 65 :1038-1043. DOI: 10.1016/j.lwt.2015.09.046 - 55.
Kirsop BH. Oxygen in brewery fermentation. Journal of the Institute of Brewing. 1974; 80 (3):252-259. DOI: 10.1002/j.2050-0416.1974.tb03614.x - 56.
Virkajärvi I, Lindborg K, Jukka Kronlöf EP. Effects of aeration on flavor compounds in immobilized primary fermentation. Monatsschrift für Brauwiss. 1999; 52 (1):9-28 - 57.
Kucharczyk K, Tuszyński T. The effect of wort aeration on fermentation, maturation and volatile components of beer produced on an industrial scale. Journal of the Institute of Brewing. 2017; 123 (1):31-38. DOI: 10.1002/jib.392 - 58.
Postigo V, O’Sullivan T, Elink Schuurman T, Arroyo T. Non-conventional yeast: Behavior under pure culture, sequential and aeration conditions in beer fermentation. Food. 2022; 11 (22):3717. DOI: 10.3390/foods11223717 - 59.
Fukuhara H. The Kluyver effect revisited. FEMS Yeast Research. 2003; 3 (4):327-331. DOI: 10.1016/S1567-1356(03)00112-0 - 60.
Sims AP, Barnett JA. The requirement of oxygen for the utilization of maltose, cellobiose and D-galactose by certain anaerobically fermenting yeasts (Kluyver effect). Journal of General Microbiology. 1978; 106 (2):277-288. DOI: 10.1099/00221287-106-2-277 - 61.
Mauricio JC, Moreno J, Zea L, Ortega JM, Medina M. The effects of grape must fermentation conditions on volatile alcohols and esters formed by Saccharomyces cerevisiae . Journal of the Science of Food and Agriculture. 1997;75 (2):155-160. DOI: 10.1002/(SICI)1097-0010(199710)75:2<155::AID-JSFA853>3.0.CO;2-S - 62.
Saerens SMG, Delvaux FR, Verstrepen KJ, Thevelein JM. Production and biological function of volatile esters in Saccharomyces cerevisiae . Microbial Biotechnology. 2010;3 (2):165-177. DOI: 10.1111/j.1751-7915.2009.00106.x - 63.
Vanderhaegen B, Neven H, Coghe S, Verstrepen KJ, Verachtert H, Derdelinckx G. Evolution of chemical and sensory properties during aging of top-fermented beer. Journal of Agricultural and Food Chemistry. 2003; 51 (23):6782-6790. DOI: 10.1021/jf034631z - 64.
Meilgaard MC, Dalgliesh CE, Clapperton JF. Beer flavour terminology. Journal of the Institute of Brewing. 1979; 85 (1):38-42. DOI: 10.1002/j.2050-0416.1979.tb06826.x - 65.
Dvořák J, Dostálek P, Štěrba K, Čejka P, Kellner V, Čulík J, et al. Determination of total sulphur dioxide in beer samples by flow-through chronopotentiometry. Journal of the Institute of Brewing. 2006; 112 (4):308-313. DOI: 10.1002/j.2050-0416.2006.tb00736.x - 66.
Nagami K. Hydrogen sulfide in brewing. MBAA Technical Quarterly. 1980; 17 :64-68 - 67.
Guido LF. Sulfites in beer: Reviewing regulation, analysis and role. Science in Agriculture. 2016; 73 (2):189-197. DOI: 10.1590/0103-9016-2015-0290 - 68.
Meilgaard MC. Individual differences in sensory threshold for aroma chemicals added to beer. Food Quality and Preference. 1993; 4 (3):153-167. DOI: 10.1016/0950-3293(93)90158-3 - 69.
Ilet DR. Aspects of the analysis, role, and fate of sulphur dioxide in beer—A review. Tech Quaterly. Master Brewers Association of the America. 1995; 32 :213-221 - 70.
Oka K, Hayashi T, Matsumoto N, Yanase H. Decrease in hydrogen sulfide content during the final stage of beer fermentation due to involvement of yeast and not carbon dioxide gas purging. Journal of Bioscience and Bioengineering. 2008; 106 (3):253-257. DOI: 10.1263/jbb.106.253 - 71.
Anderson RJ, Howard GA. The origin and occurrence of volatile sulphur compounds in British ales and lagers. Journal of the Institute of Brewing. 1974; 80 (4):357-370. DOI: 10.1002/j.2050-0416.1974.tb03630.x - 72.
Meilgaard M, Elizondo A, Moya E. A study of carbonyl compounds in beer, part II. Flavor and flavor thresholds of aldehydes and ketones added to beer. Technical Quarterly - Master Brewers Association. 1970; 7 (3):143-149 - 73.
Krogerus K, Gibson BR. 125th anniversary review: Diacetyl and its control during brewery fermentation. Journal of the Institute of Brewing. 2013; 119 (3):86-97. DOI: 10.1002/jib.84 - 74.
Ryan ED, Kohlhaw GB. Subcellular localization of isoleucine-valine biosynthetic enzymes in yeast. Journal of Bacteriology. 1974; 120 (2):631-637. DOI: 10.1128/jb.120.2.631-637.1974 - 75.
Liu SQ. Impact of yeast and bacteria on beer appearance and flavor [Internet]. In: Hill AE, editor. Brewing Microbiology. Oxford, UK: Woodhead Publishing; 2015. pp. 357-374. DOI: 10.1016/B978-1-78242-331-7.00017-4 - 76.
Canonico L, Agarbati A, Comitini F, Ciani M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiology. 2016;56 :45-51. DOI: 10.1016/j.fm.2015.12.005 - 77.
Eßlinger HM. Handbook of Brewing Processes Technology Markets-Wiley. Weinheim: Wiley; 2009. p. 779 - 78.
Thurston P. The phenolic off-flavour test: A method for confirming the presence of wild yeasts. Journal of the Institute of Brewing. 1986; 92 (1):9-10 - 79.
Scholtes C, Nizet S, Collin S. Guaiacol and 4-Methylphenol as specific markers of torrefied malts. Fate of volatile phenols in special beers through aging. Journal of Agricultural and Food Chemistry. 2014; 62 (39):9522-9528. DOI: 10.1021/jf5015654 - 80.
Vanbeneden N, Van Roey T, Willems F, Delvaux F, Delvaux FR. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chemistry. 2008; 111 (1):83-91. DOI: 10.1016/j.foodchem.2008.03.029 - 81.
Thurston PA, Tubb RS. Screening yeast strains for their ability to produce phenolic off-flavours: A simple method for determining phenols in wort and beer. Journal of the Institute of Brewing. 1981; 87 (3):177-179. DOI: 10.1002/j.2050-0416.1981.tb04012.x - 82.
Rodrigues JEA, Erny GL, Barros AS, Esteves VI, Brandão T, Ferreira AA, et al. Quantification of organic acids in beer by nuclear magnetic resonance (NMR)-based methods. Analytica Chimica Acta. 2010; 674 (2):166-175. DOI: 10.1016/j.aca.2010.06.029 - 83.
Clapperton JF, Brown DGW. Caprylic flavour as a feature of beer flavour. Journal of the Institute of Brewing. 1978; 84 (2):90-92. DOI: 10.1002/j.2050-0416.1978.tb03844.x - 84.
Siebert KJ. Modeling the flavor thresholds of organic acids in beer as a function of their molecular properties. Food Quality and Preference. 1999; 10 (2):129-137. DOI: 10.1016/S0950-3293(98)00059-7 - 85.
Montanari L, Perretti G, Natella F, Guidi A, Fantozzi P. Organic and phenolic acids in beer. LWT - Food Science and Technology. 1999; 32 (8):535-539. DOI: 10.1006/fstl.1999.0593 - 86.
Amata AI, Germain P. The effect of pitching yeast aeration on the production of acetic acid during fermentations with brewers’ yeast: An enzymatic approach. Journal of the Institute of Brewing. 1990; 96 (3):131-134. DOI: 10.1002/j.2050-0416.1990.tb01023.x - 87.
Li H, Liu F. The chemistry of sour taste and the strategy to reduce the sour taste of beer. Food Chemistry. 2015; 185 :200-204. DOI: 10.1016/j.foodchem.2015.03.135 - 88.
Coote N, Kirsop BH. The content of some organic acids in beer and other fermented media. Journal of the Institute of Brewing. 1974; 80 (5):474-483. DOI: 10.1002/j.2050-0416.1974.tb06797.x - 89.
Eden A, Simchen G, Benvenisty N. Two yeast homologs of ECA39, a target for c-myc regulation, code for cytosolic and mitochondrial branched-chain amino acid aminotransferases. The Journal of Biological Chemistry. 1996; 271 (34):20242-20245. DOI: 10.1074/jbc.271.34.20242 - 90.
Hazelwood LA, Daran JM, van Maris AJA, Pronk JT, Dickinson JR. The ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Applied and Environmental Microbiology. 2008;74 (8):2259-2266. DOI: 10.1128/AEM.02625-07 - 91.
Ehrlich F. Über die Bedingungen der Fuselölbildung und über ihren Zusammenhang mit dem Eiweissaufbau der Hefe. Berichte der Dtsch Chem Gesellschaft. 1907; 40 (1):1027-1047 - 92.
Engan S. Organoleptic threshold values of some organic acids in beer. Journal of the Institute of Brewing. 1974; 80 (2):162-163. DOI: 10.1002/j.2050-0416.1974.tb03598.x - 93.
Engan S. Organoleptic threshold values of some alcohols and esters in beer. Journal of the Institute of Brewing. 1972; 78 (1):33-36. DOI: 10.1002/j.2050-0416.1972.tb03425.x - 94.
Peddie HA. Ester formation in brewery fermentations. Journal of the Institute of Brewing. 1990; 96 (5):327-331. DOI: 10.1002/j.2050-0416.1990.tb01039.x - 95.
Ciani M, Comitini F, Mannazzu I, Domizio P. Controlled mixed culture fermentation: A new perspective on the use of non- Saccharomyces yeasts in winemaking. FEMS Yeast Research. 2010;10 (2):123-133. DOI: 10.1111/j.1567-1364.2009.00579.x - 96.
Ciani M, Comitini F. Non- Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Annales de Microbiologie. 2011;61 (1):25-32. DOI: 10.1007/s13213-010-0069-5 - 97.
Jolly NP, Varela C, Pretorius IS. Not your ordinary yeast: Non- Saccharomyces yeasts in wine production uncovered. FEMS Yeast Research. 2014;14 (2):215-237. DOI: 10.1111/1567-1364.12111 - 98.
Michel M, Meier-Dörnberg T, Jacob F, Methner F, Wagner RS, Hutzler M. Review: Pure non- Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. Journal of the Institute of Brewing. 2016;122 (4):569-587. DOI: 10.1002/jib.381 - 99.
Carrau FM, Medina K, Boido E, Farina L, Gaggero C, Dellacassa E, et al. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiology Letters. 2005;243 (1):107-115. DOI: 10.1016/j.femsle.2004.11.050 - 100.
Stribny J, Gamero A, Pérez-Torrado R, Querol A. Saccharomyces kudriavzevii andSaccharomyces uvarum differ fromSaccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. International Journal of Food Microbiology. 2015;205 :41-46. DOI: 10.1016/j.ijfoodmicro.2015.04.003 - 101.
Janssens L, De Pooter HL, Schamp NM, Vandamme EJ. Production of flavours by microorganisms. Process Biochemistry. 1992; 27 (4):195-215. DOI: 10.1016/0032-9592(92)80020-4 - 102.
Gamero A, Quintilla R, Groenewald M, Alkema W, Boekhout T, Hazelwood L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiology. 2016; 60 :147-159. DOI: 10.1016/j.fm.2016.07.006 - 103.
Postigo V, García M, Cabellos JM, Arroyo T. Wine Saccharomyces yeasts for beer fermentation. Fermentation. 2021;7 (4):290. DOI: 10.3390/fermentation7040290 - 104.
Sterckx FL, Missiaen J, Saison D, Delvaux FR. Contribution of monophenols to beer flavour based on flavour thresholds, interactions and recombination experiments. Food Chemistry. 2011; 126 (4):1679-1685. DOI: 10.1016/j.foodchem.2010.12.055 - 105.
Rossi S, Turchetti B, Sileoni V, Marconi O, Perretti G. Evaluation of Saccharomyces cerevisiae strains isolated from non-brewing environments in beer production. Journal of the Institute of Brewing. 2018;124 (4):381-388. DOI: 10.1002/jib.503 - 106.
Capece A, Romaniello R, Pietrafesa A, Siesto G, Pietrafesa R, Zambuto M, et al. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations withS. Cerevisiae for the production of craft beers with potential healthy value-added. International Journal of Food Microbiology. 2018;284 (March):22-30. DOI: 10.1016/j.ijfoodmicro.2018.06.028 - 107.
Preiss R, Tyrawa C, Krogerus K, Garshol LM, Van Der Merwe G. Traditional Norwegian Kveik are a genetically distinct group of domesticated Saccharomyces cerevisiae brewing yeasts. Frontiers in Microbiology. 2018;9 :2137 - 108.
De Francesco G, Turchetti B, Sileoni V, Marconi O, Perretti G. Screening of new strains of Saccharomycodes ludwigii andZygosaccharomyces rouxii . Journal of the Institute of Brewing. 2015;121 (1):113-121. DOI: 10.1002/jib.185 - 109.
Saison D, De Schutter DP, Vanbeneden N, Daenen L, Delvaux F, Delvaux FR. Decrease of aged beer aroma by the reducing activity of brewing yeast. Journal of Agricultural and Food Chemistry. 2010; 58 (5):3107-3115. DOI: 10.1021/jf9037387 - 110.
Hughes PS, Baxter ED. Beer: Quality, Safety and Nutritional Aspects. Cambridge: Royal Society of Chemistry; 2001. pp. 40-73 - 111.
Langstaff SA, Lewis MJ. The mouthfeel of beer—A review. Journal of the Institute of Brewing. 1993; 99 (1):31-37. DOI: 10.1002/j.2050-0416.1993.tb01143.x - 112.
Brányik T, Silva DP, Baszczyňski M, Lehnert R, Almeidae Silva JB. A review of methods of low alcohol and alcohol-free beer production. Journal of Food Engineering. 2012; 108 (4):493-506. DOI: 10.1016/j.jfoodeng.2011.09.020 - 113.
Granato D, Branco GF, Faria JDAF, Cruz AG. Characterization of Brazilian lager and brown ale beers based on color, phenolic compounds, and antioxidant activity using chemometrics. Journal of the Science of Food and Agriculture. 2011; 91 (3):563-571. DOI: 10.1002/jsfa.4222 - 114.
Moslehi-Jenabian S, Lindegaard L, Jespersen L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients. 2010; 2 (4):449-473. DOI: 10.3390/nu2040449 - 115.
Butel MJ. Probiotics, gut microbiota and health. Médecine et Maladies Infectieuses. 2014; 44 (1):1-8. DOI: 10.1016/j.medmal.2013.10.002 - 116.
Buts JP. Twenty-five years of research on saccharomyces boulardii trophic effects: Updates and perspectives. Digestive Diseases and Sciences. 2009;54 (1):15-18. DOI: 10.1007/s10620-008-0322-y - 117.
Im E, Pothoulakis C. Progrès récents dans la recherche sur Saccharomyces boulardii . Gastroentérologie Clinique et Biologique. 2010;34 (SUPPL. 1):S62-S70. DOI: 10.1016/S0399-8320(10)70023-3 - 118.
Mulero-Cerezo J, Briz-Redón Á, Serrano-Aroca Á. Saccharomyces cerevisiae Var.Boulardii : Valuable probiotic starter for craft beer production. Applied Sciences. 2019;9 (16):3250. DOI: 10.3390/app9163250 - 119.
Canonico L, Zannini E, Ciani M, Comitini F. Assessment of non-conventional yeasts with potential probiotic for protein-fortified craft beer production. LWT. 2021; 145 (March):111361. DOI: 10.1016/j.lwt.2021.111361 - 120.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature reviews. Gastroenterology & Hepatology. 2014; 11 (8):506-514. DOI: 10.1038/nrgastro.2014.66 - 121.
Iorizzo M, Coppola F, Letizia F, Testa B, Sorrentino E. Role of yeasts in the brewing process: Tradition and innovation. PRO. 2021; 9 (5):839. DOI: 10.3390/pr9050839 - 122.
Garcia-Moreno H, Calvo JR, Maldonado MD. High levels of melatonin generated during the brewing process. Journal of Pineal Research. 2013; 55 (1):26-30. DOI: 10.1111/jpi.12005 - 123.
Maldonado MD, Moreno H, Calvo JR. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clinical Nutrition. 2009; 28 (2):188-191. DOI: 10.1016/j.clnu.2009.02.001 - 124.
Rodriguez-Naranjo MI, Gil-Izquierdo A, Troncoso AM, Cantos-Villar E, Garcia-Parrilla MC. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chemistry. 2011; 126 (4):1608-1613. DOI: 10.1016/j.foodchem.2010.12.038 - 125.
Benot S, Gobema R, Reiter RJ, Garcia-Mauriño S, Osuna C, Guerrero JM. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. Journal of Pineal Research. 1999; 27 (1):59-64. DOI: 10.1111/j.1600-079X.1999.tb00597.x - 126.
Reiter RJ, Tan DX, Maldonado MD. Melatonin as an antioxidant: Physiology versus pharmacology. Journal of Pineal Research. 2005; 39 (2):215-216. DOI: 10.1111/j.1600-079X.2005.00261.x - 127.
Ramakrishna A, Giridhar P, Sankar KU, Ravishankar GA. Melatonin and serotonin profiles in beans of coffea species. Journal of Pineal Research. 2012; 52 (4):470-476. DOI: 10.1111/j.1600-079X.2011.00964.x - 128.
Rodriguez-Naranjo MI, Gil-Izquierdo A, Troncoso AM, Cantos E, Garcia-Parrilla MC. Melatonin: A new bioactive compound in wine. Journal of Food Composition and Analysis. 2011; 24 (4-5):603-608. DOI: 10.1016/j.jfca.2010.12.009 - 129.
Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, et al. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. Journal of Pineal Research. 2012; 52 (2):217-227. DOI: 10.1111/j.1600-079X.2011.00931.x - 130.
Sanchez-Barcelo EJ, Mediavilla MD, Tan DX, Reiter RJ. Clinical uses of melatonin: Evaluation of human trials. Current Medicinal Chemistry. 2010; 17 (19):2070-2095. DOI: 10.2174/092986710791233689 - 131.
Juhnevica-Radenkova K, Moreno DA, Ikase L, Drudze I, Radenkovs V. Naturally occurring melatonin: Sources and possible ways of its biosynthesis. Comprehensive Reviews in Food Science and Food Safety. 2020; 19 (6):4008-4030. DOI: 10.1111/1541-4337.12639 - 132.
Postigo V, Sánchez A, Cabellos JM, Arroyo T. New approaches for the fermentation of beer: Non- Saccharomyces yeasts from wine. Fermentation. 2022;8 (6):280. DOI: 10.3390/fermentation8060280 - 133.
Postigo V, Sanz P, García M, Arroyo T. Impact of non- Saccharomyces wine yeast strains on improving healthy characteristics and the sensory profile of beer in sequential fermentation. Food. 2022;11 (14):2029. DOI: 10.3390/foods11142029 - 134.
Valera MJ, Morcillo-Parra MÁ, Zagórska I, Mas A, Beltran G, Torija MJ. Effects of melatonin and tryptophol addition on fermentations carried out by Saccharomyces cerevisiae and non-Saccharomyces yeast species under different nitrogen conditions. International Journal of Food Microbiology. 2019;289 :174-181. DOI: 10.1016/j.ijfoodmicro.2018.09.013 - 135.
Fernández-Cruz E, Álvarez-Fernández MA, Valero E, Troncoso AM, García-Parrilla MC. Melatonin and derived l-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chemistry. 2017; 217 :431-437. DOI: 10.1016/j.foodchem.2016.08.020 - 136.
Kocadağlı T, Yılmaz C, Gökmen V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chemistry. 2014; 153 :151-156. DOI: 10.1016/j.foodchem.2013.12.036 - 137.
Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta - Bioenerg. 2006; 1757 (5-6):573-589. DOI: 10.1016/j.bbabio.2006.03.012 - 138.
Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. Journal of Pineal Research. 2011; 50 (3):261-266. DOI: 10.1111/j.1600-079X.2010.00835.x - 139.
Que Z, Ma T, Shang Y, Ge Q , Zhang Q , Xu P, et al. Microorganisms: Producers of melatonin in fermented foods and beverages. Journal of Agricultural and Food Chemistry. 2020; 68 (17):4799-4811. DOI: 10.1021/acs.jafc.0c01082 - 140.
Postigo V, García M, Arroyo T. Study of a first approach to the controlled fermentation for lambic beer production. Microorganisms. 2023; 11 (7):1681. DOI: 10.3390/microorganisms11071681 - 141.
Gerhäuser C. Beer constituents as potential cancer chemopreventive agents. European Journal of Cancer. 2005; 41 (13):1941-1954. DOI: 10.1016/j.ejca.2005.04.012 - 142.
Katalinić V, Milos M, Modun D, Musić I, Boban M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chemistry. 2004; 86 (4):593-600. DOI: 10.1016/j.foodchem.2003.10.007 - 143.
Fegredo JA, Meynell R, Lai AKH, Wong MCY, Martin CR, Wiseman H, et al. The antioxidant capacity of beer: Relationships between assays of antioxidant capacity, color and other alcoholic and non-alcoholic beverages. In: Beer in Health and Disease Prevention. Amsterdam, The Netherlands: Elsevier; 2009. pp. 475-481. DOI: 10.1016/B978-0-12-373891-2.00046-8 - 144.
Viana AC, Pimentel TC, do Borges Vale R, Clementino LS, Januario Ferreira ET, Magnani M, et al. American pale ale craft beer: Influence of brewer’s yeast strains on the chemical composition and antioxidant capacity. LWT. 2021; 152 (August):112317. DOI: 10.1016/j.lwt.2021.112317 - 145.
Pai TV, Sawant SY, Ghatak AA, Chaturvedi PA, Gupte AM, Desai NS. Characterization of Indian beers: Chemical composition and antioxidant potential. Journal of Food Science and Technology. 2015; 52 (3):1414-1423. DOI: 10.1007/s13197-013-1152-2 - 146.
Tafulo PAR, Queirós RB, Delerue-Matos CM, Sales MGF. Control and comparison of the antioxidant capacity of beers. Food Research International. 2010; 43 (6):1702-1709. DOI: 10.1016/j.foodres.2010.05.014 - 147.
Munteanu IG, Apetrei C. Analytical methods used in determining antioxidant activity: A review. International Journal of Molecular Sciences. 2021; 22 (7):3380. DOI: 10.3390/ijms22073380