The effectiveness of various methods of activation of
Abstract
The reasons why it is practically impossible to maintain optimal conditions for the development of cultural yeast populations under production conditions are briefly substantiated. A simplified classification of yeast activation methods is given: chemical, physical, and combined. In each of the mentioned groups, the varieties of the proposed technological methods and the modes of their implementation are considered. Experimental data obtained in recent years on the influence of the sound in the audible range (20–20,000 Hz) and light in the visible range on the development of Saccharomyces cerevisiae yeast used in brewing are presented. An attempt made to compare the effectiveness of various ways to improve technological indicators: the increase in the total titer of cells, the percentage of nonviable cells, the accumulation of ethyl alcohol.
Keywords
- Saccharomyces cerevisiae
- fermentation industries
- chemical
- physical and combined activation methods
- effectiveness of activation methods
1. Introduction
A wide range of productions, including food production, are based on the growth of microbial populations, such as yeast, among others. A technologist in a production of this nature should be seeking to culture a yeast population as intensively as possible. The aim is to obtain biomass or metabolism products within the shortest possible period of time with the maximum possible utilization of nutrients within the cultivation medium.
The most logical approach to address this issue would be to maintain ideal conditions for microorganisms, specifically for cultivating yeast of a particular genus and species [1]. However, achieving this objective is often unfeasible for various reasons. One clear example is the production of bottom-fermentated beer. In traditional brewing techniques, brewers cultivate a yeast population at the stage of fermentation under highly unfavorable conditions for this yeast type [2]. The process begins with a high osmotic pressure and ends with a relatively high ethanol concentration. The temperature remains too low, and the physiological condition of the inoculated yeast is suboptimal, especially in the case of pure-culture yeast or late-generations seed yeast. Furthermore, despite the technologist’s effort, the composition of the growth medium can quite often be unbalanced, with low-molecular nitrogenous compounds typically being a limiting factor. In contemporary conditions, the growth medium may also contain abiotic substances such as heavy metals, pesticides, and radionuclides that have entered the process flow from the raw materials, although their content in the latter is restricted and monitored. These factors, either individually or collectively, create suboptimal conditions for yeast population growth. Consequently, the activation of the process through additional processing methods becomes desirable or even necessary. Considerable attention has been directed toward the development of such methods. This chapter aims to provide a concise overview of these techniques (addition of activators of various natures; removal of undesirable components from a nutrient medium; physical processing of inoculum yeast, wort, or fermentation medium; or a combination of these methods) and, if possible, to compare their efficacy. It should be noted that we have intentionally excluded from the discussion any approach that relies based on enhancing the characteristics of yeast through genetic manipulation [3].
A comprehensive classification system [4, 5] for methods aimed at enhancing yeast activity has been proposed. This classification is based on various criteria, including teleological, genetic, and technological aspects. Additionally, it was suggested distinguishing activating agents based on their chemical properties, composition, origin, production stage, and intended final objectives. Nevertheless, we believe that even a complex classification fails to take into account every difference. Therefore, we find it more reasonable to categorize all yeast activation methods, whether proposed or implemented on an industrial scale, into three main groups:
Chemical methods: These involve the addition of specific agents and substances to the food medium, or conversely, the removal of unwanted components from the food medium.
Physical methods: These rely on the utilization of specialized equipment, which does not form a part of the traditional hardware circuit typically used for the given process.
Combination of the above (combined methods).
The information presented in this chapter is organized in accordance with this subdivision.
2. Chemical methods of activation
Agents employed as chemical yeast activators can be categorized based on their intended functions. These categories encompass the following:
Deficient (limiting) compounds: These agents are employed to supplement the medium with essential compounds that may be lacking for yeast growth.
Biostimulators promoting yeast activity.
Extraction of unwanted components: Specific agents are used to remove undesirable components from the medium that could hinder yeast development.
Stressors.
Antibacterial agents: These agents are introduced to create an environment conductive to the growth of the main yeast culture.
Biopolymer degraders: Certain agents are employed to break down biopolymers found in raw and semifinished products.
Adjustments to the medium composition can be made at various stages - from the preparation of the nutrient medium to the stage of cultivation of the yeast population. These additions or agents may vary in terms of their chemical nature, falling into categories such as organic, nonorganic, or mixed (complex). They can be obtained through chemical synthesis, microbiological processes, or have a natural origin. The proposed classification system enables a targeted approach in selecting agents capable of altering yeast culture metabolic activity by adjusting the composition of the culture media [4]. Below, we provide examples of agents belonging to the discussed groups.
In order to fulfill the yeast’s requirements for microelements and vitamins, various specialized preparations are currently in widespread use (“yeast feedings”, “yeast nutrition”). These preparations came in both single-component and multicomponent forms, containing amino acids, vitamins, and minerals: zinc sulfate, “Istex,” “Eastfield,” “Hi Vit,” “Eastfood,” “Alkoten,” “Rhodium Zumesit,” and others [6]. The utilization of these compounds accelerates the start of wort fermentation, prevents fermentation from slowing down and halting, reduces fermentation duration, fosters thorough fermentation of wort sugars, increases yeast growth and resistance to autolysis, and, in some instances, assists in reducing diacetyl content in beer. Typically, these feedings are added to the wort before fermentation. However, it is important to note that the composition of these feedings includes inorganic substances as mineral components (such as diammonium phosphate, manganese and zinc sulfates, and potassium metabisulfite), which are foreign to the food product and considered undesirable from a hygiene standpoint.
A study of the chemical composition of sweet potatoes revealed the presence of a complex of biologically active substances. In order to increase their yield, the use of enzyme preparations became necessary. A biologically active additive, derived from sweet potatoes, was employed as a fermentation activator, exhibiting a positive impact on brewer’s yeast and beer quality [7]. These dietary supplements were added to the wort during the fermentation stage, at concentration of 1% and 2%. Fermentation was carried out at temperatures ranging from 5 to 8° C. The research findings indicate that the use of dietary supplements in beer production positively influences several parameters of young beer, including the degree of fermentation (increased by 5.8%) and ethanol content (elevated by 3.6%). Furthermore, it was observed that the experimental sample, utilizing the sweet potato preparation, outperformed the control sample by 44% in terms of yeast biomass growth. The experiment results demonstrated that the introduction of the sweet potato preparation into the wort accelerates the fermentation process by 2–3 days compared to the control sample. Moreover, the use of this biologically active sweet potato additive during fermentation led to a more significant reduction of diacetyl, with the control and experimental beer samples having diacetyl content of 0.14 and 0.09 mg/dm3, respectively.
The organs of the Far Eastern wild plant
The study examined the results of using
The impact of succinic acid at concentrations ranging from 0.01% to 0.2% on the metabolism of the pure culture of alcohol yeast S. cerevisiae (strain XII) is demonstrated. Changes were established in the consumption of dry substances, yeast biomass accumulation, active and titrated acidity values, CO2 production, concentration of dry substances, and yeast cells titer during alcohol yeast reproduction. After 24 hours of cultivation with the introduction of 0.1% succinic acid, the number of yeast cells increased by 14%, budding cells by 71%, and cells with glycogen by 11.5% compared to the control sample [10].
The results [11] of applying an amino acid-vitamin activator (AVA), a natural stimulant obtained from residual brewer’s yeast cells of the first-generation strain Saflager S-189, were also studied. AVA serves as a source of amino acids, vitamins, and other essential compounds required for cell development. The addition of the activator to a 12% wort in quantities of 0.2 and 0.5% stimulated the growth and accumulation of reserve nutrients by yeast cells. After 5 days of fermentation, the increase in cell count in the control sample was 2.46 million/cm3, whereas in samples with 0.2% AVA, it reached 4.6 million/cm3, and with 0.5%, it was 1.9 million/cm3. After 7 days of fermentation, the percentage of budding cells in the aforementioned samples was 52%, 74%, and 57%, respectively. Additionally, the proportion of cells containing glycogen was 62%, 83%, and 70%, respectively. However, it was observed that an excessive amount of AVA in a dosage of 1.0% somewhat suppressed yeast growth and activity. During testing in a small-scale brewery, the activator was found to be more effective as the generation number of seed yeast increased. When fermenting 12% wort with seed yeast of the third generation, the experimental sample with 0.2% AVA achieved a visible extract of 3.8% and a visible degree of fermentation of 68.3% after 6 days. In contrast, the control sample reached similar values of 4.3% and 64.1%, respectively, but only after 7 days. Similar results were obtained when assessing the effectiveness of using the AVA activator in combination with a preparation derived from the microalgae
A method has been developed to increase the viability of brewing yeast using exclusively preparations derived from
The impact of baking yeast hydrolysate on fermentation and the physiological state of
Yeast extracts also exhibit effectiveness in fermentation high-gravity beer wort with dry substances concentration ranging from 16.2% to 20.3%. The addition of 1% of the extract, on average, reduced the fermentation duration by 1.5 days. Furthermore, it was found to stimulate yeast flocculation ability, fermentation activity, and the number of budding cells [15].
Yeast extracts have proven to be efficient in activating
Complex yeast feeding (CYF) was developed, and its impact on the wort fermentation process and beer quality using dry yeast of the Saflager strain W-34/70 was evaluated. CYF can be added to yeast or to the wort before yeast introduction. CYF consists of a mixture of coarsely ground natural zeolite-containing tuffs and yeast. The use of CYF was found to significantly increase the activity of yeast cell enzymes compared to the control sample: α-glucosidase increased by 1.7–2.7 times, zymase by 1.6–2.4 times, and invertase by 9–30%. The positive impact of CYF on the physiological state of the yeast culture was observed even at a dosage of 0.05 g per 100 cm3 and further improved with higher doses. The number of budding cells increases by 1.5–2.0 times compared to the control sample, and cells containing glycogen increased by 1.6–1.7 times. Additionally, there was a reduction in the concentration of dead cells, ranging from 8 to 30%. In order to study the wort fermentation process, yeast was added to the wort at a rate of 20 million cells/cm3. The fermentation was conducted at a temperature of 12° C for 7–8 days, followed by secondary fermentation lasting 30 days at a temperature of 2–3° C. The following samples were compared: experiment sample 1—fermentation of wort with yeast pre-activated using CYF at a dose of 0.1 g/100 cm3; experiment sample 2—fermentation of wort into which CYF was previously introduced at a rate of 0.05 g/100 cm3, with reactivated yeast without prior treatment; control sample - wort fermented with yeast starter without prior activation and without CYF application. In the experiment samples, the processes of yeast propagation and consumption of extract and amino nitrogen were notably more vigorous. In the experiment samples of young beer, the concentration of yeast cells in the suspended state by the end of fermentation was significantly lower than in the control sample. This is expected to have a positive impact on the beer clarification process during secondary fermentation and filtration and potentially enhance the colloidal stability of the finished drink. In the samples initially enriched with the yeast feeding, several favorable outcomes were observed. These include a higher degree of attenuation and increased volume fraction of alcohol, a reduced content of polyphenolic compounds, and a lower presence of high-molecular-weight proteins. In addition, in the samples where CYF was applied, the beer exhibited a harmonious and full flavor profile, accompanied by a gentle hop bitterness [18].
Many small beer production enterprises employ active dry brewing yeast for pitching the wort, but in most cases, the viability of the yeast is reduced. Therefore, yeast activation is necessary before fermentation. The effectiveness of increasing the activity of brewing dry yeast [19] Saflager W-34/70 was established through the use of preparations of natural organic and inorganic origin. Yeast activation involved the utilization of antler-containing raw materials in the form of a dry preparation as a source of various biologically active organic substances. The antler-containing preparation composes lipids, nitrogenous compounds, calcium, phosphorus, and other components. The lipid complex includes phospholipids; mono-, di-, and triglycerides; sterols; fatty acids; and sterol esters. The medium for processing the yeast culture, as well as for fermentation, was industrial hopped beer wort with an extra activity of 12%. Biochemical parameters of the culture were assessed both in the initial yeast and during its activation, focusing on the activity of α-glucosidase (maltase), invertase (β-fructofuronosidase), and the zymase complex. The results indicate that adding the antler preparation to the yeast starter during its preparation for wort fermentation leads to a significant increase in the activity of the yeast cell enzymes under investigation when compared to the control sample: α-glucosidase (maltase) by 2.0–6.8 times, zymase by 1.5–2.5 times, and invertase by 4.5–6.2 times. The introduction of the antler-containing preparation, either separately or in combination with CYF, during both yeast preparation for fermentation and directly into the wort before yeast pitching, ensures high yeast viability and high biochemical and physiological activity of the culture. The effectiveness of these feedings is further enhanced when applied under aeration conditions during the dry yeast preparation stage for wort fermentation. Recommended doses of the antlers-containing preparation (% by volume of the medium) are (0.10–0.75) × 10−3, and CYF should be in the range of 0.05–0.075.
The study presents the results demonstrating the positive impact of multifunctional components of geothermal water in the yeast culture medium on ethanol biosynthesis [20]. It was observed that an increase in carbohydrate content and the fermentation duration in a nutrient medium with geothermal water resulted in a 20% increase in alcohol synthesis compared to the previously established technology. Morphophysiological parameters of the cells further validate the active state of the experimental yeast.
The positive effects of silicon on the carbohydrate and nitrogen metabolism of
The research also explored the potential for activating dry brewing yeast using an energy exchange regulator: a mixture of organic acids from the Krebs cycle, including succinic, malic, fumaric, citric, and oxalic acid in a 1:1:1:1:1 ratio, at concentrations ranging from 10−8 to 10−10 mol/dm3). The influence of various concentrations of this acid mixture was evaluated, demonstrating a positive effect on yeast cell enzymes activity and the physiological state of the yeast culture due to increased cell membranes permeability. The use of yeast activated in the acids solution at a concentration of 1x10−10 mol/dm3 positively impacted the beer wort fermentation process, as evidenced by production tests. This resulted in a reduction of the fermentation process duration by 1 day and improved physiological parameters of the yeast culture compared to the control sample without special treatment. Additionally, the quality indicators and organoleptic characteristics of the beer met the standard requirements [22].
The utilization of milk whey as an activator of brewer’s yeast was investigated [23]. The efficacy of yeast treatment through exposure in a medium consisting of whey, as well as a mixture of whey and beer wort, at a temperature of 4–6° C, was also defined. During the exposure, the number of budding cells was shown to increase twofold, cells with glycogen by 1.3 times, the zymase activity of yeast by over 2-fold, and maltase activity by 21–42.4%. This led to a reduction in the main fermentation duration by 1 day. At the same time, in experimental beer samples, higher degree of attenuation and alcohol content were achieved, with reduced diacetyl formation. An additional effect is achieved through a joint rotary-pulsation process, as described in the section on combined processing methods.
Furthermore, the optimization of the nutrient medium composition for yeast cultivation can be achieved not only by introducing components with a positive impact but also by eliminating those with a negative influence [24]. This task can be addressed, in part, by employing sorbents.
A proposal is made to employed oyster mushroom mycelium (
Biosorbent “OD-2,” derived from initiated autolysis of sedimentary brewer’s yeast, when added to the wort before pitching at dosages ranging from 0.1% to 0.5% by weight/vol., led to a significant increase in the concentration of ethanol in young beer by 17–46% compared to the control sample [26].
While baking may not be directly related to the fermentation industry, one of the crucial stages in bread production involves fermenting dough using
Biologically active components and acids found in fruit and berry extracts can either inhibit or activate
A method [28] has been proposed for the preliminary activation of baking yeast to enhance their resistance to acid stress. It was observed that yeast treated with solutions of hydrogen peroxide in concentrations ranging from 0.5 to 3.0 mM retained higher fermentation activity when subjected to stressful conditions (such as a 2% lactic acid solution) compared to the control sample.
The effectiveness of several chemical activation methods was compared by increasing in the concentration of ethyl alcohol and decreasing in the content of the apparent extract (Figure 1).
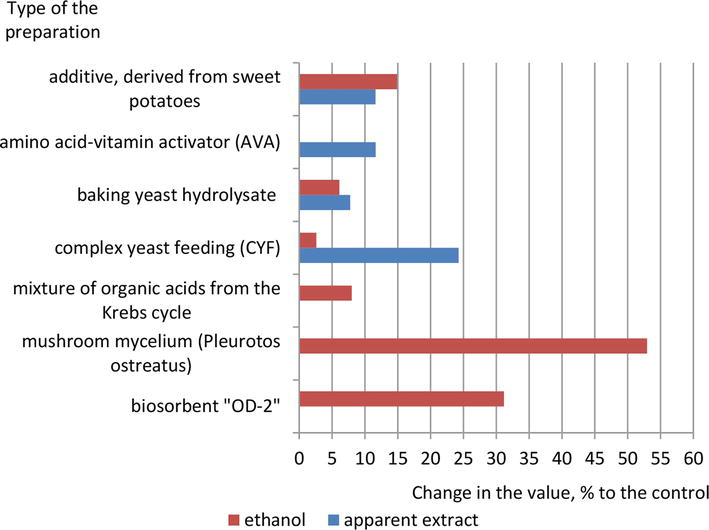
Figure 1.
Increase in ethyl alcohol content and decrease in apparent extract (%) in test samples of finished beer compared to similar values in control samples.
3. Physical activation methods
Activation methods in this group are primarily based on direct or indirect wave/field effects, with the exception of the use of mechanical vibrations (rotary pulsation action). This will be discussed below when examining the combined yeast processing method [23].
Based on the analysis of the literature, it can be concluded that the greatest interest is in the study of the effectiveness of ultrasonic treatment. Examples of such an approach are provided at the beginning of this section.
3.1 Ultrasound and audible range sound processing
Ultrasonic treatment is widely employed for the destruction or inactivation of treated objects, including yeast cells [29]. However, altering the parameters of such treatment can yield the opposite effect and activate yeast cell population, as confirmed by the data presented below.
The same physical and mechanical effects utilized in sonochemistry, such as strong shear forces, particle fragmentation, and increased mass and heat transfer, are also applied in the food industry. Powerful ultrasound is employed to influence the development of living cells, aiming to boost sterilization efficiency, impact enzyme activity [30], and enhance the overall quality of food products [31].
Fermentation processes involve enzymes produced by microorganisms’ cells to carry out chemical transformations. Ultrasound can be employed in such processes for monitoring the course of fermentation or influencing its. High-frequency ultrasound (>2 mHz) is well-known as a tool for measuring changes in chemical composition during the fermentation process, offering real-time insights into the reaction’s progress. Low-frequency ultrasound (20–50 kHz) can affect the fermentation process by improving mass transfer and cell permeability. This leads to increased process efficiency and productivity. Additionally, this type of ultrasound can be used to eliminate microorganisms that may disrupt the fermentation process [32].
The activation method of Safale T-58 brewer’s top fermentation yeast
In their later work, the same group of authors [34] researched the activating and disintegrating effect of ultrasound at a frequency of 44 kHz and intensity of 1.0 W/cm2 on brewer’s yeast. It was found that a 10-minute ultrasonic treatment of yeast is sufficient to achieve a stimulating effect. Further ultrasound treatment is impractical, since the percentage of dead cells in the yeast suspension exceeds the permissible level (more than 10%). The experiment showed that two-minute ultrasound treatment improved the physiological activity of the seed yeast. The beer obtained using this technique had a higher quality.
The effect of ultrasound on the results of fermentation of beer wort from Korean six-row barley was also studied. Beer samples were processed in an ultrasonic bath for 4 days during the primary fermentation. The ultrasound frequency was 40 kHz, and the input power was 120, 160 and 200 watts. The ultrasound treatment was carried out for 2, 6, and 12 hours for each input power. The physicochemical and organoleptic properties were measured, and the quality of the beer was evaluated. Ultrasound with a power of 160 W increased the yield of ethanol by 13.18% [35].
The study examined the impact of ultrasound at a frequency of 22 kHz and an intensity of 1.0 W/cm2 on the outcomes of water–heat treatment of wort derived from winter triticale grain [36]. It was established that ultrasonic exposure results in increased activity of grain α-amylases enzymes. The wort obtained was used for cultivating seed yeast with simultaneous ultrasound treatment. It was also observed that such treatment leads to an augmentation of biomass and intensification of the seed yeast growth process. The processed wort was then fermented with activated yeast to produce alcohol with a yield of 67.3 dal/t of conditional starch and a reduced content of toxic impurities.
The potential for increasing ethanol yield in alcohol production through the treatment of
The study investigated the impact of ultrasonic treatment with different operating modes and different frequencies on the accumulation of cells and metabolites of
Another study examined the effect of ultrasound treatment on the growth of
The effect of low-intensity ultrasound, varying in frequencies, processing time, and ultrasound power, on
It was also observed that the fermentation process could be activated due to the indirect impact of ultrasound: it does not process the yeast biomass at one stage or another, but the fermentation medium before pitching with yeast.
Consequently, the influence of ultrasound and thermal pretreatment on ethanol yield from cassava chips was investigated. Cassava suspensions were treated with ultrasound for 10 and 30 seconds at amplitudes of 80, 160, and 320 microns/s, corresponding to low, medium, and high power levels, respectively. Processed and untreated (control) ultrasound samples were then subjected to simultaneous liquefaction-saccharification and fermentation. Based on the efficiency of converting cassava starch into ethanol, it was concluded that higher ethanol yields are directly related to the duration of ultrasound treatment, rather than to its power level. The ethanol yield from the ultrasound-treated sample was 2.7 times higher than that from the control sample. The fermentation rate was also significantly higher, with the fermentation duration being shortened by nearly 24 hours for samples treated with ultrasound to achieve the same ethanol yield as in control samples. Thus, ultrasound pretreatment increased both the total ethanol yield and the fermentation rate. Compared to the heat-treated samples, the ethanol yield in ultrasound-treated samples was almost 29% higher. The combined heat and ultrasound treatment did not significantly affect the overall ethanol yield from cassava chips. Ultrasound was also preferred over preheat treatment due to its lower energy requirements [41].
Similar findings were reported in a study [42] where pretreatment with ultrasound during the liquefaction stage was tested in the subsequent combined saccharification/fermentation of corn flour using
Furthermore, research conducted at our university has focused on the acoustic effects on raw materials and intermediates in brewing production over several years.
3.2 Exposure to pulsed electromagnetic field
There is an opinion [45] that a pulsed electromagnetic field (PEMF) has a positive impact on yeast metabolism, cell biomass growth, and ethanol production, especially with low-frequency electromagnetic waves, which are known to have biological effects. An electromagnetic pulsed generator was used to investigate the influence of electromagnetic pulsed fields on yeast cell reproduction and ethanol accumulation in a fermenting medium, with a focus on intensifying the technological process. The fermentation of wort was carried out in a two-stage mode with treatment using a pulsed electromagnetic field (frequency - 4 Hz, power - 1 μT). The study employed beer wort with a density of 15–35% (15% unmalted raw material) and
The impact of extremely low-frequency magnetic fields on ethanol accumulation by
3.3 Electron-ion processing (EIP)
The potential for activating brewer’s yeast through EIP to enhance membrane permeability and increase nutrient and oxygen availability for cells has been confirmed. These activated cells were found to maintain their viability for 3–5 cycles after EIP, attributed to the activation of the permease system. A correlation was established between the EIP modes and glycogen content of yeast cells. When using yeast that has undergone EIP, the main fermentation cycle of beer can be shortened by 15–40%. These activated cells facilitate vigorous wort fermentation without the need for additional fermentation activators. Implementing the proposed EIP method for brewer’s yeast activation prior to its introduction into the fermentation apparatus results in beer with a fermentation degree exceeding 80%, enhancing the stability of the final product. EIP-treated yeast can be utilized for 10–11 generations, extending their operational lifespan by 1.5 times compared to untreated yeast. This also reduces the cost associated with maintaining a pure yeast culture. Production tests confirmed the effectiveness of EIP, showing that processing low-quality yeast led to a 10–60% increase in viability and a 3–8% boost in the final fermentation degree while maintaining high beer quality standards. The beer produced using EIP-treated yeast met all standards and exhibited superior physicochemical and organoleptic properties compared to beer samples produced using traditional methods [47, 48].
Glushhenko [49] provides a description of the equipment used for electron-ion processing of yeast. The impact of electron-ion treatment on yeast cells viability was investigated, revealing a reduction in nonviable yeast cells numbers The most pronounced effect was observed when yeast was processed in a “yeast + wort” medium. The efficiency of electron-ion processing is contingent on the intensity of the nonuniform electric field and the exposure time. Implementing electron-ion processing results in a significant enhancement of the yeast’s physiological state.
3.4 Processing with visible light and laser radiation in the infrared range
Another approach for yeast-saccharomycete activation is based on exposure to visible spectrum light wavelengths.
The earliest publication we found on this subject dates back to 1984 [50]. This doctoral dissertation research demonstrates that exposure to shortwave optical radiation (410–520 nm) with an intensity of 0.12–0.20 W/m2 leads to a substantial activation of metabolite transformations within the glycolytic system. Visible light exposure triggers the decarboxylation of pyruvate, facilitating the generation of a substrate for the operation of the di- and tricarboxylic acid cycle, thus alleviating substrate deficiencies in the Krebs cycle. In addition, it increases the activity of alcohol dehydrogenase, the final enzyme of alcoholic fermentation, and some respiratory enzymes. It also enhances the activity of isocitrate dehydrogenase, succinate dehydrogenase, aldehyde dehydrogenase, and the pyruvate dehydrogenase complex. Research also revealed that exposure to 410 nm light wavelength increases the rate of protein biosynthesis by 1.6 times compared to the control sample. Furthermore, an optimal parameter for applying optical radiation to influence the technological characteristics of beer seeding yeast was established. Exposure to 410 nm light at an intensity of 0.12–0.20 W/m2 for 6 hours prior to inoculation into the wort was found to boost yeast fermentation activity by 20–25%. This lead to a reduction of 1 day in the main fermentation duration without compromising the quality of the final product. Additionally, beer produced with photoactivated yeast displayed enhanced resistance to colloidal turbidity, attributed to a significant reduction in the concentration of high-molecular-weight protein substances compared to the control sample. Regrettably, in our view, this research, although promising, did not receive further attention at that time and was only resumed three decades later.
Thus Kobelev and Bagaeva [51],
For several years, our university has also been engaged in researching the effects of monochromatic light exposure within the visible spectrum, as well as near-ultraviolet and infrared spectra, on brewer’s yeast. Previously, a positive impact of such treatment on top-fermentation yeast [52] was demonstrated. This research is currently ongoing, utilizing bottom fermentation
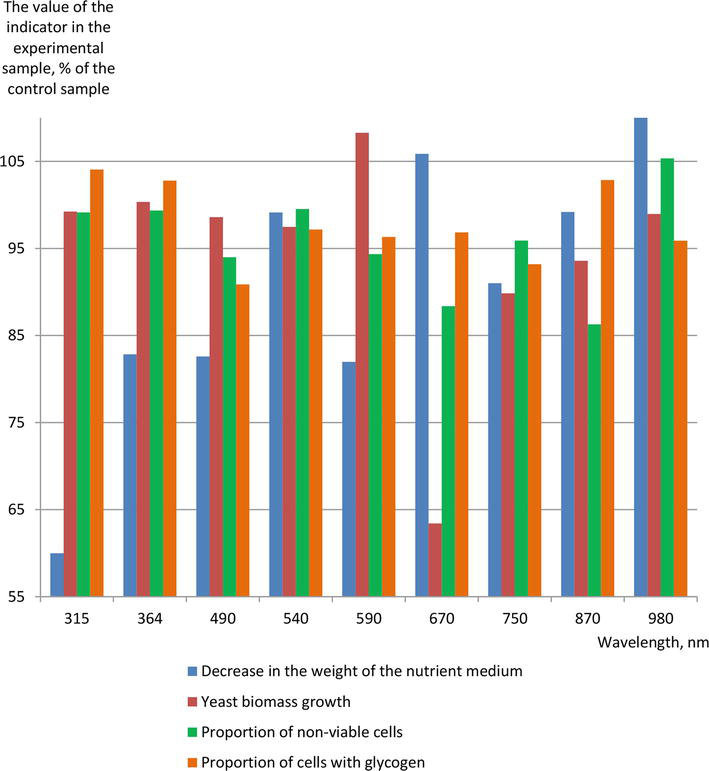
Figure 2.
The effect of fixed wavelength light treatment on the indicators of bottom fermenting brewer’s yeast population.
Based on this data, it can be concluded that light with such wavelengths has a multidirectional effect on the state of the yeast population. The processing effect is less pronounced than most of the activation methods described earlier. Nevertheless, light treatment with a wavelength of 650 nm provided a higher degree of utilization of nutrients, a smaller proportion of nonviable cells, and a significantly lower increase in biomass than in the control sample. This can be an advantage in the brewing industry, as it helps in reducing the quantity surplus yeast, which, in compliance with environmental regulations, must be disposed of in an environmentally responsible manner. We have decided to continue the research, in order to consider the possibility of optimizing the processing parameters.
The effectiveness of low-intensity laser radiation (LILR) in the infrared range on brewer’s yeast of top fermentation was evaluated as a means of intensification of fermentation processes [53]. A treatment duration equal to 2 minutes was chosen as rational. The impact of radiation with pulse frequencies was tested at 300, 600, 760, 1200, 1500, and 3000 Hz. The object of treatment was
3.5 Other physical methods of yeast activation
The effect of a magnetic field on alcoholic fermentation using
However, an opposite viewpoint was also published, suggesting that constant or variable magnetic fields have no influence on cellular processes in
The impact of electric current on yeast characteristics has also been studied. When a direct current of 10 mA or alternating current of 100 mA was applied to the culture medium, a significant increase in the rate of cell growth and accumulation of ethyl alcohol was observed. The content of higher alcohols, esters, and organic acids in culture media treated with direct and alternating current differed from that in the untreated sample. Several compounds, such as acetaldehyde and acetic acid, were formed from ethanol as a result of the electrode reaction [56].
Pulsating electromagnetic-induced currents (PEMIC, PEMF, PMF), simple alternating currents, and direct currents have been explored for their effects on cells, tissues, and organisms, stimulating membrane permeability and various metabolic processes [57].
4. Combined processing methods
A method for activating brewer’s yeast was investigated [5] and patented [58]. This method provides for the treatment of a suspension of yeast cells with an activation medium (whey, beer wort, or a mixture of whey and beer wort) in a ratio of 1:0.5 through acoustic exposure to low-frequency vibrations (20–2·104 Hz) in a rotary pulsating machine for 2 minutes. The rotor speed and the inter-cylinder clearance were 2000 min−1 (209.33 c−1) and 0.2–0.3 mm, respectively. The processing parameters were selected to avoid an excessive number of dead cells, which should be kept under 10%. This processing method significantly improved the quality of brewer’s yeast. Compared to the control sample, the total concentration of cells increased by 3.9–8.1%, the percentage of dead cells decreased by 1.7–2.3 times, and the number of budding cells increased almost twofold. After 7 days of fermentation of 11% wort in the control sample, the actual extract was 4.9%, while in the experimental samples, similar concentrations of dry substances (4.6–4.5) were achieved after 5–6 days of the main fermentation. The volume fraction of ethyl alcohol in the finished beer of the control sample was 4.58%, whereas in the experimental variants, it ranged from 5.27 to 5.37% of the volume, with the actual extract at 3.5% and 2.3–2.4%, respectively.
A patent [59] was obtained for a yeast activation method designed for the alcohol industry. In this method, a suspension of alcoholic yeast in the production grain wort is subjected to an acoustic field with an oscillation frequency of 22 kHz and an oscillation intensity of 1.0 W/cm2 for 3.5–4.5 minutes at a temperature of 28–34°C. A fraction of 5–10% of the total volume of the processed yeast suspension is then selected and undergoes a secondary treatment with an acoustic field at an oscillation frequency of 22 kHz and an oscillation intensity of 1.0 W/cm2 for 35–45 minutes at a temperature of 48–52°C. This secondary treatment is aimed at destroying yeast shells and obtaining yeast extract. The resulting extract is subsequently mixed with the suspension of processed yeast. This process leads to a significant increase in yeast biomass by 2–3 times, faster start of wort fermentation at the first day of fermentation, and a reduced fermentation duration by 10–12 hours.
5. Comparing the effectiveness of various methods of activation of Saccharomyces cerevisiae yeast
From the information provided, it is evident that various methods are employed for different types of yeast, and their effectiveness is evaluated using diverse criteria. Nevertheless, we have chosen to conduct a comparative analysis of the effectiveness of selected methods by consolidating the data from in the literature into a single table (Table 1).
| Activation method | The field of yeast use | Increasing the degree of fermentation, % | Increase in ethanol concentration, % | Increase in cell titer/yeast biomass growth, % | Shortening the duration of the stage | Increase in the proportion of budding cells, % | Increase in the proportion of cells with glycogen, % | Reduction of the proportion of non-viable cells | A source |
|---|---|---|---|---|---|---|---|---|---|
| Chemical | |||||||||
| Sweet potato supplement | Brewing | 5.8 | 3.6 in green beer | 44 | Lagering - for 2–3 days | 7 | |||
| Succinic acid | Alcohol production | 14* | 71* | 11.5* | 10 | ||||
| Amino acid-vitamin activator 0.2% | Brewing | 6.6 | 87 | The main fermentation - for 1 day | 42 | 33.9 | 11 | ||
| Complex yeast feeding | Brewing | 50–100 | 60–70 | 8–30 | 18 | ||||
| Milk whey | Brewing | The main fermentation - for 1 day | 100 | 30 | 23 | ||||
| Physical | |||||||||
| Ultrasound 23 kHz | Alcohol production | 19.3 | 38 | ||||||
| Sound at frequency of 2765 Hz | Brewing | 66 | 5 | 44 | |||||
| Light with a wavelength of 410 nm | Brewing | 20–25 | The main fermentation - for 1 day | 50 | |||||
| Low-intensity laser radiation 600 Hz | Brewing | The main fermentation - for 2 days | 42 | 75 | 53 | ||||
| Magnetic field | 240 | 150 | 54 | ||||||
| Combined | |||||||||
| Rotary pulsation treatment + whey | Brewing | 15–17 | 3.9–8.1 | The main fermentation - for 1-2 days | 100 | 70–130 | 58 | ||
| Ultrasound + yeast extract | Alcohol production | 100–200 | Fermentation of alcoholic wort - for 10–12 hours | 59 | |||||
Table 1.
After 24 hours of cultivation.
6. Conclusion
It can be seen that the proposed methods for improving the technological characteristics of
References
- 1.
Galbraith S, Bhatia H, Liu H, Yoon S. Media formulation optimization: Current and future opportunities. Current Opinion in Chemical Engineering. 2018; 22 :42-47. DOI: 10.1016/j.coche.2018.08.004 - 2.
D’amore T. Cambridge prize lecture improving yeast fermentation performance. Journal of The Institute of Brewing. 1992; 98 :375-382 - 3.
Dequin S. The potential of genetic engineering for improving brewing, wine-making and baking yeasts. Applied Microbiology and Biotechnology. 2001; 56 :577-588. DOI: 10.1007/s002530100700 - 4.
Permjakova LV. Классификация стимуляторов жизненной активности дрожжей (classification of preparations to promote yeast vital activity). Техника и технология пищевых производств. 2016; 42 (3):46-55. (in Russ.) - 5.
Pomozova VA, Permjakova LV, Safonova EA, Artemasov VV. Активация пивных дрожжей (Brewer’s yeast activation). Пиво и напитки. 2002; 2 :26-27. (In Russ.) - 6.
Meledina TV. Сырье и вспомогательные материалы в пивоварении (Raw materials and auxiliary materials in brewing). Санкт-Петербург: Издательство «Профессия»; 2003. 304 с. (In Russ.) - 7.
Gagieva LC, Cugkiev BG, Dzantieva LB, Makiev ON. Технологические аспекты использования растительного сырья в качестве активаторов бродильных процессов (Aspects of technology use vegetable raw materials as activators of fermentation processes). Пиво и напитки. 2011; 2 :28-29. (in Russ.) - 8.
Palagina MV, Drekko KA, Plehova NG. Влияние биостимуляторов дальневосточных растений на физиологическую активность пивоваренных дрожжей (Influence of biostimulators Far East plants on the physiological activity of brewing yeast). Пиво и напитки. 2011; 2 :33-35. (in Russ.) - 9.
Grebenchikov VA, Gernet MV. Использование активаторов дрожжей при производстве кваса (the use of activators of yeast in the production of kvass). Пиво и напитки. 2003; 3 :34-37. (in Russ.) - 10.
Krikunova LN, Rjabova SM, Peschanskaja VA, Urusova LM. Влияние янтарной кислоты на метаболизм дрожжей Saccharomyces cerevisiae . (effect of succinic acid on the metabolism of the yeastSaccharomyces cerevisiae ). Пиво и напитки. 2015;1 :36-38. (in Russ.) - 11.
Hnykin AM, Sadova AI, Timaev AM. Разработка метода активации сухих пивоваренных дрожжей для заводов малой мощности (development of a method of activation of dry brewing yeast for small power plants). Пиво и напитки. 2012; 2 :12-16. (in Russ.) - 12.
Gernet MV, Gribkova IN, Borisenko OA. Исследование возможности активации дрожжей при приготовлении ферментированных напитков (the yeast activation possibility study in the fermented beverages preparation). Пищевая промышленность. 2020; 8 :56-59. (In Russ.). DOI: 10.24411/0235-2486-2020-10087 - 13.
Bidihova MJ, Lavrova VL, Gernet AM, Gruzdeva AE. Повышение жизнеспособности пивоваренных дрожжей c использованием Спирулины платенсис (Increasing the viability of brewing yeast using Spirulina platensis). Пиво и напитки. 2002; 6 :10-12. (In Russ.) - 14.
Kiseleva IV, Puchkova EA, Kobelev KV, Gernet MV, Lavrova VL. Способ интенсификации процесса сбраживания сусла (Method for intensifying the wort fermentation process). Пиво и напитки. 2004; 2 :38-39. (In Russ.) - 15.
Gorelov SS. Влияние экстракта дрожжей на процесс сбраживания плотного пивного сусла (the influence of yeast extract on the fermentation process of high gravity beer wort). Пиво и напитки. 2008; 5 :38-40. (In Russ.) - 16.
Krechetnikova AN, Il’jashenko NG, Shaburova LN, Bodrova OJ. Активирующий эффект воздействия дрожжевого экстракта на клетки Saccharomyces cerevisiae (activating effect of yeast extract onSaccharomyces cerevisia e cells). Производство спирта и ликероводочных изделий. 2006;3 :29-30. (In Russ.) - 17.
Gorelov SS, Il’jashenko NG, Krechetnikova AN, Beteva EA, Gernet MV. Влияние дрожжевых экстрактов на процесс сбраживания сусла из крахмалсодержащего сырья (the influence of yeast extracts on the process of fermentation of wort from starch-containing raw materials). Производство спирта и ликероводочных изделий. 2005; 1 :22-23. (In Russ.) - 18.
Permjakova LV, Pomozova VA, Apenova DS, Russkih RV. Активация сухих пивных дрожжей с помощью комплексной дрожжевой подкормки (activation of dry brewer’s yeast using complex yeast feeding). Пиво и напитки. 2012; 1 :18-21. (in Russ.) - 19.
Permjakova LV, Pomozova VA, Pavlov AA, Horunzhina SI. Применение новых видов пищевых подкормок для дрожжей в производстве пива (application of new types of nutritional supplements for yeast in beer production). Техника и технология пищевых производств. 2013; 2 (29):46-52. (In Russ.) - 20.
JeA H, Abramov SA, Kotenko SC, JeA I. Влияние стимулятора биосинтеза этанола – геотермальной воды на морфологические особенности дрожжей Saccharomyces cerevisiae в различных условиях культивирования (the influence of the stimulator of ethanol biosynthesis - geothermal water on the morphological characteristics of the yeastSaccharomyces cerevisiae under various cultivation conditions). Хранение и переработка сельхозсырья. 2010;8 :44-46. (In Russ.) - 21.
Abramov SA, Vlasova OK, Kotenko SC. Морфофизиологические свойства дрожжей рода saccharomyces на кремнийсодержащих средах (morphophysiological properties of yeasts of the genussaccharomyces on silicon-containing media). Виноделие и виноградарство. 2008;4 :14-15. (In Russ.) - 22.
Pavlov AA, Pomozova VA, Permjakova LV, Vereshhagin AL. Активация пивных дрожжей смесью органических кислот (activation of brewer’s yeast with a mixture of organic acids). Современные проблемы науки и образования. 2013; 5 :127-134. (In Russ.) - 23.
Kozlov SG. Исследование и разработка способов активации дрожжей с использованием молочной сыворотки (Research and development of methods for activating yeast using whey) [thesis]. Кемерово: Кемеровский технологический институт пищевой промышленности; 2002. (In Russ.) - 24.
Permjakova LV. Регулирование биотехнологических свойств пивных дрожжей путем корректировки состава питательной среды : монография (Regulation of biotechnological properties of brewer’s yeast by adjusting the composition of the nutrient medium: monograph). Кемерово: КемГУ; 2017 248 с. (In Russ.) - 25.
Druzhinina ES, Gernet MV, Kolesnikova VF. Интенсификация брожения с использованием биомассы гриба Pleurotos ostreatus (вешенка) (Intensification of fermentation using biomass of the fungusPleurotos ostreatus (oyster mushroom)). Пиво и напитки. 2003;1 :26-28 (in Russ.) - 26.
Karpenko DV. Разработка технологии получения биосрбентов на основе осадочных пивных дрожжей и их применение для производства пива, этилового спирта и других пищевых продуктов (Development of Technology for Producing Biosrbents Based on Sedimentary brewer’s Yeast and their Use for the Production of Beer, Ethyl Alcohol and Other Food Products) [Thesis]. Москва: Московский государственный университет пищевых производств; 2005. (In Russ.) - 27.
Kuz’mina SS, Kozubaeva LA, Egorova EJ, Kulushtaeva BM, Smol’nikova FH. Активность дрожжей Saccharomyces cerevisiae в условиях стресс-провокации плодово-ягодными экстрактами (Effect of berry extracts onSaccharomyces cerevisiae yeast). Техника и технология пищевых производств. 2021;51 (4):819-831. DOI: 10.21603/2074-9414-2021-4-819-831. (In Russ.) - 28.
Jamashev TA, Reshetnik OA. Влияние предварительной активации дрожжей пероксидом водорода на их адаптацию к осмотическому стрессу (Effect of preliminary activation of yeast with hydrogen peroxide on their adaptation to osmotic stress). Вестник Казанского технологического университета. 2010; 11 :312-316. (In Russ.) - 29.
Tsukamoto I, Constantinoiu E, Furuta M, Nishimura R, Maeda Y. Inactivation effect of sonication and chlorination on Saccharomyces cerevisiae . Calorimetric analysis. Ultrasonic Sonochemistry. 2004;11 (3-4):167-172. DOI: 10.1016/j.ultsonch.2004.01.014 - 30.
Mason TJ, Paniwnyk L, Lorimer JP. The uses of ultrasound in food technology. Ultrasonics Sonochemistry. 1996; 3 (3):S253-S260. DOI: 10.1016/S1350-4177(96)00034-X - 31.
Chandrapala J, Oliver C, Kentish S, Ashok-kumar M. Ultrasonics in food processing – Food quality assurance and food safety. Food Science and Technology. 2012; 26 (2):88-98. DOI: 10.1016/j.tifs.2012.01.010 - 32.
Shikha Ojha K, Mason TJ, O’Donnell CP, Kerry JP, Tiwari BK. Ultrasound technology for food fermentation applications. Ultrasonics Sonochemistry. 2017; 34 :410-417. DOI: 10.1016/j.ultsonch.2016.06.001 - 33.
Kaluzhina OJ, Jakovleva KS, Kashapova RA, Chernenkov EN, Chernenkova AA, Bodrov AJ. Влияние ультразвука на пивоваренные дрожжи (the effect of ultrasound on brewing yeast). Вестник ВГУИТ. 2020; 82 :103-109. (In Russ.). DOI: 10.20914/2310-1202-2020-1-103-109 - 34.
Kalugina O, Nafikova A, Chernenkov E, Leonova S, Chernenkova A, Badamshina E, et al. Application of ultrasound for enhancing fermentation rates in brewing technology. Acta Scientiarum Polonorum, Technologia Alimentaria. 2021; 20 (3):301-312. DOI: 10.17306/J.AFS.2021.0950 - 35.
Choi EJ, Ahn H, Kim M, Han H, Kim WJ. Effect of ultrasonication on fermentation kinetics of beer using six-row barley cultivated in Korea. Journal of The Institute of Brewing. 2015; 121 (4):510-517. DOI: 10.1002/jib.262 - 36.
Kaluzhina OY, Krechetnikova AN, Smirnova IV, Gusev AN, Nafikova AR. Alcohol technology intensification with the application of ultrasound. Bulgarian Journal of Agricultural Science. 2019; 19 (Suppl.2):98-104 - 37.
Yang Y, Ren W, Xu H, Cheng L, Dapaah M, He R, et al. Incorporating transcriptomic-metabolomic analysis reveal the effect of ultrasound on ethanol production in Saccharomyces cerevisiae . Ultrasonics Sonochemistry. 2021;79 :105791. DOI: 10.1016/j.ultsonch.2021.105791 - 38.
Zhang Z, Xiong F, Wang Y, Dai C, Xing Z, Dabbour M, et al. Fermentation of Saccharomyces cerevisiae in a one liter flask coupled with an external circulation ultrasonic irradiation slot: Influence of ultrasonic mode and frequency on the bacterial growth and metabolism yield. Ultrasonics Sonochemistry. 2019;54 :39-47. DOI: 10.1016/j.ultsonch.2019.02.017 - 39.
Soro AB, Oliveira M, O’Donnell CP, Tiwari BK. Ultrasound assisted modulation of yeast growth and inactivation kinetics. Ultrasonics Sonochemistry. 2021; 80 :105819. DOI: 10.1016/j.ultsonch.2021.105819 - 40.
Dai C, Xiong F, He R, Zhang W, Ma H. Effects of low-intensity ultrasound on the growth, cell membrane permeability and ethanol tolerance of Saccharomyces cerevisiae . Ultrasonics Sonochemistry. 2017;36 :191-197. DOI: 10.1016/j.ultsonch.2016.11.035 - 41.
Nitayavardhana S, Shrestha P, Rasmussen ML, Lamsal BP, van Leeuwen J, Khanal SK. Ultrasound improved ethanol fermentation from cassava chips in cassava-based ethanol plants. Bioresource Technology. 2010; 101 (8):2741-2747. DOI: 10.1016/j.biortech.2009.10.075 - 42.
Nikolić S, Mojović L, Rakin M, Pejin D, Pejin J. Ultrasound-assisted production of bioethanol by simultaneous saccharification and fermentation of corn meal. Food Chemistry. 2010; 122 (1):216-222. DOI: 10.1016/j.foodchem.2010.02.063 - 43.
Karpenko DV, Gernet MV, Krjukova EV, Gribkova IN, Nurmukhanbetova DE, Assembayeva EK. Acoustic vibration effect on genus saccharomyces yeast population development. News of the Academy of Sciences of the Republic of Kazakhstan. Series of Geology and Technical Sciences. 2019;4 (436):103-112. DOI: 10.32014/2019.2518-170X.103 - 44.
Karpenko DV. Определение рациональных параметров акустической обработки с целью активации пивных дрожжей. Здоровье, питание и биотехнологии (Determination of rational parameters of acoustic processing in order to activate brewer’s yeast). 2020; 2 (1):140-152. DOI: 10.36107/hfb.2020.i1.s290. (In Russ.) - 45.
Mamarasulov BD, Nasirova OA, Mirzarahmetova DT. Интенсификация процесса сбраживания пивного сусла (intensification of the fermentation process of beer wort). Пиво и напитки. 2017; 5 :24-27. (In Russ.) - 46.
Perez VH, Reyes AF, Justo OR, Alvarez DC, Alegre RM. Bioreactor coupled with electromagnetic field generator: Effects of extremely low frequency electromagnetic fields on ethanol production by Saccharomyces cerevisiae . Biotechnology Progress. 2007;23 (5):1091-1094. DOI: 10.1021/bp070078k - 47.
Osipova MV. Интенсификация процесса брожения методом электронно-ионной обработки (ЭИО) пивных дрожжей (Intensification of the Fermentation Process by Electron-Ion Processing (EIP) of brewer’s Yeast) [Thesis]. Москва: Новгородский Государственный Университет имени Ярослава Мудрого; 2007. (In Russ.) - 48.
Osipova MV, Glushhenko LF. Интенсификация брожения пива посредством электронно-ионной обработки (ЭИО) пивных дрожжей (intensification of beer fermentation through electron-ion processing (EIP) of brewer’s yeast). Пиво и напитки. 2006; 5 :22-24. (In Russ.) - 49.
Glushhenko NA. О некоторых эффектах влияния электронно-ионной обработки на дрожжевые микроорганизмы (About some effects of electron-ion treatment on yeast microorganisms). Вестник Новгородского государственного университета им. Ярослава Мудрого. 2013; 2 (71):36-40 - 50.
Shaburova GV. Интенсификация производства пива путем фотостимуляции метаболизма дрожжей (Intensification of beer production by photostimulation of yeast metabolism) [thesis]. Кемерово: Кемеровский технологический институт пищевой промышленности; 1984. (In Russ.) - 51.
Kobelev AV, Bagaeva TV. Влияние разного светового спектра на рост дрожжей Saccharomyces cerevisiae (effect of different light spectrum on the growth of yeastSaccharomyces cerevisiae ). Ученые записки Казанского университета: Естественные науки. 2012;154 :98-102. (In Russ.) - 52.
Suprunjuk AJ, Karpenko DV. Влияние обработки монохроматическим светом на характеристики пивных дрожжей (Effect of monochromatic light treatment on the characteristics of brewer’s yeast). In: В сборнике: Общеуниверситетская научная конференция молодых учёных и специалистов «День Науки». Часть II. 16 апреля 2016. Москва: МГУПП; 2016. pp. 134-138. (In Russ.) - 53.
Shaburova LN, Danilova AN, Ponomareva MS, Gernet MV. Действие импульсной частоты лазерного излучения на дрожжи верхового брожения (Effect of pulsed frequency laser radiation on top-fermenting yeast). Пиво и напитки. 2019; 2 :16-19. (In Russ.) - 54.
da Motta MA, Muniz JB, Schuler A, Da Motta M. Static magnetic fields enhancement of saccharomyces cerevisae ethanolic fermentation. Biotechnology Progress. 2004;20 (1):393-396. DOI: 10.1021/bp034263j - 55.
Anton-Leberre V, Haanappel E, Marsaud N, Trouilh L, Benbadis L, Boucherie H, et al. Exposure to high static or pulsed magnetic fields does not affect cellular processes in the yeast Saccharomyces cerevisiae . Bioelectromagnetics. 2010;31 (1):28-38. DOI: 10.1002/bem.20523 - 56.
Nakanishi K, Tokuda H, Soga T, Yoshinaga T, Takeda M. Effect of electric current on growth and alcohol production by yeast cells. Journal of Fermentation and Bioengineering. 1998; 85 (2):250-253 - 57.
Grosse H-H, Bauer E, Berg H. Electrostimulation during fermentation. Bioelectrochemistry and Bioenergetics. 1988; 20 (1-3):279-285. DOI: 10.1016/S0302-4598(98)80024-X - 58.
Pomozova VA, Permjakova LV, Plotnikov VA, Safonova EA, Kozlov SG, Artemasov VV, et al. Способ активации пивных дрожжей (Brewer’s yeast activation method). Патент РФ (Patent) RU 2 234 529 C2. 2004. 8 p (In Russ.) - 59.
Bodrova OJ, Krechetnikova AN, Il’jashenko NG, Shaburova LN, Gernet MV. Способ активации спиртовых дрожжей (Method for activating alcoholic yeast). Патент на изобретение (Patent) RU 2288262 C1. 2006. 5 p (In Russ.)