Abstract
Leishmaniasis is a parasitic disease caused by protozoa belonging to the genus Leishmania. Over one billion people are living in areas endemic to leishmaniasis and are at risk of infection. Each year, more than one million new cases are reported. Although few drugs are available for the treatment of leishmaniasis, none of them are ideal due to their high resistance and toxicity risk. Many compounds with quinazoline scaffold were synthesized and reported to have promising antiparasitic and antileishmanial activities. This review aims to evaluate the reported antileishmanial activities of quinazoline and its derivatives with a special focus on their structure-activity relationships.
Keywords
- quinazoline
- privileged scaffold
- antileishmanial
- heterocyclic compounds
- medicinal chemistry
1. Introduction
Quinazolines are aza-derivative of quinolone and represent a large group of heterocyclic compounds that are composed of a fused benzene ring and a pyrimidine ring, also known as 1,3-diazanaphthalene. Quinazolinones and quinazolinediones are quinazolines in which one and two carbonyl groups, respectively, are present on the pyrimidine ring and are the most commonly encountered quinazoline derivatives. Quinazolinone has two isomers: quinazolin-2-one and quinazolin-4-one (Figure 1).
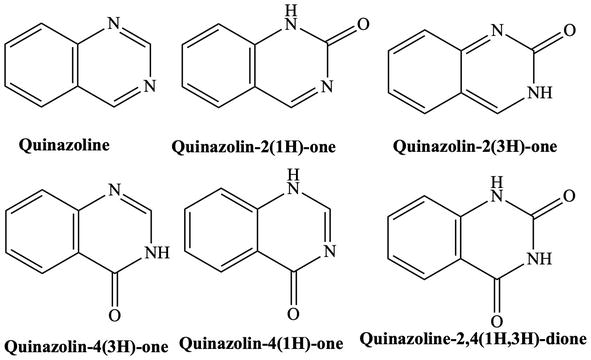
Figure 1.
Quinazoline, quinazolinones, and quinazolinedione structures.
2. Antileishmanial quinazoline derivatives
One of the first publications on the therapeutic potential of quinazolines was the development of
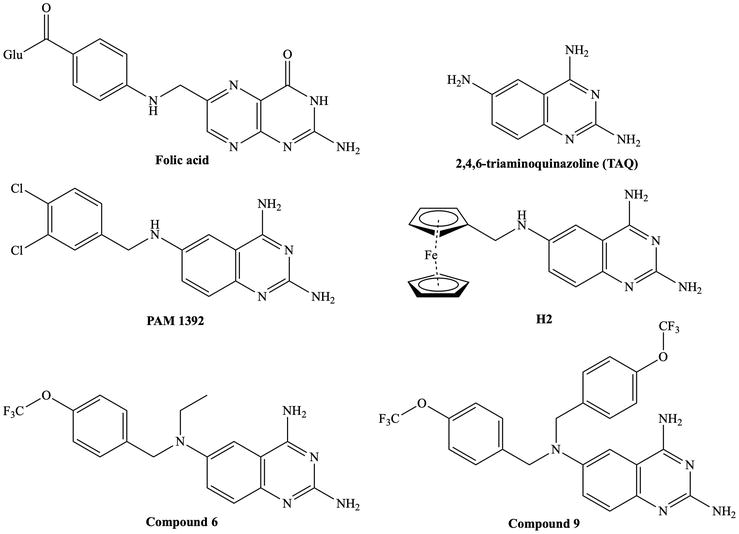
Figure 2.
Folic acid and reported quinazoline derivatives with antitrypanosomal and antileishmanial activity [
Mendoza-Martínez et al. reported a series of
Berman et al. synthesized 2,4-diaminoquinazoline derivatives and evaluated their antileishmanial activity against
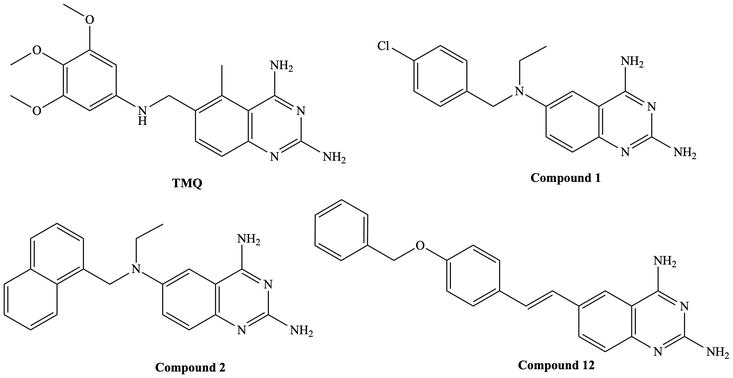
Figure 3.
TMQ and reported quinazoline derivatives with antileishmanial activity [
Khabnadideh et al. investigated the inhibitory effects of a series of 2,4-diaminoquinazolines against the
Since several studies have identified trypanothione and the trypanothione system and its role in the oxidative stress defense mechanisms of the Kinetoplastida
A series of 2-piperazin-1-yl-quinazolin-4-ylamine derivatives were reported and tested as antitrypanosomal and antileishmanial lead drug candidates against trypanothione reductase (TR) by Cavalli et al. (
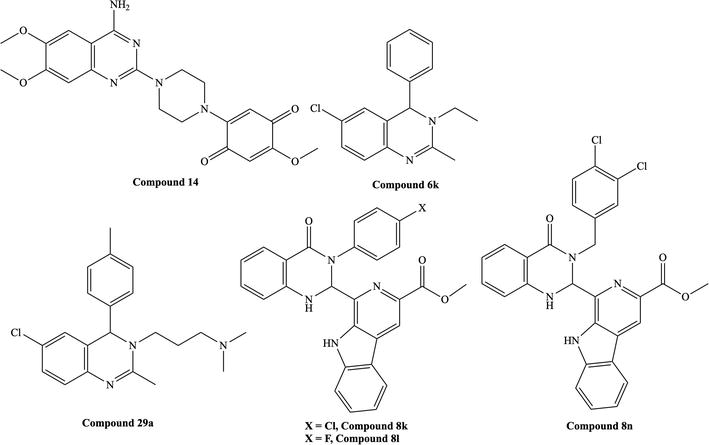
Figure 4.
Reported quinazoline derivatives with antitrypanosomal and antileishmanial activity [
Patterson et al. reported 3,4-dihydroquinazoline analogs as TR inhibitors as new antitrypanosomal agents. The compounds were tested against the bloodstream form of
Chauhan et al. reported new
Kumar et al. reported a series of a new class of 4-(hetero)aryl-2-piperazino quinazolines and assessed their
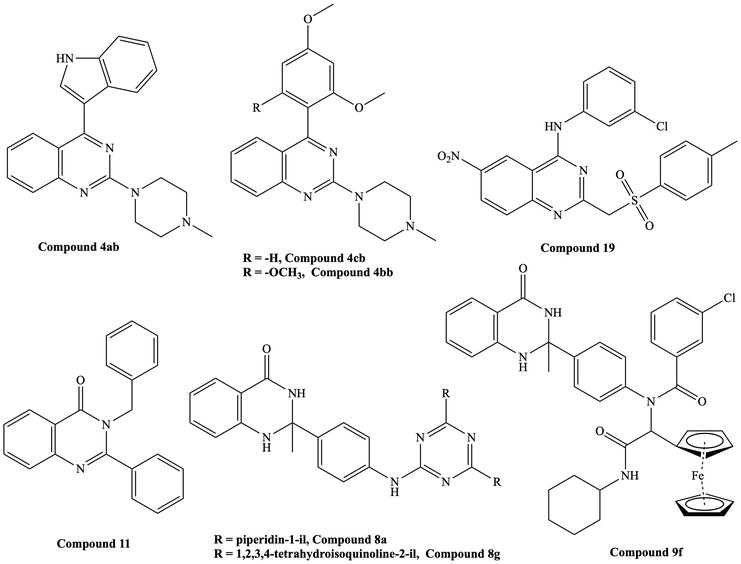
Figure 5.
Reported quinazoline derivatives with antileishmanial activity [
Kabri et al. reported quinazoline derivatives with antiplasmodial, anti-toxoplasmic, and antileishmanial activity.
Arfan et al. reported the antileishmanial activity of 2,3-disubstituted-3H-quinazolin-4-one derivatives [16]. The compound 3-benzyl-2-phenylquinazolin-4(3H)-one (
Sharma et al. carried out studies on 2,3-dihydroquinazoline, tetrahydroquinazoline, and their ferrocene derivatives [17].
Birhan et al. synthesized compounds that showed significant antileishmanial activities compared to standard drugs [18]. (
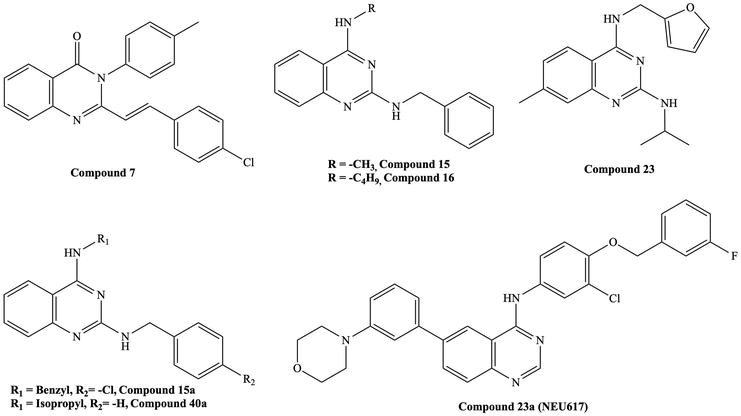
Figure 6.
Reported quinazoline derivatives with antitrypanosomal and antileishmanial activity [
Van Horn et al. reported the antileishmanial activity of a series of
Zhu et al. investigated
Katiyar et al. reported that the 4-anilinoquinazolines canertinib and lapatinib, which are kinase inhibitors, killed bloodstream
Woodring et al. also investigated lapatinib analogs [23]. They replaced the quinazoline scaffold with [3,2-d] and [2,3-d] thienopyrimidine. They found that the compounds were active against
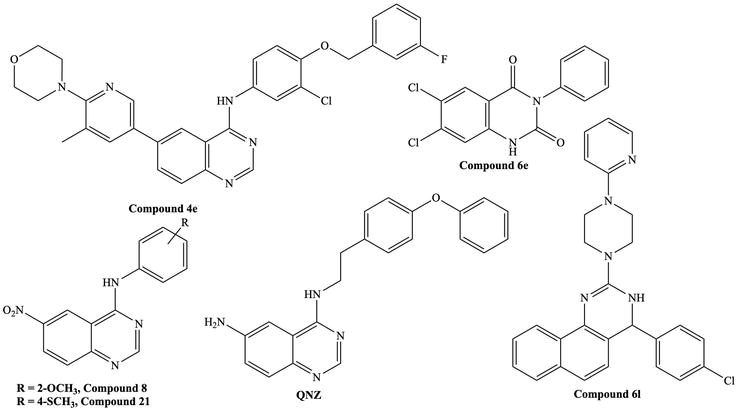
Figure 7.
Reported quinazoline derivatives with antitrypanosomal and antileishmanial activity [
Saad et al. reported 4-arylamino-6-nitroquinazoline derivatives with antileishmanial activities [24]. Among all the derivatives,
Enciso et al. have studied quinazolin-2,4-diones as new antileishmanial agents [25].
Macedo et al. reported that when Glucantime® was incubated with the quinazoline derivative
Agarwal et al. reported fused quinazoline derivatives and tested them against
3. Conclusions
Quinazoline and quinazolinone scaffolds are one of the privileged scaffolds of medicinal chemistry. Among the various activity reports, we tried to summarize the reports that showed antiparasitic activity, especially antileishmanial activity. According to these reports, compounds containing quinazoline-quinazolinone have promising antileishmanial activity. Compounds with this scaffold are an important starting point in the search for antileishmanial drug candidates.
References
- 1.
Davoll J, Johnson AM. Quinazoline analogues of folic acid. Journal of the Chemical Society C: Organic. 1970; 8 :997-1002. DOI: 10.1039/J39700000997 - 2.
Davoll J, Johnson AM, Davies HJ, Bird OD, Elslager EF. Folate antagonists. 2. 2,4-Diamino-6-{[aralkyl and (heterocyclic)methyl]amino} quinazolines, a novel class of antimetabolites of interest in drug-resistant malaria and Chagas’ disease. Journal of Medicinal Chemistry. 1972; 15 (8):812-826. DOI: 10.1021/jm00278a007 - 3.
Thompson PE, Bayles A, Olszewski B. PAM 1392 [2,4-diamino-6-(3,4-dichlorobenzylamino) Quinazoline] as a chemotherapeutic agent: Plasmodium berghei, P. Cynomolgi, P. Knowlesi, and Trypanosoma cruzi. Experimental Parasitology. 1969; 25 :32-49. DOI: 10.1016/0014-4894(69)90050-2 - 4.
Davoll J. Quinazoline Derivatives. London: The Patent Office; 1966. GB1045180A - 5.
McLuskey K, Gibellini F, Carvalho P, Avery MA, Hunter WN. Inhibition of Leishmania major pteridine reductase by 2,4,6-triaminoquinazoline: Structure of the NADPH ternary complex. Acta Crystallographica. Section D, Biological Crystallography. 2004; 60 (Pt. 10):1780-1785. DOI: 10.1107/S0907444904018955 - 6.
Mendoza-Martínez C, Galindo- Sevilla N, Correa-Basurto J, Ugalde- Saldivar VM, Rodríguez-Delgado RG, Hernández-Pineda J, et al. Antileishmanial activity of quinazoline derivatives: Synthesis, docking screens, molecular dynamic simulations and electrochemical studies. European Journal of Medicinal Chemistry. 2015; 6 (92):314-331. DOI: 10.1016/j.ejmech.2014.12.051 - 7.
Mendoza-Martínez C, Correa-Basurto J, Nieto-Meneses R, Márquez-Navarro A, Aguilar-Suárez R, Montero-Cortes MD, et al. Design, synthesis and biological evaluation of quinazoline derivatives as anti-trypanosomatid and anti-plasmodial agents. European Journal of Medicinal Chemistry. 2015; 96 :296-307. DOI: 10.1016/j.ejmech.2015.04.028 - 8.
Berman JD, King M, Edwards N. Antileishmanial activities of 2,4-diaminoquinazoline putative dihydrofolate reductase inhibitors. Antimicrobial Agents and Chemotherapy. 1989; 33 (11):1860-1863. DOI: 10.1128/AAC.33.11.1860 - 9.
Khabnadideh S, Pez D, Musso A, Brun R, Pérez LM, González-Pacanowska D, et al. Design, synthesis and evaluation of 2,4-diaminoquinazolines as inhibitors of trypanosomal and leishmanial dihydrofolate reductase. Bioorganic & Medicinal Chemistry. 2005; 13 (7):2637-2649. DOI: 10.1016/j.bmc.2005.01.025 - 10.
Augustyns K, Amssoms K, Yamani A, Rajan PK, Haemers A. Trypanothione as a target in the design of antitrypanosomal and antileishmanial agents. Current Pharmaceutical Design. 2001; 7 (12):1117-1141. DOI: 10.2174/1381612013397564 - 11.
Cavalli A, Lizzi F, Bongarzone S, Belluti F, Piazzi L, Bolognesi ML. Complementary medicinal chemistry-driven strategies toward new antitrypanosomal and antileishmanial lead drug candidates. FEMS Immunology and Medical Microbiology. 2010; 58 (1):51-60. DOI: 10.1111/j.1574-695X.2009.00615.x - 12.
Patterson S, Alphey MS, Jones DC, Shanks EJ, Street IP, Frearson JA, et al. Dihydroquinazolines as a novel class of Trypanosoma brucei trypanothione reductase inhibitors: Discovery, synthesis, and characterization of their binding mode by protein crystallography. Journal of Medicinal Chemistry. 2011; 54 (19):6514-6530. DOI: 10.1021/jm200312v - 13.
Chauhan SS, Pandey S, Shivahare R, Ramalingam K, Krishna S, Vishwakarma P, et al. Novel β-carboline–quinazolinone hybrid as an inhibitor of Leishmania donovanitrypanothione reductase: Synthesis, molecular docking and bioevaluation. MedChemComm. 2015; 6 :351-356. DOI: 10.1039/C4MD00298A - 14.
Kumar S, Shakya N, Gupta S, Sarkar J, Sahu DP. Synthesis and biological evaluation of novel 4-(hetero) aryl-2-piperazino quinazolines as anti-leishmanial and anti-proliferative agents. Bioorganic & Medicinal Chemistry Letters. 2009; 19 (9):2542-2545. DOI: 10.1016/j.bmcl.2009.03.020 - 15.
Kabri Y, Azas N, Dumètre A, Hutter S, Laget M, Verhaeghe P, et al. Original quinazoline derivatives displaying antiplasmodial properties. European Journal of Medicinal Chemistry. 2010; 45 (2):616-622. DOI: 10.1016/j.ejmech.2009.11.005 - 16.
Arfan M, Khan R, Khan MA, Anjum S, Choudhary MI, Ahmad M. Synthesis and antileishmanial and antimicrobial activities of some 2,3-disubstituted 3H-quinazolin-4-ones. Journal of Enzyme Inhibition and Medicinal Chemistry. Aug 2010; 25 (4):451-458. DOI: 10.3109/14756360903309412 - 17.
Sharma M, Chauhan K, Shivahare R, Vishwakarma P, Suthar MK, Sharma A, et al. Discovery of a new class of natural product-inspired quinazolinone hybrid as potent antileishmanial agents. Journal of Medicinal Chemistry. 2013; 56 (11):4374-4392. DOI: 10.1021/jm400053v - 18.
Birhan YS, Bekhit AA, Hymete A. Synthesis and antileishmanial evaluation of some 2,3-disubstituted-4(3H)-quinazolinone derivatives. Organic and Medicinal Chemistry Letters. 2014; 4 (1):10. DOI: 10.1186/s13588-014-0010-1 - 19.
Van Horn KS, Zhu X, Pandharkar T, Yang S, Vesely B, Vanaerschot M, et al. Antileishmanial activity of a series of N2,N4-disubstituted quinazoline-2,4-diamines. Journal of Medicinal Chemistry. 2014; 57 (12):5141-5156. DOI: 10.1021/jm5000408 - 20.
Zhu X, Van Horn KS, Barber MM, Yang S, Wang MZ, Manetsch R, et al. SAR refinement of antileishmanial N(2),N(4)-disubstituted quinazoline-2,4-diamines. Bioorganic & Medicinal Chemistry. 2015; 23 (16):5182-5189. DOI: 10.1016/j.bmc.2015.02.020 - 21.
Katiyar S, Kufareva I, Behera R, Thomas SM, Ogata Y, Pollastri M, et al. Lapatinib-binding protein kinases in the African trypanosome: Identification of cellular targets for kinase-directed chemical scaffolds. PLoS One. 2013; 8 (2):e56150. DOI: 10.1371/journal.pone.0056150 - 22.
Patel G, Karver CE, Behera R, Guyett PJ, Sullenberger C, Edwards P, et al. Kinase scaffold repurposing for neglected disease drug discovery: Discovery of an efficacious, lapatinib-derived lead compound for trypanosomiasis. Journal of Medicinal Chemistry. 2013; 56 (10):3820-3832. DOI: 10.1021/jm400349k - 23.
Woodring JL, Patel G, Erath J, Behera R, Lee PJ, Leed SE, et al. Evaluation of aromatic 6-substituted thienopyrimidines as scaffolds against parasites that cause trypanosomiasis, leishmaniasis, and malaria. Medchemcomm. 2015; 6 (2):339-346. DOI: 10.1039/C4MD00441H - 24.
Saad SM, Ghouri N, Perveen S, Khan KM, Choudhary MI. 4-Arylamino-6-nitroquinazolines: Synthesis and their activities against neglected disease leishmaniasis. European Journal of Medicinal Chemistry. 2016; 27 (108):13-20. DOI: 10.1016/j.ejmech.2015.11.016 - 25.
Enciso E, Sarmiento-Sánchez JI, López-Moreno HS, Ochoa-Terán A, Osuna-Martínez U, Beltrán-López E. Synthesis of new quinazolin-2,4-diones as anti-Leishmania mexicana agents. Molecular Diversity. 2016; 20 (4):821-828. DOI: 10.1007/s11030-016-9693-8 - 26.
Macedo SR, de Figueiredo Nicolete LD, Ferreira Ados S, de Barros NB, Nicolete R. The pentavalent antimonial therapy against experimental Leishmania amazonensis infection is more effective under the inhibition of the NF-κB pathway. International Immunopharmacology. 2015; 28 (1):554-559. DOI: 10.1016/j.intimp.2015.07.020 - 27.
Agarwal KC, Sharma V, Shakya N, Gupta S. Design and synthesis of novel substituted quinazoline derivatives as antileishmanial agents. Bioorganic & Medicinal Chemistry Letters. 2009; 19 (18):5474-5477. DOI: 10.1016/j.bmcl.2009.07.081