Abstract
Osteoporosis management is effective in decreasing vertebral fracture risk. The assessment of vertebral fracture risk is used to identify patients with high fracture risk for anti-osteoporotic treatment, especially for those who have not yet fractured. Several pharmacological agents are available to lower vertebral fracture risk by reducing bone resorption or/and stimulating bone formation. Aside from surgical treatment for fresh vertebral fracture or fracture nonunion in elderly patients, recent studies indicated that management of osteoporosis plays a vital role in boosting vertebral fracture union, preventing progressive vertebral collapse and decreasing the refracture risk. In this chapter, we focus on the treatment of osteoporosis, acute vertebral fractures and nonunion, as well as the evaluation of clinical efficacy by bone quality and bone turnover markers after treatment.
Keywords
- osteoporosis
- spine
- vertebral fracture
- drug therapies
- anti-resorptive treatment
- anabolic treatment
1. Introduction
Osteoporosis (OP), an age-related metabolic bone disease characterized by low bone mass and microarchitectural deterioration of bone tissue, increases the risk of fractures in the elderly population [1]. The prevalence of osteoporosis over the age of 50 years is 23% in men and 7% in women. It is emerging as a serious public health problem worldwide and brings severe challenges to the global health and social service sector because of aging of the population, especially in developing countries [2].
Osteoporotic vertebral compression fractures (OVCFs), the most common single fragility fractures, account for almost 50% of all osteoporotic fractures [3]. OVCFs are the most common type of osteoporotic fractures and often occur in the absence of trauma or following a low-energy trauma (a fall from a standing height or less) [4, 5, 6]. Worldwide, the incidence of OVCFs was 30–50% of people over age 50 and increased with age in both men and women [3, 7]. Acute OVCF patients commonly suffer from significant and long-lasting back pain, substantially impacting the patients’ Health-related quality of life (HRQoL) [8, 9, 10, 11, 12]. Magnetic resonance imaging (MRI) features are crucial to achieving an accurate and early diagnosis, on which the bone marrow signals displayed hypointensity on T1-weighted and hyperintensity on T2-weighted and fat-suppressed sequences within a deformed vertebral body [13, 14, 15]. OVCFs were acute if the interval between the trauma or symptom onset and MRI was less than 2 weeks [16, 17]. It is also a major risk factor to subsequent refractures, which are also called vertebral fracture cascade [18, 19]. In the first year after an OVCF, risks of refractures increase by 4–7 times [20, 21]. The progressive loss of anterior height leads to spinal kyphotic deformities and imposes more stress on the anterior spinal column, increasing the risk of new OVCFs [22]. In addition, kyphotic deformity also disrupts body balance, resulting in an increased risk of falls and other fractures [22].
Not all OVCFs could heal following conservative treatment. Once nonunion occurs, the intractable back pain and even neurological deficits further decrease the HRQoL [23, 24]. In addition to increased morbidity and mortality, it imposes a significant economic burden on the public health systems worldwide and patient families [2, 23, 25].
The anti-osteoporosis (anti-OP) management to prevent OVCFs is one of the most important objectives, a chronic and long-term condition even requiring lifelong management [26]. The advice in this passage provides a framework for healthcare professionals. The detailed treatment plans should be based on the individual situations in clinical practice.
2. Risk assessment of OVCFs
2.1 Background
Osteoporosis is a significant risk factor for OVCFs. In postmenopausal women and men aged more than 50 years, the risk factors of OVCFs are assessed to determine whether anti-OP therapy is needed to reduce the fracture risks. If at high risk of fracture, the anti-OP to prevent subsequent fractures is advised as early as possible. Fractures are common in severe osteoporosis patients, particularly among older women. Generally, anti-OP therapy is advised in patients having a bone mineral density (BMD) T-score of −2.5 or less, a history of spine or hip fracture or a Fracture Risk Assessment Tool (FRAX) score indicating increased fracture risk [16, 27]. In addition, the refracture risk is multiplied following the initial fracture in OVCF patients, indicating the need for anti-OP therapy to prevent subsequent fractures [20, 28].
Several fracture risk assessment tools, including the BMD, FRAX, QFracture and Garvan fracture risk calculator, have been developed to estimate the fracture risk in OP patients. Unfortunately, fracture risk prediction remains an imprecise science.
2.2 BMD
Bone mineral density could be an option for the prediction of OVCFs risk. In clinical practice, BMD measured with dual-energy X-ray absorptiometry (DXA) is closely related to OVCFs risk and incorporated in most risk assessment paradigms. Each 1-standard deviation (1-SD) reduction is associated with an increased fracture risk of 1.5–2-fold. However, the BMD is not sensitive in the assessment of osteoporosis. OVCFs may occur in individuals with a BMD above this threshold (T-score > −2.5), reflecting the other factors contributing to fracture risk [29, 30]. Such clinical risk factors as age, sex, smoking, weight loss and glucocorticoid use have been noted with OVCFs risk and included in the risk assessment, even in the absence of BMD.
2.3 FRAX
Fracture Risk Assessment score (http://www.shef.ac.uk/FRAX), the most widely used fracture risk assessment tool, allows an accurate assessment of 10-year OVCFs risk without BMD T-score and could help to make treatment decisions [31]. Its significant feature is that it can be directly calibrated to fracture incidence rates in the target population and considers death a competing risk. Most clinical practice guidelines incorporated it for assessing the OVCFs risk in many countries. High fracture risk patients are recommended anti-OP treatment, especially for those who have not yet sustained an OVCF. Treatment is unnecessary for those patients with very low fracture risk, and an intermediate group of patients undergoes BMD testing to refine their fracture risk. Therefore, an osteopenic individual with a BMD T-score of −2.5 to −1.0 but with high OVCF risk should be administered anti-OP therapy.
However, the FRAX was only used in patients with a BMD T-score ≤ −1.0 and without anti-OP treatment. Neither follow-up patients nor those patients treated for osteoporosis or osteopenia are appropriate for taking up this risk assessment tool. In addition, such limitations, including the inability to identify the imminent fracture risk, underestimated OVCFs risk in diabetic patients, fracture risk stratification for the exposure of different dose glucocorticoids and fall risk, have not been settled [32, 33].
It is important to emphasize that FRAX is not a substitute for BMD, which can be reserved for osteoporosis diagnosis.
2.4 QFracture
QFracture (www.qfracture.org), primarily applicable to the UK population, could predict the 1–10-year OVCFs, independent of BMD in primary care. Like the FRAX, it includes several causes of osteoporosis, such as a history of smoking, alcohol, corticosteroid use, parental history of osteoporosis and several secondary causes of osteoporosis. Besides, a history of falls was included, utilizing a large number of clinical risk factors. A feature of QFracture is that it contains more questions and does not accommodate the inclusion of BMD for assessing fracture risk [34].
2.5 Garvan scale
Garvan scale (www.garvan.org.au) was devised to predict the absolute risk of OVCFs in 5 or 10 years in a given patient [35]. This tool does include age, sex, fractures after the age of 50, history of falls in the previous year, femoral neck BMD or T-score and weight. It differs from FRAX by including a history of falls and the number of previous fragility fractures. In addition to falls, multiple risk factors of OVCFs, including cardiovascular disease, type 2 diabetes, asthma, tricyclic antidepressants’ usage and history of falls or liver disease, may make QFracture more advantageous in predicting individuals with internal medicine diseases.
2.6 Computed tomography (CT)
The risk assessment methods for OVCFs mentioned above remain underutilized. Computed tomography (CT) has been used to identify undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases [36, 37]. The trabecular BMD assessed by CT provides a rapid and effective opportunistic screen for detecting individuals at increased risk for fragility fractures [38].
Recently, routine abdomen or chest CT scans were used for automatically assessing the feasibility of OVCFs risk [39]. Compared with FRAX for 5-year fracture risk, the CT-based predictor of major osteoporotic fracture presented better receiver operating characteristic area under curve (ROC AUC), sensitivity and positive predictive value.
In a word, the main task of risk assessment tools is to identify the individuals with a fracture risk high enough to justify anti-OP treatment, especially for those who did not yet suffer from a fracture and by administering anti-OP treatment to prevent OVCFs [40]. However, no optimal risk assessment tool available quantificationally identifies the OVCFs risk. More precise evaluation tools are needed to develop and quantitatively evaluate and predict the OVCFs risks in the future.
3. Primary drug treatment
3.1 Vitamin D
Vitamin D is essential for bone health and the functioning of systems of the human body. Vitamin D sufficiency refers to the serum concentration of vitamin D from 30 to 50 ng/ml (nanograms per milliliter) [41, 42, 43]. Endocrine Society suggested administering a daily vitamin D dose of 1500–2000 IU (international units), to reach the desired value of 30 ng/ml [44]. The association between serum concentration of vitamin D and bone health is described as follows: vitamin D deficiency (<25 nmol/L (nanomole per liter) produces a mineralization defect; vitamin D insufficiency (<50 nmol/L) produces secondary hyperparathyroidism and an increase in bone turnover [45, 46, 47]. Individuals who do not achieve the recommended dietary intake of calcium or vitamin D, especially those taking anti-OP medication, are advised to take supplements.
There is no single, fixed dose for all subjects to supplement. Most studies have investigated the efficacy of calcium and vitamin D on fracture risk but with inconsistent outcomes. Correcting vitamin D deficiencies and a serum concentration of 25-hydroxyvitamin D > 50 nmol/L can be beneficial [48]. However, there are little or no benefits in the supplements in individuals with sufficient vitamin D. A single oral high dose of vitamin D (such as 500,000 IU in autumn or winter annually) for 3 to 5 years was related to an increased risk of falls and fractures [49]. Except for malabsorption, the recommended dose should not exceed 4000 IU/day [49, 50, 51]. According to the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases, the use of an initial loading dose (3000–10,000 IU/day, average 5000 IU/day for 1–2 months) was followed by the maintenance dose (2000 IU/day) in patients with serum 25(OH)D < 10 ng/ml, or in patients starting anti-OP therapy with intravenous bisphosphonates or denosumab with serum 25(OH)D < 20 ng/ml.
3.2 Calcium
Calcium is the main constituent of bone. Recommend a diet with adequate total calcium intake (1000 mg/day for men aged 50–70 years; 1200 mg/day for women ≥51 years and men≥71 years), incorporating calcium supplements if intake is insufficient [52]. Excessive intake of calcium (>1500 mg/day) might be associated with an increased risk of renal stones and cardiovascular events [53]. Calcium intake should be recommended in combination with vitamin D, and calcium supplementation alone does not affect increased BMD or decreased fracture risk [54].
4. Anti-OP therapy
4.1 Background
Either anti-resorptive or anabolic treatment is recommended by the US Food and Drug Administration for high-risk patients without contraindications following a risk assessment. Adequate intake of vitamin D and calcium is required in the anti-OP treatment. Both bisphosphonates (bone remodeling inhibitor) and denosumab (receptor activator of nuclear factor kappa-B ligand (RANKL inhibitor) are anti-resorptive agents to reduce the risk of hip, nonvertebral and vertebral fractures. Anabolic drugs, teriparatide and abaloparatide, are given by daily subcutaneous injection to stimulate bone formation. OVCFs risk is usually reduced by 30–70% following anti-OP treatment [26]. The benefit-to-risk ratio for anti-OP therapy is strongly positive for patients with osteoporosis.
4.2 Anti-resorptive therapies
4.2.1 Bisphosphonates
Bisphosphonates, the most used anti-OP drugs, reduce fracture risk by inhibiting bone remodeling. Generally, it is the first-line anti-OP agent in osteoporosis patients without contraindications. The progressively increased BMD has reached a plateau following 3–4 years of treatment [55, 56]. Other than zoledronic acid, the efficacy of anti-resorptive agents on the increased BMD is similar in both men and women. The benefits of decreasing fracture risks are retained after discontinuing alendronate or zoledronic acid. After 5 years of alendronate therapy and 3 years of zoledronic acid therapy, drug holidays are considered in patients with lower OVCFs risk [27]. Zoledronic acid treatment every 18 months for 6 years could effectively reduce the OVCFs risk [57]. A small note on bisphosphonate use should be limited to patients with a creatinine clearance >35 ml/min and serum vitamin D levels of 30–50 nmol/L to prevent symptomatic hypocalcemia [27, 58, 59]. Such adverse events as jaw osteonecrosis and atypical femoral fractures are related to bisphosphonates.
4.2.2 RANK ligand inhibitor
Denosumab, an osteocyte-derived regulator of osteoclast development binding to and inhibiting RANK ligand, reduces osteoclast activity to inhibit bone remodeling [60]. In postmenopausal women and men, a single dosage of 60 mg half-yearly administered by subcutaneous injection could effectively prevent the fracture risk of vertebrae, nonvertebrae and hip. The mineralization degree reaches a maximum by year 5 of treatment but by 10 years of treatment it results in greater BMD gains for sustainably protecting the vertebrae from fracture [61]. However, the BMD returns to baseline 18 months after the last injection [62, 63]. Treatment adherence is vital to maintaining the BMD, and note that alendronate or zoledronic acid administration is recommended for maintaining the efficacy of the prevention of OVCFs if denosumab treatment is discontinued [64, 65]. Rapid decreases in BMD were related to the loss of vertebral fracture protection, and multiple vertebral fractures have been reported to occur 3–18 months after stopping denosumab treatment [65].
Denosumab offers an alternative approach to the treatment of osteoporosis by decreasing bone resorption and increasing BMD through the inhibition of RANKL. Compared to bisphosphate, denosumab showed a stronger effect on inhibiting bone resorption and increasing BMD [66]. Denosumab rapidly inhibits bone resorption markers, which leads to an early and rapid decrease (within 24 h of treatment) in C-terminal telopeptide of type I collagen (CTX), followed by a later and smaller decrease in bone formation markers such as the N-terminal propeptide of type I procollagen (PINP) [67]. A reduction of CTX by 70–85% and PINP by 60–70% was found with anti-resorptive therapy [68, 69]. PINP and CTX were continuously suppressed with continuous administration for up to 8 years with a greater extent of PINP than CTX [70]. Compared with placebo, denosumab reduced the risk of vertebral fracture by 68%, hip fracture by 40% and nonvertebral fracture by 20% [71]. No serious adverse reaction, including cancer, infection, cardiovascular disease, fracture nonunion, hypocalcemia or jaw osteonecrosis, was found during the treatment period.
4.2.3 Hormone replacement therapy
The aim of estrogen treatment, with or without progestin, is to increase the BMD to reduce fracture risk at the hip, vertebrae and other sites in postmenopausal women [72]. The Women’s Health Initiative randomized controlled trial indicated that the combined estrogen plus progestin could reduce the OVCFs by 34% [73]. A decreased use of estrogen is attributed to a balance of benefits and overall health risks between the efficacy of anti-OP and such extraskeletal effects as breast, endometrial, colorectal cancers, coronary heart disease, stroke and deep vein thrombosis. Guidelines recommend that the benefit-to-risk ratio is most favorable in women with elevated risk for bone loss or fracture in the age younger than 60 years or those who are within 10 years of menopause onset [74]. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, and it is advised to periodically reevaluate both the benefits and risks of continuing or discontinuing hormone replacement therapy.
4.2.4 Anabolic medications
Compared to anti-resorptive drugs, the anabolic agents of teriparatide and abaloparatide exert larger increases in vertebral BMD and have a stronger clinical efficacy in reducing OVCFs risk [75].
Teriparatide, a human parathyroid hormone (1–34), is used in patients with severe osteoporosis or complication to reduce the risk of new OVCFs by 65% [76]. It is advised to give the patient daily administration of teriparatide 20 μg by subcutaneous injection, and the longest medication duration time was 24 months because of the concerns of osteosarcoma. However, the US Food and Drug Administration now permits teriparatide use >2 years in patients at continuing high fracture risk, based on a study without increasing adult osteosarcoma incidence in a 15-Year US Postmarketing Surveillance Study [75, 77]. Increased spinal BMD with little effect on proximal femoral and distal radius BMD is the most striking feature (about 7%) following 2 years of treatment [78, 79]. In clinical trials in women with osteoporosis following teriparatide treatment for 21 months, vertebral and nonvertebral fractures were, respectively, reduced by two-thirds and one third, but with no significant reduction in hip fractures. In glucocorticoid-induced osteoporosis, it produces larger BMD increases than alendronate and substantially reduces vertebral, but not nonvertebral, fracture risk [80]. Upon discontinuing teriparatide therapy, an anti-resorptive agent, such as bisphosphonate or denosumab, is recommended to preserve the progressive increases in BMD [81]. PINP was a biological response marker during teriparatide treatment for osteoporosis and was used to assess treatment response [82, 83]. The changes in PINP are apparent as early as 3 days following the start of treatment [84]. A 30–50% increase in PINP with anabolic therapy is observed within months [85]. PINP levels increased following the teriparatide treatment and can be a surrogate marker of bone formation and strength [85]. PINP returned to around or below baseline levels by 28 days after stopping treatment [84]. A PINP value on treatment that is low (<35 μg/L) for anti-resorptive treatment or above 69 μg/L for anabolic therapy may be presumed to indicate adequate response [67].
Abaloparatide, available in the USA and Japan, is a selective activator of the Parathyroid Hormone 1 Receptor (PTH1R) and has a similar mechanism of action to teriparatide. Similar to teriparatide, abaloparatide is administered 80 μg daily by subcutaneous injection for up to 24 months.
4.2.5 Anti-sclerostin antibody therapies
Sclerostin, an osteocyte protein released at the bone surface, stimulates bone formation and inhibits bone resorption by inhibiting the wingless-type (Wnt) signaling in osteoblasts. The anabolic effect is achieved by an early and transient bone formation increase and a sustained bone resorption decrease [86].
Romosozumab, an anti-sclerostin monoclonal antibody, stimulates bone formation and inhibits resorption to increase BMD [86]. Subcutaneous injections of 210 mg/month for 12-month increase the BMD by 11.3% at the lumbar spine, compared to 7.1% with teriparatide (20 μg daily) and 4.1% with alendronate (70 mg weekly) [86]. Treatment for 12 months reduced the risk of vertebral and clinical fractures in postmenopausal osteoporosis women compared to a placebo [87]. And sequential treatment with denosumab further decreased the spinal fracture risk, providing a foundation for an ongoing reduction in the OVCFs risk during sequential treatment with denosumab. As to the risk of hip fractures, romosozumab followed by alendronate treatment resulted in a significantly lower risk than alendronate alone, suggesting an important benefit and challenging the common treatment practice of first-line use of alendronate [88].
5. Monitoring anti-OP treatment
5.1 Background
Anti-OP treatment aims to improve the BMD to reduce OVCFs risk. Prevention of fracture is the predominant outcome following anti-OP treatment. Hence, monitoring anti-OP treatment is vital for successful osteoporosis management. The changes in BMD are affected by bone turnover. Bone turnover is comprised of two processes including bone resorption and bone formation. The decreased bone resorption or/and increased bone formation could affect the changes in BMD. Bone turnover markers (BTMs) are used to assess the changes in bone resorption and formation. Fractures are the gold standard for measuring efficacy and changes in BMD, and BTMs could be served as surrogates [89]. Hence, the assessment of changes in bone turnover as well as BMD was used to monitor anti-OP treatment.
In an attempt to better monitor the efficacy of anti-OP treatment, requirements for anti-OP treatment are listed below [89]:
Anti-OP therapy was well tolerated.
Intake of adequate calcium and vitamin D.
A treatment period of at least 1 year to become fully effective.
5.2 BMD
Osteoporosis is characterized by progressive loss of bone, and BMD is a predictor of fracture risk. There is a strong, continuous relationship between the BMD and OVCFs, the greater the increased BMD, the lower the fracture risk [90, 91]. Early monitoring of anti-OP treatment efficacy is very important to guide anti-OP treatment. However, the changes in BMD are difficult to detect at an early treatment stage. According to the clinical circumstances, BMD testing is commonly performed 1 year after the anti-OP therapy and at appropriate intervals thereafter.
Based on the changes in BMD and new fracture, treatment response was clarified as three categories [89]: inadequate response (incident fracture and a decrease in BMD greater than 2%), possible inadequate response (incident fracture and a decrease in BMD greater than 2%) and adequate response (no fracture and no decrease in BMD greater than 2%). The Committee of Scientific Advisors of International Osteoporosis Foundation (IOF) recommended that the treatment should be changed to 4% decline in BMD at the lumbar spine after at least two serial BMD measurements [92].
5.3 BTMs
Anti-OP agents exert their effect by influencing the bone formation and/or bone resorption. The International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry (IFCC) have recommended serum PINP and CTX as reference bone turnover markers. Bone turnover markers hold promise in fracture risk prediction and monitoring treatment [93, 94]. The use of BTMs for the monitoring of treatment requires a baseline assessment with a repeat measurement at some defined point during treatment. In people with osteoporosis, bone turnover markers might be useful to assess the response to anabolic and anti-resorptive therapies, assess compliance to therapy or indicate possible secondary osteoporosis. Several studies have described the relationship between the reduction in bone turnover markers following anti-resorptive therapy and the reduction in vertebral and nonvertebral fracture risk [95, 96]. Increased concentrations of bone turnover markers can be associated with increased rates of bone loss and fracture risk [93]. In patients receiving teriparatide treatment, the changes in serum levels of PINP were significantly correlated with vertebral bone strength, which indicates that serum PINP monitoring could serve as a surrogate marker of biomechanical properties in clinical practice [85].
The Committee of Scientific Advisors of IOF recommended that a significant response is a decline of 25% from baseline levels for anti-resorptive treatments and a 25% increase for anabolic agents after 6 months [92]. For anti-resorptive treatments, if baseline levels are not known, a positive response is a decrease below the average value of young healthy adults. It is assumed that the response is similar between men and women [92].
5.4 CT
The CT Hounsfield unit values of the vertebra have been used for the assessment of vertebral BMD [38]. In the previous studies, the vertebral CT values increase significantly following anti-OP treatment [17]. Here, we suggest that vertebral CT values could be used as a method for monitoring anti-OP treatment.
6. OVCFs therapy
6.1 Treatment goal
The primary treatment goal is to improve spinal BMD to boost fracture union, prevent vertebral collapse, relieve back pain and prevent new OVCFs. Improving the HRQoL is the ultimate aim following OVCFs.
6.2 Conservative treatment
Traditional conservative treatment, including brace treatment and pain relief, is an option for symptomatic OVCFs [97]. Bracing is the standard conservative treatment for acute OVCFs to immobilize the fracture site to manage pain and boost the OVCFs healing, resulting in the least residual pain and disability in the long term [98]. Routine use of a custom-made rigid brace for acute OVCFs is not recommended [99]. For acute OVCFs patients, rigid-brace treatment did not result in better prevention of spinal kyphotic deformities, better HRQoL or lesser back pain than soft brace. Bed rest was not recommended during OVCFs treatment [52].
However, approximately 30–40% of patients still experience severe low back pain [100, 101]. Calcitonin salmon has been shown to dramatically reduce acute pain due to acute OVCFs. Recent studies indicate that the paravertebral muscles play a substantial role in back pain, sagittal balance and HRQoL [102]. Low back pain at the 6-month follow-up was higher in patients with paravertebral muscle decline. Once the acute pain improves, patients are recommended to exercise paravertebral muscles for chronic back pain.
Conservative treatment for OVCF carries inherent risks. Treatment failure was not rare in clinical practice, such as the progression of collapse, kyphotic deformity, fracture nonunion and neurologic impairment. BMD T value less than −3.45 and the presence of intravertebral cleft were high-risk factors for conservative treatment failure [103]. In addition, the shape of the OVCFs vertebrae, the middle column damage and the involvement of thoracolumbar junction can also be predictors for vertebral collapse and nonunion of OVCFs [104]. A diffuse low-type pattern on T1-weighted MRI and diffuse low- and fluid-type patterns on T2-weighted MRI were independent risk factors for nonunion of acute OVCFs [105]. Acute OVCFs with these risk factors should be actively observed for nonunion and vertebral collapse.
6.3 Surgery treatment
Vertebral augmentation procedures (VAPs) were recommended for patients with severe back pain or no response to conservative treatment [106]. Being the immediate postoperative pain relief, VAP was widely applied to restore intravertebral stability following the injection of bone cement into the fractured vertebrae and recommended by the Cardiovascular and Interventional Radiological Society of Europe for OVCF treatment [107]. Detailed surgical techniques will not be presented in this section. Based on a meta-analysis, the clinical efficacy showed that OVCFs patients who underwent VAP treatment were 22% less likely to die at up to 10 years than those receiving nonsurgical treatment [106]. However, the prevalence of severe complications related to VAP could be as high as 12.5–36.8% [108, 109, 110, 111, 112]. The most common complications encountered are cement leakage and new OVCFs at the adjacent level. Higher viscosity and less cement were recommended to reduce the leakage risk and related complications [113]. New OVCFs of cemented vertebrae after VAP were about 63%, and the greater the anterior vertebral height obtained, the greater the risk of refracture occurring [18]. Other cement-related complications, including pulmonary embolism, spinal cord burn, adjacent vertebrae collapse and cement migration, were not rare [110, 114, 115]. Besides, patients who received VAP should be informed about the clinical effect and the subsequent anti-OP treatment was also administered to reduce the risk of new OVCFs [116].
However, VAP remains a controversial treatment. Although extensively used for OVCFs, VAP was not recommended based on recent studies [116, 117]. Compared with placebo control, the efficacy of VAP in pain control was less than 6 weeks [118]. Recent high- to moderate-quality evidence indicates that VAP does not offer significant clinical benefits compared with the sham procedure [117, 119, 120]. One challenge was that VAP treatment only targeted the restoration of stability of the fractured vertebrae and did not improve spinal BMD to decrease the fracture risk of the treated or nontreated vertebrae [18]. Another challenge is that VAP is not suitable for patients with surgical contraindications [121]. The anti-osteoporotic treatment administered following VAP could reduce the risk of refracture by 40–70% [116].
Obviously, VAP was not suitable for OVCFs. The goal of OVCFs treatment is to relieve back pain, boost OVCFs union, improve spinal BMD to decrease the risk of refracture and improve the HRQoL. In addition, the fracture healing vertebrae significantly related to relieved back pain, compared to the patients without bone union [122].
Now that anti-OP therapy is still needed after VAP, selecting an anti-OP therapy method that can promote bone healing as well as prevent refracture is a better treatment. Thus, obtaining similar therapeutic effects to VAP with lesser complication and cost, no matter patients with or without surgical contraindications [123].
6.4 Teriparatide treatment
As far as the clinical efficacy of anti-OP treatment is concerned, the clinical benefits of teriparatide treatment had been confirmed by stimulating new bone formation to increase BMD and strength to prevent OVCFs [76]. Bisphosphonate use does not significantly affect the clinical results during conservative treatment for OVCFs, as well as the occurrence of the nonunion was related to medication history [124]. So, we do not recommend bisphosphonate use during the fracture union period. Recently, teriparatide treatment gradually showed its clinical advantage on OVCFs in clinical practice [125, 126]. In patients with neurological deficits following new unstable OVCF and surgical contraindications, teriparatide treatment was better than alendronate at improving the BMD and preventing aggravation of spinal cord compromise [121]. In postmenopausal women with OVCFs, teriparatide treatment was related to the consequent back pain alleviation and improved HRQoL [1]. The signal changes in bone marrow following treatment on magnetic resonance imaging (MRI) could reflect the role of enhancing fracture healing at 3 months posttreatment.
The Union of OVCFs following teriparatide treatment was first quantitatively assessed with CT images in our previous study [17, 127, 128]. Teriparatide treatment for OVCFs proved effective in remission of back pain by boosting fracture union, prevention of progressive vertebral collapse and new OVCFs and improvement in the HRQoL [17]. At least 1 year of treatment indicated that the clinical effect on back pain, spinal function, BMD and new OVCFs was superior to that of VAP, but with no requirements for hospitalization [17]. A typical acute OVCF case following teriparatide treatment is shown in Figure 1.
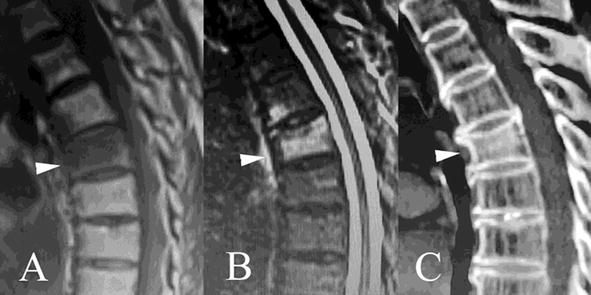
Figure 1.
Teriparatide treatment boosts T7 OVCF union in a 70-year-old female patient. The hypointensity on sagittal T1-weighted (A, white arrow) and hyperintensity on fat-suppressed sequence (B, white arrow) within the T7 show acute OVCF. Sagittal CT image of T7 vertebral body (C, white arrow) at month 4 following teriparatide treatment shows intravertebral bone formation connecting the upper and lower endplates, indicating the fracture union.
7. Nonunion of OVCFs therapy
7.1 Background
Like other fractures, OVCFs may develop nonunion with an incidence of 10–48% [129, 130, 131]. The fracture nonunion was defined as an intravertebral vacuum cleft detected within a deformed vertebral body at least 4 months after the fracture event, in which the signals displayed hypointensity on T1- and T2-weighted and fat-suppressed sequences [127, 132, 133, 134]. Once nonunion occurs, patients may suffer from intractable back pain and neurological deficits caused by intravertebral instability, leading to a further reduced HRQoL and increased mortality [23, 24].
Although the nonunion of OVCFs is called by most scholars as Kümmell’s disease, research showed that they are two different diseases now [135, 136, 137]. The main cornerstones of Kümmell’s disease must be a history of trauma with a negative spinal radiological investigation, an asymptomatic period followed by the intravertebral vacuum cleft [138]. Based on our research, the initial factor for Kümmell’s disease was not undisplaced OVCFs but a magnetic resonance imaging of negative spine trauma that progressed into a delayed vertebral collapse with intravertebral vacuum cleft [128].
7.2 Treatment goal
The treatment goal of fracture nonunion treatment is to restore intravertebral stability by cement technique, vertebral body replacement, long- or short-level screw fixation or the promotion of fracture union. The OVCFs nonunion was often refractory to conservative treatment. So far, no standard or single treatment is yet available.
7.3 Surgery treatment
Open operation or VAP was recommended in patients with no response to conservative treatment, including severe back pain, worsening neurological deficits or progressive spinal kyphosis [106].
Traditional open internal fixation, including a posterior, anterior and a combined approach, was used to relieve the clinical symptoms. However, severe osteoporosis often accompanies this fracture nonunion in these aged patients, and medical complications may also be present. The osteoporotic vertebrae could not hold the implanted pedicle screws and titanium cage, leading to internal fixation or intervertebral fusion failure.
Less invasive VAP was widely applied for OVCFs nonunion, recommended by the Cardiovascular and Interventional Radiological Society of Europe for OVCF treatment [107]. VAP could relieve back pain, restore vertebral height and correct spinal kyphosis to an extent. However, patients with nerve damage from spinal canal stenosis were likely not to benefit from invasive treatment, with the risk of cement leakage and neurological damage.
Some studies have found that patients may not benefit from VAP. VAP for nonunion of OVCFs, the same as OVCFs, remains a controversial treatment. In addition, more complications are another challenge. Like OVCFs, anti-OP is still needed after VP.
7.4 Teriparatide treatment
The main clinical symptom, including persistent pain and spinal cord deficits, is intravertebral instability from the nonunion [129, 139, 140]. We believe that restoration of the intravertebral stability by enhancing the fracture union may be more suitable for the nonunion of OVCFs. Assessment of fracture union following teriparatide treatment for at least 6 months was quantified on sagittal CT images in our previous study [127]. Our research indicated that teriparatide treatment could boot fracture union and prevent progressive vertebral collapse [127, 132]. All the clinical symptoms were relieved following the fracture union. Besides, the increased spinal BMD to prevent new OVCFs and improve the HRQoL was also observed in the study. At least 6 months of teriparatide treatment may be a choice for the nonunion of OVCF.
8. Conclusions
Osteoporosis is a chronic and long-term condition that even requires lifelong management. The anti-OP management to prevent OVCFs is one of the most important objectives. The fracture risk assessment tools were used to assess the fracture risk and direct subsequent anti-OP therapy. In elderly populations with a high risk of fracture, early anti-OP with bisphosphonates or denosumab is efficient in decreasing the OVCFs risk. Teriparatide or abaloparatide treatment followed by anti-resorptive agent treatment may be more suitable for those with high or imminent fracture risk. Although VAP is extensively used either in OVCFs or nonunion patients, anabolic medications gradually showed clinical efficacy in enhancing fracture union. Despite substantial advances in treatment options to reduce OVCF risk, many high-risk individuals have not given enough attention. Offering individualized, efficient and safe treatment regimens remains a challenge for the future.
Funding
The Basic Research Program of Shanxi Province, China (grants 202203021221264) supported this research.
References
- 1.
Chen Z, Lin W, Zhao S, Mo X, Yuan W, Cheung WH, et al. Effect of teriparatide on pain relief, and quality of life in postmenopausal females with osteoporotic vertebral compression fractures, a retrospective cohort study. Annals of Palliative Medicine. 2021; 10 :4000-4007. DOI: 10.21037/apm-20-2333 - 2.
Ruiz-Adame M, Correa M. A systematic review of the indirect and social costs studies in fragility fractures. Osteoporosis International. 2020; 31 :1205-1216. DOI: 10.1007/s00198-020-05319-x - 3.
Ballane G, Cauley JA, Luckey MM, El-Hajj FG. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporosis International. 2017; 28 :1531-1542. DOI: 10.1007/s00198-017-3909-3 - 4.
Hoffmann DB, Popescu C, Komrakova M, Welte L, Saul D, Lehmann W, et al. Chronic hyponatremia in patients with proximal femoral fractures after low energy trauma: A retrospective study in a level-1 trauma center. Bone Report. 2020; 12 :100234. DOI: 10.1016/j.bonr.2019.100234 - 5.
Kim SM, Yeom JW, Song HK, Hwang KT, Hwang JH, Yoo JH. Lateral locked plating for distal femur fractures by low-energy trauma: What makes a difference in healing? International Orthopaedics. 2018; 42 :2907-2914. DOI: 10.1007/s00264-018-3881-3 - 6.
Genant HK, Cooper C, Poor G, et al. Interim report and recommendations of the World Health Organization task-force for osteoporosis. Osteoporosis International. 1999; 10 :259-264. DOI: 10.1007/s001980050224 - 7.
Karmakar A, Acharya S, Biswas D, Sau A. Evaluation of percutaneous vertebroplasty for management of symptomatic osteoporotic compression fracture. Journal of Clinical and Diagnostic Research. 2017; 11 :RC07-RC10. DOI: 10.7860/JCDR/2017/25886.10461 - 8.
Schousboe JT. Epidemiology of vertebral fractures. Journal of Clinical Densitometry. 2016; 19 :8-22. DOI: 10.1016/j.jocd.2015.08.004 - 9.
Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017; 104 :54-65. DOI: 10.1016/j.bone.2017.03.004 - 10.
Borgstrom F, Olafsson G, Strom O, Tillman JB, Wardlaw D, Boonen S, et al. The impact of different health dimensions on overall quality of life related to kyphoplasty and non-surgical management. Osteoporosis International. 2013; 24 :1991-1999. DOI: 10.1007/s00198-012-2237-x - 11.
Si L, Winzenberg TM, de Graaff B, Palmer AJ. A systematic review and meta-analysis of utility-based quality of life for osteoporosis-related conditions. Osteoporosis International. 2014; 25 :1987-1997. DOI: 10.1007/s00198-014-2636-2 - 12.
Ross PD, Ettinger B, Davis JW, Melton LJ 3rd, Wasnich RD. Evaluation of adverse health outcomes associated with vertebral fractures. Osteoporosis International. 1991; 1 :134-140. DOI: 10.1007/BF01625442 - 13.
Mauch JT, Carr CM, Cloft H, Diehn FE. Review of the imaging features of benign osteoporotic and malignant vertebral compression fractures. AJNR. American Journal of Neuroradiology. 2018; 39 :1584-1592. DOI: 10.3174/ajnr.A5528 - 14.
Takahashi S, Hoshino M, Takayama K, et al. Predicting delayed union in osteoporotic vertebral fractures with consecutive magnetic resonance imaging in the acute phase: A multicenter cohort study. Osteoporosis International. 2016; 27 :3567-3575. DOI: 10.1007/s00198-016-3687-3 - 15.
Marongiu G, Congia S, Verona M, Lombardo M, Podda D, Capone A. The impact of magnetic resonance imaging in the diagnostic and classification process of osteoporotic vertebral fractures. Injury. 2018; 49 (Suppl. 3):S26-S31. DOI: 10.1016/j.injury.2018.10.006 - 16.
Miller PD. Management of severe osteoporosis. Expert Opinion on Pharmacotherapy. 2016; 17 :473-488. DOI: 10.1517/14656566.2016.1124856 - 17.
Gou P, Zhao Z, Yu C, Hou X, Gao G, Zhang T, et al. Efficacy of recombinant human parathyroid hormone versus vertebral augmentation procedure on patients with acute osteoporotic vertebral compression fracture. Orthopaedic Surgery. 2022; 14 :2510-2518. DOI: 10.1111/os.13470 - 18.
Lin WC, Lee YC, Lee CH, Kuo YL, Cheng YF, Lui CC, et al. Refractures in cemented vertebrae after percutaneous vertebroplasty: A retrospective analysis. European Spine Journal. 2008; 17 :592-599. DOI: 10.1007/s00586-007-0564-y - 19.
Lavelle WF, Cheney R. Recurrent fracture after vertebral kyphoplasty. The Spine Journal. 2006; 6 :488-493. DOI: 10.1016/j.spinee.2005.10.013 - 20.
Lunt M, O’Neill TW, Felsenberg D, Reeve J, Kanis JA, Cooper C, et al. Characteristics of a prevalent vertebral deformity predict subsequent vertebral fracture: Results from the European Prospective Osteoporosis Study (EPOS). Bone. 2003; 33 :505-513. DOI: 10.1016/s8756-3282(03)00248-5 - 21.
Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. Journal of the American Medical Association. 2001; 285 :320-323. DOI: 10.1001/jama.285.3.320 - 22.
Wong CC, McGirt MJ. Vertebral compression fractures: A review of current management and multimodal therapy. Journal of Multidisciplinary Healthcare. 2013; 6 :205-214. DOI: 10.2147/JMDH.S31659 - 23.
Edidin AA, Ong KL, Lau E, Kurtz SM. Morbidity and mortality after vertebral fractures: Comparison of vertebral augmentation and nonoperative management in the medicare population. Spine (Phila Pa 1976). 2015; 40 :1228-1241. DOI: 10.1097/BRS.0000000000000992 - 24.
Lim J, Choi SW, Youm JY, Kwon HJ, Kim SH, Koh HS. Posttraumatic delayed vertebral collapse: Kummell’s disease. Journal of Korean Neurosurgical Association. 2018; 61 :1-9. DOI: 10.3340/jkns.2017.0505.010 - 25.
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. Journal of the American Medical Association. 2009; 301 :513-521. DOI: 10.1001/jama.2009.50 - 26.
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019; 393 :364-376. DOI: 10.1016/S0140-6736(18)32112-3 - 27.
Black DM, Rosen CJ. Clinical practice: Postmenopausal osteoporosis. New England Journal of Medicine. 2016; 374 :254-262. DOI: 10.1056/NEJMcp1513724 - 28.
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Annals of the Rheumatic Diseases. 2009; 68 :99-102. DOI: 10.1136/ard.2008.092775 - 29.
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Archives of Internal Medicine. 2004; 164 :1108-1112. DOI: 10.1001/archinte.164.10.1108 - 30.
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam study. Bone. 2004; 34 :195-202. DOI: 10.1016/j.bone.2003.10.001 - 31.
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporosis International. 2008; 19 :385-397. DOI: 10.1007/s00198-007-0543-5 - 32.
Middleton RG, Shabani F, Uzoigwe CE, Shoaib A, Moqsith M, Venkatesan M. FRAX and the assessment of the risk of developing a fragility fracture. Journal of Bone and Joint Surgery. British Volume (London). 2012; 94 :1313-1320. DOI: 10.1302/0301-620X.94B10.28889 - 33.
Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporosis International. 2011; 22 :2395-2411. DOI: 10.1007/s00198-011-1713-z - 34.
Kanis JA, Harvey NC, Johansson H, Oden A, McCloskey EV, Leslie WD. Overview of fracture prediction tools. Journal of Clinical Densitometry. 2017; 20 :444-450. DOI: 10.1016/j.jocd.2017.06.013 - 35.
Crandall CJ. Risk assessment tools for osteoporosis screening in postmenopausal women: A systematic review. Current Osteoporosis Reports. 2015; 13 :287-301. DOI: 10.1007/s11914-015-0282-z - 36.
Engelke K, Chaudry O, Bartenschlager S. Opportunistic screening techniques for analysis of CT scans. Current Osteoporosis Reports. 2023; 21 :65-76. DOI: 10.1007/s11914-022-00764-5 - 37.
Zou D, Li W, Deng C, Du G, Xu N. The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. European Spine Journal. 2019; 28 :1758-1766. DOI: 10.1007/s00586-018-5776-9 - 38.
Lee S, Binkley N, Lubner M, Bruce R, Ziemlewicz T, Pickhardt P. Opportunistic screening for osteoporosis using the sagittal reconstruction from routine abdominal CT for combined assessment of vertebral fractures and density. Osteoporosis International. 2016; 27 :1131-1136. DOI: 10.1007/s00198-015-3318-4 - 39.
Dagan N, Elnekave E, Barda N, Bregman-Amitai O, Bar A, Orlovsky M, et al. Automated opportunistic osteoporotic fracture risk assessment using computed tomography scans to aid in FRAX underutilization. Nature Medicine. 2020; 26 :77-82. DOI: 10.1038/s41591-019-0720-z - 40.
Cozadd AJ, Schroder LK, Switzer JA. Fracture risk assessment: An update. The Journal of Bone and Joint Surgery. American Volume. 2021; 103 :1238-1246. DOI: 10.2106/JBJS.20.01071 - 41.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism. 2011; 96 :1911-1930. DOI: 10.1210/jc.2011-0385 - 42.
Dudenkov DV, Yawn BP, Oberhelman SS, Fischer PR, Singh RJ, Cha SS, et al. Changing incidence of serum 25-hydroxyvitamin D values above 50 ng/mL: A 10-year population-based study. Mayo Clinic Proceedings. 2015; 90 :577-586. DOI: 10.1016/j.mayocp.2015.02.012 - 43.
Bertoldo F, Cianferotti L, Di Monaco M, et al. Definition, assessment, and management of vitamin D inadequacy: Suggestions, recommendations, and warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients. 2022; 14 :4148. DOI: 10.3390/nu14194148 - 44.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. The Journal of Clinical Endocrinology and Metabolism. 2012; 97 :1153-1158. DOI: 10.1210/jc.2011-2601 - 45.
Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evidence Report/Technology Assessment. 2007;(158):1-235 - 46.
Fernandez H, Mohammed HT, Patel T. Vitamin D supplementation for bone health in adults with epilepsy: A systematic review. Epilepsia. 2018; 59 :885-896. DOI: 10.1111/epi.14015 - 47.
Jean G, Souberbielle JC, Chazot C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients. 2017; 9 :328. DOI: 10.3390/nu9040328 - 48.
Bouillon R. Safety of high-dose vitamin D supplementation. The Journal of Clinical Endocrinology and Metabolism. 2020; 105 :1290-1291. DOI: 10.1210/clinem/dgz282 - 49.
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. Journal of the American Medical Association. 2010; 303 :1815-1822. DOI: 10.1001/jama.2010.594 - 50.
Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. The Journal of Clinical Endocrinology and Metabolism. 2011; 96 :53-58. DOI: 10.1210/jc.2010-2704 - 51.
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: A randomized clinical trial. JAMA Internal Medicine. 2016; 176 :175-183. DOI: 10.1001/jamainternmed.2015.7148 - 52.
LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis International. 2022; 33 :2049-2102. DOI: 10.1007/s00198-021-05900-y - 53.
Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. The New England Journal of Medicine. 2006; 354 :669-683. DOI: 10.1056/NEJMoa055218 - 54.
Bailey RL, Zou P, Wallace TC, McCabe GP, Craig BA, Jun S, et al. Calcium supplement use is associated with less bone mineral density loss, but does not lessen the risk of bone fracture across the menopause transition: Data from the study of women’s health across the Nation. JBMR Plus. 2020; 4 :e10246. DOI: 10.1002/jbm4.10246 - 55.
Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: The fracture intervention trial long-term extension (FLEX): A randomized trial. Journal of the American Medical Association. 2006; 296 :2927-2938. DOI: 10.1001/jama.296.24.2927 - 56.
Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: A randomized second extension to the HORIZON-pivotal fracture trial (PFT). Journal of Bone and Mineral Research. 2015; 30 :934-944. DOI: 10.1002/jbmr.2442 - 57.
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, et al. Fracture prevention with zoledronate in older women with osteopenia. The New England Journal of Medicine. 2018; 379 :2407-2416. DOI: 10.1056/NEJMoa1808082 - 58.
Robinson DE, Ali MS, Pallares N, et al. Safety of oral bisphosphonates in moderate-to-severe chronic kidney disease: A binational cohort analysis. Journal of Bone and Mineral Research. 2021; 36 :820-832. DOI: 10.1002/jbmr.4235 - 59.
Evenepoel P, Cunningham J, Ferrari S, et al. European consensus statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrology, Dialysis, Transplantation. 2021; 36 :42-59. DOI: 10.1093/ndt/gfaa192 - 60.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: Now and the future. Lancet. 2011; 377 :1276-1287. DOI: 10.1016/S0140-6736(10)62349-5 - 61.
Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. The Lancet Diabetes and Endocrinology. 2017; 5 :513-523. DOI: 10.1016/S2213-8587(17)30138-9 - 62.
Popp AW, Varathan N, Buffat H, Senn C, Perrelet R, Lippuner K. Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long-term denosumab treatment for osteoporosis. Calcified Tissue International. 2018; 103 :50-54. DOI: 10.1007/s00223-018-0394-4 - 63.
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. The Journal of Clinical Endocrinology and Metabolism. 2011; 96 :972-980. DOI: 10.1210/jc.2010-1502 - 64.
Tutaworn T, Nieves JW, Wang Z, Levin JE, Yoo JE, Lane JM. Bone loss after denosumab discontinuation is prevented by alendronate and zoledronic acid but not risedronate: A retrospective study. Osteoporosis International. 2023; 34 :573-584. DOI: 10.1007/s00198-022-06648-9 - 65.
Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: A post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. Journal of Bone and Mineral Research. 2018; 33 :190-198. DOI: 10.1002/jbmr.3337 - 66.
Miller PD, Pannacciulli N, Brown JP, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. The Journal of Clinical Endocrinology and Metabolism. 2016; 101 :3163-3170. DOI: 10.1210/jc.2016-1801 - 67.
Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA. Diagnosis of endocrine disease: Bone turnover markers: Are they clinically useful? European Journal of Endocrinology. 2018; 178 :R19-R31. DOI: 10.1530/EJE-17-0585 - 68.
Camacho PM, Lopez NA. Use of biochemical markers of bone turnover in the management of postmenopausal osteoporosis. Clinical Chemistry and Laboratory Medicine. 2008; 46 :1345-1357. DOI: 10.1515/CCLM.2008.310 - 69.
Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET, National Bone Health Alliance Bone Turnover Marker P. Use of CTX-I and PINP as bone turnover markers: National bone health alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporosis International. 2017; 28 :2541-2556. DOI: 10.1007/s00198-017-4082-4 - 70.
Papapoulos S, Lippuner K, Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the FREEDOM extension study. Osteoporosis International. 2015; 26 :2773-2783. DOI: 10.1007/s00198-015-3234-7 - 71.
Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. The New England Journal of Medicine. 2009; 361 :756-765. DOI: 10.1056/NEJMoa0809493 - 72.
Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The women’s health initiative randomized trial. Journal of the American Medical Association. 2003; 290 :1729-1738. DOI: 10.1001/jama.290.13.1729 - 73.
Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. Journal of the American Medical Association. 2002; 288 :321-333. DOI: 10.1001/jama.288.3.321 - 74.
The NHTPSAP. Hormone therapy position statement of the North American Menopause Society. Menopause. 2017; 24 :728-753. DOI: 10.1097/GME.0000000000000921 - 75.
Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022; 399 :1080-1092. DOI: 10.1016/S0140-6736(21)02646-5 - 76.
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England Journal of Medicine. 2001; 344 :1434-1441. DOI: 10.1056/NEJM200105103441904 - 77.
Gilsenan A, Midkiff K, Harris D, Kellier-Steele N, McSorley D, Andrews EB. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. Journal of Bone and Mineral Research. 2021; 36 :244-251. DOI: 10.1002/jbmr.4188 - 78.
Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet. 2017; 390 :1585-1594. DOI: 10.1016/S0140-6736(17)31613-6 - 79.
Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-switch study): Extension of a randomised controlled trial. Lancet. 2015; 386 :1147-1155. DOI: 10.1016/S0140-6736(15)61120-5 - 80.
Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. The New England Journal of Medicine. 2007; 357 :2028-2039. DOI: 10.1056/NEJMoa071408 - 81.
McClung MR. Using osteoporosis therapies in combination. Current Osteoporosis Reports. 2017; 15 :343-352. DOI: 10.1007/s11914-017-0376-x - 82.
Recker RR, Marin F, Ish-Shalom S, et al. Comparative effects of teriparatide and strontium ranelate on bone biopsies and biochemical markers of bone turnover in postmenopausal women with osteoporosis. Journal of Bone and Mineral Research. 2009; 24 :1358-1368. DOI: 10.1359/jbmr.090315 - 83.
Krege JH, Lane NE, Harris JM, Miller PD. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporosis International. 2014; 25 :2159-2171. DOI: 10.1007/s00198-014-2646-0 - 84.
Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, et al. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009; 45 :1053-1058. DOI: 10.1016/j.bone.2009.07.091 - 85.
Farahmand P, Marin F, Hawkins F, et al. Early changes in biochemical markers of bone formation during teriparatide therapy correlate with improvements in vertebral strength in men with glucocorticoid-induced osteoporosis. Osteoporosis International. 2013; 24 :2971-2981. DOI: 10.1007/s00198-013-2379-5 - 86.
McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. The New England Journal of Medicine. 2014; 370 :412-420. DOI: 10.1056/NEJMoa1305224 - 87.
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. The New England Journal of Medicine. 2016; 375 :1532-1543. DOI: 10.1056/NEJMoa1607948 - 88.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. The New England Journal of Medicine. 2017; 377 :1417-1427. DOI: 10.1056/NEJMoa1708322 - 89.
Diez-Perez A, Gonzalez-Macias J. Inadequate responders to osteoporosis treatment: Proposal for an operational definition. Osteoporosis International. 2008; 19 :1511-1516. DOI: 10.1007/s00198-008-0659-2 - 90.
Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. Journal of Bone and Mineral Research. 2002; 17 :1-10. DOI: 10.1359/jbmr.2002.17.1.1 - 91.
Khosla S. Surrogates for fracture endpoints in clinical trials. Journal of Bone and Mineral Research. 2003; 18 :1146-1149. DOI: 10.1359/jbmr.2003.18.6.1146 - 92.
Diez-Perez A, Adachi JD, Agnusdei D, et al. Treatment failure in osteoporosis. Osteoporosis International. 2012; 23 :2769-2774. DOI: 10.1007/s00198-012-2093-8 - 93.
Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporosis International. 2011; 22 :391-420. DOI: 10.1007/s00198-010-1501-1 - 94.
Szulc P. The role of bone turnover markers in monitoring treatment in postmenopausal osteoporosis. Clinical Biochemistry. 2012; 45 :907-919. DOI: 10.1016/j.clinbiochem.2012.01.022 - 95.
Delmas PD, Munoz F, Black DM, et al. Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. Journal of Bone and Mineral Research. 2009; 24 :1544-1551. DOI: 10.1359/jbmr.090310 - 96.
Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: The fracture intervention trial. Journal of Bone and Mineral Research. 2004; 19 :1250-1258. DOI: 10.1359/JBMR.040512 - 97.
Ameis A, Randhawa K, Yu H, et al. The global spine care initiative: A review of reviews and recommendations for the non-invasive management of acute osteoporotic vertebral compression fracture pain in low- and middle-income communities. European Spine Journal. 2018; 27 :861-869. DOI: 10.1007/s00586-017-5273-6 - 98.
Stadhouder A, Buskens E, Vergroesen DA, Fidler MW, de Nies F, Oner FC. Nonoperative treatment of thoracic and lumbar spine fractures: A prospective randomized study of different treatment options. Journal of Orthopaedic Trauma. 2009; 23 :588-594. DOI: 10.1097/BOT.0b013e3181a18728 - 99.
Kato T, Inose H, Ichimura S, et al. Comparison of rigid and soft-brace treatments for acute osteoporotic vertebral compression fracture: A prospective, randomized, multicenter study. Journal of Clinical Medicine. 2019; 8 :198. DOI: 10.3390/jcm8020198 - 100.
Klazen CA, Verhaar HJ, Lohle PN, Lampmann LE, Juttmann JR, Schoemaker MC, et al. Clinical course of pain in acute osteoporotic vertebral compression fractures. Journal of Vascular and Interventional Radiology. 2010; 21 :1405-1409. DOI: 10.1016/j.jvir.2010.05.018 - 101.
Venmans A, Klazen CA, Lohle PN, Mali WP, van Rooij WJ. Natural history of pain in patients with conservatively treated osteoporotic vertebral compression fractures: Results from VERTOS II. AJNR. American Journal of Neuroradiology. 2012; 33 :519-521. DOI: 10.3174/ajnr.A2817 - 102.
Takahashi S, Hoshino M, Takayama K, et al. The natural course of the paravertebral muscles after the onset of osteoporotic vertebral fracture. Osteoporosis International. 2020; 31 :1089-1095. DOI: 10.1007/s00198-020-05338-8 - 103.
Zhang J, He X, Fan Y, Du J, Hao D. Risk factors for conservative treatment failure in acute osteoporotic vertebral compression fractures (OVCFs). Archives of Osteoporosis. 2019; 14 :24. DOI: 10.1007/s11657-019-0563-8 - 104.
Muratore M, Ferrera A, Masse A, Bistolfi A. Osteoporotic vertebral fractures: Predictive factors for conservative treatment failure. A systematic review. European Spine Journal. 2018; 27 :2565-2576. DOI: 10.1007/s00586-017-5340-z - 105.
Inose H, Kato T, Ichimura S, et al. Risk factors of nonunion after acute osteoporotic vertebral fractures: A prospective multicenter cohort study. Spine (Phila Pa 1976). 2020; 45 :895-902. DOI: 10.1097/BRS.0000000000003413 - 106.
Hinde K, Maingard J, Hirsch JA, Phan K, Asadi H, Chandra RV. Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: A systematic review and meta-analysis. Radiology. 2020; 295 :96-103. DOI: 10.1148/radiol.2020191294 - 107.
Tsoumakidou G, Too CW, Koch G, Caudrelier J, Cazzato RL, Garnon J, et al. CIRSE guidelines on percutaneous vertebral augmentation. Cardiovascular and Interventional Radiology. 2017; 40 :331-342. DOI: 10.1007/s00270-017-1574-8 - 108.
Hayashi T, Maeda T, Masuda M, Ueta T, Shiba K. Morphology of the injured posterior wall causing spinal canal encroachment in osteoporotic vertebral fractures. The Spine Journal. 2016; 16 :946-950. DOI: 10.1016/j.spinee.2016.03.021 - 109.
Morghen I, Borrelli M, Saletti A, Zoppellari R. Percutaneous vertebroplasty and spinal cord compression: A case report. Journal of Radiological Case Report. 2009; 3 :17-20 - 110.
Morris O, Mathai J, Weller K. Polymethylmethacrylate pulmonary embolism following kyphoplasty. Clinical Practicing Cases Emergency Medicine. 2019; 3 :226-228 - 111.
Kim YY, Rhyu KW. Recompression of vertebral body after balloon kyphoplasty for osteoporotic vertebral compression fracture. European Spine Journal. 2010; 19 :1907-1912. DOI: 10.1007/s00586-010-1479-6 - 112.
Li YX, Guo DQ , Zhang SC, Liang YK, Mo GY, Li DX, et al. Risk factor analysis for re-collapse of cemented vertebrae after percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP). International Orthopaedics. 2018; 42 :2131-2139. DOI: 10.1007/s00264-018-3838-6 - 113.
Anselmetti GC, Zoarski G, Manca A, Masala S, Eminefendic H, Russo F, et al. Percutaneous vertebroplasty and bone cement leakage: Clinical experience with a new high-viscosity bone cement and delivery system for vertebral augmentation in benign and malignant compression fractures. Cardiovascular and Interventional Radiology. 2008; 31 :937-947. DOI: 10.1007/s00270-008-9324-6 - 114.
Yu WB, Jiang XB, Liang D, Xu WX, Ye LQ , Wang J. Risk factors and score for recollapse of the augmented vertebrae after percutaneous vertebroplasty in osteoporotic vertebral compression fractures. Osteoporosis International. 2018; 30 :423-430. DOI: 10.1007/s00198-018-4754-8 - 115.
Mudano AS, Bian J, Cope JU, et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: A population-based cohort study. Osteoporosis International. 2009; 20 :819-826. DOI: 10.1007/s00198-008-0745-5 - 116.
Ebeling PR, Akesson K, Bauer DC, et al. The efficacy and safety of vertebral augmentation: A second ASBMR task force report. Journal of Bone and Mineral Research. 2019; 34 :3-21. DOI: 10.1002/jbmr.3653 - 117.
Dyer O. Vertebral augmentation in osteoporosis: Common procedures for spinal fractures show no benefit. BMJ. 2019; 364 :l515. DOI: 10.1136/bmj.l515 - 118.
Clark W, Bird P, Gonski P, Diamond TH, Smerdely P, McNeil HP, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 388 :1408-1416. DOI: 10.1016/S0140-6736(16)31341-1 - 119.
Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database of Systematic Reviews. 2018; 4 :CD006349. DOI: 10.1002/14651858.CD006349.pub3 - 120.
Buchbinder R, Busija L. Why we should stop performing vertebroplasties for osteoporotic spinal fractures. Internal Medicine Journal. 2019; 49 :1367-1371. DOI: 10.1111/imj.14628 - 121.
Zhao Y, Xue R, Shi N, Xue Y, Zong Y, Lin W, et al. Aggravation of spinal cord compromise following new osteoporotic vertebral compression fracture prevented by teriparatide in patients with surgical contraindications. Osteoporosis International. 2016; 27 :3309-3317. DOI: 10.1007/s00198-016-3651-2 - 122.
Omi H, Yokoyama T, Ono A, Numasawa T, Wada K, Fujisawa Y. Can MRI predict subsequent pseudarthrosis resulting from osteoporotic thoracolumbar vertebral fractures? European Spine Journal. 2014; 23 :2705-2710. DOI: 10.1007/s00586-014-3490-9 - 123.
Ma Y, Wu X, Xiao X, Ma Y, Feng L, Yan W, et al. Effects of teriparatide versus percutaneous vertebroplasty on pain relief, quality of life and cost-effectiveness in postmenopausal females with acute osteoporotic vertebral compression fracture: A prospective cohort study. Bone. 2020; 131 :115154. DOI: 10.1016/j.bone.2019.115154 - 124.
Ha KY, Park KS, Kim SI, Kim YH. Does bisphosphonate-based anti-osteoporosis medication affect osteoporotic spinal fracture healing? Osteoporosis International. 2016; 27 :483-488. DOI: 10.1007/s00198-015-3243-6 - 125.
Min H-K, Ahn J-H, Ha K-Y, et al. Effects of anti-osteoporosis medications on radiological and clinical results after acute osteoporotic spinal fractures: A retrospective analysis of prospectively designed study. Osteoporosis International. 2019; 30 :2249-2256 - 126.
Iwata A, Kanayama M, Oha F, Hashimoto T, Iwasaki N. Effect of teriparatide (rh-PTH 1-34) versus bisphosphonate on the healing of osteoporotic vertebral compression fracture: A retrospective comparative study. BMC Musculoskeletal Disorders. 2017; 18 :148. DOI: 10.1186/s12891-017-1509-1 - 127.
Gou P, Wang Z, Zhao Z, Wang Y, Jiang Y, Xue Y. Restoration of the intravertebral stability in Kummell’s disease following the treatment of severe postmenopausal osteoporosis by 1-34PTH-a retrospective study. Osteoporosis International. 2021; 32 :1451-1459. DOI: 10.1007/s00198-020-05761-x - 128.
Gou P, Jing W, Zhou J, Wang R, Wang Z, Chang F, et al. Magnetic resonance imaging negative spine trauma followed by a delayed intravertebral vacuum cleft-Kummell’s disease: A case report and literature review. Orthopaedic Surgery. 2023; 15 :366-370. DOI: 10.1111/os.13559 - 129.
Kim DY, Lee SH, Jang JS, Chung SK, Lee HY. Intravertebral vacuum phenomenon in osteoporotic compression fracture: Report of 67 cases with quantitative evaluation of intravertebral instability. Journal of Neurosurgery. 2004; 100 :24-31. DOI: 10.3171/spi.2004.100.1.0024 - 130.
McKiernan F, Faciszewski T. Intravertebral clefts in osteoporotic vertebral compression fractures. Arthritis and Rheumatism. 2003; 48 :1414-1419. DOI: 10.1002/art.10984 - 131.
Tsujio T, Nakamura H, Terai H, et al. Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: A prospective multicenter study. Spine (Phila Pa 1976). 2011; 36 :1229-1235. DOI: 10.1097/BRS.0b013e3181f29e8d - 132.
Gou PG, Zhao ZH, Zhou JM, Ren LH, Wang XY, Mu YF, et al. Vertebral collapse prevented following teriparatide treatment in postmenopausal Kummell’s disease patients with severe osteoporosis. Orthopaedic Surgery. 2021; 13 :506-516. DOI: 10.1111/os.12959 - 133.
Diamond T, Clark W, Kumar S. Histomorphometric analysis of fracture healing cascade in acute osteoporotic vertebral body fractures. Bone. 2007; 40 :775-780. DOI: 10.1016/j.bone.2006.10.009 - 134.
Formica M, Zanirato A, Cavagnaro L, Basso M, Divano S, Lamartina C, et al. Vertebral body osteonecrosis: Proposal of a treatment-oriented classification system. European Spine Journal. 2018; 27 :190-197. DOI: 10.1007/s00586-018-5600-6 - 135.
Yang H, Pan J, Wang G. A review of osteoporotic vertebral fracture nonunion management. Spine (Phila Pa 1976). 2014; 39 :B4-B6. DOI: 10.1097/BRS.0000000000000538 - 136.
Laredo JD. Expert’s comment concerning grand rounds case entitled “Kummell’s disease: Delayed post-traumatic osteonecrosis of the vertebral body” (by Ma R, Chow R, Shen FH). European Spine Journal. 2010; 19 :1071-1072. DOI: 10.1007/s00586-009-1204-5 - 137.
Matzaroglou C, Georgiou CS, Wilke HJ, Assimakopoulos K, Karageorgos A, Konstantinou D, et al. Kümmell’s disease: Is ischemic necrosis or vertebral “microcracking” the first step in the sequence? Medical Hypotheses. 2013; 80 :505. DOI: 10.1016/j.mehy.2012.12.003 - 138.
Formica M, Basso M, Cavagnaro L, Formica C, Zanirato A, Felli L. Kümmell’s disease: Illustrative case for definition criteria. The Spine Journal. 2016; 16 :e707-e708. DOI: 10.1016/j.spinee.2016.03.035 - 139.
Nakamae T, Fujimoto Y, Yamada K, Takata H, Shimbo T, Tsuchida Y. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture with intravertebral cleft associated with delayed neurologic deficit. European Spine Journal. 2013; 22 :1624-1632. DOI: 10.1007/s00586-013-2686-8 - 140.
Kawaguchi S et al. Symptomatic relevance of intravertebral cleft in patients with osteoporotic vertebral fracture. Journal of Neurosurgery: Spine. 2010; 13 :267-275. DOI: 10.3171/2010.3.SPINE09364