1. Introduction
1.1 History of polyethylene
It is interesting to know that the most utility item of modern day was discovered accidentally approximately 90 years ago. In 1933, Reginald Gibson and Eric Fawcett at the Imperial Chemical Industries (ICI) in the UK were trying to react ethylene with benzaldehyde under high pressure but ended up with a white waxy substance instead [1]. The Scientist discovered that the compound on heating was not exploding, which was quite surprising. He further heated it and let it cool down overnight and further noticed that the compound had turned into a white paste, which later on was known as “Polytheylene.” The term “plastic” is given to polyethylene (PE), as the plastic signifies the ability of a substance to mold itself into a variety of shapes. Its unique ability to get into different shapes is attributed to its composition that possesses C, N, Si, H, O, and N.
In the early 1940s, researchers at ICI and later at DuPont in the United States of America continued to develop polyethylene [2]. They realized that the process required high pressure and worked on improving production methods. In 1939, Paul Hogan and Robert Banks at ICI developed the first practical method for producing polyethylene by using a high-pressure process. This marked the beginning of large-scale production [3].
1.2 Applications of polyethylene
From there till date, this polyethylene and plastic have changed our life to a great extent. Polyethylene is widely used in packaging materials, including plastic bags, plastic films, and containers. In the medical field, polyethylene is used to manufacture various devices such as syringes, catheters, joint implants, and medical tubing [4]. Its biocompatibility and resistance to chemicals make it a valuable material for these applications [5]. Polyethylene is used in the construction industry for pipes, sheets, and insulation materials. Its durability, resistance to corrosion, and flexibility make it a popular choice for water and gas distribution systems. Many everyday items, such as toys, household items, and kitchenware, are made from polyethylene. Its versatility allows for a wide range of shapes and sizes. In automobiles, polyethylene is used for various components, including bumpers, fuel tanks, interior trims, and electrical insulation. It provides lightweight, impact-resistant solutions. Agricultural films made from polyethylene are used to cover crops, protecting them from pests, weeds, and adverse weather conditions. Polyethylene fibers are used in the production of a variety of textiles, including ropes, nets, and fabrics. Polyethylene is used in the production of various personal care items such as disposable diapers, sanitary napkins, and medical gloves. Overall, polyethylene’s versatility, durability, and cost-effectiveness have made it a fundamental material in modern society, influencing a wide range of industries and aspects of daily life (Figure 1).
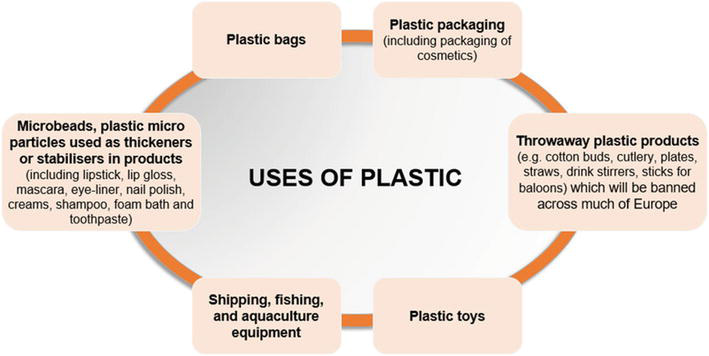
Figure 1.
Uses of plastics in various applications.
2. Structure, characteristics, and properties of polyethylene
Polyethylene is a polymer made up of repeating units of ethylene. It is a member of the polyolefin family of polymers. The polyethylene polymers are formed by condensation reaction, which was first produced by the scientist Von Pechmann in 1898 from the ether solution of diazomethane [6]. A similar product was observed by the scientist Bamberger in 1900 [7]. Just by observing the chemical structure of polyethylene, it seems quite simple. It includes the repetition of the same unit methylene along with some inclusion of comonomer at different places. This simple looking structure exhibits diversity, the largest diversity in the products formed by them.
There are three important or we can say fundamental molecular forces that contribute to its versatility and they are (1) the molecular weight and its distribution, (2) amount of comonomer and its distribution, and (3) short-chain/long-chain branching of monomers. Considering these factors, there are a variety of PEs available in the market. They are classified as:
2.1 High-density polyethylene (HDPE)
It is among the mostly used thermoplastic, as it is available in plenty, is pocket friendly, and possesses superior properties [8]. HDPE is known for its high strength-to-density ratio and is widely used in a variety of applications due to its versatile properties. It is a type of thermoplastic polymer made from petroleum. It possesses lesser branching, thus having stronger forces and high tensile strength. The HDPE has found its industrial applications in toys, packaging, construction, etc. HDPE is popular because it is relatively inexpensive, chemically resistant, tough, and can be easily molded or extruded into various shapes. It also has a lower environmental impact compared to some other plastics, as it is recyclable and can be reused in a variety of applications.
2.2 Low-density polyethylene (LDPE)
LDPE stands for low-density polyethylene. It is a type of thermoplastic polymer that is commonly used in a wide range of applications. LDPE is known for its flexibility, transparency, and resistance to moisture. It is commonly used in packaging materials such as plastic bags, shrink wraps, and containers. Additionally, LDPE is used in various consumer products like toys, squeeze bottles, and some types of medical devices.
Low-density polyethylene (LDPE) is created through the polymerization of ethylene, a basic hydrocarbon compound, under high pressure. This process produces a material with a lower density compared to other types of polyethylene, which gives LDPE its characteristic flexibility.
2.3 Linear low-density polyethylene (LLDPE)
LLDPE is a type of thermoplastic polymer made from the monomer ethylene. It is called “linear” because it has a more linear structure compared to other forms of polyethylene, which gives it certain unique properties. LLDPE is known for its flexibility, toughness, and relatively low density. It is commonly used in applications where a combination of these properties is desired.
2.4 Ultra-low density polyethylene (ULDPE)
ULDPE is a type of thermoplastic material known for its exceptionally low density compared to other forms of polyethylene. This characteristic makes ULDPE particularly suitable for applications where lightweight and flexible materials are required.
3. Conclusion
In conclusion, polyethylene (PE) stands as a remarkable material with a wide range of applications both in industry and households, owing to its exceptional properties. Its versatility, durability, and cost-effectiveness make it a cornerstone of modern manufacturing and consumer goods.
In industry, PE’s high chemical resistance and excellent electrical insulating properties make it a preferred material for various applications. It serves as a fundamental component in pipelines, cable insulation, and a variety of packaging materials. Its ability to be easily molded and extruded allows for intricate designs and customized solutions in a multitude of sectors.
Within households, PE plays an integral role in everyday life. From packaging materials like bags and containers to essential items like bottles, it provides convenience and protection for a wide array of goods. Its use in insulation materials, flooring, and furniture underscores its importance in enhancing the comfort and functionality of living spaces. Furthermore, in medical and personal care applications, PE ensures safety and hygiene.
However, it is crucial to recognize the environmental challenges associated with PE production and disposal. As a petroleum-based product, its production contributes to resource consumption and pollution. Proper recycling and responsible disposal practices are imperative to mitigate its impact on the environment.
In light of these considerations, ongoing efforts to develop sustainable alternatives and improve recycling technologies are essential. Despite its widespread use, the responsible use and management of polyethylene remain paramount in achieving a more sustainable and environmentally conscious future.
References
- 1.
Fawcett EWG, Gibson RO, Perrin MW, Patton JG, Williams EGB. Patent, 471,590. 1937 - 2.
Fawcett EW. Transactions of the Faraday Society. 1936; 32 :119 - 3.
Hoff R, Mathers RT. Chapter 10. Review of Phillips chromium catalyst for ethylene polymerization. In: Hoff R, Mathers RT, editors. Handbook of Transition Metal Polymerization Catalysts. John Wiley & Sons; 2010. DOI: 10.1002/9780470504437.ch10 - 4.
Biomedical applications of polyethylene. European Polymer Journal. 2019; 118 (3). DOI: 10.1016/j.eurpolymj.2019.05.037 - 5.
Plastics packaging for pharmaceutical products. 2021. DOI: 10.1016/B978-0-12-820352-1.00088-2 - 6.
Von Pechmann H. Chemische Berichte. 1898; 31 :2643 - 7.
Bamberger E, Tschirner F. Chemische Berichte. 1900; 33 :955 - 8.
Singh N, Hui D, Singh R, Ahuja I, Feo L, Fraternali F. Recycling of plastic solid waste: A state of art review and future applications. Composites. Part B, Engineering. 2017; 115 :409-422