Bacteria employed in research on the biodegradation of polyethylene (PE).
Abstract
Due to its adaptability and affordability, polyethylene, a synthetic polymer that is often utilized, has made a substantial contribution to modern civilization. However, due to its widespread usage, there is concern about its environmental persistence and potential ecological effects. This article seeks to present a thorough explanation of the mechanisms involved in polyethylene degradation, the environmental repercussions of its buildup, and proposed remediation techniques to lessen those effects. The study examines the fundamental processes of several degradation routes, such as biological degradation etc.. Efforts to address the ecological consequences of polythene use include reducing plastic waste management, developing biodegradation products.
Keywords
- polythene
- degradation
- microbial enzymes
- plastic pollution
- environmental impact
1. Introduction
The five most common petroleum-based polymers used to make single-use plastic products are polyethylene terephthalate (PET), polypropylene (PP), high density polyethylene (HDPE), polyvinyl chloride (PVC), and low density polyethylene (LDPE). The most prevalent petroleum-polymer on earth, LDPE, is responsible for up to 64% of single-use plastics that are discarded shortly after use, causing a massive and fast buildup in the environment [1, 2]. The negative impacts of basically “non-biodegradable” LDPE rubbish buildup in landfills and seas are growing despite recycling and energy recovery measures. Micro-plastics may now be found everywhere on the planet, including in the arctic snow, according to mounting evidence [3]. Finding an environmentally appropriate disposal method is thus required [4]. Contrary to biological waste that is dumped, polyethylene (PE) and other petroleum-based polymers are particularly resistant to natural biodegradation processes. The scientific literature has several studies on the biodegradation of synthetic polymers, including PE in particular. Thirteen evaluations of the microbes involved in the physical and microbial biodegradation processes have been published since 2008.
Although microbial breakdown of PE has been observed in various studies, significant degradation of PE wastes at usable sizes has not yet been achieved. We have been limited in our ability to develop a biochemically based knowledge of the mechanisms and processes involved in PE degradation due to the lack of a concrete definition of polyethylene biodegradation that may lead to testable hypotheses. Early investigations on microbial biodegradation attempted to demonstrate how microbial activity may change the tensile strength, water absorption, and crystallinity of plastics [5].
Pirt (1980) [6] conducted the first investigation of the microbial biodegradation of polymers. Ten years later, 0.2% less PE weight was present, according to Albertsson and Karlsson [1]. Otake et al. [7] found that PE polymers exhibited surface alterations after being buried in soil for 10 to 32 years. It was discovered that LDPE thin film deterioration was relatively high. Despite the fact that parts of the PE films with considerable deterioration were characterized by whitening with tiny holes, the overall rate of degradation was still fairly low even after years of contact with soil microorganisms.
Some researchers have investigated the aerobic biodegradation of treated polyethylene and/or polyethylene modified by the addition of additives (“addivitated”) PE in simulated soil burial and mature compost [3, 8], as well as in natural aquatic environments under laboratory conditions [9, 10]. Living microbial consortia are present in several kinds of soil [11]. Others looked at the microorganisms that cause LDPE to biodegrade in soil [12]. The biodegradability of thermally and photochemically damaged addiviated LDPE films by microorganisms adsorbed on the surface of PE films buried in agricultural soil was assessed in a research by Abrusci et al. [13] whiteness with tiny holes that defines it.
Typically, as part of microbial degradation test investigations, microorganisms from diverse sources are isolated to ascertain the optimal microbial power to degrade polymeric PE chains. Researchers have isolated potential microorganisms from a range of soil types, including garden soil, forest soil, waste soil, mangrove soil, and soil covered in agricultural PE films for soil mulching [14, 15, 16, 17]. Alternative sources for the isolation of high potential microorganisms that deteriorate PE included landfills, solid waste dumps, and plastic garbage (municipal solid soil) [4, 18, 19, 20, 21, 22, 23], water [2], waste water or sewage sludge, oil-contaminated soil, and even waxworm larvae [23].
Numerous bacteria from a small number of text were found to be present in these trials; however, not all of them were involved in the breakdown of PE (Table 1). Following the bacteria’s initial isolation, the capacity of each isolate to use treated and/or untreated polyethylene was examined in isolated shake-flask cultures throughout a range of time periods. The majority of these bacteria were identified using the sequencing of 16S ribosomal RNA genes following PCR amplification. The third phase was estimating biodegradation using PE-degrading bacteria on polyethylene particles or films using various approaches.
| Genus (and Species) | Source | Biodegradation result |
|---|---|---|
| Municipal landfill | Biomass production | |
| Dumped soil area | 20–30% W.L. | |
| Pelagic waters | 1.5%–1.75% W.L. | |
| Waste coal, a forest and an extinct volcano crater | Reduction of mechanical properties by 98% | |
| No W.L. detected | ||
| Shallow waters of ocean | 3.5% and 10% | |
| 9% and 19% | ||
| Soil | 7–10% mineralization | |
| Solid waste dumped | 11–16% | |
| MCC No. 2183 | 9.26% W.L. | |
| Pelagic waters | 1.5–1.75 W.L. % | |
| DSMZ | 17% W.L. | |
| Waste disposal site | 37.5% W.L. | |
| Waste water activated sludge soil | — | |
| Plastic debris in soil | Changing in chemical properties | |
| Plastic debris in soil | Changing in chemical properties | |
| Pelagic waters | 1% | |
| Having Gene bank ID | 61% W.L. | |
| Mangrove soil | 20.54% W.L. | |
| Petroleum contaminated beach soil | 40.8% W.L. | |
| Beach soil contaminated with crude oil | 4.9%–28.6% CO2 production | |
| Garbage soil | 37.09% W.L. | |
| Municipal Landfill | 17.8% W.L. | |
| Having Gene bank ID | 50.5% W.L. | |
| ATCC | 9–20% | |
| Waste disposal site | 40.5% W.L. | |
| PE agricultural waste in soil | Up to 8% W.L. | |
| PE agricultural waste in soil | 0.86% W.L./week | |
| PE agricultural waste in soil | 1.5%–2.5% W.L. | |
| Reduction of 20%.in Mw and 15%.in Mn | ||
| ATCC | 60% mineralization | |
| ATCC 29672 | Different amount of mineralization | |
| Waste disposal site | 33% W.L. | |
| Three forest soil | Confirmation of Adhering | |
| Various soil environments | 13.6% W.L. | |
| Plastic debris in soil | Changing in chemical properties | |
| Solid waste dump site | Confirmed by FTIR | |
| Nile River Delta | 3 species showed slight W.L. |
Table 1.
Comparisons of the various biodegradation results are not significant due to the large diversity of PE materials employed and the vast range of growth conditions. This emphasizes the requirement for standardized approaches and procedures to comprehensively investigate the biodegradation of synthetic polymers. We need to identify the differences between degradation and deterioration as well as what the biodegradation process entails in order to resolve any difficulties brought up by stories of attempts at microbial biodegradation of PE that failed. The conditions that promote the microbial destruction of PE are discussed in the sections that follow, along with how these factors led to reports of incorrect PE biodegradation percentages. Then, we provide an appropriate explanation of the biodegradation process that will make it possible to interpret the findings of biodegradation in an accurate manner.
2. Biological degradation of PE
There are four steps to the whole biodegradation process: biodeterioration, biofragmentation, bioassimilation, and mineralization. However, access sites in the PE structure are necessary for microorganisms to start fragmenting before they can start attacking PE. As a result, before the presence of microorganisms, oxidation of PE polymers happens by abiotic processes such as ultraviolet (UV) radiation exposure combined with heat and/or environmental chemicals. It is well known that thermal aging frequently occurs in conjunction with PE oxidation, particularly UV-induced PE oxidation. The mechanisms of polymer change have also been well shown. According to earlier studies, when PE is exposed to UV radiation or oxidizing agents, carbonyl groups are produced in the alkane chains. These carbonyl groups are then further hydrolyzed by microorganisms, which catabolize the shorter PE chain reaction products (fragmentation). In this method, the polymer chain initially absorbs UV light, which causes radical production. At some point, oxygen is taken in, hydroperoxides are created, and carbonyl groups are created (Figure 1). The carbonyl groups proceed through Norrish Type I and/or Type II degradation with additional UV exposure. Additionally, pro-oxidants or contaminants might start photo-oxidation. Additionally, UV-degradation might start at spots where minute amounts of ketone or hydroperoxide groups were added during fabrication or production.
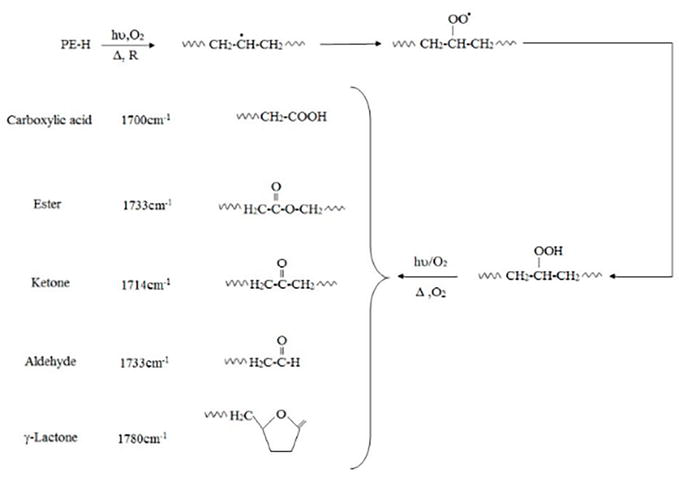
Figure 1.
Degradation pathways of polyethylene containing pro-oxidant additives.
Infrared spectroscopy (IR) measurements of the degree of carbonyl group adsorption can be used to monitor the oxidative degradation of polyolefins. The concentration levels of carbonyl compounds determined by ATR-FTIR were often represented as a carbonyl index (C.I.), which is defined as the ratio of carbonyl and methylene absorbances. The ratio of the methylene absorption band at 1435 cm1 (the CH2 scissoring peak) to the carbonyl peak at 1714 cm1 taken as an internal thickness band (CI = A1714/A1435). Even after storage in an abiotic environment, photo-oxidation and increased stress both accelerate the production of carbonyl groups.
3. Biodeterioration of PE
Some microbes can start the oxidation process on their own, via the process of “hydroperoxidation,” in addition to the abiotic degradation of PE materials. “Biodeterioration” is the word used to describe this. However, it is currently unclear whether PE that has been oxidized in this way can eventually be broken down by microbes [9]. Different pro-oxidation additives (prodegradants) have been added to the structure of polyethylene products to make them “oxo-degradable” in various investigations of the microbial breakdown of PE. “Addiviated” polymers are PE polymers that include additives that make them oxo-degradable. Materials used to make addiviated PE polymers oxo-degradable include polyunsaturated compounds, transition metals like iron, cobalt, manganese, and calcium, totally degradable plastic additives (TDPA) with different commercial names [7, 30, 31, 40], natural polymers (e.g., starch, cellulose, or chitosan), food grade dyes, or synthetic polymers containing ester, hydroxyl or ether groups [33, 35, 40] that are prone to hydrolytic cleavage by microorganisms. Abiotic factors like sunlight, heat, or both, as well as the addition of oxidizing chemical agents like nitric acid are used in some comparative studies of the microbial degradation of PE to start the degradation of raw and addiviated PE polymers and make the plastic more susceptible to microbial degradation. Following this, the impacts of various treatments on PE structure and microbial degradation were examined and compared to samples that had not been processed. The development of oxidized oligomers and alteration of the polymer are caused by a change in the fundamental structure of PE during the degrading process. The PE becomes brittle and vulnerable to additional oxidation by enzymes released by the microorganisms as a result of deterioration caused by physical, biological, or chemical factors. While PE’s molecular structure is changing at this point, the polymer is not fragmenting or losing structure. An increase in entry locations for enzymes released by microbes and a decline in the polymer’s mechanical or other physical qualities are two main characteristics of the degradation phase overall.
4. Experiments on microbial degradation of PE: contributing factors
The outcome and findings of PE biodegradation tests are significantly influenced by a variety of parameters in the microbial breakdown of PE polymers. Unfortunately, while planning and designing the trials that were described in the literature, these considerations were frequently ignored. As a result, the information provided in these papers about PE biodegradation has been inconsistent and inconclusive. Following is a description of these elements.
4.1 Polyethylene shape and structure
The ability of the microorganisms’ secreted enzymes to reach the PE carbon chain is crucial for microbial breakdown. All PE materials have a simple linear carbon chain microstructure that is joined by hydrogen bonds. But polyethylene polymers can have a variety of densities and three-dimensional (3-D) structures (Figure 2), including low molecular weight polyethylene (LMWPE), linear low-density polyethylene (LLDPE), low-density polyethylene (LDPE), and high-density polyethylene (HDPE), depending on the manufacturing processes used.
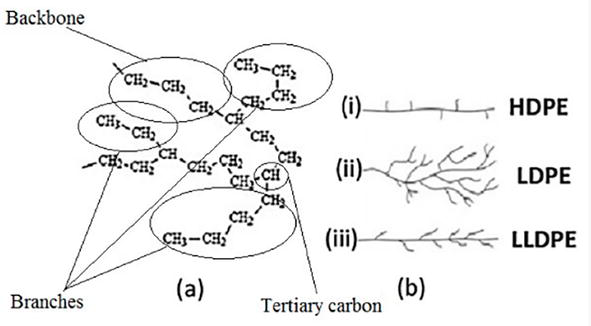
Figure 2.
Polyethylene structure.
PE often has a semi-crystalline structure as well. LDPE crystallinity ranges from 45–65%, depending on the type of processing. Short branches (10–30 CH3 groups per 1000 C-atoms) made comprised of one or more co-monomers like 1-butene, 1-hexene, and 1-octene are typically found in amorphous regions of LDPE. The LDPE chains near the surface are made more accessible by the branching system’s prevention of the PE molecules from stacking closely together, and the tertiary carbon atoms at the branch sites are left more vulnerable to assault. Additionally, amorphous areas are more likely to contain contaminants.
So that it is feasible to determine how much polymer is present, it is crucial that the structure and percentage of amorphous and crystalline areas in the polymer be recorded.
4.2 Modification of polyethylene
PE often has a semi-crystalline structure as well. LDPE crystallinity ranges from 45–65%, depending on the type of processing. Short branches (10–30 CH3 groups per 1000 C-atoms) made comprised of one or more co-monomers like 1-butene, 1-hexene, and 1-octene are typically found in amorphous regions of LDPE. The LDPE chains near the surface are made more accessible by the branching system’s prevention of the PE molecules from stacking closely together, and the tertiary carbon atoms at the branch sites are left more vulnerable to assault. Additionally, amorphous areas are more likely to contain contaminants. So that it is feasible to determine how much polymer is present, it is crucial that the structure and percentage of amorphous and crystalline areas in the polymer be recorded.
But the primary goal of LDPE modification is to cause the polyethylene structure to deteriorate, allowing more access to the enzymes released by microorganisms during the biodegradation stage. Treatments alter the structure of PE, and as a result, investigations using various forms of PE with varying Mw, Mn, and/or molecular distributions have produced varying biodegradation outcomes. To effectively quantify microbial degradation and ascertain the only impact of microorganisms’ activities, these changes in the biodegradation process need to be identified and documented.
4.3 Partial biodegradation versus complete degradation
The consumption and mineralization of whole, unaltered polymers, including the polymer’s backbone, might be considered complete biodegradation of PE polymers. Microbes that can totally breakdown and mineralize virgin polyethylene have not yet been discovered, according to Yoon et al. Even so, it’s possible to classify the numerous cases of PE biodegradation in the literature as incomplete biodegradation. As previously mentioned, PE polymers are composed of a complex of linear carbon chains held together by van-der-Waals interactions, accessible short side-chains with tertiary carbon that contain amorphous sections, terminal methyl-groups at the ends of chains, short branches, and small oxidative products, as well as numerous linear and branched n-alkane side-chains. Because the side-chains of PE mimic linear n-alkanes, they may serve as the first site of contact for bacterial enzymes that cause the polymers to partially degrade. Without fragmenting the polymer’s backbone, low molar mass molecules and/or amorphous segments are removed from its surface. In contrast to the fragmentation of the backbone or pure PE polymers, weight loss during the early stages of PE degradation may be explained by the enzymatic hydrolysis of these readily accessible side chains. It is insufficient to conclude that polyethylene has completely degraded by looking at the development of microbes on agar plates containing the material. This has been one of the main issues with biodegradation experiments since it is necessary to establish complete biodegradation.
4.4 Other carbon sources’ influence on biodegradation
There are various carbon sources that, in biodegradation tests, are frequently absorbed by bacteria during the initial phases of microbial breakdown and may interfere with the only carbon supply of PE. Establishing a growth curve for the bacteria under research using PE as the carbon source is advised as a solution to the issue. Changes in the development curve might signify the use of various carbon sources with varying degrees of accessibility to microbes [18]. Impurities that are integrated into PE chains or that adhere to the PE surface may include substances that bacteria can use as a source of carbon. Consumption of these contaminants can compete with or obstruct the use of PE as a carbon source. Incubating non-PE degrading bacteria, such as
Generally speaking, two distinct groups of researchers have carried out experiments for the microbial breakdown of PE. Environmentalists in general are the groups of researchers who have studied the degradation of bulk PE materials of various types (LMWPE, LLDPE, LDPE, or HDPE) in natural settings such as soil, compost, or aquatic systems with mixed, undefined populations of microorganisms, without paying attention to microbial type. Any change seen is referred to as “biodegradation” whether it relates to appearance, weight loss, or mechanical qualities of the PE. The mechanisms influencing changes in the PE are unclear, and this strategy is mostly based on “trial and error.” The distinction between deterioration and partial degradation is misunderstood by the authors of these works. On the other hand, these tests have the benefit of being carried out in the real world under actual environmental circumstances, and the outcomes accurately represent the deterioration of PE. The final conversion of PE to CO2 and biomass (mineralization via genuine biodegradation), is a topic of interest to microbiologists who have also studied PE breakdown. The biodegradation tests are carried out with specific species of microorganisms isolated using specialized medium from collections. In general, the tests’ many components are clearly specified, and the authors are aware of how biodegradation works. The use of molecular biology and genomic sciences has started to pinpoint the precise genes and gene products involved in the breakdown of polyethylene in this regard. PE biodegradation is a complicated process that is impacted by a wide range of variables. Before being subjected to microbial treatment, a PE polymer chain may be exposed to various manufacturing, treatment, and sample preparation operations. The biodegradation process is complicated and uncertain since it involves a vast variety of bacteria with diverse behaviors and released chemicals. However, studies of polyethylene biodegradation experiments may be conducted from both chemical and microbiological perspectives.
5. Conclusion
The four steps of PE’s biodegradation process are biodeterioration, biofragmentation, bioassimilation, and mineralization. Complete biodegradation of PE necessitates a decrease in the polymer’s molar mass and molecular mass number as a result of fragmentation into smaller molecules that are then metabolized by microorganisms. However, the majority of investigations on the purported biodegradation of PE by microorganisms show biodeterioration and just a small number report biofragmentation. Furthermore, there is not enough proof to support bioassimilation and mineralization. Understanding the molecular processes of polyethylene biodegradation may be improved by investigating the genes and gene products that oxidize the alkane chains of polyethylene.
References
- 1.
Albertsson AC, Karlsson S. The influence of biotic and abiotic environments on the degradation of polyethylene. Progress in Polymer Science. 1990; 15 :177-192. DOI: 10.1016/0079-6700(90)90027-X - 2.
Bergmann M, Mützel S, Primpke S, Tekman MB, Trachsel J, Gerdts G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Science Advances. 2019; 5 :eaax1157. DOI: 10.1126/sciadv.aax1157 - 3.
Chiellini E, Cortia A, Swift G. Biodegradation of thermally-oxidized, fragmented low-density polyethylenes. Polymer Degradation and Stability. 2003; 81 :341-351. DOI: 10.1016/S0141-3910(03)00105-8 - 4.
Das MP, Kumar S. An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens , 3 Biotech. 2015;5 :81-86. DOI: 10.1007/s13205-014-0205-1 - 5.
Celina M, Linde E, Brunson D, Quintana A, Giron N. Overview of accelerated aging and polymer degradation kinetics for combined radiation-thermal environments. Polymer Degradation and Stability. 2019; 166 :353-378. DOI: 10.1016/j.polymdegradstab. 2019.06.007 - 6.
Chiellini E, Corti A, D’Antone S. Oxo-biodegradable full carbon backbone polymers biodegradation behaviour of thermally oxidized polyethylene in an aqueous medium. Polymer Degradation and Stability. 2007; 92 :1378-1383. DOI: 10.1016/j.polymdegradstab. 2007.03.007 - 7.
Otake Y, Kobayashi T, Asabe H, Murakami N. Biodegradation of low density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. Applied Polymer Science. 1995; 56 :1789-1796. DOI: 10.1002/app.1995.070561309 - 8.
Divyalakshmi S, Subhashini A. Screening and isolation of polyethylene degrading bacteria from various soil environments. IOSR Journal of Environmental Science Toxicology and Food Technology. 2016; 10 :1-7. DOI: 10.9790/2402-1012040107 - 9.
El-Shafei H, Nasser NHA, Kansoh AL, Ali AM. Biodegradation of disposable polyethylene by fungi Streptomyces species. Polymer Degradation and Stability. 1998; 62 :361-365. DOI: 10.1016/S0141-3910(98)00019-6 - 10.
Fontanella S, Bonhomme S, Koutny M, Husarova L, Brusso JM, Courdavault JP, et al. Comparison of the biodegradability of various polyethylene films containing pro-oxidant additives. Polymer Degradation and Stability. 2010; 95 :1011-1021. DOI: 10.1186/s13765-020-00511-3 - 11.
Gilan I, Hadar Y, Sivan A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber . Applied Microbiology and Biotechnology. 2004;65 :97-104. DOI: 10.1007/s00253-004-1584-8 - 12.
Hadad D, Geresh S, Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis . Journal of Applied Microbiology. 2005;98 :1093-1100. DOI: 10.1111/j.1365-2672.2005.02553.x - 13.
Harshvardhan K, Jha B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters Arabian Sea, India. Marine Pollution Bulletin. 2013; 77 :100-106. DOI: 10.1016/j.marpolbul.2013.10.025 - 14.
Hassan F, Shah AA, Hameed A, Ahmed S. Synergistic effect of photo and chemical treatment on the rate of biodegradation of low density polyethylene by Fusarium sp. AF4. Journal of Applied Polymer Science. 2007; 105 :1466-1470. DOI: 10.1002/app.26328 - 15.
Jeon HJ, Kim MN. Degradation of linear low density polyethylene (LLDPE) exposed to UV-irradiation. European Polymer Journal. 2014; 52 :146-153. DOI: 10.1016/j.eurpolymj.2014.01.007 - 16.
Jeon HJ, Kim MN. Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation. International Biodeterioration and Biodegradation. 2015; 103 :141-146. DOI: 10.1128/JB.184.6.1733-1742.2002 - 17.
Kawai K, Watanabe M, Shibata M, Yokoyama S, Sudate Y, Hayashi S. Comparative study on biodegradability of polyethylene wax by bacteria and fungi. Polymer Degradation and Stability. 2004; 86 :105-114. DOI: 10.1016/j.polymdegradstab. 2004.03.015 - 18.
Usha R, Sangeetha T, Palaniswamy M. Screening of polyethylene degrading microorganisms from garbage soil. Libyan Agricultural Research Central Journal of International. 2011; 2 :200-204. DOI: 10.9790/2402-1012040107 - 19.
Veethahavya KS, Rajath BS, Noobia S, Kumar MB. Biodegradation of low density polyethylene in aqueous media. Procedia Environmental Sciences. 2016; 35 :709-713 - 20.
Vimala PP, Mathew L. Biodegradation of polyethylene using Bacillus subtilis . Procedia Technology. 2016;24 :232-239. DOI: 10.1016/j.protcy.2016.05.031 - 21.
Yang J, Yang Y, Wu WM, Zhao J, Jiang L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environmental Science & Technology. 2014; 48 :13776-13784. DOI: 10.1021/es504038a - 22.
Yashchuk O, Portillo FS, Hermida EB. Degradation of polyethylene film samples containing oxodegradable additives. Procedia Materials Science. 2012; 1 :439-445. DOI: 10.1016/j.mspro.2012.06.059 - 23.
Yoon MG, Jeon JH, Kim MN. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. Journal of Bioremediation & Biodegradation. 2012; 3 :145. DOI: 10.4172/2155-6199.1000145 - 24.
Kelkar VP, Rolsky CB, Pant A, Green MD, Tongay S, Halden RU. Chemical and physical changes of microplastics during sterilization by chlorination. Water Research. 2019; 163 :114871. DOI: 10.1016/j.watres.2019.114871 - 25.
Koutny M, Amato P, Muchova M, Ruzicka J, Delort AM. Soil bacterial strains able to grow on the surface of oxidized polyethylene film containing prooxidant additives. International Biodeterioration and Biodegradation. 2009; 63 :354-357. DOI: 10.1016/j.ibiod.2008.11.003 - 26.
Kyaw BM, Champakalakshmi R, Sakharkar MK, Lim CS, Sakharkar KR. Biodegradation of low-density polythene (LDPE) by Pseudomonas species. Indian Journal of Microbiology. 2012; 52 :411-419. DOI: 10.1007%2Fs12088-012-0250-6 - 27.
Mehmood CT, Qazi IA, Hashmi I, Bhargava S, Deepa S. Biodegradation of low density polyethylene (LDPE) modified with dye sensitized titania and starch blend using Stenotrophomonas pavanii . International Biodeterioration and Biodegradation. 2016;113 :276-286. DOI: 10.1016/j.ibiod.2016.01.025 - 28.
Montazer Z, Habibi Najafi MB, Levin DB. Challenges with verifying microbial degradation of polyethylene. Polymers. 2020; 12 (1):123. DOI: 10.3390/polym12010123 - 29.
Montazer Z, Habibi-Najafi MB, Mohebbi M, Oromiehei A. Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading bacteria isolated from plastic-dump soil. Journal of Polymers and the Environment. 2018; 26 :3613-3625. DOI: 10.1007/s10924-018-1245-0 - 30.
Jeon JM, Park SJ, Choi TR, Park JH, Yang YH, Yoon JJ. Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polymer Degradation and Stability. 2021;191 :109662. DOI: 10.1016/j.polymdegradstab. 2021.109662 - 31.
Nowak B, Pajak J, Drozd-Bratkowicz M, Rymarz G. Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. International Biodeterioration and Biodegradation. 2011; 65 :757-767. DOI: 10.1016/j.ibiod.2011.04.007 - 32.
Peixoto J, Silva PL, Krüger RH. Brazilian Cerrado soil reveals an untapped microbial potential forunpretreated polyethylene biodegradation. Journal of Hazardous Materials. 2017; 324 :634-644. DOI: 10.1016/j.jhazmat.2016.11.037 - 33.
Pramila R, Ramesh KV. Potential biodegradation of low-density polyethylene (LDPE) by Acinetobacter bumannii . Africa Journal of Bacteriology Research. 2015;7 :24-28. DOI: 10.5897/JBR2015.0152 - 34.
Ragaert K, Delva L, Van Geem K. Mechanical and chemical recycling of solid plastic waste. Waste Management. 2017; 69 :24-58. DOI: 10.1016/j.wasman.2017.07.044 - 35.
Rajandas H, Parimannan S, Sathasivam K, Ravichandran M, Yin LS. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polymer Testing. 2012; 3 :1094-1099. DOI: 10.1016/j.polymertesting.2012.07.015 - 36.
Ranjan VP, Goel S. Degradation of low-density polyethylene film exposed to UV radiation in four environments. Journal of Hazard Toxic Radioactive Waste. 2019; 23 :04019015. DOI: 10.1061/(ASCE)HZ.2153-5515.0000453 - 37.
Santo M, Weitsman R, Sivan A. The role of the copper-binding enzyme, laccase, in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber . International Biodeterioration and Biodegradation. 2013;84 :204-210. DOI: 10.1016/j.ibiod.2012.03.001 - 38.
Sivan A, Santo M, Pavlov V. Biofilm development of the polyethylene-degrading bacterium Rhodococcus ruber . Applied Microbiology and Biotechnology. 2006;72 :346-352. DOI: 10.1007/s00253-005-0259-4 - 39.
Sudhakar M, Doble M, Sriyutha Murthy P, Venkatesan R. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. International Biodeterioration and Biodegradation. 2008; 61 :203-213. DOI: 10.1016/j.ibiod.2007.07.011 - 40.
Thakur P. Screening of Plastic Degrading Bacteria from Dumped Soil Area. Odisha, India: National Institute of Technology of Rourkela; 2012. DOI: 10.9790/2402-1105029398 - 41.
Bhatia M, Girdhar A, Tiwari A, Nayarisseri A. Implications of a novel Pseudomonas species on low density polyethylene biodegradation: An in vitro to in silico approach. Springer Plus. 2014;3 :497. DOI: 10.1186/2193-1801-3-497 - 42.
Bonhomme S, Cuer A, Delort AM, Lemaire J, Sancelme M, Scott C. Environmental biodegradation of polyethylene. Polymer Degradation and Stability. 2003; 81 :441-452. DOI: 10.1016/S0141-3910(03)00129-0