Abstract
Objective: To study the application value of multi-modal image fusion combined with navigation for precise localization of spinal cord lesions. Methods: From November 2015 to January 2022, a total of 28 patients with spinal cord lesions were enrolled in the study, and intraoperative CT images were obtained by O-arm, and preoperative MRI and intraoperative CT were fused by the navigation system to reconstruct 3D models of the spine and lesion. Complete the localization and resection of spinal cord lesions during navigation, and record basic patient information, image fusion time, and navigation accuracy. Results: All 28 patients successfully localized spinal cord lesions with multi-modal image fusion combined with navigation. The time of image processing was between 7 min and 19 min, and the mean value was 15.3 ± 2.2 min. The navigation error was between 0.9 mm and 5.3 mm, and the mean value was 1.8 ± 0.9 mm. Conclusion: The multi-modal image fusion combined with navigation can be used to precisely localize spinal lesions, providing a more objective basis and a new approach for individualized and precise surgical planning.
Keywords
- O-arm
- navigation
- multi-modal
- image fusion
- spinal cord lesions
1. Introduction
Spinal cord lesions, also known as spinal canal lesions, account for about 4.3% ~ 10.4% of all central nervous system lesions and an annual incidence of about 0.97/100,000 people. Lesions can occur in the spinal cord, nerve roots, and meninges [1, 2], and surgical resection is the preferred treatment. The intraoperative precise localization of spinal cord lesions is one of the crucial factors affecting the quality of surgery. Determining the lesion location mainly relies on the vertebral body being inferred indirectly, while the localization of spinal segments lacks consistent standards. It has been reported that the risk of surgical exposure or exploration in the wrong segment is between 0.032 and 15% [3, 4, 5, 6, 7]. This is likely to cause unnecessary spinal cord injuries, especially for lesions that extend into the foramen area. Due to the difficulty of exposure and the complex spatial structure of lesions, a large amount of lamina milling is required to remove part of the small joints, which may lead to accelerated spinal degeneration and instability in the long term. Glioma and intramedullary tiny lesions of the spinal cord can be difficult to locate and require the surgeon’s experience to determine the extent of resection. However, the spinal cord lacks a functional dummy area, and blind exploration is bound to cause nerve function injury, which is far from the standard of accurate neurosurgery.
In 2015, the study team started using multi-modal image fusion techniques to identify intraoperative spinal cord lesions. The O-arm was used to capture intraoperative CT images of the patients, which were then uploaded to the navigation system and combined with other images for intraoperative localization of spinal cord lesions. Previous investigations [8, 9, 10] have confirmed the technique’s viability and accuracy. The intangible surgical experience can be made concrete through preoperative MRI images that clearly show the lesions. The use of multi-modal image fusion technology in treating spinal cord lesions can be encouraged by summarizing and updating existing data.
2. Methods
28 patients with spinal cord lesions who underwent surgical treatment in our hospital’s neurosurgery department between November 2015 and January 2022 were selected, including 13 men and 15 women, with cases affecting the cervical segment 6, thoracic segment 10, and lumbar segment 12. Patients’ ages ranged from 14 to 78, with an average age of 41. During postoperative pathology, 8 cases of neurofibroma, 3 cases of schwannoma, 2 cases of meningioma, 3 cases of capillary hemangioma, 6 cases of spinal cuff cyst of nerve roots, 1 case of intramedullary vascular malformation of the spinal cord, 3 cases of cavernous hemangioma, and the remaining 4 cases of ependymoma were identified.
Prior to surgery, patients underwent regular CT (64-slice spiral CT, GE, USA) and MRI (3.0 T, GE, USA) scans. After scanning, axial, sagittal, and crown pictures were captured, recorded in DICOM format, and stored at the Stealth station 7 navigation workstation. The enhanced inspection is selected based on the relationship between the lesions and significant blood vessels.
Before surgery at Synergy Cranial, the intraoperative O-arm was used to take CT images of the patient’s spinal segments, which were then registered and merged with CT and MRI images sent to the navigation workstation. Operate in the Stealth Merge module, select the O-arm CT images as the reference, verify the fusion image’s accuracy by combining or separating Windows, choose the appropriate transparency, and ensure that the bone cortex is consistently aligned on both pictures. Additionally, the key to a successful fusion was to maintain the continuous union of vertebrae and facet joints in the diseased segment due to intraoperative spinal flexion and rotation, which could not align with preoperative imaging. Two senior neurosurgeons collaborated to achieve image fusion.
In the Synergy Cranial software’s building 3D model module, the target structure’s 3D model is reconstructed. Blood vessels and the spine can be reconstructed using modules including bone and vessel using preoperative CT and O-arm scans as data sources. and enhanced by tools such as Push, Threshold, and others. Based on preoperative MRI data that can be reconstructed using programs like Lasso, the 3D model of spinal cord occupation was created. Once the 3D model reconstruction is complete, it is possible to display the integrated model of the spine, blood vessel, and lesion in the same spatial coordinate system by varying the model’s size and transparency. At this stage, the operation path can be virtually created and planned at any level.
With reference to multi-modal 3D fusion images, surgical incisions, laminae milling, facet joint grinding, and other precise locations were designed. Prior to the surgical exploration of the spinal cord using anatomical markers on the bones, such as the spinous process and facet joints, the precision of navigation was examined. The degree of lesion excision was determined, the lesion location was found, navigation accuracy was validated, and CT or MRI images were switched to display in accordance with the operation’s goals.
The time for image processing included the time for acquiring the image using O-arm and the time for image fusion. Navigation errors were evaluated by measuring the maximum distance between the end of the lesion in MRI images and its real position. Descriptive statistics were used to calculate the mean and standard deviation. SPSS (version 13.0 SPSS Inc) was used for the analysis.
3. Results
The findings showed that only a small portion (2.29%–15.66%) of the entire operation time (83–400 min) was accounted for by the operating time for O-arm scanning and image fusion (7–19 min). For the high cervical-level spinal cord lesion, O-arm scanning was not necessary. The posterior fossa bone can usually be used for automatic fusion, and the procedure is quick overall. Patients with lumbar lesions undergo O-arm scanning and image fusion in a shorter time (10–17 minutes) than those with cervical and thoracic lesions (15–19 minutes).
MRI and CT image fusion for patients with spinal cord lesions in the high cervical segment was successful (Figure 1). O-arm scanning was omitted, and intraoperative contour registration was performed using MRI data. The intraoperative O-arm scanning was performed according to the schedule of all the other patients. During the procedure, one patient with a cervical 5–6 ganglion lesion was lying on their side with their neck partially extended to the healthy side. The preoperative CT and MRI images and the intraoperative O-arm image did not integrate well, and the image fusion was finished using the principle of maintaining continuous fusion of vertebral body and joint bone in lesion segments (Figure 2). A patient with a thoracic spinal cord intramedullary vascular malformation paraplegic had an acute intramedullary hemorrhage and only an emergency thoracic MRI scan was performed before surgery. Sagittal T2-weighted sequences were used to fuse the MRI with intraoperative O-arm images (Figure 3).
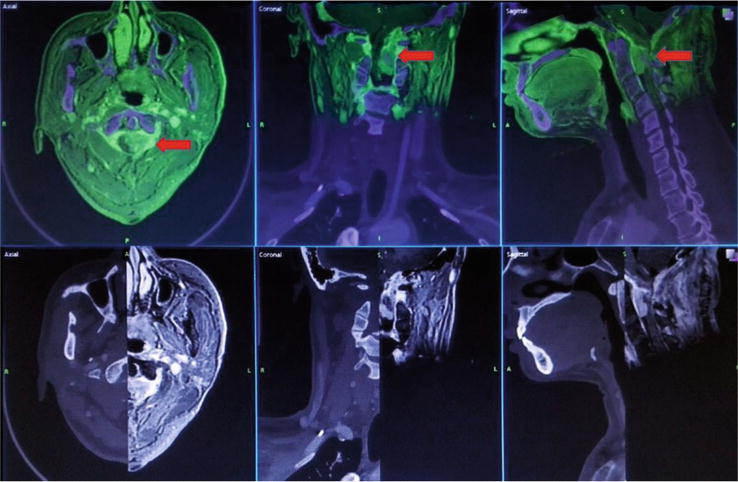
Figure 1.
Shows the image fusion of patients with meningioma from the foramen magnum to C2. The MRI T1WI axial image was combined with the CT image and the red arrow shows the location of C1–2. The accuracy of the fusion was verified by adjusting transparency and separate windows.
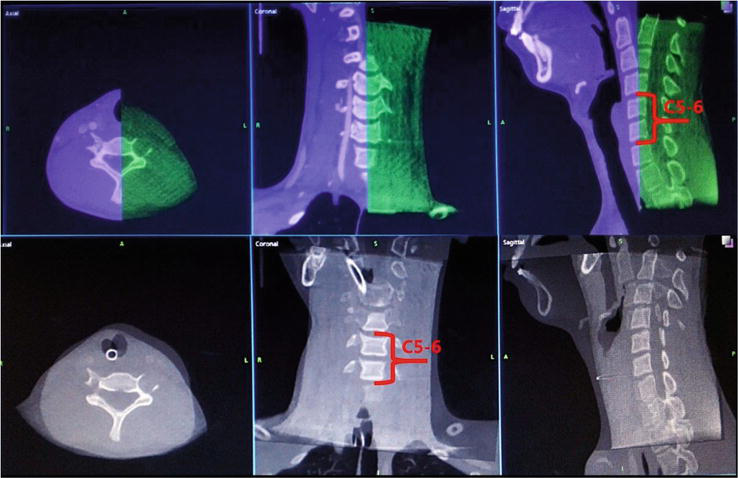
Figure 2.
Image fusion of patients with C5–6 ganglion lesion: Preoperative enhanced CT was combined with intraoperative O-arm images. The red figure indicated the C5–6 vertebral body where the lesion was located. The registration time maximized the C5–6 fusion.
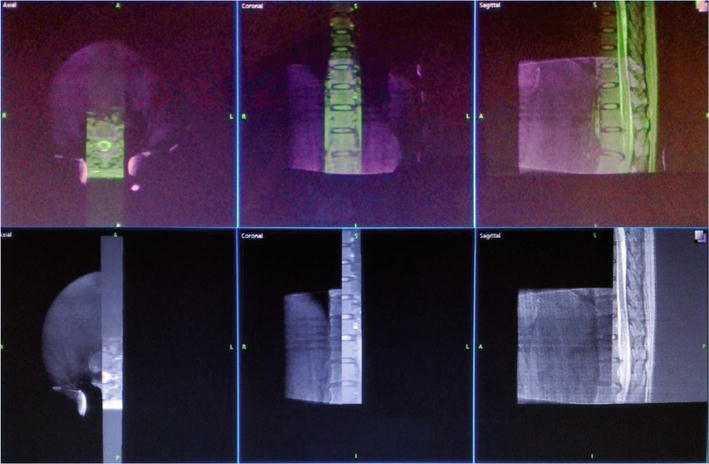
Figure 3.
Image registration of patients with intramedullary vascular malformation of the thoracic spinal cord. Sagittal images of patients with MRI T2WI were fused with intraoperative 3D images of an O-arm, and the accuracy of fusion was verified by adjusting transparency and separate windows, respectively.
After the image fusion was completed, Synergy Cranial software was used to reconstruct the patient’s 3D model and plan the surgical path. To completely remove the lesion and minimize spinal and paravertebral muscle damage, minimally invasive surgical incisions were made in 2 cases of lumbar schwannoma and 1 case of thoracic nerve root rotator cuff cyst. Figure 4 depicts a case of minimally invasive image-assisted navigation for lumbar 3–4 schwannoma resection. Under the microscope, the lesion was completely removed while only a part of the patient’s lamina was ground. The minimally invasive incision is based on the body surface projection of the lesion recorded by navigation, and the accurate finding of the lesion through minimally invasive channels is defined by letters A, B, and C. A is the navigational interface of CT images during O-arm surgery; B is the navigational interface of synchronous T2WI imaging on MRI.
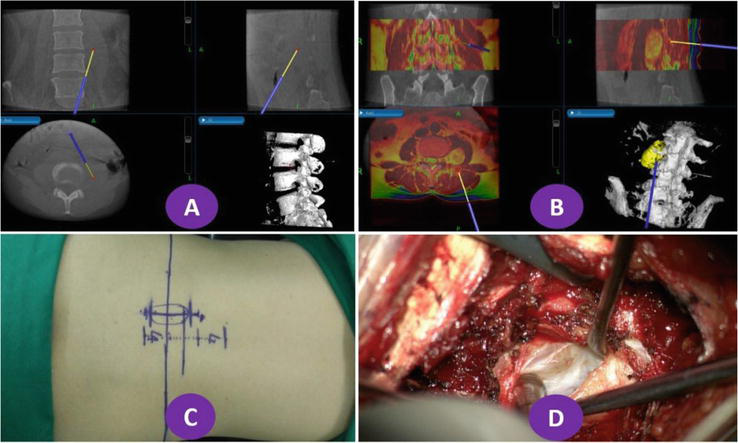
Figure 4.
Navigation and operation of lumbar 3–4 schwannoma using rainbow mode for MRI images, greyscale mode for O-arm images, yellow for lesion models, and white for lumbar models.
An example of lumbar schwannoma with L3–5 posterior pedicle screw placement due to injury to the L4 vertebrae. The O-arm scan immediately confirmed that all screws were in their proper places (Figure 5). During intraoperative 3D model reconstruction, a patient with ganglioblastoma at the C5–6 level (Figure 6) had a satisfactory union of the C5–6 vertebrae and tiny joints, while the remaining vertebrae showed significant deviation due to changes in body position. A and B used data from intraoperative O-arm scanning to create a 3D reconstruction of the cervical spine, while C and D used preoperative CT data to create a reconstruction of the cervical spine. To reach the base of the skull, the left vertebral artery may be seen passing through the neck’s transverse foramen 1–6. According to figures A and B, the vertebral artery outside of the C5–6 vertebrae was shifted significantly from the cervical vertebrae. Surgical navigation requirements can be met due to intraoperative navigation, which also confirmed the high accuracy of navigation within the neck’s fifth and sixth vertebrae. A navigation stick was used to assess the extent of the lesion in a patient with intramedullary spinal vascular malformations at the T10–12 level before exploring the spinal cord, and the position of the upper and lower poles of the lesion was consistent with the position shown by the navigation stick (Figure 7).
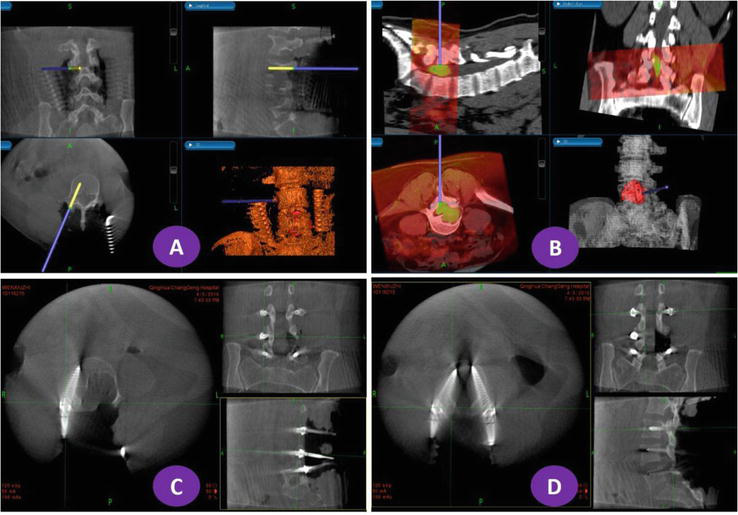
Figure 5.
O-arm scanning of patients with lumbar 4–5 schwannoma after navigation and screw placement. The lesion model was red, the orange spinal model was reconstructed from intraoperative O-arm 3D CT data, and the white spinal model was reconstructed from preoperative CT images.
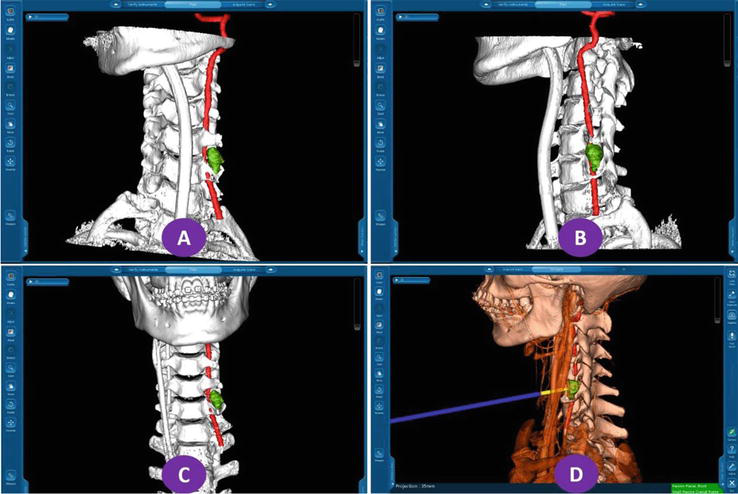
Figure 6.
3D model reconstruction and navigation of patients with C5–6 ganglioblastoma: The spine and other bony models were set in white, the left vertebral artery was set in red, and the lesion was set in green.
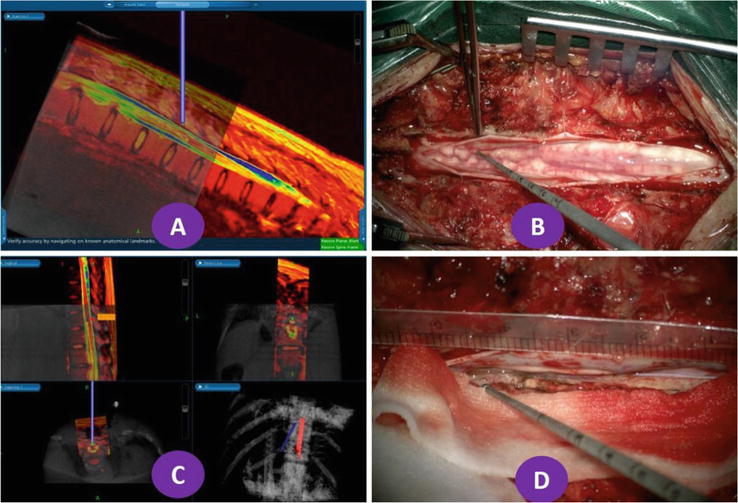
Figure 7.
Navigation and operation of patients with 10–12 intramedullary vascular malformations of the spinal cord in the chest. A and B show the location of intramedullary lesions under navigation; C and D show the extent of lesions during surgery to verify the accuracy of navigation. The spine and lesion are reconstructed in white and red, respectively.
4. Discussion
Intraoperative localization of spinal segments is a unique problem in spinal surgery. Jillian E [11] et al., in a survey of 2338 members of the North American Spinal Association, which included 173 physicians, showed that fluoroscopy was the most commonly used method for thoracic and lumbar spine localization (89% and 86%, respectively). This was followed by intraoperative plain radiographs (54% vs. 58%, respectively), and fluoroscopy plus plain radiographs were used by 43.9% of physicians, even though 68% of the physicians reported having misidentified the segment. Currently, the chance of incorrect exposure and milling of incorrect segments is estimated to be between 0.032 and 15% [3, 4, 5, 6, 7]. Although the probability is low, incorrect localization can not only increase the trauma and cost of patients, but also prolong their hospital stay, and sometimes even lead to legal disputes for doctors.
The O-arm can collect intraoperative 3D CT images of the spine and then transfers them to the neural navigation system to complete real-time spine navigation. It changes the traditional localization based on intraoperative plain X-rays and fluoroscopy, with extremely high localization accuracy. In recent years, reports on its clinical application in spinal cord surgery have been increasing year after year [12, 13, 14, 15, 16]. Jang, Sang Hoon et al. [12] reported in 2015 that 31 patients undergoing anterior cervical surgery used an O-arm image navigation system during surgery. The statistical navigation errors showed that the horizontal deviation was 0.49+/− 0.71 mm, and the vertical deviation was 0.88+/− 0.93 mm. The sagittal plane deviation Angle is 0.59+/−0.55°. Silbermann, J et al. [13] conducted a study of 187 screws inserted under an O-arm image-guided navigation in 37 patients, with an accuracy of 99%. Hand insertion was performed in 30 patients in the control group, with an accuracy of 94.1%, while 187 screws were inserted by hand in 37 patients, with an accuracy of 94.1%. In conclusion, the O-arm image navigation system is fully qualified for its requirements and provides accurate localization of spinal segments, eliminating the risk of radiation [14], and reducing the long-term cost of patients [15, 16]. However, it is challenging to display spinal cord lesions using O-arm scanning.
The multi-modal image fusion technology is based on the information fusion of images from two or more sources, which is helpful in obtaining a more accurate, comprehensive, and reliable image description of the same scene [17, 18, 19]. With the advancements in medical imaging and computer technology, neural navigation systems have evolved from simple anatomical navigation to functional navigation. Through multi-modal image fusion technology, functional brain images are combined with anatomical images such as CT and MRI, 3D reconstruction is performed, and space occupation, functional cortex, white matter conduction tract, and blood vessels are visually recorded. Assisted in creating virtual surgical plans before surgery and continued with localization and navigation during surgery. In the past 20 years, studies on multi-modal image fusion technology and neural navigation systems have mainly been seen in cases of craniocerebral lesions [20, 21, 22, 23, 24, 25, 26, 27], with glioma being the most common. Glioma is an invasive growth that lacks a clear boundary, especially in middle and low-grade lesions. It is often difficult to distinguish the boundary between glioma and normal brain tissue during surgery. Studies [25, 27, 28] have shown that this technique can significantly improve the lesion resection rate and reduce postoperative complications in patients.
In this study, the innovative Synergy Cranial software was used to integrate intraoperative O-arm 3D CT images with patients’ preoperative high-definition CT and MRI for image fusion and navigation surgery, reducing navigation errors caused by spinal flexion and rotation and ensuring accurate localization of spinal segments while completing spinal lesions development. All 28 patients successfully underwent image fusion and underwent guided lesion resection. The intraoperative findings were completely in line with those indicated by navigation. Navigation localization was accurate, giving objective reliance on the operation, reducing unnecessary spinal cord exploration, and improving the surgeon’s confidence. In the study, no patient opened the lamina by mistake and no patient damaged important blood vessels. It is worth mentioning that the results of the study on intramedullary spinal cord lesions showed that the sagittal and navigational boundaries under the microscope were completely consistent, which is of great significance for the accurate localization of intramedullary spinal cord lesions, the improvement of resection rate, and the reduction of reducing nerve injury.
The 3D lesion model, which is based on multi-modal image fusion reconstruction, can adjust transparency to meet the needs of the operation, perform free cutting, measure the distance between the lesion and any location, observe the direction and branch of blood vessels throughout the entire process, and assist the surgeon in creating a surgical plan and evaluating the degree of lesion resection. The digital model can help junior doctors understand the 3D anatomy of lesions, overcome the limitations of traditional anatomy teaching, and provide a good learning platform for young doctors to grow.
In conclusion, this study conducted multi-modal image fusion and precision navigation surgery on spinal cord lesions, which established the clinical operation process of multi-modal image fusion technology in precision surgical treatment and the modern surgical treatment concept of minimally invasive, individualized treatment [29, 30].
Acknowledgments
This work was supported by Tsinghua Precision Medicine Foundation (20219990008), Tsinghua University, Beijing, China.
References
- 1.
Duong LM, McCarthy BJ, McLendon RE, et al. Descriptive epidemiology of malignant and nonmalignant and primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004-2007. Cancer. 2012; 118 :4220-4227 - 2.
Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncology. 2012; 14 (Suppl. 5):v1-v49 - 3.
Siminski CP, Carr CM, et al. Fluoroscopy- and CT-guided gold fiducial marker placement for intraoperative localization during spinal surgery: Review of 179 cases at a single institution-technique and safety profile. AJNR. American Journal of Neuroradiology. 2023; 44 (5):618-622 - 4.
Hsu W, Kretzer RM, Dorsi MJ, et al. Strategies to avoid wrong-site surgery during spinal procedures. Neurosurgical Focus. 2011; 31 :E5 - 5.
Hsu W, Sciubba DM, Sasson AD, et al. Intraoperative localization of thoracic spine level with preoperative percutaneous placement of intravertebral polymethylmethacrylate. Journal of Spinal Disorders & Techniques. 2008; 21 :72-75 - 6.
Paolini S, Ciappetta P, Missori P, et al. Spinous process marking: A reliable method for preoperative surface localization of intradural lesion of the high thoracic spine. British Journal of Neurosurgery. 2005; 19 :74-76 - 7.
Mayer JE, Dang RP, Duarte Prieto GF, Cho SK, Qureshi SA, Hecht AC. Analysis of the techniques for thoracic- and lumbar-level localization during posterior spine surgery and the occurrence of wrong-level surgery: Results from a national survey. Spine J. 1 May 2014; 14 (5):741-748 - 8.
Zhang P, Sun Z, et al. Preliminary application of multimodal image fusion technique to treatment of lesions within the spinal canal. Chinese Journal of Neurosurgery. 2016; 32 (12):1239-1243 - 9.
Zhang P, Wang G, Sun Z, Lv X, Guo Y, Wang J, et al. Application of multimodal image fusion to precisely localize small intramedullary spinal cord Tumors. World Neurosurgery. 2018; 118 :246-249 - 10.
Zhang P, Liu H, Sun Z, Wang J, Wang G. The application of O-arm and navigation system in precise localization of spinal cord lesions: A case series study. Clinical Neurology and Neurosurgery. 2020; 196 :105922 - 11.
Jillian E, Mayer BA, et al. Analysis of the techniques for thoracic- and lumbar-level localization during posterior spine surgery and the occurrence of wrong-level surgery: Results from a national survey. The Spine Journal. 2014; 14 :741-748 - 12.
Jang SH, Cho JY, et al. Novel method for setting up 3D navigation system with skin-fixed dynamic reference frame in anterior cervical surgery. Computer Aided Surgery. 2015; 20 (1):24-28 - 13.
Silbermann, J Riese, F, et al. Computer tomography assessment of pedicle screw placement in lumbar and sacral spine: Comparison between free-hand and O-arm based navigation techniques. European Spine Journal. 2011; 20 (6):875-881 - 14.
Grelat, M. Zairi, F, et al. Assessment of the surgeon radiation exposure during a minimally invasive TLIF: Comparison between fluoroscopy and O-arm system. Neurochirurgie. 2015; 61 (4):255-259 - 15.
Dea N, Fisher CG, et al. Economic evaluation comparing intraoperative cone beam CT-based navigation and conventional fluoroscopy for the placement of spinal pedicle screws: A patient-level data cost-effectiveness analysis. The Spine Journal. 2016; 16 (1):23-31 - 16.
Sclafani JA, Regev GJ, et al. Use of a quantitative pedicle screw accuracy system to assess new technology: Initial studies on O-arm navigation and its effect on the learning curve of percutaneous pedicle screw insertion. SAS Journal. 2011; 5 (3):57-62 - 17.
Maintz JBA, Viergever MA. A survey of medical image registration. Medical Image Analysis. 1998; 1 :1-36 - 18.
Moche M, Busse H, Dannenberg C, et al. Fusion of MRI, fMRI and intraoperative MRI data: Methods and clinical signance examplified by neurosugical interventions. Radiologe. 2001; 41 :993-1000 - 19.
Nimsky C, Kuhnt D, Ganslandt O, et al. Multimodal navigation integrated with imaging. Acta Neurochirurgica. Supplement. 2011; 109 :207-214 - 20.
Baum KG et al. Fusion viewer: A new tool for fusion and visualization of multimodal medical data sets. Journal of Digital Imaging. 2008; 21 (Suppl. 1):S59-S68 - 21.
Inoue A et al. Usefulness of an image fusion model using three-dimensional CT and MRI with Indocyanine green fluorescence endoscopy as a multimodal assistant system in endoscopic Transsphenoidal surgery. International Journal of Endocrinology. 2015; 2015 :694273 - 22.
Kin T et al. A new strategic neurosurgical planning tool for brainstem cavernous malformations using interactive computer graphics with multimodal fusion images. Journal of Neurosurgery. 2012; 117 (1):78-88 - 23.
Mirzadeh Z et al. Validation of CT-MRI fusion for intraoperative assessment of stereotactic accuracy in DBS surgery. Movement Disorders. 2014; 29 (14):1788-1795 - 24.
Pichler BJ et al. Multimodal imaging approaches: PET/CT and PET/MRI. Handbook of Experimental Pharmacology. 2008; 185 Pt 1 :109-132 - 25.
Rajasekar D et al. Multimodality image fusion in dose escalation studies of brain tumors. Journal of Applied Clinical Medical Physics. 2003; 4 (1):8-16 - 26.
Wakabayashi T et al. Advanced new neurosurgical procedure using integrated system of intraoperative MRI and neuronavigation with multimodal neuroradiological images. Nagoya Journal of Medical Science. 2009; 71 (3-4):101-107 - 27.
Xiao BX, Xu BN, Chen XI, et al. Assessment of complete resection of gliomas by intraprative high-field magnetic resonance imaging. Zhonghua Yi Xue Za Zhi. 2010; 26 :320-322 - 28.
Zhang JS, Chen XL, Li FY, et al. Influences of high-field intraoperative magnetic resonance imaging on the extent of resection in low-grade gliomas. Zhonghua Yi Xue Za Zhi. 2012; 92 :1738-1741 - 29.
Stellato TA. Humanism and the art of surgery. Surgery. 2007; 142 (4):433-438 - 30.
Brooke B, Nathan H, Pawlik TM. Trends in the quality of highly cited surgical research over the past 20 years. Annals of Surgery. 2009; 249 (1):162-167