Effect of aging on lees (L) on the aroma compound levels of Malvasia
Abstract
The loss of aromatic and sensory quality in wines because of climate change in traditional winemaking areas is a challenge for winemakers. Aging on lees of the wine fermented with Saccharomyces native yeasts has been tested as a technique to try to improve the sensory characteristics of Malvasia aromatica white wines in PDO “Vinos de Madrid.” The grapes were pre-cold macerated and fermented with S. cerevisiae CLI 271 and CLI 889 (native yeast strains). Then, commercial lees were used for aging of wines for a five-month at low temperature in order to compare with the effect of S. cerevisiae CLI 271 and CLI 889 without lees treatment. Aromatic and organoleptic properties of wines aged on lees were studied using GC-FID and HS-SPME/GC–MS to quantify volatile compounds and a taster panel to sensorial analysis. There was a significant decrease in the ester family in wines aged on lees being more pronounced in CLI 889 wines. The treatment contributed to enhance the fruity and floral aromatic properties in CLI 271 and CLI 889 wines, respectively according to tasting panel, which showed a hedonic preference for CLI 271 wines without lees treatment and CLI 889 wines aged on lees.
Keywords
- lees
- native yeast
- aroma
- climate change
- Malvasia aromatica
1. Introduction
Aging on lees is a technique used after fermentation associated with the improvement of the sensory properties of wine through yeast autolysis via enzymatic self-degradation of cellular constituents that begins after the death of the yeast. It is a technique used for decades mainly in the production of white wines. Its main objective is to reduce astringency and bitterness, increase body, structure and roundness in the mouth and improve aromatic persistence and complexity of wines [1, 2, 3] in addition to contributing to the reduction of undesirable flavors [4]. The main conditioning and essential factor to allow cell autolysis is the time. It is estimated that the process begins as soon as the cell dies and increases significantly after the second month. The autolysis process is slow and involves risks of microbiological spoilage, reduction and organoleptic off-flavors [1, 5]. Therefore, aging on lees is combined with battonnages to improve the contact of lees with the wine and help the faster release of polysaccharides from the yeasts [6] and compounds such as amino acids and lipids that can be aroma precursors.
Aroma is one of the main quality attributes of white wines and a very important aspect for consumers. The volatile compounds provided by the grapes, more recognized as varietal aromas, are responsible for their aromatic typicity and their presence is influenced by factors such as variety, cultural practices, terroir, geographical location and climate [7]. The compounds released in the course of alcoholic fermentation also have a decisive influence on the volatile composition of wines and their synthesis depends mainly on the microorganisms involved.
PDO “Vinos de Madrid” is located in the center of the Iberian Peninsula with a prevalence of hot summers, cold winters and low levels of precipitation. Climate predictions indicate a gradual increase in temperatures, a decrease in precipitation and a greater frequency of severe phenomena such as frosts, storms and heat waves with greater incidence in the center of Spain [8]. These events could compromise the correct development of the technological and aromatic ripening of the grapes, preventing the production of intense aromatic white wines. On the other hand, the loss of aromatic and sensory typicity attached to a particular region is also a challenge for winemakers. The yeast activity during a spontaneous fermentation could contribute to fewer desirable attributes to the wine and the quality can be variable between seasons. Thus, the use of commercial yeasts could be employed to obtain a product of uniform quality [9, 10]. However, the typical character of the harvest as well as its aromatic properties could be lost.
In order to adapt to the climate change-related effects, as well as the preservation of the typical organoleptic characteristics of the Madrid region, our laboratories have proposed the use of oenological practices such as skin contact treatment and the use of indigenous yeast strains better adapted to climatic conditions and terroir [11, 12]. As a final step, based on the results of our previous research, this study has focused on the effect of using lees during the aging period in white wines of Malvasia

Figure 1.
2. Material and methods
2.1 Vintage, yeast strains and fermentation procedure
Grapes from the white-berried cv. Malvasia
2.2 Aging on lees
After the end of the alcoholic fermentation, samples of each wine were racked into 5 L screw-capped glass bottles. Commercial lees (Super Bouquet MN from Agrovin) were used at a concentration of 30 g/hl for a five-month contact period with wine lees (L) at low temperature (8–11°C). Five liters of each wine (without lees) were used as a control. Battonnages in wines aging on lees were carried out twice per week, for 10 minutes per bottle with the aid of a magnetic stirrer. At the end of this treatment, wines were racked to eliminate lees. Wines were also clarified and bottled in the same way as those without lees. Prior to bottling, samples were taken for analysis.
2.3 Wine volatile composition
Wine volatile composition was defined with: major aroma compounds and free varietal minor volatiles. The first were analyzed using gas chromatography coupled with a flame ionization detector (GC-FID). The second, by undertaking a headspace-solid phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME/GC–MS).
The analysis of major aromatic compounds included the extraction and detection of alcohols, acids, lactones, esters, aldehydes and ketones, according to the method described by Ortega [17], using dichloromethane and a DB-Wax column (60 m × 0.32 mm × 0.5 μm film thickness) from J&W Scientific (Folsom, CA, USA). For sample preparation, conical bottom glass tubes were used, adding 3.9 g of ammonium sulfate, 6.3 mL of milli-Q grade deionized water, 2.7 mL of wine, 20 μL of an internal standard solution (2-Butanol, 4-Methyl-2-pentanol, 4-hydroxy-4-methyl-2-pentanone and 2-Octanol) and 250 μL of dichloromethane. Chromatography conditions included an oven temperature initially programmed at 40°C for 5 min, and then ramped to 200°C. A constant helium flow of 2 mL/min was used. Two mL of aroma extract were injected at 250°C in splitless mode. The total run time was 75 min per sample.
Minor volatiles (terpenoids and C13-norisoprenoids) were determined using headspace-solid phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME/GC–MS), following the method proposed by Yuan and Qian [18] on an Agilent 6890 gas chromatograph equipped with an Agilent 5973 mass selective detector (Agilent). A volume of two mL of the wine sample was diluted with 8 mL of citric acid (0.5 g/L citric acid, pH 3 saturated with sodium chloride) and 20 μL of 4-octanol (100 μg/L), used as internal standard. The extraction was done by stirring the sample in vials for chromatography (20 mL of volume, Agilent Technologies) tightly capped with a magnetic stir bar for 10 min at 50°C in a thermostatic bath and using SPME fiber (50/30 μm DVB/CAR/PDMS fiber from Supelco Inc., Bellefonte, PA, USA) for 50 min at the same temperature with stirring (1000 rpm) to capture the volatiles. The fiber was manually inserted into the injection port of the GC at 230°C to desorb the analytes. A DB-Wax column from J&W Scientific (Folsom, CA, USA) (60 m × 0.32 mm × 0.5 μm film thickness, Phenomenex, Torrance, CA, USA) was employed to separate the analytes. Carrier gas (helium) was set at a constant flow rate of 1 mL/min. The oven temperature was initially set at 40°C for 2 min, and raised to 230°C at 5°C per min for 15 min. All samples were analyzed in duplicate.
2.4 Incidence of aroma composition
To estimate the contribution of volatile compounds to wine aroma, the odor activity value (OAV) was calculated by estimating the ratio between the concentration of each compound and its perception threshold. A compound was considered to contribute to wine aroma if OAV ≥ 1. The perception threshold used in this work were those found in the literature [19, 20, 21, 22, 23, 24, 25].
2.5 Sensorial analysis
Wines were tasted at the tasting room of Experimental Winery from IMIDRA Institute by a sensory panel of eight trained evaluators. Descriptive sensory analyses and triangle tests were performed following the indications of the ISO 4120:2004 to assess the effect of experimental treatments (use of lees) on wine aroma. Sensory descriptive analysis was performed to describe and quantify wine attributes from 1 (low intensity) to 10 (high intensity). The evaluation included a visual phase, an olfactory phase and a gustatory phase, which was interpreted by graphical representation. A hedonic classification was also carried out to determine the order of preference of the wines. A final score for each wine was obtained as the mean with their respective standard deviation.
2.6 Statistical analysis
Statistical analyses were performed using SPSS ver. 20.0 (SPSS, Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was made to examine the differences between treatments in terms of volatile compounds and sensory attributes of the wines. In order to assess the significance (p < 0.05) of differences between means, Tukey honestly significant difference (HSD) post-hoc tests were used to establish the significance of differences between means to assess significance (p < 0.05).
3. Results and discussion
3.1 Wine volatile composition of Malvasia aromatica elaborated wines
An analysis of the aroma profile of Malvasia wines elaborated with the indigenous yeast strains CLI 271 and CLI 889 was made in both conditions, with and without aging on lees, to determine the effects of aging on lees on the volatile composition. Figure 2 and Table 1 show the evolution of volatile compounds in wines from the two yeasts after treatment on lees, grouped into chemical families.
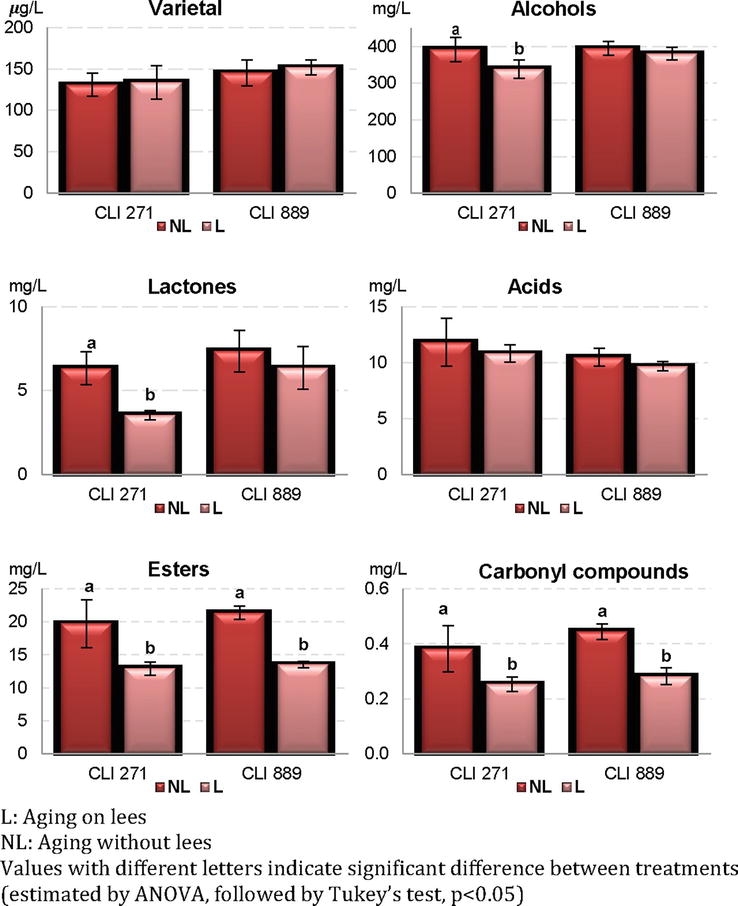
Figure 2.
Effect of aging on lees on the levels of aromatic compounds grouped by families in Malvasia
| Compounds | OTH | CLI 271 | CLI 271 L | Siga | CLI 889 | CLI 889 L | Sig.a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| — | 1.17 | ± | 0.22 | 1.86 | ± | 0.06 | 2.17 | ± | 0.27 | Ns | |||||
| α-Terpinene | — | 0.22 | ± | 0.03 | 0.22 | ± | 0.06 | Ns | 0.17 | ± | 0.06 | 0.26 | ± | 0.02 | Ns |
| Limonene | 15b | 0.53 | ± | 0.08 | 0.56 | ± | 0.08 | Ns | 0.49 | ± | 0.08 | ||||
| — | 1.23 | ± | 0.12 | 1.89 | ± | 0.10 | |||||||||
| Linalool | 25c | 69.89 | ± | 5.52 | 75.14 | ± | 8.29 | Ns | 78.55 | ± | 8.29 | 84.17 | ± | 4.79 | Ns |
| α-Terpineol | 250c | 25.21 | ± | 4.12 | 25.26 | ± | 2.06 | Ns | 23.39 | ± | 2.06 | 23.86 | ± | 1.31 | Ns |
| β-Citronellol | 100d | 12.07 | ± | 1.12 | 11.43 | ± | 2.05 | Ns | 17.31 | ± | 2.05 | 16.99 | ± | 0.96 | Ns |
| Geraniol | 30b | 19.17 | ± | 2.78 | 16.65 | ± | 3.01 | Ns | 20.74 | ± | 3.01 | 19.69 | ± | 1.53 | Ns |
| 129.48 | ± | 13.97 | 132.63 | ± | 15.71 | 144.40 | ± | 15.71 | 150.41 | ± | 9.22 | ||||
| 0.05d | 1.24 | ± | 0.06 | 1.16 | ± | 0.14 | Ns | 1.11 | ± | 0.02 | |||||
| 1.24 | ± | 0.06 | 1.16 | ± | 0.14 | 1.11 | ± | 0.02 | 1.33 | ± | 0.04 | ||||
| 40c | 35.58 | ± | 1.74 | 42.30 | ± | 0.44 | |||||||||
| 150b | 1.03 | ± | 0.05 | 0.40 | ± | 0.02 | 0.35 | ± | 0.03 | Ns | |||||
| Isoamyl alcohol | 30c | 296.05 | ± | 27.63 | 262.31 | ± | 22.26 | Ns | 315.63 | ± | 16.13 | 309.26 | ± | 16.92 | Ns |
| 8c | 1.61 | ± | 0.01 | 1.58 | ± | 0.03 | |||||||||
| 1c | 1.80 | ± | 0.19 | 0.85 | ± | 0.07 | |||||||||
| 14c | 56.33 | ± | 3.21 | 34.30 | ± | 2.20 | 31.11 | ± | 0.53 | Ns | |||||
| 392.41 | ± | 32.82 | 338.95 | ± | 25.31 | 395.09 | ± | 18.90 | 380.62 | ± | 17.70 | ||||
| 35e | 6.33 | ± | 0.98 | 7.35 | ± | 1.25 | 6.34 | ± | 1.27 | Ns | |||||
| 6.33 | ± | 0.98 | 3.53 | ± | 0.28 | 7.35 | ± | 1.25 | 6.34 | ± | 1.27 | ||||
| 2.30f | 1.39 | ± | 0.21 | 1.72 | ± | 0.11 | |||||||||
| 0.17c | 0.64 | ± | 0.08 | 0.73 | ± | 0.11 | 0.65 | ± | 0.11 | Ns | |||||
| 0.03c | 2.43 | ± | 0.30 | 2.09 | ± | 0.13 | |||||||||
| Hexanoic acid | 0.42c | 2.92 | ± | 0.62 | 3.03 | ± | 0.22 | Ns | 2.31 | ± | 0.17 | 2.44 | ± | 0.11 | Ns |
| Octanoic acid | 0.50c | 4.01 | ± | 0.81 | 4.22 | ± | 0.22 | Ns | 3.27 | ± | 0.26 | 3.23 | ± | 0.02 | Ns |
| 1c | 0.41 | ± | 0.12 | 0.37 | ± | 0.00 | Ns | 0.34 | ± | 0.02 | |||||
| 11.80 | ± | 2.14 | 10.81 | ± | 0.76 | 10.47 | ± | 0.80 | 9.67 | ± | 0.42 | ||||
| 0.02c | 0.27 | ± | 0.01 | 0.31 | ± | 0.02 | 0.28 | ± | 0.01 | Ns | |||||
| 0.003c | 0.26 | ± | 0.04 | 0.26 | ± | 0.01 | 0.22 | ± | 0.03 | Ns | |||||
| 0.03c | 1.68 | ± | 0.19 | 1.40 | ± | 0.01 | Ns | 1.11 | ± | 0.03 | |||||
| 0.01c | 0.54 | ± | 0.12 | 0.38 | ± | 0.01 | Ns | 0.52 | ± | 0.03 | |||||
| 1g | 0.10 | ± | 0.01 | 0.10 | ± | 0.00 | Ns | 0.10 | ± | 0.00 | |||||
| 154b | 15.48 | ± | 2.86 | 17.16 | ± | 0.84 | |||||||||
| 0.58b | 0.52 | ± | 0.08 | 0.51 | ± | 0.03 | |||||||||
| 20e | 0.03 | ± | 0.06 | 0.04 | ± | 0.04 | Ns | 0.11 | ± | 0.00 | |||||
| 1.20c | 0.56 | ± | 0.22 | 0.24 | ± | 0.01 | Ns | 1.17 | ± | 0.00 | |||||
| 0.25c | 0.26 | ± | 0.01 | 0.12 | ± | 0.00 | |||||||||
| 19.71 | ± | 3.62 | 12.93 | ± | 1.00 | 21.38 | ± | 0.98 | 13.51 | ± | 0.49 | ||||
| 0.10d | 0.16 | ± | 0.05 | 0.11 | ± | 0.01 | Ns | 0.23 | ± | 0.02 | |||||
| 150h | 0.04 | ± | 0.00 | Ns | 0.30 | ± | 0.07 | ||||||||
| Benzaldehyde | 5c | 0.18 | ± | 0.04 | 0.14 | ± | 0.02 | Ns | 0.21 | ± | 0.01 | 0.17 | ± | 0.01 | |
| 0.38 | ± | 0.08 | 0.25 | ± | 0.03 | 0.44 | ± | 0.03 | 0.28 | ± | 0.03 | ||||
| 430.77 | ± | 39.6 | 366.62 | ± | 27.41 | 434.88 | ± | 21.97 | 410.57 | ± | 19.93 | ||||
Table 1.
Significance at which means differ as shown by analysis of variance:
[18]
[19]
[20]
[21]
[22]
[23]
[24].
OTH*: Odor threshold value.
Ns: not significant.
Tr: traces.
In bold the compounds that seem to have a clear connection with aging on lees treatment.
Thirty-five volatiles were quantified, and distributed in varietal aromas (terpenols and C13-norisoprenoids), alcohols, acids, lactones, esters, aldehydes and ketones; their total content is given in μg/L for varietal aromas and mg/L for the rest of the compounds. Table 1 also shows the results of the analysis of variance (ANOVA) for each fermentation to determine the compounds that show significant individual differences due to the effect of aging on lees.
The effect of lees treatment was significant in 57% of the compounds identified in wines fermented with CLI 889 strain (20 of 35 compounds identified) and 45% (16 of 35 compounds) in wines from CLI 271 strain (Table 1).
Aging on lees caused a significant decrease in the total content of esters, aldehydes and carbonyl compounds in the two winemaking processes (Figure 2). The same behavior for the ester family was reported by Bueno et al. [26] and Del Barrio-Galán et al. [27] in Macabeo and Verdejo varieties respectively after a period of aging on lees.
The ANOVA analysis in Table 1 shows major differences in the CLI 889 wines, 8 of the 10 esters identified decreased significantly. Some studies have shown how the interactions between macromolecules released by the lees and aromatic compounds present in the wine could modify the volatility and aromatic intensity of these compounds; thus, the lower content of some esters compared to the control wine (without lees) could be explained by their association with mannoproteins and polysaccharides released, reducing volatility. These results are in agreement with other authors on model wines [28, 29]. On the other hand, in the esters where there was a notable decrease in terms of concentration, it was not decisive in terms of aroma contribution. Despite the loss, they continued to exceed their perception threshold (isoamyl acetate and ethyl hexanoate) or did not reach the threshold before lees treatment (hexyl acetate, ethyl lactate, ethyl octanoate, diethyl succinate and ethyl 3-hydroxy butyrate). Some of these compounds also showed lower concentrations in Tempranillo wines aged on lees [30]. The authors explained that the lower content of these volatiles can be due to an interaction between esters, acetates and mannoproteins from yeast autolysis and/or the sorption phenomenon onto yeast cell walls. This behavior was also denoted in model wines [28, 29, 31] and in white wines [26]. It could be happened in Malvasia wines from the present work. Wines fermented with CLI 271 showed a significant decrease in 4 of the 10 esters quantified. This decrease was not a disadvantage in terms of aroma contribution because ethyl butyrate and ethyl isovalerate still exceeded their perception threshold despite the loss of concentration and ethyl lactate and ethyl octanoate did not reach the threshold before lees treatment. It is important to note the role of 2-phenylethyl acetate in CLI 271 wines. Its concentration increases significantly with lees treatment. This compound is related to fruity attributes.
Regarding carbonyl compounds, the significant decrease of acetoin (creamy and buttery aromas) in CLI 889 L wines is noteworthy. Acetoin on its own does not have a significant aromatic impact due to its high detection threshold, although it does contribute to the wine bouquet due to its interrelation in the synthesis of diacetyl [32], a compound that, although it decreased significantly in CLI 889 wines, exceeds its perception threshold, contributing buttery aromas. Benzaldehyde also decreased significantly in CLI 889 wines but in all cases, like acetoin, it was far from its threshold.
The effect of aging on lees was different for the lactone family (only represented by γ-butyrolactone). The treatment resulted in a significant decrease in CLI 271 wines and no variations were observed in CLI 889 wines. In all cases, the concentrations of this compound were far from its perception threshold.
The increase of terpenes and norisoprenoids on lees presence is likely related to the release during autolysis of β-glucosidases. These enzymes are able to break the glycoside bound of these volatile compounds releasing the free aromatic form, which has an influence on final aroma of wine [33]. In wines aged on lees, varietals tended to increase in agreement with other works [26, 33]. Individually (Table 1), the varietal compounds β-myrcene, limonene and γ-terpinene varied significantly but were found in low concentrations. As for β-damascenone, a significant increase was only seen in CLI 889 wines with lees treatment always exceeding their olfactory perception threshold.
Regarding to acids content, the decrease in their concentration as a consequence of contact with lees had no effect on the aroma of the wines. The maximum values correspond to octanoic acid, and after that, to hexanoic acid in wines aging on lees in good agreement with other studies in white wines [26, 34].
According to the family of alcohols (Figure 2) only the wines made with strain CLI 271 aged on lees showed a notable decrease, 5 of the 6 compounds identified (Table 1) decreased significantly. Methionol and β-phenylethanol seem to actively contribute to the aroma of these wines with a concentration above their perception threshold even while decreasing in presence of lees. Isobutanol decreased significantly and was found at levels below its threshold in CLI 889 wines aged on lees. These changes in alcohol content compounds were similar to obtained in Macabeo wines [26].
3.2 Odor activity values of Malvasia aromatica wines
In order to estimate the sensory contribution of aromatic compounds to overall aroma of wine, the odor activity value (OAV) was calculated for all aroma compounds in study (Table 2). An aromatic compound contributes actively in wine when its concentration is higher than its olfactory perception threshold (OTH). The concentration/threshold ratio, known as odor activity value (OAV), shows the contribution of a specific compound to wine aroma properties. Those compounds with an OAV ≥ 1 can be considered active odorants [21].
| Linalool | Floral, citric | 25 | 4.66 | 5.01 | 5.24 | 5.61 |
| Floral, lilac | 0.05 | 24.80 | 23.27 | 22.13 | ||
| Isoamyl alcohol | Vegetal/herbaceous | 30 | 9.87 | 8.74 | 10.52 | 10.31 |
| Alcohol | 40 | <1 | <1 | 1.06 | ||
| Methionol | Cooked vegetable | 1 | 1.80 | 1.36 | <1 | <1 |
| β-Phenylethanol | Roses | 14 | 4.02 | 3.21 | 2.45 | 2.22 |
| Acid fruit, apple | 0.02 | 13.68 | 15.33 | |||
| Sweet fruit, orange, blackberry | 0.003 | 87.17 | 87.52 | |||
| Banana | 0.03 | 56.14 | 37.07 | |||
| Fruit, green apple | 0.01 | 53.80 | 51.90 | |||
| Green apple | 0.25 | 1.04 | <1 | <1 | ||
| Butyric acid | Cheese | 0.17 | 3.79 | 2.38 | 4.30 | 3.79 |
| Blue cheese | 0.03 | 81.04 | 59.82 | 69.70 | 55.59 | |
| Hexanoic acid | Cheese | 0.42 | 6.95 | 7.22 | 5.51 | 5.80 |
| Octanoic acid | Butter, sour | 0.50 | 8.01 | 8.43 | 6.55 | 6.46 |
| Butter | 0.10 | 1.62 | 2.31 |
Table 2.
Odor threshold values and odor activity values of the volatile compounds with the greatest influence on the aroma of Malvasia wines fermented with
*OTH: Odor threshold values
aOAV: Odor activity values calculated by dividing concentration by odor threshold value of the compound.
OTH and OAV are given in mg l−1 except linalool and β-damascenone which are in μg l−1. Sensory descriptor and OTH values according to the references included in Table 1.
In bold the compounds that seem to have a clear connection with aging on lees treatment.
Sixteen of thirty-five identified compounds presented OAV > 1, contributing to the aroma of the wines studied (Table 2). The varietal compounds linalool and β-damascenone had an active contribution to the aroma of all resulting wines providing floral aromas with citrus and lilac attributes respectively. The OAV was always higher than 1. β-damascenone also presented concentrations higher than OTH in all wines showing interesting correlations between the increase in OAVs of wines fermented with CLI 889 yeast strain aged on lees. A study realized by Gallardo-Chacón et al. [35] in Cava aged in contact with lees, they detected some norisoprenoids as β-damascenone more retained in lees surface and thus its presence in the volatile profile of Cava was significant.
The alcohols, characterized by vegetal and bitter aromatic descriptors with the exception of β-phenylethanol (roses), did not show significant differences according to aroma contribution. OAVs were higher than 1 in all wines except isobutanol in CLI 889 L wines.
The wine elaborated with
A significant decrease is observed in CLI 889 wines regarding diacetyl during aging on lees (Table 1). This compound is related to buttery aroma descriptors although their OAV is still higher than 1 (Table 2).
Finally, butyric, isovaleric, hexanoic and octanoic acids, characterized by their unpleasant odors (cheese and rancid attributes) influenced all the wines equally, all wines had OAV > 1. They cannot be considered determinants of the differences in the overall aromatic quality of the wines.
3.3 Sensory characteristics of Malvasia aromatica wines
A descriptive tasting of wines was performed at visual, olfactory and gustative levels in order to determine the main organoleptic differences among wines after aging on lees (Figure 3A–C). The sensory characterization of the Malvasia
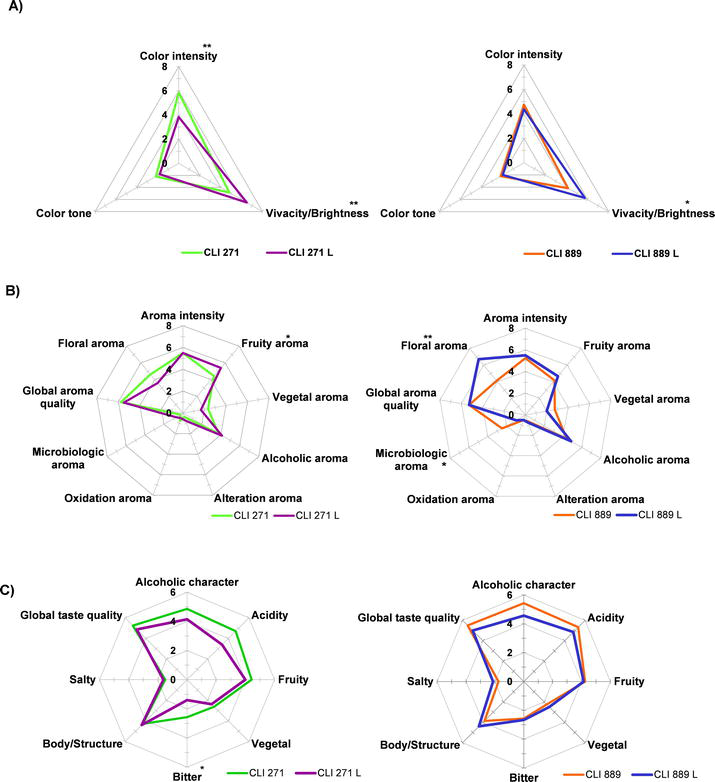
Figure 3.
Descriptive analysis of Malvasia wines elaborated with
In the visual phase (Figure 3A), aging on lees (L) resulted in Malvasia
In the olfactory phase (Figure 3B), CLI 889L wines obtained significantly higher scores on floral aroma attribute. As shown in Table 1, wines elaborated with CLI 889 strain presented higher concentrations of varietals compounds and lees treatment could have contributed to enhance the floral characteristics of this family of volatiles. According to the results in Table 2, there was a noticeable increment of linalool and β-damascenone compounds. Regarding to CLI 271L wines were considered significantly fruitier than CLI 271 control (non lees). These results are in line with those obtained by Del Barrio-Galán et al. [27] in the sensory analysis of the Verdejo variety where the treatment with lees would have displayed stronger fruity and floral aromas and higher olfactory intensity than the control wines which could indicate that these initially retained aromatic compounds are released over time, increasing aroma intensities. Based on the analysis of Table 2, 2-phenylethyl acetate with fruit descriptor increased significantly after lees aging. Aging on lees decreased the vegetal character of Malvasia
Finally, in the gustatory phase (Figure 3C), there are almost no differences between treated wines and their controls. Wines not aged on lees were evaluated as alcoholic and scored higher in global taste quality without significant differences. Aging on lees apparently reduced bitterness in Malvasia wines, but the decrease was only significant in CLI 271. Additionally, there was a tendency for the lees-treated wines to have greater structure and less acidity, although this was not statistically significant. This trend toward a bitterness and acidity reduction and an increment of mouthfeel and balance in wines treated with lees has been described in previous publications [27, 44].
With the aim of completing the sensory study, triangular tests were done to determine whether descriptive analysis was determinant to differentiating the samples. Discriminant triangular tastings were carried out using dark glasses and two series of tastings were performed by each type of wine: CLI 889 vs. CLI 889 L, CLI 271 vs. CLI 271 L. The panel clearly differentiated CLI 271 and CLI 889 wines aged on lees from their respective controls with statistical significance levels of 1% and 0.1% in the two tests performed for CLI 271 wines, and 5% and 1% in the two tests performed for CLI 889 wines. In addition, tasters were asked to assess their hedonic preference for wines in the tests presented, taking into account the preferences of the taster who answered correctly. The tasting panel showed preference by CLI 271 wines in 57% of cases in the first test and 62% in the second. Preferences for the CLI 889 wines were equally divided. In the first test, 50% of the correct judges chose CLI 889 L samples. In the second test, 57% of the correct tasters preferred CLI 889 L.
4. Conclusions
According to the results, we can affirm that lees treatment has had an effect on the result of aroma and sensory composition of Malvasia
Acknowledgments
JMC acknowledges INIA for a PhD grant (FPI-INIA 2012 call). The authors acknowledge IMIDRA colleagues at the El Socorro experimental farm and the Microbiology lab for providing material of Malvasia
Acronyms and abbreviations
Protected Designation of Origen | |
lees | |
divinylbenzene | |
carboxen | |
polydimethylsiloxane | |
gas chromatography with flame-ionization detection | |
gas chromatography–mass spectrometry | |
headspace-solid phase microextraction | |
C13-norisoprenoids | |
analysis of variance | |
olfactory perception threshold | |
odor activity value |
References
- 1.
Fornairon-Bonnefond C, Camarasa C, Moutounet M, Salmon JM. New trends on yeast autolysis and wine aging on lees: A bibliographic review. Journal International des Sciences de la Vigne et du Vin. 2002; 36 :49-69. DOI: 10.20870/oeno-one.2002.36.2.974 - 2.
Charpentier C, Dos Santos AM, Feuillat M. Release of macromolecules by Saccharomyces cerevisiae during aging of French flor sherry wine “Vin jaune”. International Journal of Food Microbiology. 2004;96 :253-262. DOI: 10.1016/j.ijfoodmicro.2004.03.019 - 3.
Pérez-Bibbins B, Torrado-Agrasar A, Salgado JM, Oliveira RP, Domínguez JM. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Management. 2015; 40 :72-81. DOI: 10.1016/j.wasman.2015.03.009 - 4.
Lavigne V, Pons A, Dubourdieu D. Assay of glutathione in must and wines using capillary electrophoresis and laser-induced fluorescence detection. Changes in concentration in dry white wines during alcoholic fermentation and aging. Journal of Chromatography A. 2007; 1139 :130-135. DOI: 10.1016/j.chroma.2006.10.083 - 5.
Chattonnet P. La contamination des vins par Brettanomyces au cours de la vinification et de l’élevage Incidence, détection et moyens de lutte. Revue des OEnologues. 2000;2000 :23-26 - 6.
Doco T, Vuchot P, Cheynier V, Moutounet M. Structural modification of wine arabinogalactans during aging on lees. American Journal of Enology and Viticulture. 2003; 53 :150-157 - 7.
Bureau SM, Razungles AJ, Baumes RL. The aroma of Muscat of Frontignan grapes: Effect of the light environment of vine or bunch on volatiles and glycoconjugates. Journal of the Science of Food and Agriculture. 2000; 80 :2012-2020. DOI: 10.1002/1097-0010(200011)80:14<2012: AID-JSFA738>3.0.CO; 2-X - 8.
Compés R, Sotés V. El sector vitivinícola frente al desafío del cambio climático. Estrategias públicas y privadas de mitigación y adaptación en el Mediterráneo. Almería, Spain: Cajamar Caja Rural; 2018. p. 372 - 9.
Ribéreau-Gayon P. New developments in wine microbiology. American Journal of Enology and Viticulture. 1985; 36 :1-10. DOI: 10.5344/ajev.1985.36.1.1 - 10.
Heard GM, Fleet GH. Growth of natural yeast flora during the fermentation of inoculated wines. Applied and Environmental Microbiology. 1985; 50 :727-728. DOI: 10.1128/aem.50.3.727-728.1985 - 11.
Crespo J, Romero V, García M, Arroyo T, Cabellos JM. Influence of skin-contact treatment on aroma profile of Malvasia aromatica wines in D.O. “Vinos de Madrid”. In: Morata A, editor. Grapes Wine. London, UK: IntechOpen; 2021. pp. 137-144. DOI: 10.5772/intechopen.99216 - 12.
Crespo J, García M, Arroyo T, Romero V, Cabellos JM. Influence of native Saccharomyces cerevisiae strains on Malvasiaaromatica wines. Frontiers in Bioscience-Elite. 2023;15 :18. DOI: 10.31083/j.fbe1503018 - 13.
Cabello F, Ortiz J, Muñoz G, Rodríguez I, Benito A. Variedades de vid en España. 1st ed. Madrid, España: Agrícola Española; 2012. p. 504 - 14.
Arroyo T. Estudio de la influencia de diferentes tratamientos enológicos en la evolución de la microbiota y en la calidad de los vinos elaborados con la variedad “Airén”, en la D.O. “Vinos de Madrid.” Alcalá de Henares: University of Alcalá; 2000 - 15.
Balboa-Lagunero T, Arroyo T, Cabellos JM, Aznar M. Yeast selection as a tool for reducing key oxidation notes in organic wines. Food Research International. 2013; 53 :252-259. DOI: 10.1016/j.foodres.2013.04.006 - 16.
Cordero-Bueso G, Esteve-Zarzoso B, Gil-Díaz M, García M, Cabellos J, Arroyo T. Improvement of Malvar wine quality by use of locally-selected Saccharomyces cerevisiae strains. Fermentation. 2016;2 :7. DOI: 10.3390/fermentation2010007 - 17.
Ortega C, López R, Cacho J, Ferreira V. Fast analysis of important wine volatile compounds-development and validation of a new method based on gas chromatographic-flame ionisation detection analysis of dichloromethane microextracts. Journal of Chromatography A. 2001; 923 :205-214. DOI: 10.1016/S0021-9673(01)00972-4 - 18.
Yuan F, Qian MC. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food Chemistry. 2016;192 :633-641. DOI: 10.1016/j.foodchem.2015.07.050 - 19.
Etiévant XP. Wine. In: Maarse H, editor. Volatile Compounds in Foods and Beverages. New York: Marcel Dekker; 1991. pp. 483-546 - 20.
Ferreira V, López R, Cacho JF. Quantitative determination of the odorants of young red wines from different grape varieties. Journal of the Science of Food and Agriculture. 2000; 80 :1659-1667. DOI: 10.1002/1097-0010(20000901)80:11<1659: AID-JSFA693>3.0.CO; 2-6 - 21.
Guth H. Quantitation and sensory studies of character impact odorants of different white wine varieties. Journal of Agricultural and Food Chemistry. 1997; 45 :3027-3032. DOI: 10.1021/jf9608433 - 22.
Aznar M, López R, Cacho J, Ferreira V. Prediction of aged red wine aroma properties from aroma chemical composition. Partial least squares regression models. Journal of Agricultural and Food Chemistry. 2003; 51 :2700-2707. DOI: 10.1021/jf026115z - 23.
Van Gemert LJ, Nettenbreijer AH. Compilation of Odour Threshold Values in Air and Water. The Netherlands; 1977 - 24.
Gil M, Cabellos JM, Arroyo T, Prodanov M. Characterization of the volatile fraction of young wines from the denomination of origin “Vinos de Madrid” (Spain). Analytica Chimica Acta. 2006; 563 :145-153. DOI: 10.1016/j.aca.2005.11.060 - 25.
Ferreira V, Ortín N, Escudero A,López R, Cacho J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. Journal of Agricultural and Food Chemistry. 2002; 50 :4048-4054. DOI: 10.1021/jf0115645 - 26.
Bueno JE, Peinado RA, Medina M, Moreno J. Effect of a short contact time with lees on volatile composition of Airen and Macabeo wines. Biotechnology Letters. 2006; 28 :1007-1011. DOI: 10.1007/s10529-006-9038-2 - 27.
Del Barrio-Galán R, Pérez-Magariño S, Ortega-Heras M, Williams P, Doco T. Effect of aging on lees and of three different dry yeast derivative products on Verdejo white wine composition and sensorial characteristics. Journal of Agricultural and Food Chemistry. 2011; 59 :12433-12442. DOI: 10.1021/jf204055u - 28.
Lubbers S, Voilley A, Feuillat M, Charpentier C. Influence of mannaproteins from yeast on the aroma intensity of a model wine. LWT – Food Science and Technology. 1994; 27 :108-114. DOI: 10.1006/fstl.1994.1025 - 29.
Chalier P, Angot B, Delteil D, Doco T, Gunata Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chemistry. 2007;100 :22-30. DOI: 10.1016/j.foodchem.2005.09.004 - 30.
Rodríguez-Bencomo JJ, Ortega-Heras M, Pérez-Magariño S. Effect of alternative techniques to aging on lees and use of non-toasted oak chips in alcoholic fermentation on the aromatic composition of red wine. European Food Research and Technology. 2010; 230 :485-496. DOI: 10.1007/s00217-009-1189-7 - 31.
Voilley A, Beghin V, Charpentier C, Peyron D. Interaction between aroma substances and macromolecules in a model wine. LWT – Food Science and Technology. 1991; 24 :469-472 - 32.
Romano P, Suzzi G. Origin and production of acetoin during wine yeast fermentation. Applied and Environmental Microbiology. 1996; 62 :309-315. DOI: 10.1128/aem.62.2.309-315.1996 - 33.
Liberatore MT, Pati S, Del NMA, La NE. Aroma quality improvement of chardonnay white wine by fermentation and aging in barrique on lees. Food Research International. 2010; 43 :996-1002. DOI: 10.1016/j.foodres.2010.01.007 - 34.
Bautista R, Fernández E, Falqué E. Effect of the contact with fermentation-lees or commercial-lees on the volatile composition of white wines. European Food Research and Technology. 2007; 224 :405-413. DOI: 10.1007/s00217-006-0336-7 - 35.
Gallardo-Chacón J, Vichi S, López-Tamames E, Buxaderas S. Analysis of sparkling wine lees surface volatiles by optimized headspace solid-phase microextraction. Journal of Agricultural and Food Chemistry. 2009; 57 :3279-3285. DOI: 10.1021/jf803493s - 36.
Waters EJ, Pellerin P, Brillouet JM. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohydrate Polymers. 1994;23 :185-191. DOI: 10.1016/0144-8617(94)90101-5 - 37.
Waters E. A review of current knowledge on polysaccharides which “protect” against protein haze en white wine. Australian Grapegrower Winemaker. 2000; 438 :13-17 - 38.
Mazauric JP, Salmon JM. Interactions between yeast lees and wine polyphenols during simulation of wine aging. Journal of Agricultural and Food Chemistry. 2006; 54 :3876-3881. DOI: 10.1021/jf060037o - 39.
Márquez T, Millán C, Souquet JM, Salmon JM. Effect of different yeast strains and their culture conditions on the prevention of wine model solution browning by yeast lees. Journal of Agricultural and Food Chemistry. 2009; 57 :3771-3779. DOI: 10.1021/jf803839s - 40.
Razmkhab S, Lopez-Toledano A, Ortega JM, Mayen M, Merida J, Medina M. Adsorption of phenolic compounds and browning products in white wines by yeasts and their cell walls. Journal of Agricultural and Food Chemistry. 2002; 50 :7432-7437. DOI: 10.1021/jf025733c - 41.
Lopez-Toledano A, Mayen M, Merida J, Medina M. Yeasts used to delay browning in white wines. Food Chemistry. 2006; 97 :498-504. DOI: 10.1016/j.foodchem.2005.05.030 - 42.
Guadalupe Z, Palacios A, Ayestarán B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and color extraction in red wines. Journal of Agricultural and Food Chemistry. 2007; 55 :4854-4862. DOI: 10.1021/jf063585a - 43.
Poncet-Legrand C, Doco T, Williams P, Vernhet A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. American Journal of Enology and Viticulture. 2007; 58 :87-91 - 44.
Liu L, Loira I, Morata A, Suárez-Lepe JA, González MC, Rauhut D. Shortening the aging on lees process in wines by using ultrasound and microwave treatments both combined with stirring and abrasion techniques. European Food Research and Technology. 2016; 242 :559-569. DOI: 10.1007/s00217-015-2566-z