Possible number of genotypes of multiple alleles.
Abstract
Mendelian genetics revealed only two alternative forms of a gene called alleles. The concept has evolved with the identification of more than two alternative forms of a gene, commonly referred to as multiple alleles. There are several traits that are governed by multiple alleles, such as ABO blood group system in humans, coat color in rabbits, and self-incompatibility in crop plants. The test of allelism is a very common practice to establish the relationship between alleles of the same or different genes. The inter-mating among different mutants helps to confirm whether mutations are allelic or non-allelic. The structural allelism determines whether two mutations are present at the same or different site in DNA and functional allelism determines whether two mutations are present in the same gene or in different genes. The concept of multiple alleles should not be confused with pseudoalleles and with pseudogenes. Pseudoalleles are two genetically linked genes with similar effects located close to each other on the chromosome, on the other hand, pseudogenes are nonfunctional copies of the functional genes. To understand the allelic relationships among and between genes is always a subject of interest. Therefore, in this chapter, the concept, function, and importance of multiple alleles are discussed.
Keywords
- multiple alleles
- mutation
- pseudoalleles
- pseudogene
- allelism
1. Introduction
The extensive research of Gregor Johann Mendel on pea led to the discovery of the law of segregation and law of independent assortment, the two important principles of genetics. He studied seven traits with two contrasting phenotypes (wild and mutant) and indicated the presence of only two alleles for each gene [1]. Allele is referred to as an alternative form of a gene and locus is the location of allele in the genome or on chromosome in an organism. The concept of two alleles for each gene has changed with the discovery of more than two alleles for a gene as there is no restriction on number of alleles in a population. The presence of more than two alleles in a group of individuals is designated as multiple alleles (also referred to as allelic series). The concept of multiple alleles holds true for a population and it should not be misinterpreted at the individual level. A particular diploid organism can have at most two alleles from different alleles of the same gene on each homologous chromosome. However, many alternative forms of a gene can exist between different members of species. Multiple alleles have a similar inheritance pattern as that of two alleles but multiple alleles may have a large number of genotypes and phenotypes. The general formula to calculate the possible number of genotypes is n(n + 1)/2, where n stands for number of alleles involved. The possible number of homozygotes and heterozygotes are n and n(n-1)/2, respectively (Table 1).
| Total number of alleles | Number of genotypes | Number of homozygotes | Number of heterozygotes |
|---|---|---|---|
| 1 | 1 | 1 | 0 |
| 2 | 3 | 2 | 1 |
| 3 | 6 | 3 | 3 |
Table 1.
Let us have a hypothetical example to understand the concept of multiple alleles at the molecular level. With understanding that an organism has a pair of homologous chromosomes each from male and female parent, and genes are present on the chromosomes and their positions on the chromosomes are referred to as locus/loci. Gene, on the other hand, is a sequence of nucleotides performing specific functions. The mutation causes a change in DNA sequence of a gene, leading to the emergence of an alternative form of a gene (allele/mutant). Multiple alleles, are thus, changed DNA sequences of a gene, arises due to mutation at a locus. In the following example, wild-type allele
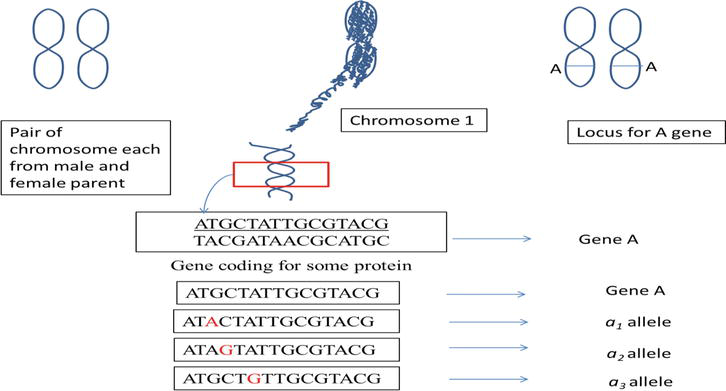
Figure 1.
Concept of gene, allele, and locus.
2. Symbols for mutant alleles
William Bateson designated dominant and recessive alleles of a particular gene with a single letter. He assigned the symbol
3. Important examples of multiple alleles
There are several examples of multiple alleles, such as blood group in human, coat color in rabbit, and self-incompatibility in plant, which helps in understanding this concept with ease.
3.1 ABO blood group system
Study on human blood type led to the discovery of ABO blood group system by Karl Landsteiner in the early 1900s [2]. He awarded with Nobel Prize in Physiology or medicine for the discovery of the human blood group in 1930. Serological studies were used to identify the A, B, and O antigens in which blood samples were tested with the different types of sera. Out of different sera, one serum specifically detects A antigen, and another serum detects B antigen. The presence of only “A” antigen is responsible for “A” blood group, “B” antigen is responsible for B blood type, AB blood type contains both A and B antigen and O blood type neither have A nor B antigen. A single gene with multiple alleles
Biosynthesis of ABO blood group system required H substance precursor made up of galactose (Gal), N-acetylglucosamine (AcGluNH) [4]. ABO genes encode different types of glycotransferase enzymes. The function of glycotransferase enzymes is to add sugar groups to the preexisting polysaccharides. These polysaccharides with added sugar combined with lipids to form glycolipids and the association of red blood cells with glycolipids led to the formation of blood group antigens.
3.1.1 Synthesis of H/O antigen
Almost all individuals having glycolipids are called as H antigen. It is made up of chemically linked galactose (Gal), N-acetylglucosamine (AcGluNH), and Fucose. Addition of Fucose to the precursor was done by α 1,2 fucosyltranferase (H transferase) enzyme led to the formation of H antigen [5]. As it is commonly found in all individuals, one, two, or more different types of sugar groups can be added to it for the synthesis of other antigens. As

Figure 2.
Flow chart represents the biosynthesis of A, B, and O antigens [
3.1.2 Synthesis of “A” antigen
3.1.3 Synthesis of “B” antigen
Due to the difference in the DNA sequence of IA and IB alleles, they encode functionally different but highly related glycosyltransferase enzymes. The formation of AB blood group type is possible only when IA and IB alleles are present in a heterozygote state. As both alleles act co-dominantly, both enzymes are produced and a person with AB blood group type will be having both A and B antigens [7]. Some of the H antigens will be converted to the A antigens by the activity of A transferase and some of the H antigens will be converted to B antigens by the activity of B transferase.
The inheritance of a blood group is used to solve cases of disputed maternity or paternity. For example, parents with O blood type cannot have a child with AB blood type (alone these data are insufficient for legal purposes). Based on blood group inheritance, we cannot prove that an individual is a parent instead, we can only determine that an individual is not a parent of a particular child. Antigen-antibody relationship in the ABO blood group system is an important aspect in determining the protocol for blood transfusion [8].
Person with blood group A having genotypes as
| Blood group phenotype | Genotype/alleles | Antigen present | Antibody produced | Special properties |
|---|---|---|---|---|
| A | A antigen | B antibody | ||
| B | B antigen | A antibody | ||
| AB | A and B antigen | No antibodies | Universal recipient | |
| O | — | Both A and B antibody | Universal donor |
Table 2.
ABO blood type system.
Individuals with A blood type can donate blood to people with blood type A or AB as they do not have antibodies against “A” antigen. The person with B blood type can donate blood to the ones with blood type B or AB as they do not have antibodies against B. Person with AB blood type can only donate blood to the ones with only blood type of AB because of the presence of both A and B antigen [9]. However, they can accept blood from any of the other blood types as they do not have antibodies against A and B antigens. Therefore, individuals with AB blood type are referred to as universal recipients. Individuals with O blood type can donate blood to a person with any of the other blood types because they are devoid of A and B antigens in their blood serum. But they can receive blood only from the people with O blood type only as both antigen A and B acts as foreign molecule in the blood serum of O blood type. Therefore, individuals with O blood type are regarded as universal donors (Table 2).
3.2 Bombay blood type
The H antigen is an important precursor for the ABO blood group system. It is produced by the activity of the α 1,2 fucosyltransferase (H transferase) enzyme, which is encoded by the dominant H gene. The H gene is distinct from the ABO blood group gene [10]. In rare cases, individuals may inherit two copies of a rare mutation in the dominant H gene, resulting in the inability to produce the H transferase enzyme, which leads to the absence of H antigen [11]. This is known as the Bombay phenotype or the h/h genotype. Individuals with the Bombay phenotype cannot produce A or B antigens, regardless of the presence of IA and IB alleles [12]. The absence of H antigen means that IA and IB alleles cannot recognize H substance, leading to the lack of A and B antigen synthesis. Individuals with the Bombay phenotype are similar to the O blood group type, but they are different in that they produce anti-O antibodies, whereas individuals with O blood type do not [13]. This information is useful in solving cases of disputed maternity or paternity, as it provides insight into the likelihood of biological relationships between individuals based on their blood group inheritance [14] (Figure 3).

Figure 3.
Pedigree showing Bombay blood type [
3.3 Coat color in rabbits
Coat color in rabbits is governed by color determining gene (
3.4 Self-incompatibility in plant
The self-incompatibility in the plant has been reported to be governed by multiple alleles [17]. Self-sterility or self-incompatibility is referred to as pollen of the plant unable to germinate on its own stigma and no fertilization in ovules takes place [18]. East and Mangelsdorf [19] observed multiple alleles are responsible for self-incompatibility in
The pollen produced on a plant unable to fertilize the ovule of the same plant and cross combination is completely self-sterile, such as
| Female parent (Stigma) | Male parent (Pollen) | ||
|---|---|---|---|
| Self-sterile | |||
| Self-sterile | |||
| Self-sterile | |||
Table 3.
Different cross combinations of self-incompatibility in plant.
4. Test of allelism
The alternate form of a gene is called as allele/mutant allele. The change in the base pair of the DNA sequence of a gene lead to the development of mutant allele. Mutant allele may or may not have predictable phenotype always. If a trait is governed by several genes and mutation occurs in one of the genes governing the trait, there will be a reduction or abolishment of phenotype. Prediction of a gene that has mutated on the basis of phenotype remains a challenge. Therefore, a test has to be conducted to predict which gene has mutated, provided mutation is recessive in nature. For this, an unknown recessive mutation is crossed with other recessive mutations of known genes. If we get wild-type phenotype as a product of crossing over, it means two mutations used in crossing were non-allelic in nature and if we get mutant type, then two mutations used in crossing were allele of the same gene [20]. It explains the principle that mutation for the same gene affects the same genetic function (Table 4).
| Unknown recessive mutation | Known tester recessive mutation | Phenotype of hybrid (F1) | Remarks |
|---|---|---|---|
| aa | bb | wild | a and b are non-allelic |
| cc | mutant | a and c are allelic | |
| dd | wild | a and d are non-allelic |
Table 4.
General scheme for the test of allelism.
Let us have an example of eye color mutants of fruit fly,
| Crosses | Phenotype of hybrid | Remarks |
|---|---|---|
| Wild type (dark eye color) | ||
| Mutant type (bright eye color) | ||
| Wild type (dark eye color) |
Table 5.
A test of allelism involving recessive eye color mutations in Drosophila.
5. Structural and functional basis of allelism
Generally, the allelic relationship between different alleles can be established on two major aspects, such as structure and function, and it is named as structural allelism and functional allelism, respectively [22]. In the case of structural allelism, the occurrence of two or more mutations (governing the same trait) on the same site of the nucleotide sequence in DNA and is based on a recombination test whether two mutations can recombine or not at the DNA level. If the two mutations can recombine with each other through crossing over and producing wild-type allele, then they are structurally non-allelic, whereas, if two mutations do not recombine and are unable to produce wild-type DNA sequence, they are known to be structurally allelic in nature. Mutations on different sites can undergo the process of recombination, referred to as structurally non-allelic and mutations on the same site in DNA cannot recombine, referred to as structurally allelic (Figure 4). Functional allelism determines whether two mutations, governing the same trait, are present in the same gene or in the two different genes [23]. It is done by a complementation test (complementation at product level). If two individuals have mutations in the same gene, they are referred to as functionally allelic and wild-type phenotype will not develop after intermating of these mutants while if two individuals have mutations in two different genes, then wild-type phenotype will be produced through complementation [24], are referred as functionally non-allelic (Table 6, Figure 4). Therefore, if two mutations are structurally and functionally allelic, they are called as homoalleles and if two mutations are functionally allelic but structurally non-allelic are referred to as heteroalleles.
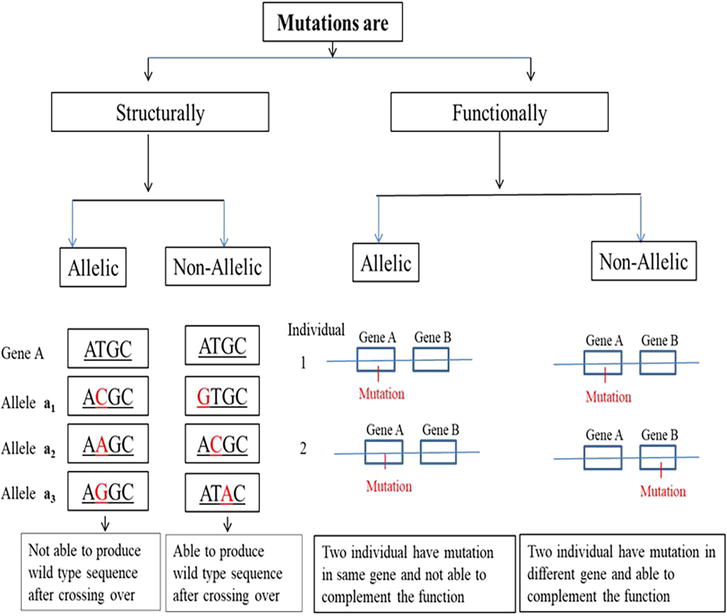
Figure 4.
Allelic relationship of different mutations.
| Trait | Structural allelism | Functional allelism |
|---|---|---|
| Function | It determines location of two mutation in DNA sequences, help to understand whether two mutations can recombine to produce wild types | It determines whether two mutations present in the same gene or in two different genes and help to understand their complementation |
| Detection | Based on the recombination test | Based on the complementation test |
| Occurs at | DNA level | Product level |
| Breakage of chromosome | Yes | No |
Table 6.
Distinguishing features of structural and functional allelism.
6. Pseudoalleles vs. multiple alleles
Pseudoalleles are defined as two genetically linked genes with similar effects located close to each other on the chromosome [25, 26]. As two genes (pseudoalleles) are genetically linked, genes always tend to inherit together and may appear to act as a single gene [27]. Pseudoalleles should not be confused with pseudogenes, which are nonfunctional copies of functional genes, and arises directly through duplication or indirectly by reverse transcription of mRNA transcripts, while pseudoalleles are linked loci. The pseudoalleles differed from multiple alleles that former is linked loci situated close to each other while later is alternative form of single gene [28]. To distinguish pseudoalleles from multiple alleles, few shreds of evidence were shown in past such as (1) many crosses have been made between the mutants available at that time to resolve allelic series by crossing over resulted into failure (2) heterozygotes for the two different mutant genes have phenotypes intermediate of two respective homozygotes, but expected phenotype was non-allelic/wild type. Some cases have been reported where test for allelism comes positive for non-allelic genes due to the position effect. This phenomenon has been considered as positional pseudoallelism, as two genes occupy separate loci along with the position effect [29]. In positional pseudoallelism, the coupling phase heterozygote gives a wild type or nearly wild type (++/ab) phenotype and the repulsion phase (+a/b+) heterozygote gives mutant phenotypes.
Red eye color mutants of
Pseudoalleles originated from the process of gene duplication. Two copies of the gene have been created by gene duplication, which remains closely associated on the chromosome but progressively diverges in structure and function [25]. Some of the distinguishing features of multiple alleles, pseudoallele, and pseudogene have been given in Table 7.
| Trait | Pseudoallele | Pseudogene | Multiple allele |
|---|---|---|---|
| Definition | Pseudoalleles are defined as two genetically linked genes with similar effects located close to each other on the chromosome | Pseudogenes are non-functional copies of functional genes | The presence of more than two alleles in a group of individuals, designated as multiple alleles |
| Origin | By gene duplication | Duplication or indirectly by reverse transcription of mRNA | By mutation |
| Crossing over | Possible | Possible | Not possible |
| Final product | Functional | Non-functional | Functional |
| Locus involved | More than one locus | More than one locus | Only one locus |
| Trait affected | Same trait | Same trait | Same trait |
| Example | Red eye colour of Drosophila has different mutants like white and apricot | ABO blood group system in humans |
Table 7.
Distinguishing features of multiple alleles, pseudoallele, and pseudogene.
7. Conclusion
In this text, we have discussed the concept, function, and importance of multiple alleles. Multiple alleles arise due to mutations and result in different forms of a gene that can affect the traits of an organism. The study of allelic relationships among genes is essential for understanding genetic diversity and evolution. We have also discussed the differences between multiple alleles, pseudoalleles, and pseudogenes, and the methods used to distinguish them. Furthermore, we have explored the phenomenon of positional pseudoallelism and its effects on genetic inheritance. In conclusion, multiple alleles are a significant source of genetic variation that plays a crucial role in the evolution and genetic diversity. Understanding the allelic relationships between genes is essential for studying the genetics of organisms and for developing methods for genetic manipulation and disease prevention.
References
- 1.
Dunn LC. A Short History of Genetics. New York: McGraw-Hill; 1966 - 2.
Landsteiner K. Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymphe. Zentralblatt Bak-teriologie. 1900; 27 :357-362 - 3.
Yoshida A. Biochemical genetics of the human blood group ABO system. American Journal of Human Genetics. 1982; 34 :1-14 - 4.
Ginsburg V. Enzymatic basis for blood groups. Methods Enzymology. 1972; 36 :131-149 - 5.
Misevic G. ABO blood group system. Asia-Pacific Journal of Blood Types and Genes. 2018; 2 (2):71-84 - 6.
Hosoi E. Biological and clinical aspects of ABO blood group system. Journal of Medical Investigation. 2008; 55 :174-182 - 7.
Peters JA. Classic Papers in Genetics. Englewood Cliffs, NJ, London: Prentice-Hall Inc; 1959 - 8.
Race RR, Sanger R. Blood Groups in Man. 6th ed. Oxford: Blackwell; 1975 - 9.
Landsteiner K, Levine P. Further observations on individual differences of human blood. Proceedings of the Society for Experimental Biology and Medicine. 1927; 24 :941-942 - 10.
Yunis EJ, Svardal JM, Bridges RA. Genetics of the Bombay phenotype. Blood. 1969; 33 (1):124-132 - 11.
Kelly RJ, Ernst LK, Larsen RD, Bryant JG, Robinson JS, Lowe JB. Molecular basis for H blood group deficiency in Bombay (Oh) and Para-Bombay individuals. Proceedings of the National Academy of Sciences. 1994; 91 (13):5843-5847 - 12.
Dipta TF, Hossain AZ. The Bombay blood group: Are we out of risk? Mymensingh Medical Journal: MMJ. 2011; 20 (3):536-540 - 13.
Bhar S, De A, Saha A, Bhattacharyya C. Pediatric patient with Bombay blood group: A rare case report. Saudi Journal of Anaesthesia. 2015; 9 (3):318 - 14.
Klug WS, Cummings MR, Spencer CA, Palladino MA, Ward SM. Concepts of Genetics. 10th ed. Vol. 9. USA: Pearson Education, Inc; 2009 - 15.
Punnett RC. Inheritance of coat-colour in rabbits. Journal of Genetics. 1912; 2 (3):221-238 - 16.
Foster M. Mammalian pigment genetics. Advance. Genetics. 1965; 13 :311-339 - 17.
Charlesworth D. Self-incompatibility. F1000 Biology Reports. 2010; 2 :68 - 18.
Castric V, Vekemans X. Invited review: Plant self-incompatibility in natural populations: A critical assessment of recent theoretical and empirical advances. Molecular Ecology. 2004; 13 (10):2873-2889 - 19.
East EM, Mangelsdorf AJ. A new interpretation of the hereditary behavior of self-sterile plants. Proceedings of the National Academy of Sciences of the United States of America. 1925; 11 (2):166 - 20.
Demerec M, Ozeki H. Tests for allelism among auxotrophs of Salmonella typhimurium. Genetics. 1959; 44 (2):269 - 21.
Ephrussi B, Beadle GW. Development of eye colors in Drosophila: Production and release of CN+ substance by the eyes of different eye color mutants. Genetics. 1937; 22 (5):479 - 22.
Laughnan JR. Structural and functional bases for the action of the A alleles in maize. The American Naturalist. 1955; 89 (845):91-103 - 23.
Girard LR, Fiedler TJ, Harris TW, Carvalho F, Antoshechkin I, Han M, et al. WormBook: The online review of Caenorhabditis elegans biology. Nucleic Acids Research. 2007;35 :D472-D475 - 24.
Rédei GP. Allelism Test. In: Encyclopedia of Genetics, Genomics, Proteomics, and Informatics. Dordrecht, Netherlands: Springer Science & Business Media; 2008. DOI: 10.1007/978-1-4020-6754-9_513 - 25.
Morange M. Pseudoalleles and gene complexes. Perspectives in Biology and Medicine. 2015; 58 (2):196-204 - 26.
Welshons WJ, Von Halle ES. Pseudoallelism at the notch locus in drosophila. Genetics. 1962; 47 (6):743 - 27.
Lewis EB. Pseudoallelism and gene evolution. In: Cold Spring Harbor Symposia on Quantitative Biology. New York, USA: Cold Spring Harbor Laboratory Press; 1951; 16 :159-174. DOI: 10.1101/sqb.1951.016.01.014 - 28.
Carlson EA. Allelism, complementation, and pseudoallelism at the dumpy locus in Drosophila melanogaster. Genetics. 1959; 44 :347 - 29.
Lewis EB. Some aspects of position pseudoallelism. The American Naturalist. 1955; 89 (845):73-89 - 30.
Lewis EB. The Pseudoallelism of white and apricot in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America. 1952;38 (11):953-961