Thermal characteristics of LLDPE-g-MA/gelatin blends.
Abstract
In this work, a biodegradable graft copolymer based on linear low-density polyethylene grafted maleic anhydride and gelatin (LLDPE-g-MA/Gel) was formed by reactive mixing of functionalized polyethylene with gelatin to achieve finely dispersed blend morphology. Using a selection of components of the mixture, we studied its morphology and thermal properties. It was found that the thermal stability (initial temperature) of the composition decreases as the amount of gelatin increases due to the degradation of gelatin. In the temperature range of 400–500°C, the maximum rate of destruction of the graft copolymer increases significantly with increasing gelatin content. Samples with the same composition were selected using a Brabender plastograph, and a mechanical mixer, and when taken at different speeds, the morphological structure of the samples was determined to depend on their mixing speed. It was found that the morphological structure, with the increase in speed, the two components react well, and the particles become smaller.
Keywords
- TGA
- DSC
- biodegradation
- gelatin
- glycerin
- polyethylene
- maleic anhydride
1. Introduction
The mixture of polyolefin with natural thermoplastics is of great interest not only from a scientific point of view but also because of the possibility of their practical application. Polymers are blended to produce polymer materials with new improved properties and to expand the range of polymer materials.
The unregulated advancement of morphology during the creation of biodegradable compositions using both synthetic and natural polymers hinders the potential for attaining satisfactory physical, mechanical, and operational traits. Several studies have reported the successful preparation of PE/gelatin blends using various techniques, such as solution casting, melt blending, and electrospinning. These studies have demonstrated that the addition of gelatin can improve the mechanical, thermal, and barrier properties of the blends [1, 2, 3, 4, 5, 6, 7]. Blending can improve a wide variety of polymer properties. However, while polymer blending is attractive for producing new materials, most polymer blends are incompatible. This is the reason for the difficulties of processing processes and the deterioration of the performance properties of such polymer mixtures [8, 9].
The packaging sector requires biodegradable materials. Despite the advantages such as production technology, flexibility in physical and mechanical properties, and cost-effectiveness, traditional synthetic polymers, especially polyolefin, present notable ecological challenges for the environment. To address this issue, researchers have investigated the use of biodegradable additives to improve the biodegradability of PE while maintaining its properties [10, 11, 12, 13, 14, 15]. An examination of scientific literature suggests that addressing this issue involves employing biopolymers or combining them with synthetic polymers.
From this perspective, the enthusiasm for developing blends of gelatin with synthetic polymers remains strong [16, 17]. Simultaneously, it has been observed that the conventional mixing of gelatin with polyolefin in different ratios results in very low rates of biodegradability (up to 10%). Optimizing the blend’s morphology by transitioning gelatin to a thermoplastic state slightly enhances this indicator.
Amidst the escalating environmental pollution crisis, significant research focus in academia and industry over the last two decades has been directed toward biodegradable polymers. These environmentally friendly polymers are acknowledged as promising substitutes for conventional polymers due to their ability to biodegrade, thereby mitigating waste pollution. Biopolymers, such as gelatin, chitosan, and starch, have attracted significant attention due to their biodegradability, biocompatibility, and renewability. Gelatin, in particular, is a protein-based biopolymer derived from animal collagen and has been extensively used in various applications, including food, pharmaceuticals, and cosmetics [18, 19, 20, 21]. Biodegradable polymer-based blends/composites have been manufactured to enhance the various properties of original polymer components notably [22, 23]. The rate of crystallization and the level of crystallinity in crystalline polymers significantly influence their properties and ultimate applications. By altering the crystallization conditions of polymers capable of crystallizing, a wide range of morphologies and supramolecular structures can be achieved [24, 25, 26, 27, 28, 29].
In this research work, LLDPE-g-MA/Gel copolymers based on maleic anhydride (MA) grafted polyethylene (PE), (LLDPE-g-MA) and gelatin (Gel) were obtained. Crystallinity structure, thermal stability, morphological structure, and hydrolytic degradation of neat PE, LLDPE-g-MA (5%), and LLDPE-g-MA/Gel composites and components were investigated and compared.
2. Materials and research methods
2.1 Materials
Research and experiments were carried out by the authors of the article in the laboratory of nanostructured polymer composite materials of the Institute of Polymer Chemistry and Physics within the framework of the scientific project aimed at the fundamental goal of “Nanocomposite polymer based on polyolefins - polymer blends materials produced in Uzbekistan” in 2022–2023.
Used in the work: linear low-density polyethylene (LLDPE) grade F-0320, d = 0.920 g/cm3, MFI = 2.5 g/10 min (at a load of 2.16 kg). Producer—Shurtan Gas Chemical Complex of the Republic of Uzbekistan; edible gelatin (GEL) grade P-200 (GOST 11293–2019). Producer JSC “MOGELIT,” Belarus; maleic anhydride (MA) C4H2O3, analytical grade, colorless rhombic crystals, Mr = 98.06 g/mol, was distilled at Tbp = 84.0°C/14 mm Hg, Tm = 60°C, ρ60 = 1.3140 g/cm3.
2.2 Preparation of thermoplastic gelatin
To dissolve gelatin granules and make them thermoplastic, glycerin was added to distilled water and stirred until the same mixture was obtained; gelatin was added to the resulting mixture and mixed again, then heated in an oven at 80°C for 2 hours.
2.3 Functionalization
Functionalization of LLDPE with maleic anhydride (LLDPE-g-MA) was carried out on a Brabender plastograph (Plasticorder Brabender OHGDUISBURG Germany), with a cam speed of 98 rpm and at a temperature of 180 ± 5°C [30]. The concentration of maleic anhydride in the weld is 5.0% by weight.
Polymer blends based on LLDPE-g-MA and gelatin were obtained on a Brabender plastograph, for 20 min, at 50 rpm and 180 ± 5°C by adding plasticized gelatin to the LLDPE-g-MA melt.
2.4 DSC and TGA measurements
Thermal analyses of DSC and TGA of polymer blends were carried out in dynamic mode on a LINSEIS THERMAL ANALYSIS PT1600 [thermogravimetric analysis (TGA)] instrument (in air atmosphere) in the range from room temperature to 1000°C, guided by the requirements established by the ASTM E 1131 standard. The heating rate was 10°C/min, and the sample weight for analysis was from 2 to 100 mg.
2.5 Atomic force microscope (AFM)
The morphology of polymer mixtures was studied using atomic force (scanning probe microscope Agilent 5500) microscopy at room temperature. We used silicon cantilevers with a stiffness of 9.5 N/m and a frequency of 145 kHz. The maximum scanning area on AFM along X, Y is 15 × 15 μm2, along Z it is 1 μm.
3. Results and discussion
3.1 TGA and DSC measurements
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) are commonly used techniques to investigate the thermal properties of materials. The results obtained from these measurements can provide valuable information on the thermal stability, decomposition behavior, and phase transitions of the materials under investigation.
The statement in the text suggests that the TGA measurements have revealed the presence of reaction products in the mixtures and initial components under investigation. The changes in the thermogravimetric characteristics, such as the initial degradation temperature (Ti) and the temperature of the maximum degradation rate (Tmax), can indicate the formation of new compounds or the degradation of the original components.
Table 1 provides a summary of the Ti and Tmax values for various compositions of mixtures and initial components. These values can be used to compare the thermal stability of different materials and to assess the effect of mixing on the thermal properties of the composite materials (Figure 1).
| No | Sample name and component ratio (wt%) | The temperature corresponding to the loss of mass is °C | Degree of crystallinity % | |||
|---|---|---|---|---|---|---|
| Initial degradation temperature Ti, °C | Maximum thermal decomposition rates (Tmax), °C | |||||
| first stage | second stage | third stage | ||||
| 1 | LLDPE | 186 | 100–200°C m(mg) ≤ 10% | 275–400°C 10 ≤ m(mg) ≤ 50% | 400–500°C m(mg) ≥ 50% | 19.85 |
| 2 | LLDPE-g-MA (5%) | 242 | 18.15 | |||
| 3 | LLDPE-g-MA/GEL 70/30 | 233 | 14.85 | |||
| 4 | LLDPE-g-MA/GEL 60/40 | 223 | 13.32 | |||
| 5 | LLDPE-g-MA/GEL 50/50 | 190 | 12.02 | |||
| 6 | LLDPE-g-MA/GEL 40/60 | 201 | 10.4 | |||
| 7 | GELATIN | 97 | — | |||
Table 1.
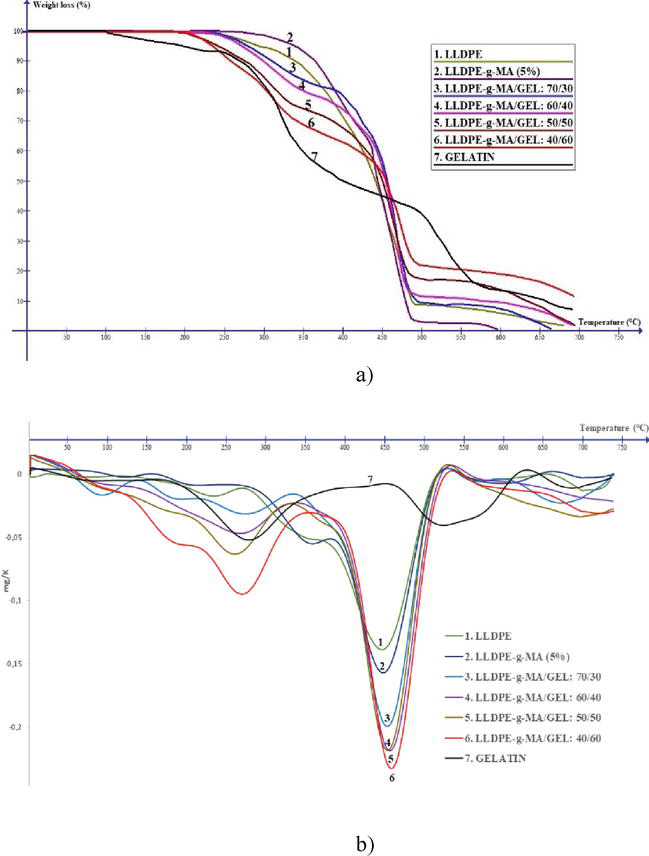
Figure 1.
Thermogravimetric curves (a) and the first derivative of the TGA curves (b) of the film sample.
The TGA curves for the LLDPE-g-MA/gelatin blend reveal a thermal decomposition process occurring in three successive stages. The initial stage, within the temperature range of 100–200°C, involves the removal of absorbed and bound water molecules, resulting in a weight loss of less than 10%. The subsequent stage, occurring between 275 and 400°C with a weight loss ranging from 10 to 50 wt%, corresponds to the cleavage of peptide bonds within the gelatin macro chain [31]. The temperatures corresponding to the maximum rate of thermal decomposition of the gelatinous phase are within 250–280°C. A partial contribution to the weight loss of the composition in this temperature range is also made by the onset of LLDPE-g-MA dehydrogenation, the maximum rate of thermal decomposition (third stage) of which falls at a temperature of 450°C. The position of this peak practically does not depend on the content of gelatin in the composition; as the content of gelatin increases, it slightly shifts toward higher temperatures (up to 7°C). Temperatures, contingent on the anticipated rate of thermal decomposition of the gelatin phase, are identified within the range of 250–280°C. Within this interval, the initial stages involve LLDPE-g-MA dehydration, and an escalated rate of thermal decomposition (third stage) is observed at 450°C. The position of this peak remains essentially constant irrespective of the gelatin content; however, as the gelatin content rises, it notably increases at higher temperatures (up to 7°C). To sum up the TGA data, it is evident that the formation of the graft copolymer LLDPE-g-MA/gelatin results in a reduction in thermal stability, with a Ti decrease ranging from 10 to 40°C depending on the gelatin content (30–60 wt%). Simultaneously, the maximum rate of thermal decomposition in the third stage substantially increases with higher biopolymer content (from −0.14 to −0.23 mg/K) due to the degradation of the grafted gelatin.
3.2 Atomic force microscope (AFM)
The use of atomic force microscopy (AFM) in the study of the morphology of the dispersed phase particles in polyethylene gelatin composites is a valuable technique as it allows for high-resolution imaging of the surface topography and can provide information on the size, shape, and distribution of the particles.
The results presented in Figure 2 suggest that the mixing speed has a significant effect on the size and shape of the dispersed phase particles. Specifically, increasing the mixing speed from 50 to 150 rpm leads to a reduction in particle size and a change in particle shape. This suggests that the use of higher mixing speeds may be beneficial in achieving a more uniform distribution of the dispersed phase particles in the composite material, which can ultimately lead to improved physical and mechanical properties.
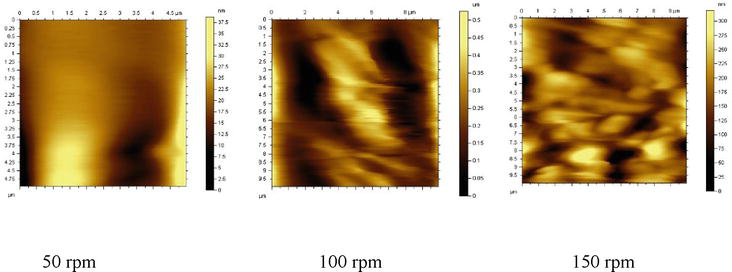
Figure 2.
Atomic stress microscope images of samples taken at 50 rpm, 100 rpm, and 150 rpm.
Overall, the use of AFM in conjunction with varying mixing speeds provides valuable insights into the morphology of composite materials and can aid in the development of improved processing methods for these materials.
In order to prevent the coalescence of particles of the dispersed phase of gelatin in polyethylene and to ensure uniform morphological distribution, the samples were mixed at three different speeds and studied under atomic force microscopy (AFM). Coordinating speeds varied from 50, 100, and 150 rpm (Figure 2). The phase analysis of the size and distribution of the dispersed phase particles shows that the dispersed phase particles become smaller in size and have different shapes when the speed is increased.
AFM studies show that in the structure of the drawn samples, the dispersed phase particles of the second component increased in velocity, and the distribution improved with less coalescence. Thus, microscopic studies have shown that the obtained polymer mixtures have a structural morphology that helps to improve the physical and mechanical properties of the obtained materials.
4. Conclusion
Grafting thermoplastic gelatin onto maleated polyethylene facilitates the creation of uniform compositions with a more refined dispersion of the gelatin phase within the polyethylene matrix. The thermal decomposition of the LLDPE-g-MA/gelatin blend manifests in three stages linked to the presence of water molecules, degradation of the gelatin, and degradation of the polyethylene phases.
The introduction of gelatin induces a significant reduction in the onset temperature of decomposition, shifting it from 242–201°C, while the temperature range for the degradation of the polyethylene matrix remains relatively unchanged. Notably, the maximum degradation rate of the graft copolymer within the 400–500°C range sees a noticeable increase, moving from −0.14 to −0.23 mg/K with higher gelatin content. Microscopic examinations reveal a structural morphology in the obtained polymer mixtures that contributes to enhancing the physical-mechanical properties of the resulting materials.
Overall, the findings of this study demonstrate the potential of using reactive mixing to achieve finely dispersed blend morphology in biodegradable copolymers. The observations regarding the effect of gelatin content and mixing speed on the thermal and morphological properties of the copolymer provide valuable insights into the development of improved processing methods for these materials.
References
- 1.
Normurodov N, Berdinazarov K, Khakberdiev E, Dusiyorov N, Ashurov N. Механические свойства биоразлагаемых композитов на основе полиэтилена и желатина. Science and Innovative Development. 2022; 5 :5-12 - 2.
Normurodov NF, Berdinazarov QN, Haqberdiyev EO, Dusiyorov NK, Ashurov NR. Polietilen va jelatin asosidagi biologik parchalanadigan kompozitlarning mexanik xususiyatlari. In: Узбекско-Казахский Симпозиум Современные проблемы науки о полимерах сборник тезисов. Vol. 60. Uzbekistan Journal of Polymers; 2022 - 3.
Wollerdorfer M, Bader H. Influence of natural fibres on the mechanical properties of biodegradable polymers. Industrial Crops and Products. 1998; 8 (2):105-112 - 4.
Guo Y, He S, Yang K, Xue Y, Zuo X, Yu Y, et al. Enhancing the mechanical properties of biodegradable polymer blends using tubular nanoparticle stitching of the interfaces. ACS Applied Materials & Interfaces. 2016; 8 (27):17565-17573 - 5.
Rahman MM, Khan MA. Surface treatment of coir ( Cocos nucifera ) fibers and its influence on the fibers’ physico-mechanical properties. Composites Science and Technology. 2007;67 (11-12):2369-2376 - 6.
Willett JL, Shogren RL. Processing and properties of extruded starch/polymer foams. Polymer. 2002; 43 (22):5935-5947 - 7.
Rahmani B, Hosseini H, Khani M, Farhoodi M, Honarvar Z, Feizollahi E, et al. Development and characterisation of chitosan or alginate-coated low density polyethylene films containing Satureja hortensis extract. International Journal of Biological Macromolecules. 2017; 105 :121-130 - 8.
Chandra RUSTGI, Rustgi R. Biodegradable polymers. Progress in Polymer Science. 1998; 23 (7):1273-1335 - 9.
Nayak PL. Biodegradable polymers: Opportunities and challenges. In: Organic Polymers. Macromolecular Chemistry and Physics. 1999; C39 (3):481-505 - 10.
Tian K, Bilal M. Research progress of biodegradable materials in reducing environmental pollution. In: Abatement of Environmental Pollutants. Elsevier Inc.; 2020. pp. 313-330 - 11.
Meena PL et al. Packaging material and need of biodegradable polymers: A review. International Journal of Applied Research. 2017; 3 (7):886-896 - 12.
Bastioli C. Global status of the production of biobased packaging materials. Starch-Stärke. 2001; 53 (8):351-355 - 13.
Khan RA, Khan MA, Sarker B, Saha S, Das AK, Noor N, et al. Fabrication and characterization of gelatin fiber-based linear low-density polyethylene foamed composite. Journal of Reinforced Plastics and Composites. 2010; 29 (16):2438-2449 - 14.
Hanani ZN, Roos YH, Kerry JP. Use and application of gelatin as potential biodegradable packaging materials for food products. International Journal of Biological Macromolecules. 2014; 71 :94-102 - 15.
Wang LZ, Liu L, Holmes J, Kerry JF, Kerry JP. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. International Journal of Food Science & Technology. 2007; 42 (9):1128-1138 - 16.
Sarker B, Dey K, Khan RA. Effect of incorporation of polypropylene on the physico-mechanical and thermo-mechanical properties of gelatin fiber based linear low density polyethylene bio-foamed composite. Journal of Thermoplastic Composite Materials. 2011; 24 (5):679-694 - 17.
Kaur I et al. Biodegradation and swelling studies of gelatin-grafted polyethylene. Journal of Applied Polymer Science. 2008; 107 (6):3878-3884 - 18.
Lim LT, Auras R, Rubino M. Processing technologies for poly (lactic acid). Progress in Polymer Science. 2008; 33 (8):820-852 - 19.
Behera K, Sivanjineyulu V, Chang YH, Chiu FC. Thermal properties, phase morphology and stability of biodegradable PLA/PBSL/HAp composites. Polymer Degradation and Stability. 2018; 154 :248-260 - 20.
Suderman N, Isa MIN, Sarbon NM. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Bioscience. 2018; 24 :111-119 - 21.
Podshivalov A, Zakharova M, Glazacheva E, Uspenskaya M. Gelatin/potato starch edible biocomposite films: Correlation between morphology and physical properties. Carbohydrate Polymers. 2017; 157 :1162-1172 - 22.
Raquez J-M, Narayan R, Dubois P. Recent advances in reactive extrusion processing of biodegradable polymer-based compositions. Macromolecular Materials and Engineering. 2008; 293 (6):447-470 - 23.
Visakh PM, Nazarenko OB. Thermal Degradation of Polymer Blends, Composites and Nanocomposites. Springer International Publishing; 2015. pp. 1-16 - 24.
Vilay V, Mariatti M, Ahmad Z, Pasomsouk K, Todo M. Characterization of the mechanical and thermal properties and morphological behavior of biodegradable poly(L-lactide)/poly(ε-caprolactone) and poly(L-lactide)/poly(butylene succinate-co-L-lactate) polymeric blends. Journal of Applied Polymer Science. 2009; 114 :1784-1792 - 25.
Kalb BAJP, Pennings AJ. General crystallization behaviour of poly (L-lactic acid). Polymer. 1980; 21 (6):607-612 - 26.
Harada M, Ohya T, Iida K, Hayashi H, Hirano K, Fukuda H. Increased impact strength of biodegradable poly(lactic acid)/poly(butylene succinate) blend composites by using isocyanate as a reactive processing agent. Journal of Applied Polymer Science. 2007; 106 :1813-1820 - 27.
Mileva D, Tranchida D, Gahleitner M. Designing polymer crystallinity: An industrial perspective. Polymer Crystallization. 2018; 1 (2):e10009 - 28.
Li H, Yan S. Surface-induced polymer crystallization and the resultant structures and morphologies. Macromolecules. 2011; 44 (3):417-428 - 29.
Felder S et al. Incorporating crystallinity distributions into a thermo-mechanically coupled constitutive model for semi-crystalline polymers. International Journal of Plasticity. 2020; 135 :102751 - 30.
Ashurov NR, Sadikov SG, Khakberdiev EO, Berdinazarov KN, Normurodov NF. Preparation and properties of compositions based on polyethylene and gelatin. Uzbek Chemical Journal. 2020; 6 (3):53-60 - 31.
Moreno O, Díaz R, Atarés L, Chiralt A. Influence of the processing method and antimicrobial agents on properties of starch-gelatin biodegradable films. Polymer International. 2016; 65 (8):905-914