Stepwise approach to therapy in sarcoidosis.
Abstract
Sarcoidosis is a systemic disease of granulomatous inflammation that predominately affects the lungs. The cause is unknown. Although over half of cases spontaneously resolve, a large proportion of patients require therapy for progressive symptoms or worsening organ function. Corticosteroids remain first-line therapy, but steroid-sparing medications should be considered in high-risk cases. In this chapter, we review types of therapies targeted to the granulomatous inflammatory pathway and their role in treatment of sarcoidosis. Because of the complex interaction of patient factors and medication toxicities, appropriate clinical management should include a personalized discussion with each patient to determine the individual treatment plan. Future trials are needed to test novel drugs and establish less toxic approaches to therapy.
Keywords
- sarcoidosis
- therapeutics
- treatment
- immunosuppression
- granulomatous inflammation
1. Introduction
Sarcoidosis afflicts the lung and thoracic lymph nodes in over 90% of cases [1]. Progressive granulomatous inflammation can lead to diminished pulmonary function, lung fibrosis, and symptoms such as cough and dyspnea. Respiratory failure is the main cause of death or lung transplantation in pulmonary sarcoidosis. Treatment of granulomatous inflammation is targeted at preventing lung dysfunction, improving quality of life, and decreasing mortality. This chapter will review the current basis for therapies that impair development and propagation of granulomatous inflammation in the lung. We review current anti-inflammatory management strategies, as well as evolving research in the field which may lead to new therapeutic strategies. We will also address the difficulties in clinical trials for sarcoidosis and new methods to facilitate development of efficacious therapeutics.
2. Indications for treatment
Granulomas in sarcoidosis are tightly formed clusters of macrophages and monocytes surrounded by CD4+ T-cells and, in lesser presence, B-cells (Figure 1). Although the pathophysiology of sarcoidosis is not well-elucidated, current data suggests a predominant Th-1 immune response with abundant cytokine formation, including tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma. The driving force is not known, but evidence suggests an environmental antigen in the setting of a genetically predisposed individual. Since eradication of the unknown antigen is not yet possible, current management focuses on interruption of the uncontrolled granulomatous cascade of inflammatory cells which is thought to lead to fibrosis over time.
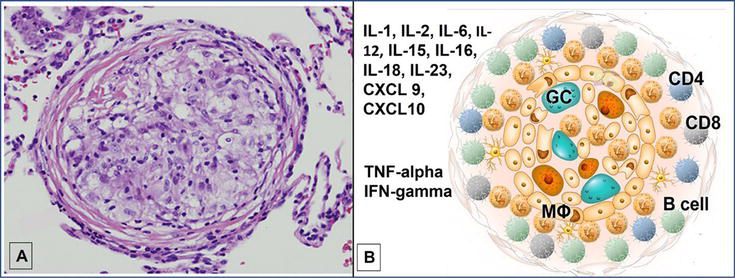
Figure 1.
Sarcoidosis granuloma and associated cytokines and chemokines. A is a histopathologic specimen of a non-necrotizing granuloma from transbronchial lung biopsy and B is a schematic of immune cells and activated cytokines reflecting the predominantly Th-1 immune response. Abbreviations: GC: multi-nucleated giant cell, MФ: macrophage, TNF: tumor necrosis factor, IFN: interferon, IL: interleukin.
Not every patient with sarcoidosis necessitates treatment; over half of cases will have spontaneous remission without therapy. Many symptoms, such as fatigue, chronic pain, and cognitive dysfunction, do not respond to immunosuppression and may be confounded by side effects. Additionally, fibrotic sarcoidosis of the lung may not respond to anti-granulomatous therapies (Figure 2). Therefore, careful selection of patients is warranted to avoid unnecessary medication use with potentially toxic side effects. Only patients with evidence of significant lung injury, progressive lung function decline, or symptom burden that alters quality life should be considered for therapy. These symptoms of pulmonary sarcoidosis often include cough, chest pain, and/or dyspnea. Given the fairly long span of time over which disability is noted in pulmonary sarcoidosis [2], a period of observation is often employed to determine clinical trajectory if there is no clear indication for immediate therapy (e.g. impending organ damage) and only mild symptoms are present.
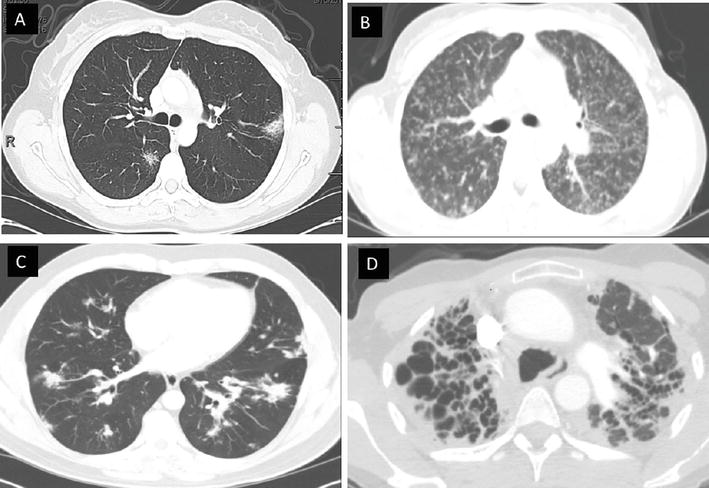
Figure 2.
Pulmonary sarcoidosis. A, B, and C reflect patients with inflammatory granulomatous infiltrates, nodules, and consolidations, whereas D reflects a patient with predominantly fibrotic sarcoidosis of the upper lobes.
3. Treatment options
3.1 Corticosteroids
Large randomized controlled trials guiding therapy are few in the field of sarcoidosis. Corticosteroids remain the mainstay of first-line therapy in the sarcoidosis population. Corticosteroids suppress granulomatous inflammation by broad suppression of inflammatory cytokines such as TNF-alpha and IFN-gamma, prominent factors in the uncontrolled immune response. Proof of long-term mortality benefit and alteration of natural history are lacking, but suppression of the inflammatory response does decrease symptoms and improve imaging and serum biomarkers of disease in many cases of pulmonary sarcoidosis [3, 4].
Corticosteroids tend to be used frequently due to their quick effect and doses can be titrated easily to desired outcomes. However, careful risk/benefit conversations should occur with providers and patients given the potential side effect profile in the long-term management of disease burden. More recent studies have shown that more modest doses of prednisone are equally efficacious as higher doses, particularly for the lung [5]. Generally, dosing of 20–40 mg per day for starting dosage is recommended for pulmonary disease, although an exact dose has not been associated with better outcomes in studies. Therefore, depending on clinical presentation and comorbidities, lower doses can be considered. Doses as low as 15 mg/day have been shown to improve lung infiltrates. Conversely, longer term use of higher doses of prednisone (greater than 40 milligrams (mg) per day) has been shown to increase morbidity and mortality whereas providing little additional physiologic benefit [5]. Additionally, higher corticosteroid use is associated with lower quality of life and increased use of health care resources such as emergency room visits [6]. Corticosteroids can then be tapered to a maintenance dose over a period of weeks to months based on clinical response. In one study, a rapid taper to a low maintenance dose over 3.5 months was effective with less side effects, and most of the pulmonary function benefit was seen within the first month [5]. If corticosteroids cannot be tapered to a dose lower than 10 mg per day, alternative therapies are often considered.
In patients requiring prolonged therapy, in those who have refractory disease, or in those with intolerance or toxicity, corticosteroid-sparing immunomodulators can be considered for additional or alternative therapy. Despite low levels of evidence, recent guidelines support use of these medications in high-risk cases of sarcoidosis [7]. Use of up-front corticosteroid-sparing agents instead of corticosteroids has not been robustly studied, but one retrospective evaluation of methotrexate monotherapy indicated non-inferiority in efficacy as compared to methylprednisolone [8]. Most strategies involving alternative therapies are derived from treatment of other rheumatologic conditions which have historically utilized these medications for suppression of autoimmune-derived inflammation. Safety and use data are often guided by larger trials in other inflammatory diseases (Table 1) [9].
Glucocorticoids |
Methotrexate Azathioprine Mycophenolate Leflunomide |
Infliximab Adalimumab |
Rituximab Repository corticotropin injection Tofacitinib Efzofitimod |
Table 1.
3.2 Second-line therapy
Methotrexate (a folic acid antagonist) is the most common second-line therapy recommended in sarcoidosis based on its long-term known safety and efficacy profile in other rheumatologic conditions. Its mechanism in autoimmune disease is likely multifactorial, likely regulating most inflammatory cells either by direct action or indirectly through interruption of various cellular functions. Primarily, the drug involves inhibition of purine and pyrimidine metabolism and suppression of polyamine and amino acid synthesis, thereby suppressing T-cells and B-cells. Other proposed mechanisms via adenosine affect the function of neutrophils and macrophages, altering cytokine function, and preventing immune cell proliferation [10]. Methotrexate has adequate response in approximately 55–80% of patients [11, 12]. The average goal dose of methotrexate is 7.5–15 mg per week, although higher doses can be used if tolerated. The main adverse effects of methotrexate include increased risk of infections, hepatotoxicity, bone marrow suppression, and gastrointestinal side effects; therefore, close monitoring and patient compliance with lab draws are imperative for safe use. Interestingly, in a ‘real-world’ survey study, patients reported fewer and less bothersome side effects while taking methotrexate as compared to prednisone (49% vs. 78%, respectively) [13].
In cases where methotrexate is not tolerated or ineffective, other corticosteroid-sparing agents are available, with efficacy supported by small trials and case series in sarcoidosis. Leflunomide, another medication used commonly for rheumatoid arthritis, is converted to teriflunomide in the body which then inhibits the mitochondrial enzyme dihydroorotate dehydrogenase. This alteration then inhibits synthesis of ribonucleotide uridine monophosphate pyrimidine (a pyrimidine), and thereby suppressing activated lymphocytes and decreasing proliferation of the T-cells. Dosing ranges from 10 to 20 mg per day. Use of leflunomide is supported by a retrospective look at 76 patients who were prescribed leflunomide after failing alternative immunosuppressives [14]. A significant improvement in forced vital capacity was seen and use allowed tapering of prednisone in these patients. This effect has also been shown in patients who failed methotrexate therapy in another smaller series of patients [15].
Azathioprine is another steroid-sparing agent that is commonly used in sarcoidosis. Its mechanism of action is a purine-mimic antimetabolite that serves to inhibit purine synthesis and diminish the number of circulating B and T lymphocytes, as well as inducing T-cell apoptosis. Dosing ranges from 50 mg per day to 200 mg per day and is often weight-based. Efficacy and side effects are like methotrexate, although azathioprine may have a higher associated rate of infections [16]. In pulmonary sarcoidosis, azathioprine has been shown to improve pulmonary function and to allow tapering of corticosteroids [17]. Similarly, mycophenolate (converted to mycophenolic acid after ingestion) is another drug which inhibits purine nucleotide synthesis in lymphocytes. It is commonly used for treatment of various interstitial lung diseases and can be considered for pulmonary sarcoidosis. Evidence supporting use is based on case series showing a positive steroid-sparing effect with minimal side effects [18, 19, 20, 21]. Mycophenolate is given at doses of 1000–3000 mg per day, and an enteric coated variation is available at slightly different dosing range. Antimalarials such as chloroquine or hydroxychloroquine have not been shown to be significantly effective in pulmonary disease but can be used in conjunction with other therapies if concurrent skin involvement or hypercalcemia. This is based upon the drugs’ immunomodulatory effect on antigen presentation, T-cell signaling, and interference with inflammatory cytokine production by the lymphocytes [22, 23].
3.3 Third-line therapy
Tumor necrosis factor (TNF) antagonists are recommended as third-line therapy in refractory pulmonary disease [24], with infliximab having the strongest recommendation in recent published guidelines [7]. In a registry cohort of patients with sarcoidosis based out of the United States rheumatology workforce, 12.1% of patients used biologics or targeted disease-modifying antirheumatic drugs [25]. The cytokine, TNF-alpha, plays a large role in the propagation of granulomatous inflammation via macrophage activation. Infliximab (chimeric) and adalimumab (human) are monoclonal antibodies that target TNF itself. Infliximab is given intravenously at a dosing range of 3–5 mg per kilogram at weeks 0, 2, and every 4–8 weeks thereafter. A randomized, placebo-controlled Phase II study of 138 patients showed an improvement of 2.5% in the forced vital capacity compared to placebo, as well as lung radiographic measures and serum biomarkers [26]. This effect was strongest in those with more severe pulmonary disease and those with concurrent extrapulmonary involvement. Controversy on whether this effect was of clinical significance has led to some limitation of its use. However, a more recent retrospective study of 26 patients has shown sustained effects of infliximab on pulmonary infiltrates on long-term follow-up [27]. Interestingly, in another small series of patients who maintained remission after use of third-line infliximab therapy, all had been able to maintain off corticosteroids for at least a year while on infliximab, indicating that perhaps the ability of corticosteroid withdrawal in combination with TNF suppression may be prognostic in the long-term [28]. These results contrast with prior reported high relapse rates after cessation of infliximab, perhaps because of concurrent corticosteroid needs or inadequate duration of therapy [29]. Additionally, because these biologics are only used in refractory cases, it is unclear if delays in effective therapy are associated with worse outcomes [30]. Biosimilars to infliximab also have promising results in small retrospective cohorts to improve lung function, quality of life, and biomarkers [31].
Adalimumab has less robust data in pulmonary disease, but small series have shown it to increase pulmonary function, six-minute walk distance, and radiographic biomarkers in patients with refractory disease [32, 33]. Additionally, it has been shown to have significant efficacy for refractory ocular sarcoidosis, increasing its appeal for multi-system involvement [34, 35]. Adalimumab holds an advantage in that it is a subcutaneous injection (40 mg every 1–2 weeks) that can be self-administered, whereas infliximab requires intravenous infusion. Anti-drug antibodies affecting efficacy can be of concern for all these TNF antagonists [36].
On the other hand, not all biologics have been equally effective. Etanercept, a TNF receptor antagonist was tested in an open label Phase II study for stage 2 and 3 pulmonary sarcoidosis and terminated early due to treatment failure and adverse events [37, 38]. This lack of efficacy in sarcoidosis is further supported by a negative placebo-controlled trial in ocular sarcoidosis [38]. Similarly, ustekinumab and golimumab were ineffective at improving FVC in patients with chronic pulmonary sarcoidosis [39].
3.4 Other potential therapies
Other biologic therapies have shown promise in treatment of granulomatous inflammation in patients with sarcoidosis [40]. Efzofitimod is a novel immunomodulatory fusion protein that selectively binds to neuropilin-2 which is a transmembrane receptor found to be expressed in sarcoid granulomas [41]. In an randomized, double blind, placebo-controlled ascending dose trial of 37 patients with pulmonary sarcoidosis, a greater steroid-sparing effect was seen in the treatment group as compared to the placebo group. Trends towards improvements were seen in quality-of-life measures and lung function that were dose-dependent [42]. The drug was well-tolerated and is being evaluated for further efficacy measures in a larger population.
B-cells in sarcoidosis have also been a plausible target of therapeutics. B-cells have been shown to be involved in granuloma formation, and indirect evidence of heightened B-cell activating factor is present in those with sarcoidosis [43]. Rituximab, a chimeric monoclonal antibody against CD20+ B-cells, was tested in a Phase I/II prospective study of 10 patients with refractory pulmonary sarcoidosis [44]. Five of those patients improved their forced vital capacity (FVC) by at least 5% and five of the patients had an improvement in 6 minute walk distance by 30 meters (seven patients total with either or both outcomes). However, in follow-up, 2 patients had progressive respiratory failure and one was hospitalized for infectious pneumonia, making broad use less clear for rituximab until further studies can be performed.
Tofacitinib, a JAK inhibitor, was found to be efficacious in an open label study of 10 patients with moderate to severe cutaneous lesions. Positron emission tomography (PET) scans in these patients also showed marked decrease (>50%) in avidity of lung lesions in five of eight patients with pulmonary sarcoidosis [45]. Repository corticotropin injection has also undergone testing as an immune modulator in sarcoidosis in a phase IV placebo-controlled trial. Its anti-inflammatory effects are thought to be mediated by the melanocortin receptors present on the immune cells which thereby decrease the production of pro-inflammatory cytokines. Steroid-sparing effect was overall like the placebo group; however, corticosteroids were tapered in a faster manner in the treatment group [46]. Non-significant trends were seen in secondary endpoints, including pulmonary function and quality of life. Smaller case series and retrospective reviews have supported a steroid-sparing effect, albeit with some toxicity [47, 48]. Larger, more robust studies are needed to determine future use of these drugs. Other anti-inflammatory agents under study include anti-GM-CSF antibodies, monoclonal antibodies against IL-18, and nicotine, a modulator of the T-cell regulatory pathways [49]. Nintedanib, an anti-fibrotic agent, has been approved for all types of progressive fibrosis of the lung via the INBUILD trial which included a small number of patients with sarcoidosis. Whether it has any anti-inflammatory properties is unknown for sarcoidosis but is a consideration for progressive fibrotic disease despite immunosuppression [50].
3.5 Inhaled therapy
Inhaled therapies are also used in pulmonary sarcoidosis, although primarily for treatment of cough, exacerbations, or more mild airway inflammation. An early randomized controlled trial showed that high dose inhaled corticosteroids were not effective in treatment of symptoms, X-rays, pulmonary function, or serum biomarkers in stage 1–3 sarcoidosis, albeit only 21 patients were included, and many had regression of disease without therapy at all [51]. Therefore, the role of inhaled therapies in certain sub-phenotypes is still unknown.
4. Considerations for treatment decisions
4.1 Comorbid conditions
The decisions of when to treat, with what medication, and for how long is based both on clinical presentation and a personalized discussion between patient and provider regarding the likelihood of clinical benefit in the setting of comorbidities, medication side effects, as well as patient preferences. The presence of comorbidities can also contribute greatly to the choice of therapy. Corticosteroids can exacerbate diabetes, glaucoma, hypertension, obesity, and fluid retention, whereas increasing risk of osteoporosis, bone fractures, and cataracts. They have also been associated with lower health-related quality of life, even when adjusted for severity of disease [52]. Concurrent medication can also significantly interact with several corticosteroid-sparing medications. Similarly, renal or liver dysfunction may require dose-adjustment of medications. Use of alcohol should also be evaluated and incorporated into therapy decisions, as alcohol can increase risk of hepatotoxicity when taking methotrexate.
4.2 Reproductive health
The possibility or desire of pregnancy should also be considered in choosing an immunosuppressive agent [53]. Methotrexate and mycophenolate are associated with increased risk of pregnancy loss and teratogenicity. Due to teratogenicity of leflunomide in mice studies, it is also not recommended in pregnancy. Mycophenolate, methotrexate, and leflunomide are contraindicated in women who are breast-feeding. Azathioprine, corticosteroids, and TNF-antagonists have more favorable profiles in terms of teratogenicity; however, risks and benefits should be discussed when prescribing this in pregnancy. Side effects such as hyperglycemia, hypertension, and increased infection risk can be heightened in pregnancy.
4.3 Pharmacogenomics
Some of the heterogenous efficacy of corticosteroid-sparing drugs such as methotrexate and leflunomide can be explained by genetic determinants of medication response [54, 55, 56]. Similarly, genetic polymorphisms of the genes responsible for metabolism of azathioprine (thiopurine S-methyltransferase and nucleoside triphosphate diphosphatase) have been associated with medication-related toxicity, including bone marrow suppression and hepatotoxicity [56]. However, testing for these polymorphisms is not universally agreed upon, just under half of patients with toxicity have no known genetic predisposition. Hence, close monitoring of liver function and blood counts are required in all patients who are prescribed azathioprine. Based on these potential pharmacogenomic traits, consideration of switching steroid-sparing medications is warranted if one has toxicity or lacks efficacy.
4.4 Treatment non-response
In addition to genetic variation in medication response, cases of ‘refractory’ disease could indicate other potential reasons for failure. If a patient is not responding, alternative diagnoses should be entertained. Additionally, non-adherence to medication can also play a role, and can occur due to financial constraints, high side effect profiles of medications, inaccessibility to health care resources (e.g. lab draws), or burdensome limitations to a patient’s work or eating schedules.
5. Duration of treatment and follow-up
Duration of treatment to maintain remission varies, but approximately 1 year is a reasonable approach and can depend greatly on severity of disease, extrapulmonary involvement, and medication tolerance. Shorter courses have been associated with increased rates of relapse, both with corticosteroids and infliximab [57]. Similarly, use of corticosteroids has been associated with increased relapse rate, bringing up the concept that the granuloma may be an adaptive response [58]. Longer courses have been associated with better outcomes in lung function, radiographs, and symptoms. There are no validated predictors of relapse, and it is unclear on how steroid-sparing agents affect relapse rate.
Ongoing efforts between a patient and provider to decrease therapy at regular intervals to lowest effective doses are warranted. Improvements of pulmonary function and symptoms after treatment appear to happen most prominently in the first month, with additional smaller effects seen over a period of 3 months, arguing that tapering of corticosteroids early and finding the lowest maintenance doses of medications thereafter may help alleviate long-term medication toxicity [5].
6. Future directions in therapeutics
Clinical trials in sarcoidosis are difficult to perform due to rarity of the disease, uncertain clinical course (many improve spontaneously), heterogeneity of disease presentation, and inadequate outcome measures of disease response. Furthermore, despite known long-term toxicities associated with corticosteroids, it is difficult to test novel therapeutics in direct comparison in placebo-controlled trials. For this reason, outcomes such as steroid-sparing effects or radiographic biomarkers have become more common. Standardization of outcomes across trials and development of personalized biomarkers to predict disease activity would greatly aid appropriate inclusion criteria to increase efficiency of clinical studies and more precisely identify clinical improvements [59]. Current work in transcriptomics and radiomics hold promise for these outcome measures [60]. Despite these difficulties, future trials are imperative to develop therapies that can alleviate the severe burden of disease and medication side effects, avoid lung transplantation, and decrease mortality.
7. Conclusions
Sarcoidosis is a complex disease with heterogeneous clinical course and limited clinical trials by which to guide therapy. Treatment should only be considered in those with significant symptoms, to prevent or alleviate organ damage, and to protect from sarcoidosis-associated mortality. Glucocorticoids continue to be first-line therapy, but corticosteroid-sparing medications should be considered for patients requiring prolonged therapy, those who have side effects or toxicity related to steroids, or for those in whom corticosteroids cannot be tapered to a reasonable dose. Despite low levels of evidence for most corticosteroid-sparing immune modulators, these options can be considered. Future trials are needed to test novel drugs and establish less toxic approaches to therapy. Last, appropriate clinical management should include a personalized discussion with each patient to determine each individual treatment plan.
References
- 1.
Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. American Journal of Respiratory and Critical Care Medicine. 2001; 164 (10 Pt 1):1885-1889 - 2.
Takada K, Ina Y, Noda M, Sato T, Yamamoto M, Morishita M. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. Journal of Clinical Epidemiology. 1993; 46 (4):359-366 - 3.
Pietinalho A, Tukiainen P, Haahtela T, Persson T, Selroos O, Finnish Pulmonary Sarcoidosis Study G. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002; 121 (1):24-31 - 4.
Gibson GJ, Prescott RJ, Muers MF, Middleton WG, Mitchell DN, Connolly CK, et al. British Thoracic Society Sarcoidosis study: Effects of long term corticosteroid treatment. Thorax. 1996; 51 (3):238-247 - 5.
Broos CE, Poell LHC, Looman CWN, In 't Veen J, Grootenboers M, Heller R, et al. No evidence found for an association between prednisone dose and FVC change in newly-treated pulmonary sarcoidosis. Respiratory Medicine. 2018; 138S :S31-SS7 - 6.
Judson MA, Chaudhry H, Louis A, Lee K, Yucel R. The effect of corticosteroids on quality of life in a sarcoidosis clinic: The results of a propensity analysis. Respiratory Medicine. 2015; 109 (4):526-531 - 7.
Baughman RP, Valeyre D, Korsten P, Mathioudakis AG, Wuyts WA, Wells A, et al. ERS clinical practice guidelines on treatment of sarcoidosis. The European Respiratory Journal. 2021; 58 (6):2004079 - 8.
Gavrysyuk V, Merenkova I, Dziublyk Y, Morska N, Pendalchuk N, Bychenko O, et al. Efficacy and tolerability of methotrexate and methylprednisolone in a comparative assessment of the primary and long-term outcomes in patients with pulmonary sarcoidosis. Diagnostics (Basel). 2021; 11 (7):1289 - 9.
Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: The treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis and Rheumatism. 2012; 64 (9):2824-2835 - 10.
Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nature Reviews Rheumatology. 2020; 16 (3):145-154 - 11.
Fang C, Zhang Q , Wang N, Jing X, Xu Z. Effectiveness and tolerability of methotrexate in pulmonary sarcoidosis: A single center real-world study. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases. 2019; 36 (3):217-227 - 12.
Goljan-Geremek A, Bednarek M, Franczuk M, Puscinska E, Nowinski A, Czystowska M, et al. Methotrexate as a single agent for treating pulmonary sarcoidosis: A single centre real-life prospective study. Pneumonologia i Alergologia Polska. 2014; 82 (6):518-533 - 13.
Kahlmann V, Moor CC, Veltkamp M, Wijsenbeek MS. Patient reported side-effects of prednisone and methotrexate in a real-world sarcoidosis population. Chronic Respiratory Disease. 2021; 18 :14799731211031935 - 14.
Sahoo DH, Bandyopadhyay D, Xu M, Pearson K, Parambil JG, Lazar CA, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. The European Respiratory Journal. 2011; 38 (5):1145-1150 - 15.
Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases. 2004; 21 (1):43-48 - 16.
Pasipanodya JG. Comparative effectiveness of methotrexate versus methylprednisolone in treatment naive pulmonary sarcoidosis patients. Diagnostics (Basel). 2021; 11 (8):1401 - 17.
Vorselaars ADM, Wuyts WA, Vorselaars VMM, Zanen P, Deneer VHM, Veltkamp M, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013; 144 (3):805-812 - 18.
Hamzeh N, Voelker A, Forssen A, Gottschall EB, Rose C, Mroz P, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respiratory Medicine. 2014; 108 (11):1663-1669 - 19.
Brill AK, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: A retrospective study. Respiration. 2013; 86 (5):376-383 - 20.
Vonk MC, Smith V, Sfikakis PP, Cutolo M, Del Galdo F, Seibold JR. Pharmacological treatments for SSc-ILD: Systematic review and critical appraisal of the evidence. Autoimmunity Reviews. 2021; 20 (12):102978 - 21.
van den Bosch L, Luppi F, Ferrara G, Mura M. Immunomodulatory treatment of interstitial lung disease. Therapeutic Advances in Respiratory Disease. 2022; 16 :17534666221117002 - 22.
Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nature Reviews Rheumatology. 2020; 16 (3):155-166 - 23.
Sharma OP. Effectiveness of chloroquine and hydroxychloroquine in treating selected patients with sarcoidosis with neurological involvement. Archives of Neurology. 1998; 55 (9):1248-1254 - 24.
Rahaghi FF, Baughman RP, Saketkoo LA, Sweiss NJ, Barney JB, Birring SS, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. European Respiratory Review. 2020; 29 (155):190146 - 25.
Hammam N, Evans M, Morgan E, Reimold A, Anastasiou C, Kay JL, et al. Treatment of sarcoidosis in US rheumatology practices: Data from the American College of Rheumatology's Rheumatology Informatics System for Effectiveness (RISE) Registry. Arthritis Care & Research (Hoboken). 2022; 74 (3):371-376 - 26.
Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. American Journal of Respiratory and Critical Care Medicine. 2006; 174 (7):795-802 - 27.
Russell E, Luk F, Manocha S, Ho T, O'Connor C, Hussain H. Long term follow-up of infliximab efficacy in pulmonary and extra-pulmonary sarcoidosis refractory to conventional therapy. Seminars in Arthritis and Rheumatism. 2013; 43 (1):119-124 - 28.
Yee AMF. Durable medication-free remission of sarcoidosis following discontinuation of anti-tumor necrosis factor-alpha therapy. Respiratory Medicine. 2023; 206 :107055 - 29.
Baughman RP, Judson MA. Relapses of sarcoidosis: What are they and can we predict who will get them? The European Respiratory Journal. 2014; 43 (2):337-339 - 30.
Vorselaars ADM, Culver DA. Hit-hard and early versus step-up treatment in severe sarcoidosis. Current Opinion in Pulmonary Medicine. 2022; 28 (5):461-467 - 31.
Schimmelpennink MC, Vorselaars ADM, van Beek FT, Crommelin HA, Deneer VHM, Keijsers RGM, et al. Efficacy and safety of infliximab biosimilar Inflectra((R)) in severe sarcoidosis. Respiratory Medicine. 2018; 138S :S7-S13 - 32.
Kamphuis LS, Lam-Tse WK, Dik WA, van Daele PL, van Biezen P, Kwekkeboom DJ, et al. Efficacy of adalimumab in chronically active and symptomatic patients with sarcoidosis. American Journal of Respiratory and Critical Care Medicine. 2011; 184 (10):1214-1216 - 33.
Sweiss NJ, Noth I, Mirsaeidi M, Zhang W, Naureckas ET, Hogarth DK, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases. 2014; 31 (1):46-54 - 34.
Erckens RJ, Mostard RL, Wijnen PA, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefe's Archive for Clinical and Experimental Ophthalmology. 2012; 250 (5):713-720 - 35.
Riancho-Zarrabeitia L, Calvo-Rio V, Blanco R, Mesquida M, Adan AM, Herreras JM, et al. Anti-TNF-alpha therapy in refractory uveitis associated with sarcoidosis: Multicenter study of 17 patients. Seminars in Arthritis and Rheumatism. 2015; 45 (3):361-368 - 36.
Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2009; 68 (11):1739-1745 - 37.
Utz JP, Limper AH, Kalra S, Specks U, Scott JP, Vuk-Pavlovic Z, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003; 124 (1):177-185 - 38.
Baughman RP, Lower EE, Bradley DA, Raymond LA, Kaufman A. Etanercept for refractory ocular sarcoidosis: Results of a double-blind randomized trial. Chest. 2005; 128 (2):1062-1047 - 39.
Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. The European Respiratory Journal. 2014; 44 (5):1296-1307 - 40.
Plichta J, Kuna P, Panek M. Biologic drugs in the treatment of chronic inflammatory pulmonary diseases: Recent developments and future perspectives. Frontiers in Immunology. 2023; 14 :1207641 - 41.
Baughman RP, Niranjan V, Walker G, Burkart C, Paz S, Chong Y, et al. Efzofitimod: A novel anti-inflammatory agent for sarcoidosis. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases. 2023; 40 (1):e2023011 - 42.
Culver DA, Aryal S, Barney J, Hsia CCW, James WE, Maier LA, et al. Efzofitimod for the treatment of pulmonary sarcoidosis. Chest. 2023; 163 (4):881-890 - 43.
Kamphuis LS, van Zelm MC, Lam KH, Rimmelzwaan GF, Baarsma GS, Dik WA, et al. Perigranuloma localization and abnormal maturation of B cells: Emerging key players in sarcoidosis? American Journal of Respiratory and Critical Care Medicine. 2013; 187 (4):406-416 - 44.
Sweiss NJ, Lower EE, Mirsaeidi M, Dudek S, Garcia JG, Perkins D, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. The European Respiratory Journal. 2014; 43 (5):1525-1528 - 45.
Damsky W, Young BD, Sloan B, Miller EJ, Obando JA, King B. Treatment of multiorgan sarcoidosis with tofacitinib. ACR Open Rheumatology. 2020; 2 (2):106-109 - 46.
Mirsaeidi M, Baughman RP. Repository corticotropin injection for the treatment of pulmonary sarcoidosis: A narrative review. Pulmonary Therapy. 2022; 8 (1):43-55 - 47.
Baughman RP, Sweiss N, Keijsers R, Birring SS, Shipley R, Saketkoo LA, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017; 195 (3):313-322 - 48.
Chopra I, Qin Y, Kranyak J, Gallagher JR, Heap K, Carroll S, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: Retrospective analysis of medical records. Therapeutic Advances in Respiratory Disease. 2019; 13 :1753466619888127 - 49.
Obi ON, Saketkoo LA, Russell AM, Baughman RP. Sarcoidosis: Updates on therapeutic drug trials and novel treatment approaches. Frontiers in Medicine. 2022; 9 :991783 - 50.
Wells AU, Flaherty KR, Brown KK, Inoue Y, Devaraj A, Richeldi L, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: A randomised, double-blind, placebo-controlled, parallel-group trial. The Lancet Respiratory Medicine. 2020; 8 (5):453-460 - 51.
Milman N, Graudal N, Grode G, Munch E. No effect of high-dose inhaled steroids in pulmonary sarcoidosis: A double-blind, placebo-controlled study. Journal of Internal Medicine. 1994; 236 (3):285-290 - 52.
Cox CE, Donohue JF, Brown CD, Kataria YP, Judson MA. Health-related quality of life of persons with sarcoidosis. Chest. 2004; 125 (3):997-1004 - 53.
Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis & Rhematology. 2020; 72 (4):529-556 - 54.
Qiu Q , Huang J, Shu X, Fan H, Zhou Y, Xiao C. Polymorphisms and pharmacogenomics for the clinical efficacy of methotrexate in patients with rheumatoid arthritis: A systematic review and meta-analysis. Scientific Reports. 2017; 7 :44015 - 55.
Bohanec Grabar P, Rozman B, Tomsic M, Suput D, Logar D, Dolzan V. Genetic polymorphism of CYP1A2 and the toxicity of leflunomide treatment in rheumatoid arthritis patients. European Journal of Clinical Pharmacology. 2008; 64 (9):871-876 - 56.
Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: Relationship to thiopurine methyltransferase genetic polymorphism. Clinical Pharmacology and Therapeutics. 1989; 46 (2):149-154 - 57.
Vorselaars AD, Verwoerd A, van Moorsel CH, Keijsers RG, Rijkers GT, Grutters JC. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. The European Respiratory Journal. 2014; 43 (2):602-609 - 58.
Judson MA, Adelstein E, Fish KM, Feustel PJ, Yucel R, Preston S, et al. Outcomes of prednisone-tapering regimens for cardiac sarcoidosis: A retrospective analysis demonstrating a benefit of infliximab. Respiratory Medicine. 2022; 203 :107004 - 59.
Hena KM, Patterson KC. Making progress in clinical trials in sarcoidosis. Chest. 2023; 164 (3):682-685 - 60.
Francesqui J, Marrades P, Sellares J. Personalized medicine in sarcoidosis: Unravelling biomarkers for targeted care. Current Opinion in Pulmonary Medicine. 2023; 29 (5):478-484