Limitations of hydrogels and possible solutions.
Abstract
Among the drug delivery systems, hydrogels and nanogels have shown a vital role because of their advantageous 3D crosslinked networks. They have the propensity to absorb water due to their hydrophilic groups, making them excellent superabsorbents that are water-insoluble. Nanogels are crosslinked nano-sized hydrogels (20–200 nm) with greater tissue permeation due to their smaller size. Hydrogels and nanogels demonstrate many advantages, including biocompatibility, hydrophilicity, controlled drug release, and smart drug delivery. They are regarded as an interesting approach for the controlled release of medications since they can encapsulate drug molecules in their water-swollen network. Recent advances in polymer chemistry and nanotechnology have resulted in several significant improvements in the field of hydrogels and nanogels as drug delivery systems. In this chapter, the properties of hydrogels and nanogels, as well as their classification, drug release mechanisms, and applications for drug delivery, will be discussed.
Keywords
- hydrogels
- nanogels
- drug delivery systems
- drug release
- tissue permeability
1. Introduction
Hydrogels are hydrophilic polymers with crosslinks that create a polymeric network. This enables them to absorb water at rates ranging from 10% to thousands of times their body weight [1, 2]. The presence of hydrophilic groups in the polymers forming hydrogel structures is attributed to their ability to absorb water. As a consequence of the contributions of various network’s domains and groups, the hydration of the polymer will be at varying extent (often higher than 90% weight) based on the nature of the polymer and the aqueous environment. Because of the crucial crosslinks that are present in the hydrogel structure, hydrogels exhibit swelling rather than dissolving in the aqueous environment [3]. The term “crosslinker” refers to substances that join molecules and enhance the characteristics of hydrogels. Crosslinking hydrogels to create a three-dimensional structure increases molecular weight, offers better stability, and impacts physical qualities including polymer elasticity, viscosity, and polymer insolubility [4]. Alginate, chitosan, hyaluronic acid, and other natural biomaterials, as well as synthetic compounds like polyvinyl alcohol, polyacrylamide, and polyethylene glycol, can be used to prepare hydrogels [5].
Hydrogels are promising, fashionable, and intelligent drug delivery systems that meet the demands of precisely directing medications to the target site and managing drug release. Environmental, hydrolytic, or enzymatic stimuli can frequently alter hydrogels for drug release at the desired location [6]. Hydrogels provide a number of benefits, including the potential for biocompatibility, hydrophilicity, controlled drug release, and intelligent drug delivery [7]. Therefore, scientists from a variety of disciplines were particularly interested in creating and improving these delivery methods [1, 2]. However, there are drawbacks to using them. The hydrophobicity of most medicines is the main drawback of drug delivery. The hydrophilic polymeric core is practically not the best place to store lipophilic medications that are incompatible with it, which is a problem because many of the pharmaceuticals that are now utilised and successful in treating diseases are hydrophobic. Additionally, certain hydrogels have lower tensile strengths, which might lead to the medication being released early before it reaches the target location [8].
Major efforts have lately been made to use nanotechnology’s potential in the delivery of various drugs. This provides a viable technique for site targeting and time-controlled delivery of different molecular weight drugs and bioactive chemicals. The nanotechnology approach is used to produce therapeutically useful compounds such as nanocapsules, nanoparticles, micellar systems, and conjugates. These preparations provide a multitude of advantages for drug administration, with the main advantages being improved medication safety and effectiveness. For instance, they can boost bioavailability, lengthen the time a drug or gene influences the target site, and improve drug stability against chemical or enzymatic degradation. They can also deliver drugs with more accuracy [3]. The hydrogel nanoparticulate materials (nanogels) would simultaneously exhibit the properties of both the nanoparticles and hydrogels that they individually possess. The hydrophilicity, adaptability, and biocompatibility of hydrogels as well as the benefits of nanoparticles, particularly their long half-life in blood and the potential to be targeted to the desired tissue, such as tumour sites, appear to benefit drug delivery [3].
2. Hydrogels
Hydrogels are comprised of 3D networks of hydrophilic polymers [9]. They have a large water absorption capacity due to the existence of hydrophilic functional groups connected to the 3D polymeric network of hydrogels [10]. The capacity to store water inside the network helps them to swell and collapse correctly, a characteristic that is helpful in medication delivery. Hydrogels are thought to be particularly promising materials for drug delivery because of their porosity and compatibility with aqueous environments [8]. Furthermore, the adjustable features of hydrogels make them ideal for certain medicinal applications such as oral drug delivery, ophthalmic, nasal, and transdermal routes [11].
2.1 Classification of hydrogels
There are several ways to categorise hydrogels, including:
Hydrogels either natural or synthetic. Natural hydrogels are a type of gel in which the polymers used to produce the gel are derived from natural sources. The utilisation of natural polymers to make hydrogels has a variety of advantages, including biocompatibility, biodegradability, and non-toxicity. Natural polymers include polysaccharides and proteins. Synthetic hydrogels, on the other hand, are hydrogels created from synthetic polymers, such as polyacrylamide and its derivatives, polyvinyl alcohol (PVA), or polyethylene glycol (PEG), which are the building blocks of synthetic hydrogels. Synthetic polymers have recently superseded natural polymers in the production of hydrogels due to the several advantages they provide, such as longer shelf life, improved gel strength, and higher water absorption capacity [12].
The hydrogels’ polymeric compositions (homo-, co-, and multi-polymer hydrogels). Homo-polymer hydrogels consist of just one kind of hydrophilic monomer; copolymer hydrogels, or network gels, consist of two types of monomers; and multi-polymer hydrogels or interpenetrating polymeric networks consist of three types of hydrophilic monomers [13].
Hydrogels’ physical structure:
Amorphous or non-crystalline
Semi-crystalline: A complicated blend of crystalline and amorphous phases
Crystalline
Lamellae, which are chain-folded layers that constitute the crystallisation of polymers, can be clustered to create spherulites, which are spherical components. Polymers have crystalline phases when the lamellar chains are ordered in well-known patterns; they display amorphous phases when the lamellae are randomly distributed [14].
According to the hydrogels’ crosslinking type (chemical or physical). Chemical crosslinking is formed by a covalent bond that occurs between polymers and molecules to generate a 3D network structure. The stability and mechanical characteristics of hydrogels are their fundamental benefits. On the other hand, physical hydrogels are formed by noncovalent interactions between linear molecules due to the electrostatic, hydrophobic, and hydrogen bonds among these molecules. While the chemically crosslinked hydrogels are irreversible, the physically crosslinked hydrogels are reversible [15].
According to the electrical charge. Based on the electric charges that are positioned across the crosslinked chains, hydrogels may be divided into three major categories: ionic, non-ionic, and ampholytic hydrogels. Ionic hydrogels can be further subdivided into cationic and anionic hydrogels based on the type of electric charge that is present on the polymeric chain. Cationic hydrogels are sensitive to pH and can interact with other anionic molecules to create complexes. These qualities of cationic hydrogels make them useful as virus-alternative vectors for gene delivery and treatment. The majority of cationic hydrogels, including si-RNA, were effectively produced by enzymatic degradation. These hydrogels work well in cancer stem cell therapy [16].
According to the hydrogels’ biodegradability. Both biodegradable and nonbiodegradable hydrogels exist. Nonbiodegradable hydrogels are hydrogels that are resistant to environmental changes and may maintain their structure and physicochemical characteristics over an extended period of time. Biodegradable hydrogels are more prevalent in natural hydrogels than nonbiodegradable hydrogels [15].
2.2 Mechanisms for hydrogel matrix release
2.2.1 Diffusion-based release
The most typical way that drugs are released from hydrogels is by diffusion-based release [17]. The hydrogel matrix’s mesh size has a significant impact on how quickly drugs diffuse out of it [18]. This is influenced by a number of factors, principally the extent of crosslinking, the monomer composition, and the nature and strength of environmental stimuli. Additionally, the size of the mesh has an important effect on the mechanical strength, degradability, diffusivity, and other physical characteristics of a hydrogel network [19, 20]. The usual mesh size of swollen hydrogels has been shown to range from 5 to 100 nm [20, 21], which is significantly bigger than the majority of small-molecule drugs. As a consequence, the diffusion of these medicines may only slightly slow in a swollen state. Macromolecules such as peptides and proteins, on the other hand, will have a prolonged release due to their hydrodynamic radii unless the structure and mesh size of the swollen hydrogels are suitably engineered to produce desired rates of macromolecular diffusion [22]. Drug release through a hydrogel mesh or water-filled hydrogel pores is made possible by diffusion-based drug delivery using either matrices or reservoirs. In the reservoir system, a core that contains the medicine is covered with a hydrogel membrane to create capsules or spheres with a high drug concentration in the system’s centre, allowing for a time-independent and continuous drug release. The matrix system operates using the macromolecular mesh, demonstrating a time-dependent (not constant) drug release [8]. Both types of drug release are illustrated in Figure 1.
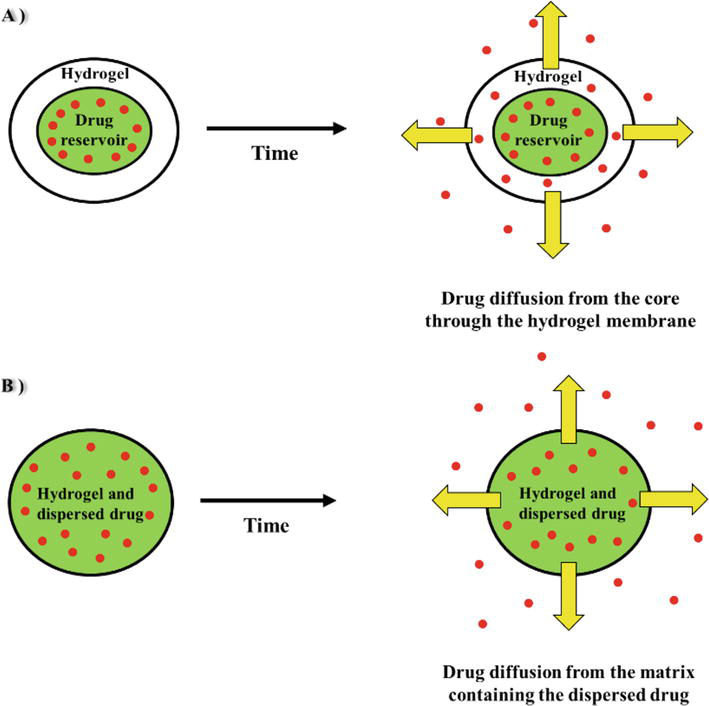
Figure 1.
(A): The core contains a drug in the Centre of the system in a higher concentration and is coated with a hydrogel membrane to produce a constant release rate in the reservoir system (time-independent), (B): Through the use of matrix delivery, the medicine is dissolved or dispersed throughout the hydrogel’s three-dimensional structure (time-dependent), modified from [
2.2.2 Swelling-based release
The hydrogels’ swelling-based drug release technology is used for pharmaceuticals that are distributed inside of glassy polymers that swell when they come into contact with biofluids. Beyond its border, swelling causes expansion that facilitates drug diffusion and polymer chain relaxation. The procedure, also known as (Case II transport), enables consistent, time-independent release kinetics. The anomalous transfer is another term for this mechanism because it mixes the procedures of swelling and diffusion to enable drug release. The active ingredient can diffuse from an area of higher concentration to a lower one due to the gradient between the drug that is distributed in the hydrogel and its surrounding environment [8].
2.2.3 Chemically based release
Chemically based release is influenced by chemical processes that occur inside the matrix of the gel. Hydrolytic or enzymatic breakdown of polymeric chains is one of these mechanisms, as well as reversible/irreversible interactions between the released medication and the polymeric network [3]. In chemically based delivery systems, drug release might occur by the breakage of polymeric chains via bulk or surface erosion, followed by the release of the entrapped drug from the hydrogels. The rate-limiting stage of chemically based release systems is polymer chain cleavage [17].
2.3 Controlled-delivery systems of hydrogel
Controlled-delivery systems are created to administer medication into the body in a certain, preset temporal and/or spatial manner to satisfy a particular therapeutic requirement. The hydrogels have special qualities that may make them one of the best controlled-release systems in the future among the various controlled-release systems that have been employed up to this point [3].
The controlled-release hydrogel systems are divided into two primary groups [3]:
Time-controlled systems
Stimulus-induced release systems (SIRS) [24, 25] are also known as stimulus-sensitive, stimulus-responsive, environmental-sensitive, environmental-responsive, or responsive hydrogel systems.
Responsive hydrogels drastically modify their structure and behaviour in response to changes in the external environment [3]. The environment-sensitive hydrogel systems, often known as smart systems, can be divided into the following categories:
Physically triggered release mechanisms
Chemically triggered release mechanisms
Other stimulus-induced release mechanisms
In this context, physical stimuli of interest include those involving temperature, electric current, pressure, sound, light, and magnetic fields. In contrast, specific molecular recognition events, ions, solvent composition, pH, and others are chemical triggers [25, 26]. Due to their capacity for recurrent conversion of swelling to deswelling in response to external temperature variations, temperature-sensitive (thermoresponsive) hydrogels have attracted a lot of attention [27, 28]. Contrarily, hydrogel systems with chemical responsiveness suggest a number of forms of hydrogels in which medicine can be released from a repository in response to variations in the amount of a particular molecule or biologically active substance in the environment. Due to their distinctive pH-dependent swelling–deswelling behaviour, hydrogels with pH-responsive systems are extremely important [3].
The main problem with stimulus-sensitive hydrogels is how slowly they react. The easiest technique to produce fast-acting responsiveness is to create hydrogels that are thinner and smaller. However, this reduces the mechanical strength of the hydrogel device and the polymer network as a whole [7]. Table 1 lists all the restrictions associated with hydrogels as well as potential remedies.
| Limitation | Solution | |
|---|---|---|
| 1 | Some hydrogels have nonbiodegradable and non-biocompatible characteristics. | Creation of hydrogels that are both biocompatible and biodegradable, such as PEG-PLGA-PEGa, or use of polymers with hydrolyzable moieties (chemical modification) [29]. |
| 2 | Response times of stimulus-sensitive hydrogels are too long. | Creating quick-acting hydrogels that are thinner and smaller [7]. |
| 3 | Fast drug release from large porous hydrogels and a quick burst release of medication during hydrogel swelling. | Prior to gelation, drugs can be physically or covalently tethered to the polymer chains (tethering approach) [30]. To combat rapid disintegration and quick drug release, covalent crosslinking is used to generate di-block or tri-block copolymers [6]. |
| 4 | In the entrapment procedure, there is a chance that the medication will become inactive and release in a burst. | Use of the tethering technique [30]. |
| 5 | possible drug inactivation during the covalent binding method’s polymer binding. | Replacing direct covalent drug binding to the polymer with suitable linkers that allow for controlled drug release [17]. |
| 6 | Hydrogel with diffusion-controlled release has an unspecific drug release mechanism. | Controlling the drug release from hydrogels using chemically and physiologically induced release triggers [30]. |
| 7 | Possibility of toxicity of small-molecule crosslinkers that are left behind after a hydrogel is created using a small-molecule crosslinking approach. | Using the Michael addition process or the Schiff base synthesis approach to crosslink polymers to other polymers [6]. |
| 8 | For oral drug delivery systems, chitosan-based hydrogel matrices quickly dissolve in stomach acid. | Crosslinking, conjugation, or the creation of polyelectrolyte complexes [17]. For the administration of oral insulin, scientists have created hydrogels based on chitosan-poly (g-glycolic acid) [31]. |
| 9 | Drug-loaded colloidal carriers are difficult to incorporate into hydrogels. | Preparation of liposomes in hydrogels as a mixed delivery system [32]. |
| 10 | Non-homogenous hydrophobic drug dispersion inside hydrogels and limited hydrophobic medication delivery using hydrogels. | Directly incorporating hydrophobic domains into the hydrogel network [17]. Creation of a weakly soluble drug’s solid molecular dispersion [17]. Using polymer nanoparticles to encapsulate the medicine allows for a well-dispersed drug slurry to be produced [17]. Preparation of drug delivery system made of hydrogel and nanoparticles [6, 33]. |
| 11 | Poloxamer-based thermosensitive hydrogels have low biodegradability and possible toxicity. | Preparation of biodegradable and biocompatible hydrophobic polyesters such as PLGA and PCLb that are crosslinked to PEG-based hydrogels [34]. A suitable in situ forming hydrogel that is temperature-sensitive is made of the PEG-PNIPAAMC copolymer. [34]. |
| 12 | Response of light-responsive hydrogels to stimuli (light) is slow and variable. | Fabrication of 2-hydroxyethyl methacrylate crosslinked polymers with azobenzene functional groups [35]. The interaction of polyacrylamide and polyacrylic acid forms an interpenetrating polymer network [36]. |
| 13 | Clogging of the needle during the injection of hydrogels that are temperature and pH sensitive. | pH/thermosensitive hydrogels are created using a dual-responsive hydrogel technique [17]. |
| 14 | Solubility, pH-related activity, and the delayed sol-gel transition phase. | Chemical change to enhance mucoadhesiveness and solubility profile [37]. |
| 15 | PEG hydrogels, as a tissue engineering scaffold, do not have any particular cell adhesive characteristics. | Using extracellular matrix proteins, PEG hydrogels may be modified to act as cell adhesives [38]. |
| 16 | Low drug loading and quick drug release. | Incorporating N-(3-aminopropyl) methacrylamide or other ionic or hydrophobic monomers into pHEMAd hydrogels to adjust drug release rate and boost drug loading capacity [39]. |
| 17 | the difficulty of surgically implanting pre-formed hydrogels or scaffolds used in tissue engineering, and the danger of infection. | To get around these scaffolding restrictions, use injectable hydrogel systems instead [40]. Hydrogels with micro-engineered structures are created [41]. |
| 18 | Low hydrogel network loading capacity for DNA or RNA and restricted transgenic expression capacity for gene transfer. | DNA/polymer polyplexes are incorporated into PEG hydrogel scaffolds [42]. Using hydrogels made of fibrin and hyaluronic acid as scaffolding for DNA/polymer polyplexes [43]. |
| 19 | The ineffectiveness and unsuitability as a carrier for hydrophobic and small-molecule active medicines. | The creation of hydrogel carriers made from copolymers of acrylic acid and methyl methacrylate serves as a unique oral drug delivery strategy for hydrophobic active medicines with tiny molecular weights [44]. |
| 20 | Hydrogels made of calcium alginate have low mechanical strength. | Agar/alginate beads’ composition, which has the advantages of improved mechanical strength and regulated drug release [45]. |
Table 1.
3. Hydrogel nanoparticles (nanogels)
Nanogels are submicron-sized, three-dimensional crosslinked polymer networks. Nanogel combines the properties of hydrogels and nanoparticles since it is made up of hydrogel particulate entities with nanometre-sized spaces [46]. Nanogels can be created through monomer-heterogeneous polymerisation or from precursors of polymers. Crosslinking, including physical and chemical crosslinking, is a crucial step in the fabrication of nanogels. Nanogels exhibit the swelling capability, or capacity, to absorb large volumes of water or biological fluids while retaining their structural integrity. Nanogels are an interesting prospect for several applications due to their special characteristics [3]. Nanogel has been found to have great drug loading capacity, biological consistency, higher stability, and sensitivity to a wide variety of environmental stimuli (such as pH, temperature, and ionic strength) in comparison to other nanocarriers [46].
Drugs loaded within nanogels can easily pass through physiological barriers due to their nanosize range, thus increasing drug bioavailability. In addition, with the use of nanogels, less medication is required, and there are fewer doses per day, which reduces the toxicity of the medication [47]. However, one should pay attention to the solvents and the type and concentration of surfactants used in the preparation of nanogels because toxicity due to surfactants can occur occasionally [47].
4. Hydrogels and nanogels applications in drug delivery
4.1 Oral drug delivery
The most popular, desired, and patient-compliant method of medication administration is oral dosing. Copolymer hydrogel networks are an appropriate drug delivery vehicle because they enhance oral absorption and bioavailability. As a mucoadhesive substance with the potential to prolong medication release and absorption, hydrogels are regarded as secure drug delivery systems for oral administration. The ability to prevent integrated drugs from enzymatic degradation is hydrogels’ additional benefit as oral drug delivery vehicles [48]. In order to avoid the difficulties involved in administering insulin parenterally, hydrogels are primarily explored for oral medication delivery of insulin [31].
4.2 Parenteral drug delivery
The parenteral method of administration is the most preferred route of administration for several medications, including peptides and proteins. For parenteral medication administration, hydrogels may be employed as controlled drug delivery systems. Drugs can be protected against enzymatic degradation, their half-life is extended, their bioavailability is increased, their frequency of administration is decreased, and as a result, their compliance with the patient’s needs is increased by using hydrogels. Temperature-sensitive injectable hydrogels are typically sol (fluid) at ambient temperature and gel (viscous) at human temperature. Drug release can be sustained, and medication bioavailability can be increased via gelation. The most popular temperature-sensitive hydrogels utilised in parenteral drug delivery systems are those based on poloxamer, although they are limited by the fact that they are not biodegradable [49].
4.3 Nasal drug delivery
High patient compliance and the avoidance of the hepatic first-pass effect, which might boost medication bioavailability, are two benefits of nasal drug administration. However, this method of administration also has its own drawbacks, such as the mucosal membranes acting as a barrier to macromolecule absorption and the short nasal residence period caused by mucosal turnover. Chitosan hydrogels, for example, are highly regarded as innovative nasal delivery systems that might lengthen the period that loaded active chemicals remain in the nasal cavity. These hydrogels have mucoadhesive, viscoelastic, and biocompatible qualities. The majority of the polymers used in nasal administration systems have a high thermal sensitivity and can gel at the site of action at body temperature [37].
4.4 Ocular drug delivery
A frequent method of topical drug administration for delivering medications to the eye is eye drops. Although this method is ineffective and might have systemic side effects, only around 5% of the incorporated drug would penetrate the intraocular tissue; the remaining 95% would be lost to tear drainage. Furthermore, the medication has a relatively brief residence duration in the eye. Because of this, it would be extremely desirable to create innovative drug delivery methods that would lengthen the period that medication is in the eye, reduce drug loss, and have fewer systemic negative effects [50]. Numerous studies have investigated contact lenses as ocular drug delivery devices, which have the benefit of extending the period that drugs are present in the body and their bioavailability. Because of their transparency and biocompatibility, hydrogel contact lenses have received a lot of attention as ocular medication delivery devices. Regarding this, drug molecules might be uniformly disseminated in matrices of hydrogels such as hydroxyethyl methacrylate (HEMA) polymer. However, this technique is only applicable to water-soluble medicines and could result in rapid drug release [50]. Hydrophobic or ionic monomers might be added to hydroxyethyl methacrylate (HEMA) hydrogels to promote hydrogel and drug interactions, regulate the rate of drug release, and boost loading capacity for drugs [39]. Dexamethasone-containing eye drops were created using either solvent evaporation or emulsification process with 2-hydroxypropyl-γ-cyclodextrin (HP-γ-CDs) medium containing cyclodextrin nanogel for sustained release. Using pH-sensitive polyvinylpyrrolidone-poly [acrylic acid] (PVP/PAAc) nanogels, pilocarpine was encapsulated to improve bioavailability, stability, and maintain a sufficient concentration of the medication at the site of action for a prolonged length of time [51].
4.5 Topical delivery
Delivering medications topically is a popular way of administration that is used to decrease side effects and localise large quantities of a medicine at the target site. Hydrogels are regarded as acceptable carriers for topical medication delivery due to their low toxicity potential and prolonged drug release. Hydrogels also offer the advantages of biocompatibility, softness, and high-water content, which can mirror the features of real tissues. Furthermore, due to hydrogels’ swelling and moisturising properties, they might prevent irritation of the enclosing tissues. Another significant feature of hydrogels is their capacity to preserve drugs from severe environmental conditions [52]. Nanogels have been used in dermatology and cosmetics as topical delivery methods for nonsteroidal anti-inflammatory medications (NSAIDs), as well as to treat psoriatic plaque and allergic contact dermatitis. For this purpose, nanogels are appropriate because they address a significant constraint of topically delivering systems: the relatively limited contact period between active medicines and the site of application. Thus, a homogenous nanogel dispersion is produced, maintaining water in the gel matrix [51].
4.6 Transdermal and subcutaneous delivery
In comparison to other administration methods, the transdermal route avoids the first-pass effect, improves therapeutic efficacy, provides plasma drug concentrations at a steady level, and increases patient adherence. Transdermal nanogels can produce an increase in permeability and stability in comparison to conventional oral administration [53]. Swollen hydrogels were employed in wound dressings as a controlled-release technique. The anti-inflammatory medication budesonide has been transported via hydrogels. To improve nicotine and hormone product penetration, these hydrogels are being investigated for transdermal iontophoresis. The favourable biodegradability of hydrogels encourages the development of biodegradable implanted hydrogels. These are mostly employed in the treatment of cancer by delivering anticancer medicines subcutaneously. It is made from crosslinked poly hydroxyethyl methacrylate (PHEMA), also used to deliver the chemotherapeutic agent cytarabine [14].
4.7 Brain drug delivery
The blood-brain barrier is still a major issue in drug delivery to the brain, which is still a difficult process. Local medication delivery via brain implants is a possibility, but it comes with the risk of infection and harm to brain tissue. Epicortical delivery utilising hydrogels is a different strategy for brain local administration that might distribute the loaded medicine directly into the brain with little tissue damage [54].
4.8 Tissue engineering
For tissue engineering, hydrogels offer several benefits including resemblance to extracellular matrices of tissues, promotion of cell proliferation, minimal irritation to surrounding tissues, and prolonged release of integrated growth factors. Injectable hydrogels are superior to other traditional scaffolds in several ways, including simplicity of handling, deeper tissue penetration, greater margin adaptability, and less invasiveness [40]. Other interesting engineering techniques to address the problems that tissue engineering is now encountering are micro-engineered hydrogels [41].
4.9 Gene delivery
Delivering DNA or RNA for the purpose of genetic alteration can be achieved using a hydrogel scaffold. Hydrogels can improve the effectiveness of gene therapy, particularly in the treatment of cancer. In cancer therapy, siRNA or fatal genes would be enclosed in hydrogel scaffolds and would encourage malignant cells to undergo cell death. However, hydrogels used for gene delivery may have some drawbacks, such as a low capacity for loading genes and a quick release of encapsulated genes. To overcome these restrictions, a number of techniques have been proposed, such as the condensation of DNA or RNA in nanoparticulate systems and subsequent encapsulation in a hydrogel scaffold [43].
4.10 Cosmetics application
The primary function of the skin is to protect the body from harmful external environmental factors like pathogens and UV radiation. Additionally, it helps keep the body hydrated and at the proper temperature. To enhance the skin’s appearance and texture, cosmetic products are frequently applied. Skin cleansers, moisturisers, and body lotions are all needed to maintain a healthy skin texture. Hydrogel-based cosmetic products are now gaining popularity in the cosmetic industry due to benefits such as biocompatibility, elasticity, softening, and increased water content. Wrinkles, cellulite, pigmentation, ageing, and skin hydration can all be efficiently treated using hydrogels. Caffeine-containing bioadhesive hydrogels, for example, are widely employed in cosmetic applications. The bioadhesive property of hydrogels aids in the gradual release of caffeine into the skin, improving skin texture and appearance [14].
4.11 Vaccines delivery
Immune responses that are specific to an antigen are induced during vaccination. Polymeric nanogels are a unique, alternative method of vaccine administration that is being used to improve the potency and effectiveness of vaccinations. Nanogels perform better than conventional immunisations in preventing the enzymatic breakdown of vaccine antigens. Vaccine administration with regard to a specified target can be considerably enhanced by using surface-altered nanogels with antibodies’ affixes and other ligands [55].
4.12 Cancer therapy
Many anticancer drugs such as doxorubicin, cisplatin, temozolamide, and 5-FU have been loaded into nanogels for the treatment of different types of cancer. For example, nanogels loaded with chitin-polymerised doxorubicin are employed for the management of lung, breast, liver, and prostate cancer. In cancer treatment, drug delivery is required to be efficient and highly specific to the target site with low toxicity to the adjacent healthy tissue. Thermosensitive and pH-sensitive nanogels containing cisplatin were utilised for the management of breast cancer [56]. Likewise, fludarabine loaded into polyplex nanogels was employed to enhance the activity and reduce the toxicity of the drug [57].
5. Conclusion
Hydrogels and nanogels can be regarded as promising carriers for drug delivery since they have shown a positive role in the delivery of various types of drugs and bioactive molecules by different routes of administration. They demonstrated superiority in drug release, targeting, and stability in comparison to conventional dosage forms due to their unique features. However, to increase their commercialisation, some issues are required to be addressed, including the development of cost-effective manufacturing methods for large-scale production and the precise control of drug pharmacokinetics to reach the target site at an adequate dose. The developments in nanotechnology and polymer sciences may resolve these challenges, which make hydrogels and nanogels an excellent platform for drug delivery in clinical settings.
References
- 1.
Hoffman AS. Hydrogels for biomedical applications. Advanced Drug Delivery Reviews. 2002; 54 (1):3-12. DOI: 10.1016/s0169-409x(01)00239-3 - 2.
Omidian H, Park K. Swelling agents and devices in oral drug delivery. Journal of Drug Delivery Science and Technology. 2008; 18 (2):83-93. DOI: 10.1016/S1773-2247(08)50016-5 - 3.
Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Advanced Drug Delivery Reviews. 2008; 60 (15):1638-1649. DOI: 10.1016/j.addr.2008.08.002 - 4.
Husnaini Zainal S, Hanisah Mohd N, Suhaili N, Hannan Anuar F, Mat Lazim A, Othaman R. Preparation of cellulose-based hydrogel: a review. Journal of Materials Research and Technology. 2021; 10 :935-952. DOI: 10.1016/j.jmrt.2020.12.012 - 5.
Su J, Li J, Liang J, Zhang K, Li J. Hydrogel preparation methods and biomaterials for wound dressing. Life. 2021; 11 (10):1016. DOI: 10.3390/life11101016 - 6.
Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008; 49 (8):1993-2007. DOI: 10.1016/j.polymer.2008.01.027 - 7.
Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews. 2001; 53 (3):321-339. DOI: 10.1016/s0169-409x(01)00203-4 - 8.
Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules. 2019; 24 (3):603. DOI: 10.3390/molecules24030603 - 9.
Bahram M, Mohseni N, Moghtader M. An introduction to hydrogels and some recent applications. In: Majee SB, editor. Emerging Concepts in Analysis and Applications of Hydrogels. Croatia: InTech; 2016, pp. 9-38. DOI: 10.5772/64301 - 10.
Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research. 2015; 6 (2):105-121. DOI: 10.1016/j.jare.2013.07.006 - 11.
Simões S. Modular hydrogels for drug delivery. Journal of Biomaterials and Nanobiotechnology. 2012; 3 (2):185-199. DOI: 10.4236/jbnb.2012.32025 - 12.
Soni RK, Kewatkar SM, Jain V, Saluja MS. A review of hydrogels, with its properties and applications in medicine. Journal of Biomedical and Pharmaceutical Research. 2023; 12 (2):20-33. DOI: 10.32553/jbpr.v12i2.970 - 13.
Vashist A, Ahmad S. Hydrogels: smart materials for drug delivery. Oriental Journal of Chemistry. 2013; 29 (3):861-870. DOI: 10.13005/ojc/290303 - 14.
Kasai RD, Radhika D, Archana S, Shanavaz H, Koutavarapu R, Lee D-Y, et al. A review on hydrogels classification and recent developments in biomedical applications. International Journal of Polymeric Materials and Polymeric Biomaterials. 2022; 72 :1-11. DOI: 10.1080/00914037.2022.2075872 - 15.
Zheng H, Li M, Wu L, Liu W, Liu Y, Gao J, et al. Progress in the application of hydrogels in immunotherapy of gastrointestinal tumors. Drug Delivery. 2023; 30 (1):2161670. DOI: 10.1080/10717544.2022.2161670 - 16.
Kaith BS, Singh A, Sharma AK, Sud D. Hydrogels: Synthesis, classification, properties and potential applications—A brief review. Journal of Polymers and the Environment. 2021; 29 (12):3827-3841. DOI: 10.1007/s10924-021-02184-5 - 17.
Ghasemiyeh P, Mohammadi-Samani S. Hydrogels as drug delivery systems; pros and cons. Trends in Pharmaceutical Sciences. 2019; 5 (1):7-24. DOI: 10.30476/tips.2019.81604.1002 - 18.
Canal T, Peppas NA. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. Journal of Biomedical Materials Research. 1989; 23 (10):1183-1193. DOI: 10.1002/jbm.820231007 - 19.
Amsden B. Solute diffusion within hydrogels. Mechanisms and Models. Macromolecules. 1998; 31 (23):8382-8395. DOI: 10.1021/ma980765f - 20.
Mason MN, Metters AT, Bowman CN, Anseth KS. Predicting controlled-release behavior of degradable PLA-b-PEG-b-PLA hydrogels. Macromolecules. 2001; 34 (13):4630-4635. DOI: 10.1021/ma010025y - 21.
Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998; 19 (14):1287-1294. DOI: 10.1016/S0142-9612(98)00025-8 - 22.
Lustig SR, Peppas NA. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. Journal of Applied Polymer Science. 1988; 36 (4):735-747. DOI: 10.1002/app.1988.070360401 - 23.
Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. European Polymer Journal. 2015; 65 :252-267. DOI: 10.1016/j.eurpolymj.2014.11.024 - 24.
Lin C-C, Metters AT. Hydrogels in controlled release formulations: Network design and mathematical modeling. Advanced Drug Delivery Reviews. 2006; 58 (12-13):1379-1408. DOI: 10.1016/j.addr.2006.09.004 - 25.
Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Advanced Drug Delivery Reviews. 2002; 54 (1):53-77. DOI: 10.1016/s0169-409x(01)00243-5 - 26.
Miyata T, Uragami T, Nakamae K. Biomolecule-sensitive hydrogels. Advanced Drug Delivery Reviews. 2002; 54 (1):79-98. DOI: 10.1016/s0169-409x(01)00241-1 - 27.
Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Advanced Drug Delivery Reviews. 1998; 31 (3):197-221. DOI: 10.1016/s0169-409x(97)00121-x - 28.
Dinarvand R, D’Emanuele A. The use of thermoresponsive hydrogels for on-off release of molecules. Journal of Controlled Release. 1995; 36 (3):221-227. DOI: 10.1016/0168-3659(95)00035-7 - 29.
Li Z, Ning W, Wang J, Choi A, Lee P-Y, Tyagi P, et al. Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharmaceutical Research. 2003; 20 (6):884-888. DOI: 10.1023/A:1023887203111 - 30.
Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Advanced Drug Delivery Reviews. 2010; 62 (1):83-99. DOI: 10.1016/j.addr.2009.07.019 - 31.
Chaturvedi K, Ganguly K, Nadagouda MN, Aminabhavi TM. Polymeric hydrogels for oral insulin delivery. Journal of Controlled Release. 2013; 165 (2):129-138. DOI: 10.1016/j.jconrel.2012.11.005 - 32.
Hurler J, Berg OA, Skar M, Conradi AH, Johnsen PJ, Škalko-Basnet N. Improved burns therapy: Liposomes-in-hydrogel delivery system for Mupirocin. Journal of Pharmaceutical Sciences. 2012; 101 (10):3906-3915. DOI: 10.1002/jps.23260 - 33.
Gou M, Li X, Dai M, Gong C, Wang X, Xie Y, et al. A novel injectable local hydrophobic drug delivery system: Biodegradable nanoparticles in thermo-sensitive hydrogel. International Journal of Pharmaceutics. 2008; 359 (1-2):228-233. DOI: 10.1016/j.ijpharm.2008.03.023 - 34.
Alexander A, Ajazuddin K, Saraf JS, Saraf S. Poly(ethylene glycol)–poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. Journal of Controlled Release. 2013; 172 (3):715-729. DOI: 10.1016/j.jconrel.2013.10.006 - 35.
Unger K, Salzmann P, Masciullo C, Cecchini M, Koller G, Coclite AM. Novel light-responsive biocompatible hydrogels produced by initiated chemical vapor deposition. ACS Applied Materials & Interfaces. 2017; 9 (20):17408-17416. DOI: 10.1021/acsami.7b01527 - 36.
Xu Y, Ghag O, Reimann M, Sitterle P, Chatterjee P, Nofen E, et al. Development of visible-light responsive and mechanically enhanced ‘smart’ UCST interpenetrating network hydrogels. Soft Matter. 2018; 14 (1):151-160. DOI: 10.1039/c7sm01851g - 37.
Nazar H, Fatouros DG, van der Merwe SM, Bouropoulos N, Avgouropoulos G, Tsibouklis J, et al. Thermosensitive hydrogels for nasal drug delivery: The formulation and characterisation of systems based on N-trimethyl chitosan chloride. European Journal of Pharmaceutics and Biopharmaceutics. 2011; 77 (2):225-232. DOI: 10.1016/j.ejpb.2010.11.022 - 38.
Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010; 31 (17):4639-4656. DOI: 10.1016/j.biomaterials.2010.02.044 - 39.
Hu X, Hao L, Wang H, Yang X, Zhang G, Wang G, et al. Hydrogel contact lens for extended delivery of ophthalmic drugs. International Journal of Polymer Science. 2011; 2011 :e814163. DOI: 10.1155/2011/814163 - 40.
Sivashanmugam A, Arun Kumar R, Vishnu Priya M, Nair SV, Jayakumar R. An overview of injectable polymeric hydrogels for tissue engineering. European Polymer Journal. 2015; 72 :543-565. DOI: 10.1016/j.eurpolymj.2015.05.014 - 41.
Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007; 28 (34):5087-5092. DOI: 10.1016/j.biomaterials.2007.07.021 - 42.
Lei Y, Huang S, Sharif-Kashani P, Chen Y, Kavehpour P, Segura T. Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds. Biomaterials. 2010; 31 (34):9106-9116. DOI: 10.1016/j.biomaterials.2010.08.016 - 43.
Lei Y, Rahim M, Ng Q , Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. Journal of Controlled Release. 2011; 153 (3):255-261. DOI: 10.1016/j.jconrel.2011.01.028 - 44.
Caldorera-Moore M, Maass K, Hegab R, Fletcher G, Peppas N. Hybrid responsive hydrogel carriers for oral delivery of low molecular weight therapeutic agents. Journal of Drug Delivery Science and Technology. 2015; 30 :352-359. DOI: 10.1016/j.jddst.2015.07.023 - 45.
Yin Z-C, Wang Y-L, Wang K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. Journal of Drug Delivery Science and Technology. 2018; 43 :12-18. DOI: 10.1016/j.jddst.2017.09.009 - 46.
Zhang H, Zhai Y, Wang J, Zhai G. New progress and prospects: The application of nanogel in drug delivery. Materials Science and Engineering:C. 2016; 60 :560-568. DOI: 10.1016/j.msec.2015.11.041 - 47.
Al Rahman F, Ibrahim Elnima E, Elhassan AM, Omar Hussein SE. Nanogel as a pharmaceutical carrier – Review article. Scholars Journal of Applied Medical Sciences. 2017; 5 (11F):4730-4736. DOI: 10.21276/sjams.2017.5.11.83 - 48.
Morishita M, Goto T, Nakamura K, Lowman AM, Takayama K, Peppas NA. Novel oral insulin delivery systems based on complexation polymer hydrogels: Single and multiple administration studies in type 1 and 2 diabetic rats. Journal of Controlled Release. 2006; 110 (3):587-594. DOI: 10.1016/j.jconrel.2005.10.029 - 49.
Alexander A, Ajazuddin K, Saraf JS, Saraf S. Polyethylene glycol (PEG)–poly (N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics. 2014; 88 (3):575-585. DOI: 10.1016/j.ejpb.2014.07.005 - 50.
Gulsen D, Chauhan A. Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. International Journal of Pharmaceutics. 2005; 292 (1-2):95-117. DOI: 10.1016/j.ijpharm.2004.11.033 - 51.
Jain S, Ancheria RK, Shrivastava S, Soni SL, Sharma M. An overview of Nanogel–novel drug delivery system. Asian Journal of Pharmaceutical Research and Development. 2019; 7 (2):47-55. DOI: 10.22270/ajprd.v7i2.482 - 52.
Morsi NM, Abdelbary GA, Ahmed MA. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: Development and in vitro/in vivo characterization. European Journal of Pharmaceutics and Biopharmaceutics. 2014; 86 (2):178-189. DOI: 10.1016/j.ejpb.2013.04.018 - 53.
Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, Yamagishi H, et al. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochemical and Biophysical Research Communications. 2008; 367 (2):330-335. DOI: 10.1016/j.bbrc.2007.12.112 - 54.
Wang Y, Cooke MJ, Morshead CM, Shoichet MS. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials. 2012; 33 (9):2681-2692. DOI: 10.1016/j.biomaterials.2011.12.031 - 55.
Wu W, Aiello M, Zhou T, Berliner A, Banerjee P, Zhou S. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials. 2010; 31 (11):3023-3031. DOI: 10.1016/j.biomaterials.2010.01.011 - 56.
Peng J, Qi T, Liao J, Chu B, Yang Q , Li W, et al. Controlled release of cisplatin from pH-thermal dual responsive nanogels. Biomaterials. 2013; 34 (34):8726-8740. DOI: 10.1016/j.biomaterials.2013.07.092 - 57.
Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. Polyplex Nanogel formulations for drug delivery of cytotoxic nucleoside analogs. Journal of Controlled Release. 2005; 107 (1):143-157. DOI: 10.1016/j.jconrel.2005.06.002