Abstract
Drug delivery systems using nanogel are extremely essential. Chemical cross-linking is used to create it and the 3D polymer network of the nanogel has the capacity to encapsulate hydrophilic or hydrophobic therapies, such as proteins, compounds containing small molecules and ultrasmall nanoparticles. They were synthesized with a precise surface area and space due to their nanoscale structure, which also increased the stability of whatever medications they contained and increased the length of time they could circulate. Through the use of pH sensitivity, redox sensitivity, and temperature sensitivity, nanogels can achieve varied responsiveness. This is accomplished by designing specific chemical structures and employing various production methods. Consequently, the development of a multifunctional nanogel-based drug delivery system has increased the efficacy of illness therapies. As nanoscopic drug carriers, nanogels have drawn a lot of interest, especially for the site- or time-specific delivery of bioactive mediators. Nanogel preparations come in a variety of useful forms because to the wide variety of polymer systems and the straightforward adjustment of their physicochemical properties. Nanogels have exceptional levels of stability, drug loading potential, biologic consistency, strong permeation potential, and responsiveness to environmental cues. Nanogels have demonstrated great promise in a variety of sectors, including the delivery of genes, chemotherapeutic treatments, diagnosis, the targeting of particular organs, and many more. This review primarily focuses on various types of nanogels, preparation techniques, including techniques for loading drugs, various modes of biodegradation mechanisms, and primary mechanisms for drug release from nanogels.
Keywords
- mediators
- nanogels
- polymer
- cleavage
- diagnosis
1. Introduction
A hydrogel is a three-dimensional network of a polymer that is cross-linked and hydrophilic, while a nanogel is a hydrogel where the particles are in the nanoscale size range. Nanogels can swell because they are hydrophilic, which explains why they can hold so much water. The ability to modify a nanogel’s qualities by changing its composition is a crucial characteristic. Nanogels have a wide range of interesting uses, including gene delivery and medical imaging; in this case, the use of triggered drug administration will be taken into consideration. Due to their substantial swelling, nanogels may often entrap at least 30% weight of medicines through a variety of interactions. As the drug is absorbed, the gel breaks down and stable nanoparticles form, trapping the drug inside. A protective barrier surrounds the gel as the hydrophilic polymer chains at the surface become exposed, and this layer can be functionalized to target certain tissue or cells. Evidently, a nanogel’s value as a triggered drug delivery system to a specific target depends on its compositional design and chemistry [1].
As nanoscopic drug carriers, nanogels have drawn a lot of interest, especially for the site- or time-specific delivery of bioactive mediators. Nanogel preparations come in a variety of useful forms because to the wide variety of polymer systems and the straightforward adjustment of their physicochemical properties. It has been demonstrated that nanogels have great potential in a variety of fields, including the delivery of genes, chemotherapy drugs, diagnosis, targeting of specific organs, and many others. This chapter will focus primarily on the various types of nanogels, methods of preparation, including methods of drug loading, and various modes of biological diffusion [2].
1.1 Types of nanogels
According to their cross-linking structure, nanogels can be divided into two main categories: chemically (covalent) cross-linked nanogels, which form cross-linking points through covalent bonds and physically cross-linked nanogels, which are cross-linked through non-covalent bonds like hydrogen bonds, electrostatic interactions, and hydrophobic interactions. They mostly resemble spheres. They can also be made with a cross-linked layer for structural integrity and either as a core-shell or core-shell-corona structure. Nanogels are extremely compatible with molecules that have a large loading capacity since they have a hydrophilic character.
1.1.1 Micelles
This type of nanogel is made from amphiphilic block or graft copolymers that have undergone supramolecular self-assembly in aqueous solutions. The centre of the micelle has adequate room to encapsulate pharmaceuticals and biomacromolecules. The effectiveness of the N-isopropylacrylamide-based micelle systems as drug delivery systems was examined [2]. It is made up of a core-shell morphological structure stabilized by hydrogen bonding, with a core hydrophobic block segment encircled by a hydrophilic polymer block shell (Figure 1).
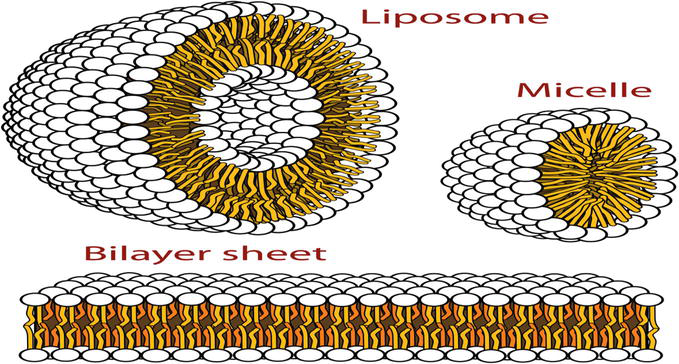
Figure 1.
Structure of micelle. Source:
1.1.2 PEG-PEI (poly(ethylene glycol)-polyethyleneimine)-based drug delivery
The initial nanogel to be introduced in 1999 was PEG-PEI. This is also an example of a nanogel. Since PEI (polyetheleneimine) had been demonstrated to be cytotoxic, PEG-PEI nanogels are often quite biocompatible. This is because of the PEG (polyethylene glycol). Due to PEG’s proved non-toxic and non-immunogenic qualities, the material is made more soluble in water and less poisonous, making it more biocompatible than if PEI were used alone [2]. Numerous characteristics, including size, are crucial to understand because they influence a nanogel’s potential applications and its efficacy as a drug release system. Due to the small size (20–220 nm) of PEG-PEI nanogel particles, penetration is probably improved. Since PEG-PEI nanogel particles are small (20–220 nm), it is expected that they will penetrate tissues and cells more readily, increasing the likelihood that the drug will affect its target.
Also, due to the charge of PEG-PEI, the amount of drug interaction between the nanogel and drug will affect how quickly the drug is released. Due to its high (positive) charge density, PEI is the ideal carrier for negatively charged pharmaceuticals and biological components [3]. Additionally, because pH can alter charge density, the release rate can be adjusted as the strength of the drug-nanogel connection changes. PEG-PEI nanogel applications in drug release systems are extremely promising and have the potential to have a significant impact on the biomedical industry.
It was reported that the encapsulation of AQ10 (6-(hydroxymethyl)-1,4-anthracenedione (AQ) analogue), a novel anti-cancer medication that targets pancreatic cancer [4]. When the medicine and nanogel were used together, cell proliferation was inhibited more efficiently, which is the desired result for cancer therapies, according to a comparison of taking the drug with or without the nanogel. The study came to the conclusion that the use of this nanogel as a drug delivery technology was successful because it made cells more receptive to the medication. PEG-PEI was employed in another investigation. In another study [3], SODN (antisense phosphorothioate oligonucleotides) distribution to multidrug-resistant (MDR) human oral epidermoid carcinoma cells was investigated using PEG-PEI nanogel. Overall, the study found that the medicine had a better chance of successfully accumulating in the cancer cells when PEG-PEI nanogel was present. This study is particularly notable because it introduces nanogels and establishes a baseline for all subsequent research on nanogels, whether it uses PEG-PEI or not [3].
1.2 Greater drug loading capacity of nanogels
The advantages of nanogels which have higher drug loading capacity are lesser carrier material, better control over the drug release and improved efficacy and safety [4]. The amount of drug loaded per unit weight of the nanoparticle is known as the loading capacity and it shows what proportion of the mass of the nanoparticle is made up of drug-encapsulated substance. By dividing the entire weight of the nanoparticles by the total amount of drug contained, one can get the loading capacity (LC%). The functional group that is present in the polymeric unit is what gives nanogels their greater drug loading capability. These functional groups play a significant role in the carrying and releasing abilities of drugs. Despite this, some functional groups have the capacity to combine a medication with an antibody for the purpose of targeting. The starting hydrogen bonding and van der Waal forces of attraction within the gel network are provided by these long functional groups of polymeric chains, as a result, reducing the carrying capacity for drugs [4].
1.3 Synthesis of nanogels
Physical covalent crosslinking or chemical covalent crosslinking was used to categorize nanogels. Some of the methods used to create nanogels include core-shell and hollow nanogel particles as well as controlled/living radical polymerization employing variable compositions, dimensions, and architectural designs. In order to introduce a high level of particular domain organization into nanogel and carry out a range of additional crosslinking operations, the core-shell self-assemblies, similar to polymer micelles, are used. Various distinctive features have recently been created in nanoscale fabrication techniques for fabricating precise nanogels. These characteristics include highly detailed control over size, shape, deformability, and surface chemistry [5] (Figure 2).
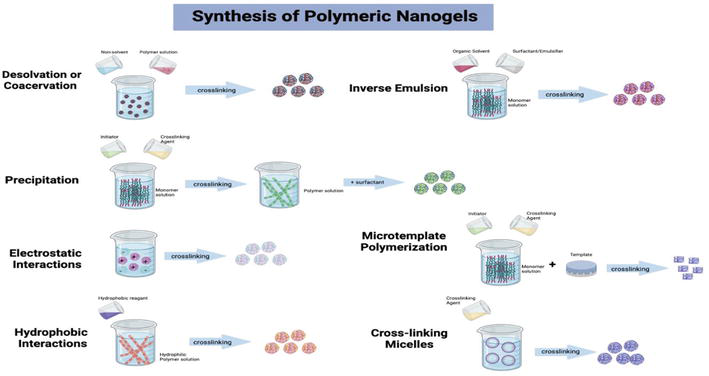
Figure 2.
Synthesis of polymeric nanogels. Source:
1.3.1 Nanogels for the delivery of small therapeutic molecules
Nanogels can quickly swell in an aqueous environment. The advanced design of the nanogel may be useful in adjusting the drug release rate, influencing carrier cell interactions, and enhancing the medication’s intended therapeutic effect. Weakly crosslinked polyelectrolyte nanogels’ capacity to assimilate molecules with the opposite charge is one of their most notable characteristics. For instance, cationic crosslinked PEG-polyethyleneimine (PEG-PEI) nanogels were investigated for the immobilization of negatively charged physiologically active compounds such retinoic acid, Indomethacine, and valproic acid [5].
It was found that these drug-loaded nanogels reduced tumor development in breast cancer cells of an animal model by delivering the active triphosphates of therapeutic nucleoside analogs [4]. Recently, the same group investigated the advantages of cationic nanogel integrated active 5′-triphosphates of nucleoside reverse transcriptase inhibitors over free medicines in the antiviral therapy of HIV-1 infection in the central nervous system (CNS). The effectiveness of cationic nanogel integrated active 5′-triphosphates of nucleoside reverse transcriptase inhibitors over free medicines in the antiviral therapy of HIV-1 infection in the central nervous system (CNS) has recently been examined by the same group. Nanogels are macromolecular systems with the capacity to distribute their cargo at the desired location and to attain lengthy circulation half lifetimes in vivo. Nanogels are created with the idea of administration route in mind, and they get past any obstacles in their path to reach the circulation unharmed.
Nanogels are very expandable and can contain 30% weight. The polymer chain interacts with the biological molecules via electrostatic, van der Waal, or hydrophobic/covalent interactions. These loading capacities are unusually high and surpass those of liposomes and polymeric micelles. Following medication loading, the nanogels disintegrate into stable nanoparticles, trapping biohazard inside. Hydrophilic polymers (like PEG) can be introduced in a very thin structure to prevent aggregation. When the drug-nanogel complex breaks down, hydrophilic polymer chains are exposed, and they surround the nanogel in a protective layer. The versatility and control of polymer chemistry enable the creation and development of a wide variety of medication formulations as well as the incorporation of several therapeutic cargos inside the same nanogel carrier [4].
Dexamethasone is administered locally to avoid acute lung inflammation. The hybrid nanogel is crosslinked, composed of partially denaturated lysozyme cores and dextran shells, and is physically biocompatible. The pulmonary vasculature’s target endothelial determinant was the target of antibodies coated on the nanogels.
1.3.2 In vivo activity
Due to their softness and deformability, splenic filtering is partially liquefied by nanogel. These are nanogel’s primary characteristics. Due to their flexibility and deformability, erythrocytes, despite having a size range in microns, are able to readily pass through the splenic filtering bed, which has pores with a size of a few hundred nanometers.
There are various small compounds, peptides, antibodies, and other nanomedicines that can be delivered specifically to tissue or cells. Numerous tiny molecules, peptides, antibodies, or antibody fragments have been shown to be effective for the targeted administration of nanogel and other nanomedicine in tissue or cells. In comparison to nontargeted nanogel, ligand-mediated target alters the nanogel’s biodistribution profile more. The strong expression of the receptor for the targeted nanogel can help to stop the over-deposition of the nanogel at the target site and so lessen the related adverse effects [5].
1.3.3 Using nanogel to distribute oligonucleotides
For the treatment and diagnostics of cancer and neurological diseases, therapeutic oligonucleotides were created for the targeted suppression of a certain mRNA (ribonucleic acid) sequence. These include the more recently identified micro RNAs (miRNAs), small interfering RNAs (siRNAs), and antisense oligodeoxynucleotides (ODNs). Realizing the ONs (oligonucleotides) delivery’s full therapeutic potential is still greatly hampered by the inability to penetrate specific cells. ONs are hydrophilic molecules with a negative charge that are difficult to pass across cell membranes. They can activate the innate immune system and can be eliminated by endogenous nuclei. Therefore, ONs require a delivery truck in order to safely go to the point of action. One of the most well-known new types of nanomaterials for overcoming in vivo ONs delivery challenges is cationic nanogels [5].
1.3.4 Nanogels for the therapeutic delivery of proteins
Nanogels have been extensively researched for the transport of proteins and peptides in addition to their self-ability to encapsulate large quantities of biomacromolecules and prevent their disintegration. The molecular mass and hydrophobicity of the protein affect the formation of nanogels. The proteins’ complexation, sizing, thermal denaturation, and ultimately aggregation shield the nanogel from enzymatic deterioration [6].
1.3.5 Delivery of nucleic acid
Small interfering RNAs, or siRNAs, have the potent capacity to selectively and effectively block gene expression. Small interfering RNA (siRNA) therapy is now a crucial component of treating diseases that are caused by genes. Due to fast enzymatic breakdown, it cannot penetrate the cell surface and has limited applications, such as modest transfection rates and brief half-lives.
In order to address these issues, siRNA can be loaded into liposomes, enhanced by the biomolecule cholesterol, or coupled to polymer nanoparticles while being processed with nucleic acids. A delivery mechanism based on nanogel recognized the siRNA treatment. Tetrahedral DNA-based nonviral vectors were introduced for siRNA assembly in nanogel, and they provide safety during delivery. This method might make it possible to effectively transfect cells both in vitro and in vivo while also stopping ribonuclease breakdown. In order to maximize productivity, this is the ideal environment for integrating different devices [7].
1.3.6 Nanogels for combined drug delivery
The nanogel structure is quickly modified to combine alternatives of different materials and, thus, provide advantages for combinatorial encapsulation of pharmaceuticals with varying chemical properties such small molecules, proteins, and nucleic acids. The development of liposomal nanogels composed of protein-encapsulating perishable polymers and drug-complexed cyclodextrins that may deliver soluble supermolecules (IL-2) and small hydrophobic molecules (TGF-). When given to mice, IL-2 and TGF- enhanced the lifespan of skin cancer tumors due to their synergistic effects [8].
When compared to single drug-loaded nanogels or free medication, binary drug combinations in nanogels demonstrated synergistic cytotoxicity against human ovarian A2780 cancer cells and exerted a stronger anticancer activity in cancer heterograft models used in-vivo [6]. The advantages of synchronic co-delivery of the platinum-taxane medication combination via a single carrier are increased by focusing on nanogels, which are overexpressed in the majority of ovarian tumors.
1.4 The use of nanogels in diagnostics and imaging
1.4.1 Nanogel for PET imaging
Researchers produced a polyacrylamide-based nanogel and crosslinked it with polydentate chelating ligands to create PET (Positron Emission Tomography) radiotracers by placing metal radionuclides on it [7]. For the purpose of enhancing the chelation stability of nanogels, a variety of crosslinkers including DTPA (diethylaminetriaminepolycarboxylic acid), DOTA (chelating agent), and 1, 4, 7-triazacyclononane-1,4,7-triacetic acid (NOTA) were produced. In contrast to the other 2 crosslinkers, NOTA-based nanogels preserved 64Cu the most consistently, according to experiments in mouse humor, with little to no trans-chelation. In comparison to DOTA-based nanogels, 64Cu-DOTA-crosslinked nanogels demonstrated substantial neoplasm accumulation as well as lesser signal in the liver and spleen. In several instances, the metastases’ accumulation of 64Cu-DOTA was higher than that of the main connective tissue neoplasm. These findings suggest that metal-chelating crosslinked nanogels may be useful as PET agents for cancer detection and treatment monitoring. Such technologies have the advantage that the isotope can be quickly and easily integrated into premade nanogels prior to their clinical application [8].
1.4.2 Nanogel for optical imaging
Imaging is a crucial component of clinical protocols because it can offer morphological, structural, metabolic, functional, and molecular data for identifying and evaluating diseases. Imaging is a crucial component of clinical protocols because it can offer morphological, structural, metabolic, functional, and molecular data for identifying and evaluating diseases. The most widely used optical imaging techniques for nanogels are computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI), and ultrasound.
High water content, structural flexibility, fluid-like transport, biocompatibility, and biodegradability are only a few of the characteristics of nanogels. By using metallic element ions to crosslink branching polyethyleneimines, gadolinium-assembled nanogels were created. To increase the duration of blood circulation, inverse microemulsion was used, followed by surface functionalization using synthetic resin glycol chains [8].
1.5 Nanogels’ applications in drugs delivery
1.5.1 Antipyretic drug transdermal drug delivery
Aceclofenac’s nanosized dispersion was created using emulsion-solvent diffusion techniques and added to a Carbopol 940. The formulation demonstrated ideal porosity characteristics, stability, and a sustained drug release.
It has been demonstrated that encapsulating magnetic nanoparticles like iron compound gives each mixture more stability and sensitivity than when these agents are delivered as unencapsulated substances [9]. Due to the cluster impact, nanogels enable the encapsulation of an excessive number of magnetic nanoparticles, which may result in the production of numerous, stronger native magnetic fields (Figure 3).
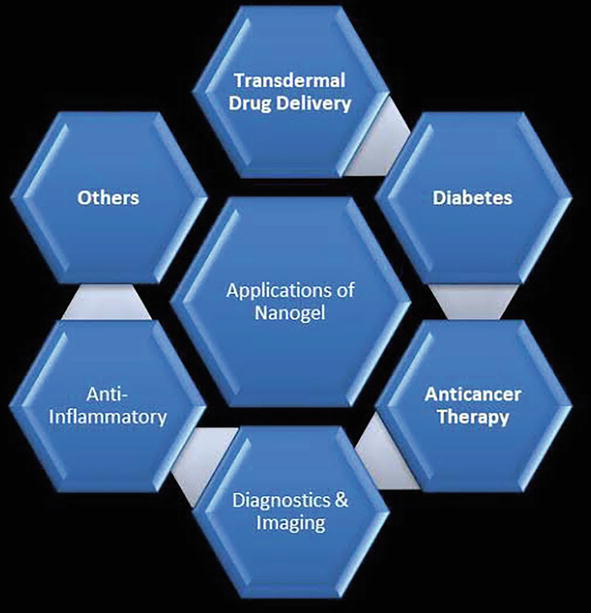
Figure 3.
Applications of nanogels. Source:
1.5.2 Vaginal medication delivery
1.5.2.1 Cancer treatment
In the recent years, researchers have looked at various drug delivery technologies, particularly nanoparticles, to overcome the drawbacks of conventional therapy agents such poor solubility, a limited therapeutic window, and toxicity [9]. An excellent benefit for the transport of anticancer drugs is expressed by nanogel, a type of unique nanoparticle. First off, the nanogel network’s consistency offers a suitable cavern for storage when loading medications since it prevents early infection and acts as a barrier against environmental threats and deterioration. For instance, nanogel significantly preserved the potency of decitabine because of their improved stability and capacity to evade glycoside transporters. Second, the form of the carrier is changed by nanogels from a spherical to an oval shape that resembles a red blood cell. Due to the lengthening of blood circulation through microcapillaries, this characteristic is essential. The nanogels may lengthen under high shear rates, which would appear to make the blood less viscous. Additionally, nanogels will only serve as passive and active targets for tumor tissue [7].
To overcome the drawbacks of conventional therapy agents, such as poor solubility, a limited therapeutic window, and toxicity to conventional tissues, numerous drug delivery technologies, particularly nanoparticles, have been studied during the past few decades. An excellent benefit for the transport of anticancer drugs is expressed by the particular nanoparticle known as nanogel. The nanogel network’s consistency firstly offers an ideal cavern for storage for loading medications in order to prevent premature unharnessing and serves as a barrier against environmental threats and degradation [8].
Additionally, the sensitivity and chargeability of nanogels are investigated as alternate targeting alternatives in cancer treatment. The effectiveness of the loaded drug will be significantly increased by pH-sensitive nanogels whether the drug acts extracellularly or intracellularly because, as was already mentioned, nanogels have a magnificent pH-responsive ability and growth may be a special tissue with a slightly acidic living thing microenvironment. Prepared PEG-chitosan nanogels for cancer treatment that are capable of being controlled by external cooling/heating as well as responding well to changes in pH scale setting [5]. Around the tumor extracellular pH scale (6.0–6.2), the 5-FU-loaded nanogels’ surface charges ranged from virtually neutral to positive. The positive-charged nanogels will aid in cell learning, and eventually, the amplified acidity in endosomes and organelles will result in a significant release of 5-FU. By using synthetic polymers that can swell at acidic pH, temperatures above their transition temperatures, and reducing environments, it was possible to create a pH, thermal, and reaction potential triple-responsive expandable nanogel system (TRN) for the delivery of photo-sensitizers to target mitochondria and tumors. The nanogels rapidly enlarge from 108 nm to over 1200 nm (in diameter) during the course of 2 hours in a very reducing environment at blood heat. Additionally, TRN was functionalized with sigma-2 receptor targeting ligand, which is capable of efficiently focusing on head and neck cancer. The majority of the cancer cells were killed by TRN 12 hours after radiation, greatly enhancing the effectiveness of photodynamic therapy (PDT) in treating necrobiosis. In addition to efficiently delivering the hydrophobic photosensitizer, nanogel is also the optimum delivery mechanism for encasing the hydrophobic photosensitizer. The nanogel is the best delivery strategy for encasing the high number molecules (such as gold and platinum) that may enhance the biological impact of X-irradiation in addition to effectively delivering the hydrophobic photosensitizer. Numerous approaches to administering a curative dosage of radiation to neoplasia using nanogels have recently been studied. Developed PEGylated poly (2- [N, N, − diethylamino] ethyl methacrylate) nanogels to encapsulate highly stabilized and neoplasm specific gold nanoparticles [9].
A nanogel with a 106 nm diameter contained 15 gold nanoparticles (GNG). In addition to having a higher accumulating potency, the GNG in the nanogels had noticeably higher stability when compared to commercially available PEGylated GNG and Citrate-stabilized GNG. The gold-containing new PEGylated nanogel’s results are conclusive. The findings demonstrate that a new PEGylated nanogel containing gold nanoparticles is a potentially effective nanomedicine for cancer photothermal treatment.
Nanoparticles will passively target cancer through EPR results, but often, their unfolding is limited to the outer region of the cancer and that they are unable to enter the deep cancer interstitial space.
1.5.2.2 Neurological conditions
A promising method for delivering oligonucleotides (ODN) to the brain may be nanogel. Systemic distribution of oligonucleotides (ODN) to the systema nervosum is necessary for the treatment of neurodegenerative illnesses. Blood-brain barrier (BBB) macromolecule injection results in poor translocation and rapid clearance from circulation. Nanogels that are certain or encapsulated with negatively charged ODN result in the creation of a stable liquid electrolyte dispersion complex with particle sizes under 100 nm that can be carried over the BBB with ease. Once siderophilin or insulin are added to the nanogel’s surface, the transport effectiveness is further increased [10].
1.5.2.3 Anti-inflammatory activity
Nanogels can be used to administer anti-inflammatory drugs to people with skin issues. A skin penetrating nanogel system with a surface made of double-layered nanostructured particles and an emulsifying agent has been developed for efficient drug delivery in dermatitis. Nanogel was created using 3-acetyl-11-keto-boswellic acid (AKBA) and nanoparticles with AKBA loaded on them. The nanogels were organized using carbopol of the appropriate consistence. Nanoparticles with AKBA were used for treatment with ease [6].
The most potent pentacyclic triterpenic acid found in the gum of Boswellia serrata, 3-acetyl-11-keto-boswellic acid (AKBA), exhibits anti-inflammatory properties. In AKBA, there is a lot of higher medicine activities. These studies shown that, in cases of skin irritation, medications can be administered successfully through the skin. Non-steroidal anti-inflammatory drugs (NSAIDs) of the diclofenac class are frequently used to treat arthritis. Diclofenac sodium self-assembling gel hydrogels produced better anti-inflammatory effects. Additionally, it has been discovered that nanogels containing cinnamon oil and cinnamaldehyde have strong antibacterial properties [10].
1.5.2.4 Additional uses
A nanogel made of polyvinyl pyrrolidone and poly(acrylic acid), or PVP/PAAc, is sensitive to the concentration of hydrogen ions. The pre-ocular retention and ocular penetration abilities of the curcumin are enhanced by using film-ultrasonic methods and thermosensitive gelling agents in curcumin-loaded cationic nanostructured supermolecule carriers (CNLC). Muscone has a colloidal gel drug loading capability, and physical studies indicated that the phase change temperature was 34°C. Based on initial in-vivo investigations, insulin-loaded nanogels seem to be 51% more successful than free insulin at preserving blood glucose levels in diabetic rats [7].
1.5.2.5 Immune disorder
In a study, a novel nanogel drug delivery system for the immunosuppressive medication mycophenolic acid (MPA) was developed and tested [6]. The findings of this study showed that nanogel-based native medication delivery is substantially more effective for treating lupus erythromatous because it specifically targets antigen-presenting cells. The innovative medicine delivery system will prolong the patient’s life and postpone the onset of renal failure.
1.5.2.6 Stopping bleeding
Major wound bleeding has been controlled using a protein molecule found in the solution that was utilized to create a nanogel. The proteins have a way for self-assembling into a biodegradable gel at the nanoscale. For instance, micronized sacchachitin promotes wound healing [8].
1.5.2.7 Nasal drug administration
Technologies for delivering drugs via nanogels have a great deal of potential for overcoming some of the problems with medication delivery. Because the nasal mucosa absorbs nanogels so quickly, they could be used to transport and distribute medications through the mucosa. An inventive method to slow the spread of disease is nasal immunization with nanogels [7]. The use of nanogels for vaccine distribution via the nasal route may be a novel way to control the spread of a disease. A composite polymeric network of nanoparticulates (NPs, microcapsules, NEs, etc.) is what gives nanogels their high viscosity.
1.6 Advantages and disadvantages of nanogels
The main benefit of nanogels is their small size since they offer a lot of specific surface area, which increases the possibility of interaction between the components. This is advantageous since it increases the likelihood that the medicine will reach the intended target and produce the desired effect. Nanogels can also be altered to be used for a variety of purposes because of their tunability. Nanogels have a wide range of applications, as shown by one study that functionalized the surface of PEG-PEI by adding an amine group, enabling the nanogel to be utilized for treating spinal cord injuries [7].
There aren’t many drawbacks to using nanogels for drug delivery. One illustration is that because it is diffusion-based, the release rate may be too rapid. Nevertheless, numerous studies have concentrated on resolving this issue by ensuring that there is a contact between the medicine and gel, delaying the release.
One major advantage of nanogels is that the research is always changing; as in the case of the example presented, as soon as a possible problem is detected, a remedy is quickly proposed and studied.
2. Conclusion
Nanogels are reassuringly modern drug delivery systems that effectively balance the drawbacks of both traditional and contemporary therapies, such as low stability and nonspecific effects. Biological ligands such proteins, peptides, and polysaccharides were produced with modifications to nanogel for optimal contact with target cells. Because of their high incorporation capacity, swelling ability, and the quantity of biological molecules interacting with the electrostatic, van der Waal interaction that occurs with polymer chains, leading to a stable nanoparticle and allowing for easy drug entrapment, nanogels are used as therapeutic drug carriers. The use of nanogels for tumor targeting appears to be quite effective. In order to promote highly selective absorption into specific cancer cells and reduce their uptake in normal cells, future objectives of this review will thus be to improve the design of nanogel with specific targeting residue.
Therefore, it can be said that because nanogel particles are flexible, they make an excellent platform for triggered drug administration. This enables them to have qualities that are advantageous for a variety of targeted release systems. As previously said, current research confirms the effectiveness of PEG-PEI nanogels and other nanogels in triggered drug delivery [8]. Although nanogel research has advanced significantly over time, more study is still required before clinical application.
References
- 1.
Vinogradov S, Batrakova E, Kabanov A. Poly(ethylene glycol)– Polyethyleneimine NanoGel™ particles: Novel drug delivery systems for antisense oligonucleotides. Colloids and Surfaces B: Biointerfaces. 1999; 16 (1-4):291-304. DOI: 10.1016/S0927-7765(99)00080-6 - 2.
Mauri E, Chincarini G, Rigamonti R, Magagnin L, Sacchetti A, Rossi F. Modulation of electrostatic interactions to improve controlled drug delivery from nanogels. Materials Science and Engineering: C. 2017; 72 :308-315. DOI: 10.1016/j.msec.2016.11.081 - 3.
Pinelli F, Pizzetti F, Ortolà Ó, Marchetti A, Rossetti A, Sacchetti A, et al. Influence of the core formulation on features and drug delivery ability of carbamate-based nanogels. International Journal of Molecular Sciences. 2020; 21 (18):6621. DOI: 10.3390/ijms21186621 - 4.
Soni K, Desale S, Bronich T. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. Journal of Controlled Release. 2016; 240 :109-126. DOI: 10.1016/j.jconrel.2015.11.009 - 5.
Pinelli F, Pizzetti F, Rossetti A, Posel Z, Masi M, Sacchetti A, et al. Effect of surface decoration on properties and drug release ability of nanogels. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2021; 614 :126164. DOI: 10.1016/j.colsurfa.2021.126164 - 6.
Ganta C, Shi A, Battina S, Pyle M, Rana S, Hua D, et al. Combination of nanogel polyethylene glycol-polyethylenimine and 6(hydroxymethyl)-1,4-anthracenedione as an anticancer nanomedicine. Journal of Nanoscience and Nanotechnology. 2008; 8 (5):2334-2340. DOI: 10.1166/jnn.2008.294 - 7.
Vismara I, Papa S, Veneruso V, Mauri E, Mariani A, De Paola M, et al. Selective modulation of A1 astrocytes by drug-loaded nano-structured gel in spinal cord injury. ACS Nano. 2019; 14 (1):360-371. DOI: 10.1021/acsnano.9b05579 - 8.
Cuggino C, Blanco E, Gugliotta L, Alvarez I, Cecilia I, Calderon M. Crossing biological barriers with nanogels to improve drug delivery performance. Journal of Controlled Release. 2019; 307 :221-246. DOI: 10.1016/j.jconrel.2019.06.005 - 9.
Suhail M, Rosenholm J, Minhas M, Badshah S, Naeem A, Khan K, et al. Nanogels as drug-delivery systems: A comprehensive overview. Therapeutic Delivery. 2019; 10 (11):697-717. DOI: 10.4155/tde-2019-0010 - 10.
Li C, Obireddy S, Lai W. Preparation and use of nanogels as carriers of drugs. Drug Delivery. 2021; 28 (1):1594-1602. DOI: 10.1080/10717544.2021.1955042