Human TLRs, their localization, ligands, and signaling adaptors.
Abstract
The connection between inflammation and cancer has been well recognized at the epidemiological, biological, and pharmacological levels. Unresolved chronic inflammation is implicated in most stages of cancer development and thus can induce certain solid tumors. The molecular regulators of these linkages are emerging and should be well-decorticated. Toll-like receptors (TLRs) recognize pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) and death–associated molecular patterns (DAMPs) secreted from dying or damaged cells of the host. TLRs can be pro and anti-tumorigenic depending on the type of TLR signaling, cancer, and its stage. Therefore, comprehensive studies are required in this direction. The current chapter supplies a concise schematic concerning the biology and the characteristics of TLRs and summarizes the major findings of the enigmatic role of TLRs and their associated signaling in the pathogenesis of human cancers. On one hand and in some neoplastic contexts, TLR activation mediates proliferation invasion, migration and correlates with poor prognosis and metastasis, and inhibits apoptosis, leading to cancer progression. On the other hand and depending on other neoplastic context, TLRs agonists enhance radiosensitivity and chemotherapy, apoptosis, immune cell infiltration, and raise the antitumor effect of T cells.
Keywords
- TLRs
- tumor progression
- tumor regression
- dichotomic role
- innate immunity
1. Introduction
The innate immune mechanisms are the first distinct defense fighting pathogen attack. They implicated many immune cell types such as monocytes, macrophages, dendritic cells (DCs), neutrophils, and natural killer (NK) cells [1]. They also comprise innate humoral components produced by the innate immune cells such as complement system, cytokines, chemokines, and antimicrobial peptides (AMPs; LL37 and Bactericidal/permeability-increasing protein (BPI), etc.) [2, 3, 4, 5]. These innate immune cells contain various intracellular or membrane-associated pattern recognition receptors (PRRs). The PRR family germline-encoded receptors include several forms of recognition receptors: nucleotide-binding oligomerization domain-like receptors (NOD)-like receptors and retinoic acid inducible gene I (RIG-I)-like receptors (RLR), C-type lectin receptors (CLR), Aim2- like receptors (ALR) and Toll-like receptors (TLRs) and intracellular DNA sensors such as cyclic GPM-AMP synthase cGAS [6, 7]. These receptors could discriminate between self and non-self-molecules. The well-known are Toll-like receptors (TLRs) to acknowledge highly conserved molecules expressed by pathogens: pathogen-associated molecular pattern (PAMP) or microbial-associated molecular pattern MAMPs (expressed/released by pathogens) or endogenous ligands released from dying, stressed and damaged cells: damaged-associated molecular pattern (DAMP) molecules released from dying cells Hsp60, Hsp70, fibronectin Host DNA from dying cells, mitochondrial DNA etc. [8, 9]. They produce inflammatory cytokines and type I interferons (IFNs) to ascertain a highly effective defense system [10]. TLRs are expressed not only by immune cells but also by epithelial cells, for defense against pathogens invading the body either through skin or mucous membranes.
Despite the host’s protective role against foreign molecules and fighting diseases, including cancer, chronic inflammation has been referred to as one of the new hallmarks of cancer since 2011 [11], besides the above settled in 2000 as proposed by Hanahan and Weinberg [11]. Further proof of the link between chronic inflammation and carcinogenesis is revealed by the diminished cancer rates in patients receiving non-steroidal anti-inflammatory drugs and elevated rate in obese patients exhibiting high adipose tissue inflammation [6]. Chronic inflammation activates constitutively signaling pathways, such as Nuclear factor kappa B (NF-kB) or mitogen-activated protein kinase (MAPK) whose pro-tumoral effect is well documented [12]. In hepatocellular carcinoma (HCC) [13] and colitis-associated cancer, the activation of NF-kB prevented tumor apoptosis and stimulated the production of pro-inflammatory cytokines in the tumor microenvironment, thereby enhancing tumor progression. In this context, NF-kB activation is implicated in both tumor initiation and progression in liver cancer [14]. Therefore, the inflammatory response can lead to carcinogenesis after NF-kB activation, by the induction of anti-apoptotic molecules [15, 16]. TLR stimulation enhances NF-kB activation and thus leads by consequence to pro-inflammatory cytokines and chemokines, growth factors and anti-apoptotic proteins release leading to tumor progression and chemoresistance. TLR overstimulation may bring about strong inflammation since it plays a part in the recruitment of inflammatory cells in the tumor microenvironment.
2. TLR structure and signaling pathway
All TLRs, type I integral membrane receptors, have a characteristic structural organization with an extracellular recognition domain (ECD), a single transmembrane (TM) helix, and cytoplasmic signal domains (Figure 1) [17].
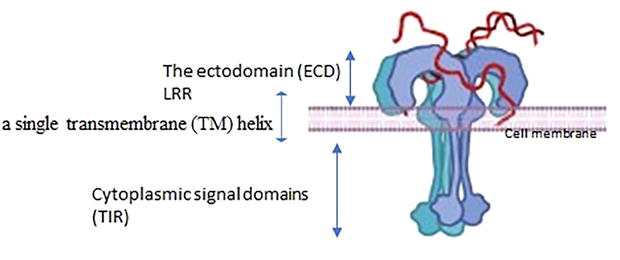
Figure 1.
Full-length TLR structure. The extracellular domain is composed of the N-terminal (LRRNT), LRRs and the C-terminal (LRR-CT) regions, a single transmembrane segment (TM), and an intracellular domain (TIR).
The ectodomain (ECD) is a terminal extracellular ligand binding domain made up of leucine-rich repeat (LRR), TLR-ECD shares a typically standard structural framework adopting horseshoe-shaped structures built from leucine-rich repeat (LRR) motif. The N-terminal extracellular ligand binding domain comprises varying numbers (19–26) hydrophobic leucine-rich repeat (LRR) modules (residues) every one of 20–30 Aa in length containing the consensus LxxLxLxx sequence in charge of ligand recognition and PAMPs and DAMPS recognition,” The LRR-NTs are disulfide linked β-hairpins, LRR-CTs are globular structures that have two α helices and are stabilized by two disulfide bonds [18, 19].
The intracellular C-terminal signaling domain of TLRs (150 Aa) is one of numerous evolutionarily conserved foundations of the immune system’ It’s named Toll IL-1 Receptor (TIR) domain since it shares homology with the signaling domain of IL-1R family members and is carboxyl-terminal to LRR [20]. TIR domain initiates downstream signaling cascades by interacting making use of its adaptor proteins such as myeloid differentiation response protein 88 (MyD88) that will be the absolute most commonly used adapter, MyD88 adaptor-like (MAL), TIR-domain-containing adapter-inducing interferon-β (TRIF), and TRIF-related adaptor molecule (TRAM). The signaling pathway activates transcription factors such as NF-κBs and interferon regulatory factors (IRFs), ultimately causing the release of proinflammatory cytokines and type I interferons, various anti-viral and anti-pathogen proteins, and initiation of the adaptive immune response [21].
Upon PAMPs or DAMPs sensing, TLRs undergo conformational changes, dimerize and reorient their TIR domains, to communicate with TIR-containing proteins, MyD88 and MAL, TRIF, and TRAM. This interaction results in undergoing a series of intracellular cascade signal transduction involving interleukin-1 receptor (IL-1R)-associated kinase 4 (IRAK4), IRAK2/1 phosphorylation resulting in the activation and dimerization of tumor necrosis factor receptor-associated factor 6 (TRAF6). TRIF interacts with TRAF3 and TRAF6 limited to TLR3 and TLR4. For endosomal TLR4, TRAM interplays with TRIF contributes to TRAF3-dependent stimulation of the kinase TBK1. These pathways promote inflammation and host defense and drive the IFN pathway activation with interferon-stimulated gene expression Table 1 [29, 30].
| TLR | Localization | Ligands | Signaling adaptator | References |
|---|---|---|---|---|
| TLR1/6 | cell membrane | lipoproteins, lipopeptides | MyD88 and TIRAP/MAL | [22] |
| TLR2/6 | cell membrane | lipopeptides peptidoglycan and lipoteichoic acid lipoarabinomannan, zymosan tGPI-mucin, hemagglutinin protein | MyD88 and TIRAP/MAL | [22] |
| TLR3 | endoplasmic reticulum, lysosomal membrane | polyinosinic-polycytidylic acid (poly(I:C)), ds RNA derived from viruses | TRIF | [23] |
| TLR4 | cell membrane, endoplasmic reticulum, lysosomal membrane | lipopolysaccharides (LPS), Mannans, Taxol | MyD88, TIRAP/MAL, Trif and TRAM | [22] |
| TLR5 | cell membrane | flagellin | MyD88 | [22, 24] |
| TLR7 | endoplsmic reticulum, lysosomal membrane | imiquimod, resiquimod (R-848), loxoribine, ssRNA (RNA viruses) and synthetic poly(U) RNA | MyD88 | [22] |
| TLR8 | endoplasmic reticulum, lysosomal membrane | R-848 and viral ssRNA | MyD88 | [24] |
| TLR9 | endoplasmic reticulum, lysosomal membrane | unmethylated 2′-deoxyribo(cytidine-phosphate-guanosine) (CpG) DNA motifs, crystal hemozoin | MyD88 | [22, 25] |
| TLR10 | Endolysosomes | HIV-1 gp41 | MyD88 | [26, 27, 28] |
Table 1.
3. TLR localization
TLRs are classified into two subfamilies depending on their localization. TLRs localize on cell/surface membrane and/or reside within intracellular compartments such as endosomes, multivesicular bodied, lysosomes, and endolysosomes. TLR1, 2, 4, 5, and 6 are located in cell. However, TLR3, 7, 8, 9 are located on endosome membrane within the cell. Their different location is ultimately linked to the type of ligand they recognize. Thus, TLRs located on the cell membrane mainly bind microbial membrane lipids and proteins, and TLR4 binds bacterial lipopolysaccharides (LPS). TLR5 recognizes bacterial flagellin, TLR 2,1 and 6 recognize peptidoglycans and zymosan, whereas TLRs located on the endosomal membranes bind nucleic acids derived from virus and bacteria but also from self-nucleic acid. Thus, compartmentalization of nucleic acids sensing TLRs in the endolysosome is primordial to control their stimulation by self-derived nucleic acids and minimizes the risk of autoimmune reactions. TLR3 recognizes viral double stranded RNA (dsRNA), whereas TLR7 recognizes single-stranded RNA (ssRNA), TLR9 binds to bacterial and viral DNA (CpG-DNA motifs) [31, 32].
4. TLR expression on immune cells
TLRs are expressed in varied cell types. Mainly they are located in innate immune cells Such as monocytes/macrophages, mast cells (MCs), neutrophils, eosinophils, basophils, natural killer (NK) cells, γδ T cells, innate lymphoid cells, DCs, platelets; brain innate immune cells like microglia and astrocytes. They are also expressed in adaptive immunity such as T and B cells. Non-immune cells, such as endothelial cells/ECs could express TLRs. TLR1 is detected in DCs and B Cells [33]. TLR2 is expressed in peripheral mononuclear leucocytes, DCs, monocytes, and T Cells [34, 35, 36]. TLR3 is mainly expressed in DCs, NK cells, and T cells [37, 38]. TLR4 in macrophages, DCs, and T Cells [36, 37, 39]. TLR5 in monocytes, DCs, and NK cells [37, 40]. TLR6 is highly expressed in B cells and DCs, but low in monocytes and NK cells [37, 41]. TLR7 is expressed in B cells, DCs, monocytes, and T cells [38, 42]. TLR8 is expressed in monocytes, DCs, and low in NK and T cells [43, 44]. TLR9 is present in DCs, B cells, macrophages, NK, and microglial cells [33, 43].
5. TLR expression and roles on cancer cells
TLRs are expressed in certain types of cancers. Their expression could be closely associated with cell proliferation invasion and the probability of metastasis promoting then cancer progression or enhancing tumor regression. In this regard, the conclusions from
| Cancer type | Tissue or cell line | Role of TLR in cancer | References |
|---|---|---|---|
| Cervical | Human cancer tissue | TLR1 and 3 in early carcinogenesis | [45] |
| HeLa cell line | TLR4 promotes tumor progression | [46] | |
| Human cancer tissue | TLR9 promotes tumor progression | [47, 48] | |
| Human cancer tissue, Hela cell line | TLR8 promotes tumor progression | [49] | |
| Human cancer tissue | TLR2 and 4 promote tumor progression | [50] | |
| Human cancer tissue | TLR3,7,8 and 9 promote tumor regression | [51] | |
| Human cancer tissue | TLR2,7 and 8 promote tumor regression | [52] | |
| Human cancer tissue | TLR3,4 and 5 promote tumor regression | [53] | |
| Ovarian | Human cancer tissue and cell line | TLR4 promotes tumor progression | [54, 55, 56] |
| Human cancer tissue | TLR9 promotes tumor progression | [57] | |
| fisher 344 rats | TLR2 and 4 promote tumor regression | [58, 59] | |
| SKOV3 cell line | TLR8 promotes tumor regression | [60] | |
| Endometrial | Human cancer tissue | TLR3 and 4 involved in adenocarcinoma | [61] |
| Human cancer cell | TLR2 and 6 promote tumor progression | [62] | |
| Human cancer cell | TLR1,2,3 and 4 promote tumor progression | [63] | |
| Melanoma | Mice | TLR7 and 8 promote tumor regression | [64] |
| Human, mice and B16 cell | TLR1 and 2 tumor regression | [65] | |
| cell line | TLR4 promotes tumor regression | [66] | |
| Hepatocellular | HUH7 HCC cells, Human samples | TLR2 promotes tumor progression | [67] |
| In vivo and in vitro | TLR9 promotes tumor progression | [68] | |
| Human samples | TLR5 predictor of poor prognostic | [69] | |
| Human samples, cell line | TLR4 promtes tumor progression | [67, 70, 71, 72] | |
| cell line | TLR2 promotes tumor regression | [73] | |
| Lung | In vivo and in vitro | TLR1/2 inhibits tumor growth | [74] |
| In vivo and in vitro | TLR2/6 promotes tumor growth | [75] | |
| In vitro | TLR3 inhibits tumor growth | [76, 77] | |
| In vivo and in vitro | TLR3 promotes tumor growth | [78] | |
| In vitro | TLR4 promotes tumor growth | [79] | |
| In vitro | TLR4 inhibits tumor growth | [80] | |
| In vitro | TLR5 has a protective role | [81] | |
| In vitro | TLR7/8 inhibits tumor growth | [82, 83] | |
| In vitro | TLR9 promotes tumor progression | [84] | |
| Human | TLR9 promotes tumor regression | [85] | |
| Breast | In vitro, Human, in vivo | TLR4,2,9 promote tumor progression | [86, 87] |
| In vitro, in vivo | TLR3,7 promotes tumor regression | [88, 89] | |
| Colorectal | In vitro, vivo, Human | TLR4 promotes tumor growth | [90, 91, 92] |
| In vivo and in vitro, Human | TLR9 promotes tumor regression | [93, 94] | |
| In vitro | TLR9 promotes tumor growth | [95, 96, 97] | |
| In vivo | TLR2 has protective role | [98] | |
| in vitro | TLR2 promotes tumor progression | [99] | |
| In vitro | TLR3 promotes tumor regression | [100] | |
| Human | TLR5 is linked to better prognostic | [101] | |
| Glioma | In vivo and in vitro | TLR2 promotes tumor progression | [102, 103, 104, 105] |
| In vitro | TLR4 promotes tumor progression | [106, 107, 108] | |
| In vitro | TLR7/8 inhibits tumor growth | [109] | |
| In vitro | TLR9 promotes tumor progression | [110] | |
| In vitro, in vivo | TLR9 promotes tumor regression | [111, 112, 113] |
Table 2.
TLR expression in human cancers.
5.1 Cervical cancer
Cervical cancer is caused essentially by High-risk human papillomavirus (HR-HPV) types. A study highlighted a down expression of TLR3 and an overexpression of TLR1 at transcriptional level in dysplastic and carcinoma epithelium. in stroma, TLR 1, 2, 5, 6, and 9 were overexpressed in association with disease severity. Thus, TLR3 and TLR1 are proposed as implicated in early and late stages of cervical carcinogenesis thus their usefulness for diagnostic and prognostic. Moreover, the stromal overexpression of TLRs may play a crucial role in cervical cancer progression [45].
TLR4 was over-expressed in cervical cancer, and its activation by LPS can accelerate the proliferation and promote anti-apoptosis in Hela cells in vitro in a dose-dependent manner. The mechanism causing this overexpression is regulated by the NF-kB/IL-6/TGF-β1 secretion [46]. Patients with persistent HR HPV exhibit over-expressed TLR9 for more than 1 year in comparison to women who cleared HPV infection and to those re-infected with low-risk LR HPV. These results speculate that overexpression of TLR9 in persistently infected women could lead to chronic inflammation thus contributing to cervical cancer risk [47]. Furthermore, TLR9 may play a role in progression of cervical neoplasia in Tunisian patients and could therefore be considered a convenient biomarker for malignant transformation of cervical cells [48]. TLR8 may be an interesting therapeutic target in cervical cancer. In this regard, overexpression of TLR8 in cervical cancer patients and Hela cells was observed. Furthermore, a strong correlation with increased expression of TLR8, VEGF, and Bcl-2 in cervical cancer patients was demonstrated. Continually, upon binding to its agonist CL075, TLR8 is able to remarkably increase the percentage of cells in G2/M + S of Hela cells, accompanied by increased COX-2, BCL-2, and VEGF mRNA levels [49]. TLR2 and TLR4 are strongly associated with TNF-α and TNF-β in cervical cancer with gradually increased expression from premalignant lesions to cervical cancer compared to normal controls [50], However, higher TLR expression is associated with HPV16 clearance revealing an important link between innate and adaptive immunity in the control of HPV infections after a persistent period [51]. It is speculated that high levels of TLR2, TLR7, and potentially TLR8 in cervical mucosa are important for CIN2 regression, suggesting their role in the clearance of HPV [52]. These studies highlight the important protective role of the innate immune system at different stages of HPV infection. A deeper understanding of host immune response toward virus factors would affect if a lesion progresses or regresses. This finding is crucial to drive the proper choice of today’s available immunotherapeutic for HPV-associated disease. Down-expression of TLR4 during the progression of cervical neoplasia is linked to P (16INK4A) expression, the crucial marker of HPV integration into host cells. These results demonstrate the crucial link between HPV infection and TLR signaling during the carcinogenesis of cervical cancer [114]. Remarkable diminution of mRNA level of TLRs 3, 4, and 5 and overexpression of TLR1 was noted in cervical squamous cell carcinoma (CSCC) as compared to controls. These results point toward TLRs 3, 4, and 5 agonists exploration as therapeutic targets to treat cervical cancer [53]. The chimeric molecule including the extracellular domain of CD200 and a murine IgG2a Fc region CD200Fc seemed to repress TLR4/NF-κB and NLRP3 inflammasome inflammatory effects in SiHa cells and Caski cells treated with LPS. It provided a novel mechanistic understanding toward the conceivable therapeutic usefulness of CD200Fc for cervical cancer [115].
Regarding TLR gene polymorphism and cervical cancer association, several studies in several ethnicities have been conducted, but results vary greatly. In like manner, Oliveira and al concluded there was not always a noteworthy association between TLR9 polymorphism and HPV clearance or persistence. Accordingly, polymorphism in the promoter region of the TLR9 gene does not appear to be primordial in the natural history of the HPV infection [116]. These authors concluded the usefulness of TLR 3 and 9 gene polymorphisms in cervical cancer susceptibility in North India [117].
In the same context, a Tunisian study searching for TLR2 (−196 to −174 del) and the TLR 9 (2848 G > A) polymorphisms and the susceptibility of cervical cancer among Tunisian women show no association. Whereas, in the same cohort study, TLR3 (c.1377 C > T) and (Asp299Gly) TLR4 polymorphism has been shown to be associated with a higher risk of cervical cancer [118]. TLR9 (−1486 T/C, rs187084)-but not TLR9 (2848G/A, rs352140)-may be a threat factor for cervical cancer [119]. Furthermore, Chinese Han patients with TLR9 rs352140-GA + AA genotype and infected with HPV have the highest cervical cancer risk, compared to no HPV-infected patients holding the rs352140-GG genotype. Furthermore, the minor alleles of TLR2-rs3775290, TLR4-rs7873784, and TLR9-rs352140, and interaction with HPV infection were linked to a high cervical cancer risk in Chinese Han populations [120]. In the Indian population, TLR4 haplotype ACAC is highly linked to multiple HR-HPV infections. In otherwise, TLR9 SNPs rs187084, rs352140, and rs352139 were linked to diminished risk of high HPV16 viral load [121]. The study gene polymorphisms in cervical cancer susceptibility in North Indian pointed toward the implication of TLR 2 (−196 to −174 del) and TLR 4 (Thr399Ile) in cervical cancer susceptibility. The TLR gene polymorphisms, may be a good tool in setting out mechanisms related to innate immunity in cervical cancer susceptibility [122]. The later Indian study points toward considering TLR4 haplotype GCAG and TLR9 haplotype GATC as threat to hrHPV infection. Further evaluation of a larger sample size covering diverse ethnic populations globally is warranted [123].
5.2 Ovarian cancer
Ovarian cancer (OC) is the deadliest in gynecological cancer. TLR4 expression was linked to ovarian cancer progression, treatment resistance, and poor prognosis. Kelly et al. demonstrated that TLR4 is overexpressed in various ovarian epithelial tumors. The high TLR4 expression correlates with increased tumor progression and leads to chemo-resistance to Paclitaxel. Thus, blocking TLR4 is proposed to be beneficial to the OC patient if targeted specifically in cancer cells that overexpress the molecule [54].
In several types of OC cells, TLR4 inhibition in synergy with standard chemotherapy may be therapeutically beneficial to decrease drug resistance. In this regard, osteopontin (OPN), brought by LPS activation, leads to the proliferation and metastasis of ovarian cancer cells. These results pave the way to decorticate novel mechanistic pathways promoting cancer progression, invasion, and metastasis. Furthermore, TLR4 combined with OPN may be considered a promising target for ovarian cancer therapy [55]. In the same issue, the TLR4/MyD88/NF-κB axis is associated with the survival of patients with ovarian epithelial cancers (OECs). MyD88 was demonstrated to be an independent prognostic predictor in patients with OECs. The TLR4/MyD88 axis may be a possible mechanism for poor prognosis in patients with clear cell types of OEC in association with drug resistance [56]. Moreover, overexpression of TLR9 is correlated with tumor-grade severity and within poorly differentiated tumors. OC patients with metastatic disease had in their serum an over level of TLR9 ligand (hypo-methylated DNA). Furthermore, the TLR/PI3K signaling axis modulated the invasion and metastasis through the production of galectin 1, suggesting that inhibition of the p110, the PI3K isoform, is a promising therapeutic approach against metastatic ovarian cancer [57]. Protein aggregate magnesium-ammonium phospholinoleate-palmitoleate anhydride (P-MAPA) upregulated TLR2 and TLR4 signaling pathway. Thus, the combination of (P-MAPA) with IL-12 improves the antitumor immunoresponse, paving the way to a novel therapeutic approach for fighting OC [58]. Another way, the association with (P-MAPA) and cisplatin (CIS) is thought to be a promising target therapy against OC cells through TLR4 signaling pathways activation [59]. Stimulation of the TLR8 abolishes glucose metabolism in CD4+ Tregs through mTOR signaling reduction, thus reverting the immunosuppressive function of the previous cells in an OC cell growth microenvironment [60].
5.3 Endometrial cancer
Uterine cancer is the second most common gynecologic cancer worldwide (globocan, 2020). Endometrial cancer (EC), originating in the epithelium is about 90% of uterine cancers. TLR3 and TLR4 protein expression level was studied during the menstrual cycle and in postmenopausal endometrium taking into account different grades of cancer from grade 1 to grade 3 (peritoneal endometriosis, hyperplasia, and endometrial adenocarcinoma specimens). The lowest TLR expression levels were shown in poorly differentiated carcinoma (grade 3). These findings propose the implication of TLR3 and TLR4 in endometrial diseases as shown by changed expression levels in endometriosis and endometrial cancer [61]. EC and endometrial hyperplasia cells overexpress TLR2 and TLR6. In addition, the expression of TLR6 marked an advanced stage of EC [62]. Wojcik-Krowiranda et al. study demonstrated a significant correlation between the expression of TLR1, TLR2, TLR3, and TLR4 and VEGFR1, VEGFR2, VEGF-A, and HIF1α on human neoplastic endometrial samples at the clinical stage and pathological grading of EC [63]. Ashton et al. reported that different alleles of TLR9 polymorphisms (The rs5743836 and rs187084 alleles of TLR9 polymorphisms in Caucasian women) were protective against EC [124]. In contrast to previous thoughts, the overexpression of TLR showed that the use of TLR agonists could be harmful in some types and stages of cancers. Indeed, TLR agonists are not only not useful in the late stages of the tumor but also may cause tumor progression. Considering this hypothesis, scientists lead various types of research using natural or synthetic inhibitors of TLRs. In the case of EC, the results showed that the use of TLR inhibitors, such as TLR4 inhibitors, could inhibit chronic inflammation. However, clinical trial research should be conducted to prove the usefulness of this therapeutic approach in comparison to other approaches in EC [125].
5.4 Melanoma
Cutaneous melanoma is one of the foremost aggressive tumors and a life-threatening skin cancer. Within the setting of rising incidence and mortality, there is a necessity to discover new prognostic markers and early diagnosis is the big challenge to improve its prognosis. When searching their transcriptional and protein expression level, TLR2, 3, 4, 7, and 9 exhibit a high amount of expression in tumor tissue and melanoma cell lines. The results are more disparate at the protein level. TLR3 and 8
More recently, vaccination with MelQbG10, an innovative vaccine that integrates three components essential for successful immunotherapy consisting of virus-like nanoparticle (VLP), A-type CpG-ODN, and a Melan-A/MART-1 peptide, was tolerated and used safely in melanoma patients by the induction of tumor-specific CD8+ T cells after MelQbG10 vaccination [128]. To further improve immune responses and increase MelQbG10 immunogenicity, additional immune stimulatory agents, such as imiquimod, a TLR7 agonist lead to enhance Melan-A-specific T-cell frequencies. Furthermore, MelQbG10 can be considered safe and well tolerated when given in combination with imiquimod [129].
Immunization against a genetically engineered tumor-specific antigen, ovalbumin, when adjuvanted with Diprovocim, which targets the innate immune receptor TLR1/TLR2 in mice and humans, inhibited the growth of B16 melanoma and prolonged survival in the presence of immune checkpoint blockade by anti–PD-L1; all mice responded to treatment. These data propose Diprovocim improving the benefit of anti–PD-L1 therapy by raising the number and activation of tumor-specific CTLs able to reply to this checkpoint inhibitor [65].
Activation of PPARγ by its agonist, pioglitazone, reduces tumor volume through LPS/TLR4/MyD-88/Nf-kb1/TNF-α axis in melanoma tumor. Moreover, treatment of melanoma cells with pioglitazone has a beneficial protective effect against melanoma through LPS-TLR4-dependent signaling pathways inhibition [66]. TLR3 polymorphism L412F was linked to an elevated mitotic index. Whereas TLR4 D299G and T399I polymorphisms were associated with melanoma severity, nodal metastases, and advanced stage III and could be then potential markers targeting the survival and prognostic of melanoma patients. TLR4 T399I polymorphism was highly correlated with worse survival [130]. However, no association between TLR7 single nucleotide G pp. ln11Leu polymorphism and susceptibility to develop melanoma was found. Further, other studies on TLR polymorphisms and their susceptibility to malignant melanoma are needed [131].
5.5 Hepatocellular carcinoma
In 2020, approximately, 830,200 people died from liver cancer globally. Despite some progress, hepatocellular carcinoma (HCC) remains a major cause of death often detected at inoperable stage. In 80%, HCC occurs often in the setting of chronic liver disease and cirrhosis [132]. New biomarkers to identify patients who could benefit from more aggressive treatment are needed. HCC cells exhibit cytoplasmic and nuclear TLR2 expression which are associated with proliferative index, Caspase-3 expression, and vascularization. Furthermore, HCC shows notably nuclear TLR2 expression; however, hepatitis and cirrhosis cell patients demonstrate a predominant cytoplasmic expression. At a functional level, upon TLR2 activation by its agonist, HUH7, HCC expressed cellular proliferation and vascularization markers CD34 and VEGF. These results propose a plausible role for TLR-2 in HCC pathogenesis [67]. The interaction of mtDNA-HMGB1 and TLR9 might contribute to tumor growth signaling pathways in response to hypoxia
Jing et al. detected positive TLR4 expression in 86% of cases of HCC patients. Other studies confirmed that LPS/TLR4/NF-κB signaling is involved in the invasion and metastasis of HCC patients [70]. In the same context, another study demonstrated that HCC patients whose tumors expressed high levels of both TLR4 and TLR9 had a poor prognosis. TLR4–MyD88 signaling pathway appears to be primordial for hepatocarcinogenesis. LPS-induced TLR4 signaling also promotes cancer cell survival and proliferation in HCC [71].
Activation of TLR4 could enhance the proliferation of HCC cells. Furthermore, TLR4/COX-2/PGE2/STAT3 axis is activated in HCC cells. Abolition of TLR4 or COX-2/PGE2/STAT3 pathway reduces LPS-induced inflammation and proliferation of HCC cells and improves the perceptivity of HCC cells to chemotherapeutics in vitro. These findings shed light on the precise molecular mechanism involved in the TLR4 signaling pathway leading to HCC progression and then pointing toward TLR4 as a promising target for HCC treatment [72].
To further investigate the expression and function of TLR2 in hepatocarcinoma proliferation recombinant plasmids expressing one of three forms of TLR2 siRNA (sh-TLR2 RNAi (A, B, and C) were transfected into BLE-7402. sh-TLR2 RNAi (B) had the most important knockdown effect. TLR2 abolition with sh-TLR2 RNAi (B) reduced cell proliferation and secretion of IL-6 and IL-8. Also, mice treated with sh-TLR2 RNAi (B) demonstrate a severe reduction in tumor volume, suggesting that TLR2 knockdown inhibits proliferation of cultured hepatocarcinoma cells and decreases the secretion of cytokines. Thus, TLR2 silencing could be proposed for siRNA-based gene therapy to treat of hepatocarcinoma patients [133]. Abolition of TLR2 by siRNA exhibited a decrease in proliferation, invasion, migration, and NF-κB/P65 expression but an increase in apoptotic ratio. In other way, rHMGB1 led to proliferation, invasion, and migration, enhanced NF-κB/P65 expression, and abolished cells apoptosis. Moreover, TLR2 reduced the role of rHMGB1. This finding proposes TLR2 and HMGB1 potential therapeutic targets against HCC [73].
Overexpression of miR-122 significantly diminished TLR4 expression in hepatoma cells. However, knocking down miR-122 overexpressed TLR4. It was found a putative miR-122 target in TLR4 3’UTR. Over-expression/down-expression of miR-122 could impact the proliferation and the expression of natural immune factors [134]. NADPH oxidase 4 (Nox4) enhanced LPS/TLR4/NF-ĸB/AP-1 signaling pathways in hepatocytes. Moreover, the effect of Nox4 abolition was time-dependent as proved in the mice model. Therefore, these data suggest LPS/TLR4/Nox4 axis is a tumor-promoting pathway as demonstrated both in human hepatoma cells and murine hepatocytes [135].
Genetic polymorphisms of TLRs are suggested to influence susceptibility to HCV infection and progression to end-stage liver disease. One haplotype (GCCCTTAG) of TLR4 was associated significantly with a decrease in the occurrence of hepatocellular carcinoma [136]. In another study, a strong association between allele C of rs3804099 of TLR2 and C allele of rs10116253 TLR4 and the risk of HCC was observed. Furthermore, A powerful link between allele T of rs1816702 of TLR2 and allele A of rs5030728 of TLR4 and the non-responder group was found. Haplotypes CAGT of TLR4 and ATAC of TLR2 showed significant association with CH and HCC groups in comparison to haplotype TGAC for TLR4 and haplotype GCGT for TLR2 [137].
5.6 Lung cancer
Lung cancer is a leading cause of cancer-related deaths with 18% of several deaths and 11.4% of new cases diagnosed in 2020 (Globocan, 2020). A better understanding of innate immunity and its related molecular mechanism in the lung could lead to the improvement and development of novel immunotherapy. TLR2 uses TLR1 and TLR6 [138]. TLR2 has been proposed to be a good target for lung cancer as the complex TLR1/2 promotes inhibition of tumor growth, decreases monocytic MDSC, and shifts macrophage toward M1 profile through JNK pathway [74]. However, the TLR2: TLR6 complex has been associated with promoting lung cancer metastasis [75]. TLR3 expression in lung cancer is a double-edged sword. Indeed, TLR3 activated by exosomal dsRN leads to chemokine liberation-inducing metastasis
5.7 Breast cancer
Breast cancer remains a major cause of death in women in the developed world. Further to their expression on immune cells, TLRs are also expressed in tumor cells. Recent evidences hold up the connection between breast cancer and inflammation. TLR4 promotes breast cancer progression and metastasis.
TLR2 and TLR9 activation in MDA-MB-231 cell lines promotes proinflammatory cytokines secretion leading to invasiveness and then metastasis. A high level of TLR9 was strongly associated with triple-negative subtypes. However, stimulation of TLR7 in combination with radiotherapy inhibits tumor growth, enhances T-cell memory, and is correlated with significant regression of spontaneous breast cancer in mice, suggesting a better prognosis [88]. TLR2 enhances breast cancer survival. Thus, preclinical
5.8 Colorectal cancer
Colorectal carcinoma (CC) is the third in cancer prevalence in both men and women and is a major leading cause of cancer morbidity and mortality worldwide. CC is among solid cancers in which inflammation is crucial in mechanistic oncogenesis. TLR4 is overexpressed in inflammatory-related colorectal cancer both in human and
5.9 Glioma
Gliomas are the deadliest type of brain tumor with a median survival time of 15 months. The current treatments are complex, offensive, and far from satisfactory. Thus, the urgent need for alternative efficient therapy. Chronic inflammation is largely implicated in glioma carcinogenesis and progression. TLR2 was found to be overexpressed in Glioma-associated microglia (GAM). This expression leads to tumor immune evasion through abolishing MCHII transcription via activation of MAPK/ERK1/2 signaling pathway thus altering the efficiency of T-cell–dependent tumor elimination in
6. Conclusion
TLRs are involved in several carcinogenic processes to promote cancer progression in some kinds of tumors and some individuals by inducing cell proliferation, invasion, and metastasis. The mechanisms behind this implicate many hallmarks in the setting of tumor cells such as inhibition of apoptosis, and stimulation of angiogenesis. On the other side, TLRs are expressed mainly on macrophages and dendritic cells (DCs). Stimulation of TLRs fundamentally starts not only innate immune response but can also induce adaptive immune response boosting the immune system to fight cancer. In this respect, TLR ligands are used as immunostimulatory molecules as anticancer treatment in numerous preclinical and clinical studies. From both points of view, one should be aware when choosing for adjuvants to treat cancer patients. In related TLR clinical trials, the dual role of TLR should be considered and further mechanistic investigations on the dual roles of TLR in tumor biology are needed [140, 141, 142]. Thus, conducting a deeper understanding of each TLR on each cancer is of crucial need. It is worth knowing whether to design a TLR inducer or inhibitor when considering TLR-targeted therapeutic. Furthermore, with the era of novel pharmacology and personalized medicine, the use of nanotechnology in combination with conventional chemotherapy and radiotherapy is of great interest as it impacts efficiently cancer treatment trials.
Acknowledgments
We thank all the members of the human and experimental pathology department at the Institut Pasteur de Tunis. This work was supported by the Tunisian Ministry of Public Health and the Tunisian Ministry of Higher Education and Scientific Research (LR16IPT04 and CIC2016IPT02).
References
- 1.
Janeway CA Jr, Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2002; 20 :197-216. DOI: 10.1146/annurev.immunol.20.083001.084359 - 2.
Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nature Reviews. Drug Discovery. 2015; 14 (12):857-877. DOI: 10.1038/nrd4657. Epub 2015 Oct 23 - 3.
Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: Key components of the innate immune system. Critical Reviews in Biotechnology. 2012; 32 (2):143-171. DOI: 10.3109/07388551.2011.594423. Epub 2011 Nov 11 - 4.
Wiesner J, Vilcinskas A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence. 2010; 1 (5):440-464. DOI: 10.4161/viru.1.5.12983 - 5.
Wagner E, Frank M. Therapeutic potential of complement modulation. Nature Reviews. Drug Discovery. 2010; 9 :43-56. DOI: 10.1038/nrd3011 - 6.
Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. International Immunology. 2009; 21 (4):317-337. DOI: 10.1093/intimm/dxp017. Epub 2009 Feb 26 - 7.
Kumar V. The trinity of cGAS, TLR9, and ALRs guardians of the cellular galaxy against host-derived self-DNA. Frontiers in Immunology. 2021; 11 :624597. DOI: 10.3389/fimmu.2020.624597 - 8.
Land WG. Prologue: About DAMPs, PAMPs, and MAMPs. In: Damage-Associated Molecular Patterns in Human Diseases. Cham: Springer; 2018. DOI: 10.1007/978-3-319-78655-1_11 - 9.
Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harbor Perspectives in Biology. 2012; 4 (3):a006049. DOI: 10.1101/ - 10.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124 (4):783-801. DOI: 10.1016/j.cell.2006.02.015 - 11.
Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011; 144 (5):646-674. DOI: 10.1016/j.cell.2011.02.013 - 12.
Trinchieri G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annual Review of Immunology. 2012; 30 :677-706. DOI: 10.1146/annurev-immunol-020711-075008 - 13.
Balkwill F, Coussens LM. Cancer: An inflammatory link. Nature. 2004; 431 (7007):405-406. DOI: 10.1038/431405a - 14.
Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004; 431 (7007):461-466. DOI: 10.1038/nature02924 - 15.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420 (6917):860-867. DOI: 10.1038/nature01322 - 16.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006; 441 (7092):431-436. DOI: 10.1038/nature04870 - 17.
Asami J, Shimizu T. Structural and functional understanding of the toll-like receptors. Protein Science. 2021; 30 (4):761-772. DOI: 10.1002/pro.4043 - 18.
Botos I, Segal DM, Davies DR. The structural biology of toll-like receptors. Structure. 2011; 19 (4):447-459. DOI: 10.1016/j.str.2011.02.004 - 19.
He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, et al. Structure of the Nogo receptor ectodomain: A recognition module implicated in myelin inhibition. Neuron. 2003; 38 :177-185 - 20.
O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in toll-like receptor signalling. Nature Reviews. Immunology. 2007; 7 (5):353-364. DOI: 10.1038/nri2079 - 21.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nature Immunology. 2010; 11 (5):373-384. DOI: 10.1038/ni.1863. Epub 2010 Apr 20 - 22.
Fleer A, Krediet TG. Innate immunity: Toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn. Neonatology. 2007; 92 (3):145-157. DOI: 10.1159/000102054. Epub 2007 Apr 27 - 23.
Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010; 32 (3):305-315. DOI: 10.1016/j.immuni.2010.03.012 - 24.
Tartey S, Takeuchi O. Pathogen recognition and toll-like receptor targeted therapeutics in innate immune cells. International Reviews of Immunology. 2017; 36 (2):57-73. DOI: 10.1080/08830185.2016.1261318. Epub 2017 Jan 6 - 25.
De Leo MG, Staiano L, Vicinanza M, Luciani A, Carissimo A, Mutarelli M, et al. Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nature Cell Biology. 2016; 18 (8):839-850. DOI: 10.1038/ncb3386. Epub 2016 Jul 11 - 26.
Henrick BM, Yao XD, Zahoor MA, Abimiku A, Osawe S, Rosenthal KL. TLR10 senses HIV-1 proteins and significantly enhances HIV-1 infection. Frontiers in Immunology. 2019; 10 :482. DOI: 10.3389/fimmu.2019.00482 - 27.
Fore F, Indriputri C, Mamutse J, Nugraha J. TLR10 and its unique anti-inflammatory properties and potential use as a target in therapeutics. Immune Network. 2020; 20 (3):e21. DOI: 10.4110/in.2020.20.e21 - 28.
Kumar V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. International Immunopharmacology. 2020; 89 (Pt B):107087. DOI: 10.1016/j.intimp.2020.107087. Epub 2020 Oct 12 - 29.
Takeda K, Akira S. TLR signaling pathways. Seminars in Immunology. 2004; 16 (1):3-9. DOI: 10.1016/j.smim.2003.10.003 - 30.
Kawai T, Akira S. TLR signaling. Seminars in Immunology. 2007; 19 (1):24-32. DOI: 10.1016/j.smim.2006.12.004. Epub 2007 Feb 1 - 31.
O’Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors - redefining innate immunity. Nature Reviews. Immunology. 2013; 13 (6):453-460. DOI: 10.1038/nri3446. Epub 2013 May 17 - 32.
Hamonic G, Pasternak JA, Wilson HL. Recognizing conserved non-canonical localization patterns of toll-like receptors in tissues and across species. Cell and Tissue Research. 2018; 372 (1):1-11. DOI: 10.1007/s00441-017-2767-9 - 33.
Kawai T, Akira S. TLR signaling. Cell Death and Differentiation. 2006; 13 (5):816-825. DOI: 10.1038/sj.cdd.4401850 - 34.
Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. The Journal of Clinical Investigation. 2006; 116 (2):485-494. DOI: 10.1172/JCI25439 - 35.
Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, et al. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. Journal of Immunology. 2005; 174 (12):7558-7563. DOI: 10.4049/jimmunol.174.12.7558 - 36.
Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. Journal of Immunology. 2005; 174 (5):2957-2963. DOI: 10.4049/jimmunol.174.5.2957 - 37.
Bellocchio S, Moretti S, Perruccio K, Fallarino F, Bozza S, Montagnoli C, et al. TLRs govern neutrophil activity in aspergillosis. Journal of Immunology. 2004; 173 (12):7406-7415. DOI: 10.4049/jimmunol.173.12.7406 - 38.
Nishiya T, Kajita E, Miwa S, Defranco AL. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. The Journal of Biological Chemistry. 2005; 280 (44):37107-37117. DOI: 10.1074/jbc.M504951200 - 39.
Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nature Immunology. 2003; 4 (5):491-496. DOI: 10.1038/ni921 - 40.
Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. Journal of Immunology. 2005; 175 (12):8051-8059. DOI: 10.4049/jimmunol.175.12.8051 - 41.
Wang RF. Regulatory T cells and toll-like receptors in cancer therapy. Cancer Research. 2006; 66 (10):4987-4990. DOI: 10.1158/0008-5472.CAN-05-4676 - 42.
Lee J, Wu CC, Lee KJ, Chuang TH, Katakura K, Liu YT, et al. Activation of anti-hepatitis C virus responses via toll-like receptor 7. Proceedings of the National Academy of Sciences of the United States of America. 2006; 103 (6):1828-1833. DOI: 10.1073/pnas.0510801103 - 43.
Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005; 22 (4):467-477. DOI: 10.1016/j.immuni.2005.02.008 - 44.
Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005; 309 (5739):1380-1384. DOI: 10.1126/science.1113401 - 45.
DeCarlo CA, Rosa B, Jackson R, Niccoli S, Escott NG, Zehbe I. Toll-like receptor transcriptome in the HPV-positive cervical cancer microenvironment. Clinical & Developmental Immunology. 2012; 2012 :785825. DOI: 10.1155/2012/785825. Epub 2011 Oct 13 - 46.
Cheng YX, Qi XY, Huang JL, Hu M, Zhou LM, Li BS, et al. Toll-like receptor 4 signaling promotes the immunosuppressive cytokine production of human cervical cancer. European Journal of Gynaecological Oncology. 2012; 33 (3):291-294 - 47.
Cannella F, Pierangeli A, Scagnolari C, Cacciotti G, Tranquilli G, Stentella P, et al. TLR9 is expressed in human papillomavirus-positive cervical cells and is overexpressed in persistent infections. Immunobiology. 2015; 220 (3):363-368. DOI: 10.1016/j.imbio.2014.10.012. Epub 2014 Oct 23 - 48.
Fehri E, Ennaifer E, Ardhaoui M, Ouerhani K, Laassili T, Bel Haj Rhouma R, et al. Expression of toll-like receptor 9 increases with progression of cervical neoplasia in Tunisian women--a comparative analysis of condyloma, cervical intraepithelial neoplasia and invasive carcinoma. Asian Pacific Journal of Cancer Prevention. 2014; 15 (15):6145-6150. DOI: 10.7314/apjcp.2014.15.15.6145 - 49.
Zhang Y, Yang H, Barnie PA, Yang P, Su Z, Chen J, et al. The expression of toll-like receptor 8 and its relationship with VEGF and Bcl-2 in cervical cancer. International Journal of Medical Sciences. 2014; 11 (6):608-613. DOI: 10.7150/ijms.8428 - 50.
de Matos LG, Cândido EB, Vidigal PV, Bordoni PH, Lamaita RM, Carneiro MM, et al. Association between toll-like receptor and tumor necrosis factor immunological pathways in uterine cervical neoplasms. Tumori. 2017; 103 (1):81-86. DOI: 10.5301/tj.5000576. Epub 2016 Nov 15 - 51.
Scott ME, Ma Y, Farhat S, Moscicki AB. Expression of nucleic acid-sensing toll-like receptors predicts HPV16 clearance associated with an E6-directed cell-mediated response. International Journal of Cancer. 2015; 136 (10):2402-2408. DOI: 10.1002/ijc.29283. Epub 2014 Oct 30 - 52.
Halec G, Scott ME, Farhat S, Darragh TM, Moscicki AB. Toll-like receptors: Important immune checkpoints in the regression of cervical intra-epithelial neoplasia 2. International Journal of Cancer. 2018; 143 (11):2884-2891. DOI: 10.1002/ijc.31814. Epub 2018 Oct 9 - 53.
Aggarwal R, Misra S, Guleria C, Suri V, Mangat N, Sharma M, et al. Characterization of toll-like receptor transcriptome in squamous cell carcinoma of cervix: A case-control study. Gynecologic Oncology. 2015; 138 (2):358-362. DOI: 10.1016/j.ygyno.2015.05.029. Epub 2015 May 27 - 54.
Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Research. 2006; 66 (7):3859-3868. DOI: 10.1158/0008-5472.CAN-05-3948 - 55.
Xu C, Li H, Yin M, Yang T, An L, Yang G. Osteopontin is involved in TLR4 pathway contributing to ovarian cancer cell proliferation and metastasis. Oncotarget. 2017; 8 (58):98394-98404. DOI: 10.18632/oncotarget.21844 - 56.
Kim KH, Jo MS, Suh DS, Yoon MS, Shin DH, Lee JH, et al. Expression and significance of the TLR4/MyD88 signaling pathway in ovarian epithelial cancers. World Journal of Surgical Oncology. 2012; 10 :193. DOI: 10.1186/1477-7819-10-193 - 57.
Park GB, Chung YH, Kim D. Induction of galectin-1 by TLR-dependent PI3K activation enhances epithelial-mesenchymal transition of metastatic ovarian cancer cells. Oncology Reports. 2017; 37 (5):3137-3145. DOI: 10.3892/or.2017.5533 - 58.
Silveira HS, Lupi LA, Romagnoli GG, Kaneno R, da Silva NI, Fávaro WJ, et al. P-MAPA activates TLR2 and TLR4 signaling while its combination with IL-12 stimulates CD4+ and CD8+ effector T cells in ovarian cancer. Life Sciences. 2020; 254 :117786. DOI: 10.1016/j.lfs.2020.117786 - 59.
de Almeida Chuffa LG, de Moura FG, Lupi LA, da Silva NI, Fávaro WJ. P-MAPA immunotherapy potentiates the effect of cisplatin on serous ovarian carcinoma through targeting TLR4 signaling. Journal of Ovarian Research. 2018; 11 (1):8. DOI: 10.1186/s13048-018-0380-5 - 60.
Wu M, Fu X, Xu R, Liu S, Li R, Xu J, et al. Glucose metabolism and function of CD4+ Tregs are regulated by the TLR8/mTOR signal in an environment of SKOV3 cell growth. Cancer Medicine. 2023; 12 (15):16310-16322. DOI: 10.1002/cam4.6247 - 61.
Allhorn S, Böing C, Koch AA, Kimmig R, Gashaw I. TLR3 and TLR4 expression in healthy and diseased human endometrium. Reproductive Biology and Endocrinology. 2008; 6 :40. DOI: 10.1186/1477-7827-6-40 - 62.
Gençoğlu Bakbak BB, Ilhan TT, Pekin A, Kerimoğlu ÖS, Yılmaz SA, Kebapçılar A, et al. Evaluation of toll-like receptor expression with clinicopathologic variables in endometrium cancer. Sisli Etfal Hastan Tip Bulletin. 2018; 52 (3):196-200 - 63.
Wojcik-Krowiranda KM, Forma E, Bienkiewicz A, Cwonda L, Wronska-Stefaniak J, Brys M. TLR family gene expression in relation to the HIF1α and the VEGFR pathway activation in endometrial cancer. Ginekologia Polska. 2020; 91 (8):439-446 - 64.
Tambunlertchai S, Geary SM, Naguib YW, Salem AK. Anti-melanoma effects of resiquimod (RSQ) in vitro and in combination with immune checkpoint blockadein vivo . The AAPS Journal. 2023;25 (4):57. DOI: 10.1208/s12248-023-00824-3 - 65.
Wang Y, Su L, Morin MD, Jones BT, Mifune Y, Shi H, et al. Adjuvant effect of the novel TLR1/TLR2 agonist diprovocim synergizes with anti-PD-L1 to eliminate melanoma in mice. Proceedings of the National Academy of Sciences of the United States of America. 2018; 115 (37):E8698-E8706. DOI: 10.1073/pnas.1809232115 - 66.
Dana N, Vaseghi G, Haghjooy JS. PPAR γ agonist, pioglitazone, suppresses melanoma cancer in mice by inhibiting TLR4 signaling. Journal of Pharmaceutical Sciences. 2019; 22 (1):418-423. DOI: 10.18433/jpps30626 - 67.
Mohamed FEA, Hammad S, Luong TV, Dewidar B, Al-Jehani R, Davies N, et al. Expression of TLR-2 in hepatocellular carcinoma is associated with tumour proliferation, angiogenesis and Caspase-3 expression. Pathology, Research and Practice. 2020; 216 (8):152980. DOI: 10.1016/j.prp.2020.152980 - 68.
Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian D, et al. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through toll-like receptor 9. Journal of Hepatology. 2015; 63 (1):114-121. DOI: 10.1016/j.jhep.2015.02.009 - 69.
Kairaluoma V, Kemi N, Huhta H, Pohjanen VM, Helminen O. Toll-like receptor 5 and 8 in hepatocellular carcinoma. APMIS. 2021; 129 (8):470-479. DOI: 10.1111/apm.13142 - 70.
Jing YY, Han ZP, Sun K, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Medicine. 2012; 10 :98 - 71.
Wang L, Zhu R, Huang Z, Li H, Zhu H. Lipopolysaccharide induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Digestive Diseases and Sciences. 2013; 58 (8):2223-2236 - 72.
Lin A, Wang G, Zhao H, Zhang Y, Han Q , Zhang C, et al. TLR4 signaling promotes a COX-2/PGE2/STAT3 positive feedback loop in hepatocellular carcinoma (HCC) cells. Oncoimmunology. 2015; 5 (2):e1074376. DOI: 10.1080/2162402X.2015.1074376 - 73.
Shi W, Su L, Li Q , Sun L, Lv J, Li J, et al. Suppression of toll-like receptor 2 expression inhibits the bioactivity of human hepatocellular carcinoma. Tumour Biology. 2014; 35 (10):9627-9637. DOI: 10.1007/s13277-014-2268-3 - 74.
Deng Y, Yang J, Qian J, Liu R, Huang E, Wang Y, et al. TLR1/TLR2 signaling blocks the suppression of monocytic myeloid-derived suppressor cell by promoting its differentiation into M1-type macrophage. Molecular Immunology. 2019; 112 :266-273. DOI: 10.1016/j.molimm.2019.06.006. Epub 2019 Jun 15 - 75.
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009; 457 (7225):102-106. DOI: 10.1038/nature07623 - 76.
Bianchi F, Alexiadis S, Camisaschi C, Truini M, Centonze G, Milione M, et al. TLR3 expression induces apoptosis in human non-small-cell lung cancer. International Journal of Molecular Sciences. 2020; 21 (4):1-14. DOI: 10.3390/ijms2104144 - 77.
He W, Liu Q , Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Molecular Immunology. 2007; 44 (11):2850-2859. DOI: 10.1016/j.molimm.2007.01.022 - 78.
Tavora B, Mederer T, Wessel KJ, Ruffing S, Sadjadi M, Missmahl M, et al. Tumoural activation of TLR3-SLIT2 axis in endothelium drives metastasis. Nature. 2020; 586 (7828):299-304. DOI: 10.1038/s41586-020-2774 - 79.
Berthon C, Driss V, Liu J, Kuranda K, Leleu X, Jouy N, et al. In acute myeloid leukemia, B7-H1 (PD-L1) protection of blasts from cytotoxic T cells is induced by TLR ligands and interferon-gamma and can be reversed using MEK inhibitors. Cancer Immunology, Immunotherapy. 2010; 59 (12):1839-1849. DOI: 10.1007/s00262-010-0909-y - 80.
Chen R, Huang M, Yang X, Chen XH, Shi MY, Li ZF, et al. CALR-TLR4 complex inhibits non-small cell lung cancer progression by regulating the migration and maturation of dendritic cells. Frontiers in Oncology. 2021; 11 :743050. DOI: 10.3389/fonc.2021.743050 - 81.
Liotti F, Marotta M, Sorriento D, Pone E, Morra F, Melillo RM, et al. Toll like receptor 7 mediates inflammation resolution and inhibition of angiogenesis in non-small cell lung cancer. Cancers (Basel). 2021; 13 (4):1-18. DOI: 10.3390/cancers13040740 - 82.
Zhou H, Chen JH, Hu J, Luo YZ, Li F, Xiao L, et al. High expression of toll like receptor 5 correlates with better prognosis in non-small-cell lung cancer: An anti-tumor effect of TLR5 signaling in non-small cell lung cancer. Journal of Cancer Research and Clinical Oncology. 2014; 140 (4):633-643. DOI: 10.1007/s00432-014-1616-4 - 83.
Koh J, Kim S, Lee SN, Kim SY, Kim JE, Lee KY, et al. Therapeutic efficacy of cancer vaccine adjuvanted with nanoemulsion loaded with TLR7/8 agonist in lung cancer model. Nanomedicine. 2021; 37 :102415. DOI: 10.1016/j.nano.2021.102415 - 84.
Ren T, Xu L, Jiao S, Wang Y, Cai Y, Liang Y, et al. TLR9 signaling promotes tumor progression of human lung cancer cell in vivo. Pathology Oncology Research. 2009; 15 (4):623-630. DOI: 10.1007/s12253-009-9162-0 - 85.
Negrao MV, Papadimitrakopoulou VA, Price AC, Tam AL, Furqan M, Laroia ST, et al. Vidutolimod in combination with Atezolizumab with and without radiation therapy in patients with programmed cell death protein 1 or programmed death-ligand 1 blockade-resistant advanced NSCLC. JTO Clinical and Research Reports. 2022; 4 (3):100423. DOI: 10.1016/j.jtocrr.2022.100423 - 86.
Yang H, Zhou H, Feng P, Zhou X, Wen H, Xie X, et al. Reduced expression of toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. Journal of Experimental & Clinical Cancer Research. 2010; 29 :92. DOI: 10.1186/1756-9966-29-92 - 87.
Di Lorenzo A, Bolli E, Ruiu R, Ferrauto G, Di Gregorio E, Avalle L, et al. Toll-like receptor 2 promotes breast cancer progression and resistance to chemotherapy. Oncoimmunology. 2022; 11 (1):2086752. DOI: 10.1080/2162402X.2022.2086752 - 88.
Dewan MZ, Vanpouille-Box C, Kawashima N, DiNapoli S, Babb JS, Formenti SC, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clinical Cancer Research. 2012; 18 (24):6668-6678. DOI: 10.1158/1078-0432.CCR-12-0984. Epub 2012 Oct 9 - 89.
Bernardo AR, Cosgaya JM, Aranda A, Jiménez-Lara AM. Synergy between RA and TLR3 promotes type I IFN-dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells. Cell Death & Disease. 2013; 4 (1):e479. DOI: 10.1038/cddis.2013.5 - 90.
Ye K, Wu Y, Sun Y, Lin J, Xu J. TLR4 siRNA inhibits proliferation and invasion in colorectal cancer cells by downregulating ACAT1 expression. Life Sciences. 2016; 155 :133-139. DOI: 10.1016/j.lfs.2016.05.012. Epub 2016 May 10 - 91.
Chen M, Zhong K, Tan J, Meng M, Liu CM, Chen B, et al. Baicalein is a novel TLR4-targeting therapeutics agent that inhibits TLR4/HIF-1α/VEGF signaling pathway in colorectal cancer. Clinical and Translational Medicine. 2021; 11 (11):e564. DOI: 10.1002/ctm2.564 - 92.
Teng Z, Sun X, Guo Y, Zhang M, Liu Y, Xu M. Curcumae longae Rhizoma (Jianghuang) extract reverses the 5-Fluoruracil resistance in colorectal cancer cells via TLR4/PI3K/Akt/mTOR pathway. Clinics and Research in Hepatology and Gastroenterology. 2022; 46 (9):101976. DOI: 10.1016/j.clinre.2022.101976. Epub 2022 Jun 13 - 93.
Damiano V, Rosa R, Formisano L, Nappi L, Gelardi T, Marciano R, et al. Toll-like receptor 9 agonist IMO cooperates with everolimus in renal cell carcinoma by interfering with tumour growth and angiogenesis. British Journal of Cancer. 2013; 108 (8):1616-1623. DOI: 10.1038/bjc.2013.153. Epub 2013 Apr 9 - 94.
Weihrauch MR, Richly H, von Bergwelt-Baildon MS, Becker HJ, Schmidt M, Hacker UT, et al. Phase I clinical study of the toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours. European Journal of Cancer. 2015; 51 (2):146-156. DOI: 10.1016/j.ejca.2014.11.002. Epub 2014 Dec 2 - 95.
Niu Z, Tang W, Liu T, Xu P, Zhu D, Ji M, et al. Cell-free DNA derived from cancer cells facilitates tumor malignancy through toll-like receptor 9 signaling-triggered interleukin-8 secretion in colorectal cancer. Acta Biochimica et Biophysica Sinica (Shanghai). 2018; 50 (10):1007-1017. DOI: 10.1093/abbs/gmy104 - 96.
Rath T, Stöckle J, Roderfeld M, Tschuschner A, Graf J, Roeb E. Matrix metalloproteinase-13 is regulated by toll-like receptor-9 in colorectal cancer cells and mediates cellular migration. Oncology Letters. 2011; 2 (3):483-488. DOI: 10.3892/ol.2011.276. Epub 2011 Mar 21 - 97.
Luo Q , Zeng L, Tang C, Zhang Z, Chen Y, Zeng C. TLR9 induces colitis-associated colorectal carcinogenesis by regulating NF-κB expression levels. Oncology Letters. 2020; 20 (4):110. DOI: 10.3892/ol.2020.11971. Epub 2020 Aug 12 - 98.
Lowe EL, Crother TR, Rabizadeh S, Hu B, Wang H, Chen S, et al. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS One. 2010; 5 (9):e13027. DOI: 10.1371/journal.pone.0013027 - 99.
Liu YD, Ji CB, Li SB, Yan F, Gu QS, Balic JJ, et al. Toll-like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-κB signaling pathways. International Immunopharmacology. 2018; 59 :375-383. DOI: 10.1016/j.intimp.2018.04.033. Epub 2018 Apr 24 - 100.
Zhao J, Xue Y, Pan Y, Yao A, Wang G, Li D, et al. Toll-like receptor 3 agonist poly I:C reinforces the potency of cytotoxic chemotherapy via the TLR3-UNC93B1-IFN-β signaling axis in paclitaxel-resistant colon cancer. Journal of Cellular Physiology. 2019; 234 (5):7051-7061. DOI: 10.1002/jcp.27459. Epub 2018 Nov 1 - 101.
Beilmann-Lehtonen I, Hagström J, Mustonen H, Koskensalo S, Haglund C, Böckelman C. High tissue TLR5 expression predicts better outcomes in colorectal cancer patients. Oncology. 2021; 99 (9):589-600. DOI: 10.1159/000516543. Epub 2021 Jun 17 - 102.
Qian J, Luo F, Yang J, Liu J, Liu R, Wang L, et al. TLR2 promotes glioma immune evasion by downregulating MHC class II molecules in microglia. Cancer Immunology Research. 2018; 6 (10):1220-1233. DOI: 10.1158/2326-6066.CIR-18-0020. Epub 2018 Aug 21 - 103.
Li C, Ma L, Liu Y, Li Z, Wang Q , Chen Z, et al. TLR2 promotes development and progression of human glioma via enhancing autophagy. Gene. 2019; 700 :52-59. DOI: 10.1016/j.gene.2019.02.084. Epub 2019 Mar 19 - 104.
Wang F, Zhang P, Yang L, Yu X, Ye X, Yang J, et al. Activation of toll-like receptor 2 promotes invasion by upregulating MMPs in glioma stem cells. American Journal of Translational Research. 2015; 7 (3):607-615 - 105.
Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Medicine. 2009; 6 (1):e10. DOI: 10.1371/journal.pmed.1000010 - 106.
Liu Y, Ju Y, Liu J, Chen Y, Huo X, Liu L. Inhibition of proliferation and migration and induction of apoptosis in glioma cells by silencing TLR4 expression levels via RNA interference. Oncology Letters. 2021; 21 (1):13. DOI: 10.3892/ol.2020.12274. Epub 2020 Nov 6 - 107.
Jiang Y, Zhou J, Luo P, Gao H, Ma Y, Chen YS, et al. Prosaposin promotes the proliferation and tumorigenesis of glioma through toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway. eBioMedicine. 2018; 37 :78-90. DOI: 10.1016/j.ebiom.2018.10.053. Epub 2018 Oct 29 - 108.
da Cruz LLP, de Souza PO, Dal Prá M, Falchetti M, de Abreu AM, Azambuja JH, et al. TLR4 expression and functionality are downregulated in glioblastoma cells and in tumor-associated macrophages: A new mechanism of immune evasion? Biochimica et Biophysica Acta - Molecular Basis of Disease. 2021; 1867 (8):166155. DOI: 10.1016/j.bbadis.2021.166155. Epub 2021 Apr 28 - 109.
Stathopoulos A, Pretto C, Devillers L, Pierre D, Hofman FM, Kruse C, et al. Development of immune memory to glial brain tumors after tumor regression induced by immunotherapeutic toll-like receptor 7/8 activation. Oncoimmunology. 2012; 1 (3):298-305. DOI: 10.4161/onci.19068 - 110.
Sandholm J, Tuomela J, Kauppila JH, Harris KW, Graves D, Selander KS. Hypoxia regulates toll-like receptor-9 expression and invasive function in human brain cancer cells in vitro. Oncology Letters. 2014; 8 (1):266-274. DOI: 10.3892/ol.2014.2095. Epub 2014 Apr 25 - 111.
Liu D, Cao G, Cen Y, Liu T, Peng W, Sun J, et al. The radiosensitizing effect of CpG ODN107 on human glioma cells is tightly related to its antiangiogenic activity via suppression of HIF-1α/VEGF pathway. International Immunopharmacology. 2013; 17 (2):237-244. DOI: 10.1016/j.intimp.2013.06.002. Epub 2013 Jun 19 - 112.
El Andaloussi A, Sonabend AM, Han Y, Lesniak MS. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006; 54 (6):526-535. DOI: 10.1002/glia.20401 - 113.
Tiwari RK, Singh S, Gupta CL, Pandey P, Singh VK, Sayyed U, et al. Repolarization of glioblastoma macrophage cells using non-agonistic dectin-1 ligand encapsulating TLR-9 agonist: Plausible role in regenerative medicine against brain tumor. The International Journal of Neuroscience. 2021; 131 (6):591-598. DOI: 10.1080/00207454.2020.1750393. Epub 2020 Apr 19 - 114.
Yu L, Wang L, Li M, Zhong J, Wang Z, Chen S. Expression of toll-like receptor 4 is down-regulated during progression of cervical neoplasia. Cancer Immunology, Immunotherapy. 2010; 59 (7):1021-1028. DOI: 10.1007/s00262-010-0825-1. Epub 2010 Feb 23 - 115.
He A, Shao J, Zhang Y, Lu H, Wu Z, Xu Y. CD200Fc reduces LPS-induced IL-1β activation in human cervical cancer cells by modulating TLR4-NF-κB and NLRP3 inflammasome pathway. Oncotarget. 2017; 8 (20):33214-33224. DOI: 10.18632/oncotarget.16596 - 116.
Oliveira LB, Louvanto K, Ramanakumar AV, Franco EL, Villa LL. For the Ludwig-McGill cohort study. Polymorphism in the promoter region of the toll-like receptor 9 gene and cervical human papillomavirus infection. The Journal of General Virology. 2013; 94 (Pt 8):1858-1864. DOI: 10.1099/vir.0.052811-0. Epub 2013 May 15 - 117.
Pandey S, Mittal B, Srivastava M, Singh S, Srivastava K, Lal P, et al. Evaluation of toll-like receptors 3 (c.1377C/T) and 9 (G2848A) gene polymorphisms in cervical cancer susceptibility. Molecular Biology Reports. 2011; 38 (7):4715-4721. DOI: 10.1007/s11033-010-0607-z. Epub 2010 Dec 5 - 118.
Zidi S, Verdi H, Yilmaz-Yalcin Y, Yazici AC, Gazouani E, Mezlini A, et al. Involvement of toll-like receptors in cervical cancer susceptibility among Tunisian women. Bulletin du Cancer. 2014; 101 (10):E31-E35. DOI: 10.1684/bdc.2014.2037 - 119.
Mu X, Zhao J, Yuan X, Zhao X, Yao K, Liu Y, et al. Gene polymorphisms of toll-like receptor 9-1486T/C and 2848G/a in cervical cancer risk. International Journal of Gynecological Cancer. 2015; 25 (7):1173-1178. DOI: 10.1097/IGC.0000000000000494 - 120.
Jin Y, Qiu S, Shao N, Zheng J. Association of toll-like receptor gene polymorphisms and its interaction with HPV infection in determining the susceptibility of cervical cancer in Chinese Han population. Mammalian Genome. 2017; 28 (5-6):213-219. DOI: 10.1007/s00335-017-9691-x - 121.
Pandey N, Chauhan A, Raithatha N, Patel P, Khandelwal R, Desai A, et al. Influence of TLR4 and TLR9 polymorphisms and haplotypes on multiple hrHPV infections and HPV16 copy number in cervical cancer and cervicitis. Microbial Pathogenesis. 2021; 159 :105149. DOI: 10.1016/j.micpath.2021.105149 - 122.
Pandey S, Mittal RD, Srivastava M, Srivastava K, Singh S, Srivastava S, et al. Impact of toll-like receptors [TLR] 2 (−196 to −174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in north Indian women. Gynecologic Oncology. 2009; 114 (3):501-505. DOI: 10.1016/j.ygyno.2009.05.032. Epub 2009 Jun 21 - 123.
Pandey NO, Chauhan AV, Raithatha NS, Patel PK, Khandelwal R, Desai AN, et al. Association of TLR4 and TLR9 polymorphisms and haplotypes with cervical cancer susceptibility. Scientific Reports. 2019; 9 (1):9729. DOI: 10.1038/s41598-019-46077-z. Erratum in: Sci Rep.;9(1):18658 - 124.
Ashton KA, Proietto A, Otton G, Symonds I, McEvoy M, Attia J, et al. Toll-like receptor (TLR) and nucleosome-binding oligomerization domain (NOD) gene polymorphisms and endometrial cancer risk. BMC Cancer. 2010; 10 :382. DOI: 10.1186/1471-2407-10-382 - 125.
Lupi LA, Cucielo MS, Silveira HS, Gaiotte LB, Cesário RC, Seiva FRF, et al. The role of toll-like receptor 4 signaling pathway in ovarian, cervical, and endometrial cancers. Life Sciences. 2020; 247 :117435. DOI: 10.1016/j.lfs.2020.117435 - 126.
Saint-Jean M, Knol AC, Nguyen JM, Khammari A, Dréno B. TLR expression in human melanoma cells. European Journal of Dermatology. 2011; 21 (6):899-905. DOI: 10.1684/ejd.2011.1526 - 127.
Mauldin IS, Wang E, Deacon DH, Olson WC, Bao Y, Slingluff CL Jr. TLR2/6 agonists and interferon-gamma induce human melanoma cells to produce CXCL10. International Journal of Cancer. 2015; 137 (6):1386-1396. DOI: 10.1002/ijc.29515 - 128.
Speiser DE, Schwarz K, Baumgaertner P, Manolova V, Devevre E, Sterry W, et al. Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients. Journal of Immunotherapy. 2010; 33 (8):848-858. DOI: 10.1097/CJI.0b013e3181f1d614 - 129.
Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. European Journal of Immunology. 2012; 42 (11):3049-3061. DOI: 10.1002/eji.201142361. Epub 2012 Aug 28. Erratum in: Eur J Immunol. 2016 Feb;46(2):493 - 130.
Ostojic N, Radevic T, Kandolf Sekulovic L, Djordjevic B, Jaukovic L, Stepic N, et al. Polymorphisms in toll-like receptor 3 and 4 genes as prognostic and outcome biomarkers in melanoma patients. Melanoma Research. 2022; 32 (5):309-317. DOI: 10.1097/CMR.0000000000000836 - 131.
Elefanti L, Sacco G, Stagni C, Rastrelli M, Menin C, Russo I, et al. TLR7 Gln11Leu single nucleotide polymorphism and susceptibility to cutaneous melanoma. Oncology Letters. 2016; 12 (1):275-280. DOI: 10.3892/ol.2016.4584 - 132.
Suresh D, Srinivas AN, Kumar DP. Etiology of hepatocellular carcinoma: Special focus on fatty liver disease. Frontiers in Oncology. 2020; 10 :601710. DOI: 10.3389/fonc.2020.601710 - 133.
Huang Y, Cai B, Xu M, Qiu Z, Tao Y, Zhang Y, et al. Gene silencing of toll-like receptor 2 inhibits proliferation of human liver cancer cells and secretion of inflammatory cytokines. PLoS One. 2012; 7 (7):e38890. DOI: 10.1371/journal.pone.0038890 - 134.
Shi L, Zheng X, Fan Y, Yang X, Li A, Qian J. The contribution of miR-122 to the innate immunity by regulating toll-like receptor 4 in hepatoma cells. BMC Gastroenterology. 2019; 19 (1):130. DOI: 10.1186/s12876-019-1048-3 - 135.
Singh A, Koduru B, Carlisle C, Akhter H, Liu RM, Schroder K, et al. NADPH oxidase 4 modulates hepatic responses to lipopolysaccharide mediated by toll-like receptor-4. Scientific Reports. 2017; 7 (1):14346. DOI: 10.1038/s41598-017-14574-8 - 136.
Minmin S, Xiaoqian X, Hao C, et al. Single nucleotide polymorphisms of toll-like receptor 4 decrease the risk of development of hepatocellular carcinoma. PLoS One. 2011; 6 (4):e19466 - 137.
Neamatallah M, El-Bendary M, Elalfy H, Besheer T, El-Maksoud MA, Elhammady D, et al. Impact of toll-like receptors 2(TLR2) and TLR 4 gene variations on HCV susceptibility, response to treatment and development of hepatocellular carcinoma in cirrhotic HCV patients. Immunological Investigations. 2020; 49 (4):462-476. DOI: 10.1080/08820139.2019.1673772. Epub 2019 Oct 15 - 138.
Bas S, Neff L, Vuillet M, Spenato U, Seya T, Matsumoto M. Gabay C the proinflammatory cytokine response to chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. Journal of Immunology. 2008; 180 (2):1158-1168. DOI: 10.4049/jimmunol.180.2.1158 - 139.
Guo H, Chen Y, Hu X, Qian G, Ge S, Zhang J. The regulation of toll-like receptor 2 by miR-143 suppresses the invasion and migration of a subset of human colorectal carcinoma cells. Molecular Cancer. 2013; 12 :77. DOI: 10.1186/1476-4598-12-77 - 140.
Dajon M, Iribarren K, Cremer I. Toll-like receptor stimulation in cancer: A pro- and anti-tumor double-edged sword. Immunobiology. 2017; 222 (1):89-100. DOI: 10.1016/j.imbio.2016.06.009 - 141.
Fehri E, Ennaifer E, Bel Haj Rhouma R, Ardhaoui M, Boubaker S. TLR9 and glioma: Friends or foes? Cell. 2022; 12 (1):152. DOI: 10.3390/cells12010152 - 142.
Fehri E, Ennaifer E, Bel Haj Rhouma R, Guizani-Tabbane L, Guizani I, Boubaker S. The role of toll-like receptor 9 in gynecologic cancer. Current Research in Translational Medicine. 2016; 64 (3):155-159. DOI: 10.1016/j.retram.2016.01.010