Different TLRs in laboratory mice and humans, their localization, ligands, and their origin.
Abstract
Toll-like receptors (TLRs) are critical components of innate immunity and serve as pattern recognition receptors (PRRs). These PRRs recognize different microbe or pathogen-associated molecular patterns (MAMPs or PAMPs) and death/danger-associated molecular patterns to initiate the pro-inflammatory immune reaction in response to foreign and internal dangers. PRRs, including TLRs, also connects innate immunity to adaptive immunity. Furthermore, TLRs expressed on both innate and adaptive (T and B cells) immune cells regulate their functions. TLRs were first discovered in the common fruit fly or Drosophila melanogaster as genes controlling dorso-ventral body patterning during embryonic development. Immunological and scientific advances have led to the discovery of different TLRs (extra and intracellular) with diverse functions. The present chapter introduces the role of TLRs in immunity and inflammation and their expansion to mammalian reproduction and embryonic development, maintenance of immune homeostasis, health, and disease, specifically neurological disorders, including neurodegeneration and cancers.
Keywords
- TLRs
- immunity
- inflammation
- immune homeostasis
- neurodegeneration
- cancer
1. Introduction
The discovery of toll-like receptors (TLRs) revolutionized the field of immunology by filling the gap that the immune system recognizes and clears pathogens to maintain immunohomeostasis or immune homeostasis. For example, the first discovery of TLR4 in human spleen, intestinal epithelial cells (IECs), and peripheral blood leukocytes (PBLs), including monocytes, macrophages, dendritic cells (DCs), T and B cells, and its downstream signaling via NF-κB upon recognizing the corresponding pathogen/microbe-associated molecular patterns (PAMPs or MAMPs) such as lipopolysaccharide (LPS) showed its involvement in the activation and regulation of the adaptive immunity [1, 2, 3]. This groundbreaking research led to the reemergence of innate immunity in mainstream immunology research and the subsequent discovery of many other TLRs. For example, 13 TLRs in laboratory mice (TLR1-TLR13) and 10 TLRs in humans (TLR1-TLR10) recognizing different ligands are known today (Table 1) [2, 5].
| TLRs | TLR Localization | Ligands (PAMPs and DAMPs) | Origin of Ligands |
|---|---|---|---|
| TLR1 | Plasma membrane | Triacyl lipopeptide soluble factors | Bacteria and mycobacteria |
| TLR2 | Plasma membrane and endosomes | Peptidoglycan (PGN), lipoteichoic acid (LTA), Lipoproteins or lipopeptides, lipoarabinomannan, glycolipids, porins, zymosan, atypical LPS, heat shock protein 70 (Hsp70), eosinophil-derived neurotoxin (EDN) acts an alarmin | Gram +ve bacteria, mycobacteria, |
| TLR3 | Endoplasmic reticulum (ER), endosomes, multivesicular bodies, lysosomes, and Endolysosomes | dsRNA and ssRNA and synthetic analog polyinosinic-polycytidylic acid (poly I:C) | dsRNA, ssRNA, and dsDNA Viruses |
| TLR4 | Plasma membrane and endosome | LPS, Taxol, Fusion protein, Envelope proteins, high mobility group box 1 protein (HMG-B1), Hsp60, Hsp70, Hsp22, Hsp96 Type III repeat extra domain A of fibronectin, hyaluronic acid, heparin sulfate, Fibrinogen, Saturated fatty-acids and Fetuin-A | Gram negative bacteria, Plant, respiratory syncytial virus (RSV), mouse mammary tumor virus (MMTV), |
| TLR5 | Plasma membrane | Flagellin | Bacteria |
| TLR6 | Plasma membrane | Di-acyl lipopeptides, Zymosan | Mycoplasma |
| TLR7 | ER, endosomes, multivesicular bodies, lysosomes, and Endolysosomes | guanosine- and uridine-rich ssRNA, Loxoribine (a guanine analog), Bropirimine, ribonucleoproteins (RNPs), siRNAs, and imidazoquinoline derivatives such as resiquimod (R848) and imiquimod | Viruses (human immunodeficiency virus-1 or HIV-1, Influenza virus and vesicular stomatitis virus or VSV), synthetic compounds |
| TLR8 | ER, endosomes, multivesicular bodies, lysosomes, and Endolysosomes | ssRNA, RNPs | Viruses |
| TLR9 | ER, endosomes, multivesicular bodies, lysosomes, and Endolysosomes | CpG oligodeoxyneucleotide (ODN), viral double stranded DNA (dsDNA), Hemozoin pigment | Bacteria and viruses (Herpes Simplex Virus-1 and -2 or HSV-1 and -2, mouse cytomegalovirus or MCMV), Malaria, and protozoa genome ( |
| TLR10 (Humans) | ER, endosomes, multivesicular bodies, lysosomes, and Endolysosomes | dsRNA [4] | Viruses |
| TLR11 | ER, endosomes, multivesicular bodies, lysosomes, and Endolysosomes | Profilin-like protein | |
| TLR12 | ER, endosomes, multivesicular bodies, lysosomes, and Endolysosomes | Profilin-like protein | |
| TLR13 (Mouse) | ER, endosomes, multivesicular bodies, lysosomes, and Endolysosomes | 23 s ribosomal RNA | Bacteria |
Table 1.
TLRs are expressed extracellularly (on the plasma membrane, TLR1, TLR2, TLR4, TLR5, TLR6, TLR10 (in humans) and TLR11, and TLR12 in mice) and intracellularly in endosomes, endolysosomes, and lysosomes (TLR3, TLR7, TLR8, and TLR9) (Table 1) [5, 6]. Mouse TLR13 resides in endosomes, but its ligand is unknown [6, 7]. However, some immune cells, such as dendritic cells (DCs), epithelial cells, and endothelial cells, also have intracellular TLR2 and TLR4 [8, 9, 10]. TLRs are members of the pattern recognition receptor (PRR) family, which frequently recognize molecules expressed and released by microbes and damaged or dying cells, called damage/death-associated molecular patterns (DAMPs), to initiate pro-inflammatory immune response for their clearance. The clearance of the invading agent proceeds to the resolution phase of the inflammation for maintaining immune homeostasis that otherwise activates the adaptive immune system later. The combined protective action of innate and adaptive immunity fights back to take care of the outer or endogenous threat, failure of which leads to chronic inflammatory diseases and many cancers.
It is important to note that TLR homolog (called
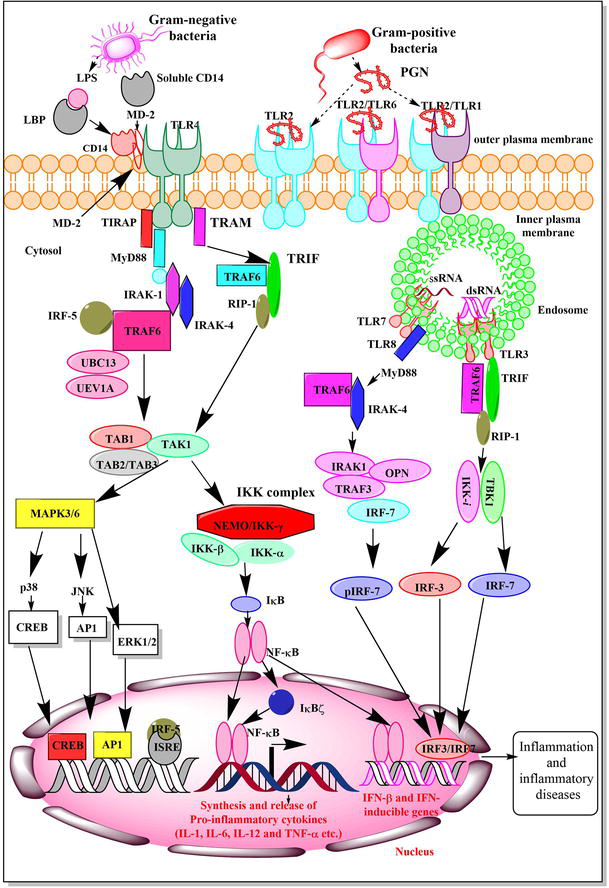
Figure 1.
TLR (MyD88-dependent and -independent or TRIF-dependent) signaling. The recognition of Gram-negative bacteria or LPS by TLR4 leads to the activation of downstream signaling pathways through the activation MyD88-dependent and -independent (TRIF-dependent) manner to activate NF-κB causing the transcription and translation of pro-inflammatory genes (cytokines and chemokines) and well as the generation of type 1 IFNs. The TRIF and MyD88-depedent signaling pathways downstream to TLR4 activation converge at transforming growth factor (TGF)-β-activated kinase 1 (TAB1), 2, and/or 3 and transforming growth factor beta-activated kinase 1 (TAK1) complex. TAK1 activation stimulates MAPK3/6 that activates AP1 and CREB along with activating NF-κB signaling via NF-κB essential modulator (NEMO). Activation of intracellular TLRs (TLR3, TLR7, TLR8, and TLR9) induces the generation of type 1 interferons (IFNs) that regulate immune response, including adaptive immunity. The activation TLR3 signaling via TRIF-dependent signaling activates TNAK-binding kinase 1 (TBK1) and IKK-1 activates interferon regulatory factor 3 (IRF3) and IRF7 to produce type 1 IFNs. On the other hand, TLR7/8 activation involves MyD88-dependent downstream signaling activating TRAF6 and IRAK4 complex formation that further activates IRAK1/
2. TLRs are critical to maintain immune homeostasis
Homeostasis maintenance and immunological well-being, called immune homeostasis, is critical for healthy life and longevity. For example, TLR-mediated commensal microbes’ recognition maintains intestinal epithelial homeostasis and protects the host from gut injury and associated mortality [17]. Furthermore, TLR3-mediated macrophage priming for subsequent TLR7 activation involves the Janus-associated kinase (JAK) and signal transducer and activator of transcription (STAT) pathway activation that controls synergistic production of cytokines, innate immune memory generation, and immune homeostasis maintenance during temporally separated subsequent infection [18]. Thus, TLR3 and TLR7 crosstalk are critical for immune homeostasis and innate immune memory generation.
Immune homeostasis disruption causes several auto-immune, auto-inflammatory, and immunodeficiency diseases. Furthermore, foreign invasion from microbes, allergens, and xenobiotics disrupts the immune homeostasis by activating different PRRs, including TLRs, which becomes detrimental to the host once it persists longer. For example, several pro-inflammatory diseases, including sepsis-associated cytokine storm, coronavirus disease 2019 (COVID-19), and neuroinflammation involve TLR overactivation [5, 12, 13, 19].
Several endogenously expressed TLR signaling negative regulators (Table 2) prevent exaggerated TLR signaling responsible for inflammation and inflammatory diseases. The details of endogenous negative regulators of TLR signaling are beyond the scope of this introductory chapter and are discussed elsewhere [13, 16, 20, 21, 22, 23]. Hence, dysregulated TLR signaling in response to external and endogenous threats (PAMPs, MAMPs, or DAMPs) impairs immune homeostasis, causing cytokine syndrome or cytokine release syndrome (CRS) seen during sepsis, acute or severe COVID-19, and other inflammatory conditions [13, 24, 25]. In addition to overactivated TLR signaling-induced inflammatory conditions, the deficiency of TLR signaling molecules causes different primary immunodeficiency diseases (PIDs) [26, 27]. For example, TLR3 signaling defect correlates well with herpes simplex virus-1 (HSV-1) encephalitis, and a dampened TLR2 and TLR4 signaling has been observed in chronic granulomatosis disease (CGD) and X-linked agammaglobulinemia (XLA) [26, 27]. Table 3 shows different TLRs and their signaling pathway molecules’ deficiency and impact on immunity or PIDs. Therefore, balanced TLR signaling is critical for immune regulation or immune homeostasis [14, 25, 28, 29, 30, 31, 32, 33, 34].
| Endogenous TLR signaling negative regulators | Classification | Mode of action |
|---|---|---|
| TRAM (Translocating chain-associated membrane protein) adaptor with Golgi dynamics (GOLD) domain) or TAG | Splice variant of TRAM | Competes with TRAM for binding to TRIF |
| Sterile alpha- and armadillo-motif-containing protein or SARM | TIR-domain containing adaptor molecule | Binds and inhibits TRIF-dependent TLR signaling |
| Interferon regulatory factor 4 or IRF4 | IRF family of transcription factors | Inhibits IRF5 binding to MyD88 and TRAF6 and its translocation to nucleus |
| Tumor necrosis factor-α-induced protein 8-like 2 or TIPE2 | Member of TNF-α-induced protein-8 (TNFAIP8) family | Inhibits TLR signaling via binding to CASP8 and inhibiting NF-κB and AP-1 activation |
| Bruton’s tyrosine kinase or Btk | Tec family tyrosine kinase | Phosphorylates MAL that activates SOCS-1 |
| DNAX activation protein of 12 kDa or DAP12, also called KARAP | Transmembrane adaptor protein | DAP12 mediated inhibition of TLR signaling involves another adaptor protein called DOK-3 |
| Downstream of kinase 1 and 2 (DOK1 and DOK2) | Adaptor Proteins | Inhibit Ras-Erk dependent signaling |
| Axl | TAM family of receptor kinases | Inhibits NF-κB mediated production of TNF-α |
| Interlukin-1 receptor-associated kinase-M (IRAK-M) or IRAK-3 | Member of serine/threonine kinase family | Inhibits dissociation of IRAK-1 and IRAK-4 form MyD88 and formation of IRAK-TRAF6 complex |
| TOLL-interacting protein (TOLLIP) | Adaptor protein that interacts with cytoplasmic TIR domain of IL-1Rs | Inhibits phosphorylation and kinase activity of IRAK1 |
| Src homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) | Intracellular tyrosine phosphatase | Inhibits MAP kinase and NF-κB activation |
| Calcineurin | A serine/threonine phosphatase | Specific pathway unknown |
| Protein tyrosine phosphatase-1 B (PTP1B) | Intracellular tyrosine phosphatase | Inhibits MAPKs, NF-κB and IRF3 |
| A20 or TNF-α-induced protein 3 (TNFAIP3) | Ubiquitin modifying enzyme | Inhibits NF-κB signaling as negative feedback by removing ubiquitin moieties from TRAF6 |
| Cylindromatosis or CYLD | Tumor suppressor deubiquitinase | Inhibits TLR2 signaling via inhibiting MyD88, TRAF2, TRAF6 TRAF7 and NEMO |
| Ubiquitin-specific protease 4 or USP4 | Deubiquitinase | Inhibits TRL4 signaling via deubiquinating TRAF6 and inhibiting its adaptor function |
| USP18 or UBP43 or ISG15 isopeptidase | Isopeptidase | It cleaves the K63-linked polyubiquitin chains of TAK1 and also targets NEMO |
| Deubiquitinating enzyme A (DUBA) | Cysteine protease | Removes K63-ubiquitin chain from TRAF3 to inhibit NF-κB and IRF3 activation |
| Nuclear receptor 4A1 or NR4A1 (Nur77) | Member of nuclear receptor 4A receptor subfamily | Prevent auto-ubiquitination of TRAF6 via binding to TRAF6 |
| NR4A2 or Nurr1 | Member of nuclear receptor 4A receptor subfamily | Inhibits NF-κB activation downstream to TLR4 signaling |
| Small heterodimer partner (SHP) or NR0B2 | Orphan nuclear receptor | Prevents Lys63-linked polyubiquitination of TRAF6 and subsequent activation of NF-κB |
| Mitogen and stress-activated kinase 1 and 2 (MSK1 and 2) | Nuclear kinase sharing homology with ribosomal S6 kinase (p90rsk) family | Phosphorylate histone H3 and CREB that negatively regulates TLR signaling and induces several anti-inflammatory genes |
| TRAF-associated NF-κB activator (TANK) | TRAF binding protein | Binding of TANK to TRAF6 inhibits NF-κB and AP-1 activation |
| PDZ and LIM domain containing protein or PDLIM-2 (Mystique and SLIM in mice) | PDZ and LIM domain containing Alkaline Phosphatase | Suppresses TLR signaling by acting as a nuclear E3 ubiquitin ligase and inhibits NF-κB activation |
| Mankorin ring finger protein 2 (MKRN2 or RNF62) | Zinc finger and RING finger domain containing nuclear protein | Binds to PDLIM2 and inhibits activation of NF-κB downstream to TLR signaling |
| PDLIM1 or CLP36 or Elfin | PDZ and LIM domain containing protein of APL subfamily | PDLIM1 inhibits NF-κB activation by sequestering p65 into cytosol |
| Tripartite motif-containing protein 30 A (TRIM30α) | A member of tripartite-motif (TRIM) protein family | Blocks TRAF6 autoubiquitination by degrading TAB2 and TAB3and suppresses NF-κB activation |
| TRIM8 | Acts as ubiquitin E3 ligase | Polyubiquitinates TRF and inhibits TRIF-TBK1 interaction |
| Triad3A or Ring finger protein 216 (RNF216) | RING finger type E3 ubiquitin ligase | Degrades TLR proteins |
| NOD-like receptor family member X1 (NLRX1) | Member of NLR family | NLRX1 binds to IKK complex causing an inhibition of IKKα and IKKβ phosphorylation and NF-κB activation |
| NLRC3 or NOD3 | Member of NLR family | NLRC3 interacts with TRAF6 to attenuate its K63-linked ubiquitination to inhibit NF-κB activation |
| NLRC5 | Member of NLR family | Blocks IKK complexes to inhibit NF-κB activation and type 1 IFN signaling pathways |
| Serum stimulation 2 factor (ST2) | Serves as a part of IL-33 receptor | ST2 binds to sequesters MyD88 and MAL without affecting TRIF and IRAK to inhibit TLR-induced NF-κB activation |
| Single immunoglobulin IL-1R-relate receptor (SIGIRR or TIR-8) | Member of TLR/IL-1R superfamily | Blocks TLR-mediated NF-κB and JNK activation via stopping the recruitment of IRAK and TRAF6 towards MyD88 |
| TLR10 | Member of TLR family | Stimulates PI3K/Akt/IL-1R antagonist pathway and inhibits MyD88 and TRIF-dependent signaling pathways |
| Activating transcription factor 3 (ATF3) | Member of activating transcription factor/cAMP response element family of bZip transcription factors | Bind to consensus c-AMP response element (CRE) sequences |
| Interleukin-37 or IL-37 | Member of IL-1 cytokine family | Blocks NF-κB and MAPK activation |
Table 2.
Endogenous negative regulators of TLR signaling.
| PIDs | Impact on TLR functions on immune cells |
|---|---|
| Reduced expression and function of TLR7 and 9 on B cells, reduced activity of TLR7 and 9 on plasmacytoid DCs (pDCs) |
| Increased TL 4 activity (increased TNF-α and IL-18 production) on neutrophils, peripheral blood monocytes (PBMCs), decreased TLR5 and TLR9 activity, TL 9 activity on B cells reduces as indicated by a decreased memory response |
| Decreased TLR2 and 4 responses by monocytes and macrophages, monocyte-derived DCs show decreased TLR2, 4, 3, and 7/8 responses, including decreased DC maturation |
| TLR2 and 4 response increases in PBMCs as indicated by increased TNF-α and IL-12 production |
| Decreased TLR7 and 9-meidated immune response by B cells |
| Abolishes TLR7, 8, and 9-mediated type 1 IFN production, leaving TLR3 and TLR4-dependent IFN production intact |
Table 3.
TLRs in different PIDs.
3. TLRs in human health, including embryonic development and disease
TLRs are responsible for dorso-ventral body patterning during the embryonic development of
The preimplantation human embryos also express TLRs [37]. Furthermore, TLR3 in the brain cells of mice in the early embryonic stages and neural stem/progenitor cells (NPCs) control the neurosphere formation. For example, NPCs from TLR3-deficient murine embryos proliferate highly and form higher numbers of neurospheres than wild-type (WT) embryos [38]. Therefore, TLR3 is a negative regulator of NPC proliferation and neurosphere formation in mice, which needs exploration in humans. TLR2 and TLR4 expression increases throughout gestation in sheep lungs, and LPS exposure increases their expression in fetal sheep lungs [39]. This study is critical as ovine and human TLR2, 3, and 4 share 83–88% homology. Human blastocysts highly express TLRs 9 and 5, and TLRs 9, 5, 2, 6, and 7 are expressed throughout embryonic development, and their stimulation
Plenty of data are available for TLRs in a vast array of infectious (bacterial, viral, fungal, and parasitic) and inflammatory diseases [5, 13, 43, 44, 45, 46, 47, 48]. We have previously mentioned TLRs’ role in autoimmune and PIDs. Therefore, this section will introduce their role in neurodegenerative diseases (NDs) and cancers. For example, Alzheimer’s and Parkinson’s disease (AD and PD) incidence has increased worldwide since their first report. PD patients’ number has more than doubled from 1999 (2.5 million) to 2016 (6.1 million) worldwide, which is further increasing [49]. For example, in the United States, 6.1 million people may have AD as per data collected by the Alzheimer’s Disease Association (ADA) in 2022, and according to PD foundation, over a million people have PD.
Inflammation plays a significant role in ND pathogenesis so as TLRs do [12, 50, 51, 52, 53, 54, 55, 56]. A novel variant p.E317D in the TLR 9 gene, co-segregating with early-onset AD (EOAD) in an autosomal dominant manner, identified in a Flander-Belgian family, increases AD risk by compromising innate immunity-mediated protection [57]. The p.E317D TLR9 variant reduces its potential to activate NF-κB by 50%, indicating that p.E317D is a loss of function mutation. The protective role of TLR9 in AD comprises the release of anti-inflammatory cytokines and upregulation of Axl (a negative regulator of TLR-mediated pro-inflammatory signaling), Run domain Beclin-1 interacting and cysteine-rich containing protein (RUBICON, an autophagy suppressor), and associated signaling pathways regulation microglial phagocytic function and inflammatory signaling [57]. For example, microglia and myeloid cell-specific deletion of RUBICON in mouse genetic models of AD induce early onset of neurotoxic amyloid-beta (Aβ) plaques, microgliosis, and increase in pro-inflammatory cytokines in cortex and hippocampus [58, 59]. AD patients’ brains express lower RUBICON, Atg16L, and Atg5 levels than non-AD brain samples [60]. Furthermore, TLR9 activation ameliorates vascular amyloid pathology in mice and provides cognitive benefits [61]. The increased TLR4 expression and its co-localization with pSer129 αSyn and Iba-1in glial cells of substantia nigra (SN) and medial temporal gyrus (GTM) in PD patients can be targeted to prevent inflammatory neuronal damage [62, 63].
Autophagy is critical for Aβ secretion and plaque formation, and TLRs are known to control autophagy [64, 65, 66]. For example, TLR7-mediated autophagy increases epileptic susceptibility by reducing kinesin family member 5 A (KIF5A)-dependent gamma-aminobutyric acid (GABA)A receptor transport in mice [67]. Furthermore, TLRs are critical players in non-NDs of the brain called neurological diseases due to their role in central nervous system (CNS) homeostasis and neurogenesis, including neuronal pruning, learning, and memory [68]. The recognition of extracellular Tau protein (one of the causal factors for PD and associated dementia) stimulates microglia to phagocytose live neurons via the TLR4/NLRP3/Caspase 1 axis and NADPH oxidase activation [69, 70]. The burden of neurological diseases is also increasing world-wide [71]. For example, neurological diseases ranked third after cardiovascular diseases and cancers in Europe’s total death [71]. Therefore, exploring TLRs’ role in NDs and other neurological diseases has the potential for designing novel therapeutics. For example, targeting TLRs may serve a promising therapeutic strategy against AD and PD [62, 63, 69, 72, 73, 74]. Furthermore,
4. Conclusion
The story of TLRs began in 1997 with the discovery of TLR4 in human spleen, immune, epithelial, and endothelial cells as a homolog of Drosophila Toll protein that filled the long-standing gap of the microbial recognition, their phagocytosis and clearance by immune cells. After almost 30 years of TLR4 discovery and revolutionizing immunology research, TLRs are still at the top as critical PRRs and immune regulators. However, in the last 30 years, they have expanded their territory from infection, immunity, and inflammation to neurosciences, mammalian reproductive biology, and cancer. Even TLRs and DAMPs are critical in heart transplant rejection [84]. TLR agonists and antagonists have a wide range of applications in different inflammatory and infectious diseases and cancers (as adjuvants). TLR-based emerging therapeutics and vaccine candidates are under clinical trials for several diseases [85, 86, 87]. Furthermore, harnessing innate immunity for cancer and ND therapy is a critical research area, and TLRs are relevant components of innate immunity [4, 88, 89]. Hence, TLRs control innate immunity as crucial members of the PRR family with diverse immune and non-immune functions.
References
- 1.
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997; 388 :394-397 - 2.
Kumar V. Toll-like receptors in adaptive immunity. Handbook of Experimental Pharmacology. 2022; 276 :95-131 - 3.
Kumar V, Barrett JE. Toll-like receptors (TLRs) in health and disease: An overview. Handbook of Experimental Pharmacology. 2022; 276 :1-21 - 4.
Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annual Review of Medicine. 2018; 69 :437-449 - 5.
Kumar V. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. International Immunopharmacology. 2018; 59 :391-412 - 6.
Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010; 32 :305-315 - 7.
Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim Y-M. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. Journal of Cell Biology. 2007; 177 :265-275 - 8.
Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. Journal of Experimental Medicine. 2003; 198 :1225-1235 - 9.
Uronen-Hansson H, Allen J, Osman M, Squires G, Klein N, Callard RE. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: Integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology. 2004; 111 :173-178 - 10.
Chen S, Wong MH, Schulte DJ, Arditi M, Michelsen KS. Differential expression of toll-like receptor 2 (TLR2) and responses to TLR2 ligands between human and murine vascular endothelial cells. Journal of Endotoxin Research. 2007; 13 :281-296 - 11.
Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980; 287 :795-801 - 12.
Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. Journal of Neuroimmunology. 2019; 332 :16-30 - 13.
Kumar V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. International Immunopharmacology. 2020; 89 :107087 - 14.
Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020; 180 :1044-1066 - 15.
Kawai T, Akira S. Signaling to NF-kappaB by toll-like receptors. Trends in Molecular Medicine. 2007; 13 :460-469 - 16.
Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004; 4 :499-511 - 17.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004; 118 :229-241 - 18.
Liu B, Liu Q , Yang L, Palaniappan SK, Bahar I, Thiagarajan PS, et al. Innate immune memory and homeostasis may be conferred through crosstalk between the TLR3 and TLR7 pathways. Science Signaling. 2016; 9 :ra70 - 19.
Alturaiki W, Alkadi H, Alamri S, Awadalla ME, Alfaez A, Mubarak A, et al. Association between the expression of toll-like receptors, cytokines, and homeostatic chemokines in SARS-CoV-2 infection and COVID-19 severity. Heliyon. 2023; 9 :e12653 - 20.
Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nature Reviews Immunology. 2005; 5 :446-458 - 21.
Liu X, Chen W, Wang Q , Li L, Wang C. Negative regulation of TLR inflammatory signaling by the SUMO-deconjugating enzyme SENP6. PLoS Pathogens. 2013; 9 :e1003480 - 22.
Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, et al. Suppressor of cytokine signaling 1 negatively regulates toll-like receptor signaling by mediating mal degradation. Nature Immunology. 2006; 7 :148-155 - 23.
Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002; 17 :677-687 - 24.
Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020; 368 :473-474 - 25.
Fajgenbaum DC, June CH. Cytokine storm. New England Journal of Medicine. 2020; 383 :2255-2273 - 26.
Maglione PJ, Simchoni N, Cunningham-Rundles C. Toll-like receptor signaling in primary immune deficiencies. Annals of the New York Academy of Sciences. 2015; 1356 :1-21 - 27.
Mortaz E, Adcock IM, Tabarsi P, Darazam IA, Movassaghi M, Garssen J, et al. Pattern recognitions receptors in immunodeficiency disorders. European Journal of Pharmacology. 2017; 808 :49-56 - 28.
Duffy L, O’Reilly SC. Toll-like receptors in the pathogenesis of autoimmune diseases: Recent and emerging translational developments. ImmunoTargets and Therapy. 2016; 5 :69-80 - 29.
Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. European Journal of Immunology. 2008; 38 :1971-1978 - 30.
Farrugia M, Baron B. The role of toll-like receptors in autoimmune diseases through failure of the self-recognition mechanism. International Journal of Inflammation. 2017; 2017 :8391230-8391230 - 31.
Mohammad Hosseini A, Majidi J, Baradaran B, Yousefi M. Toll-like receptors in the pathogenesis of autoimmune diseases. Advanced Pharmaceutical Bulletin. 2015; 5 :605-614 - 32.
Zhang Y, Liu J, Wang C, Liu J, Lu W. Toll-like receptors gene polymorphisms in autoimmune disease. Frontiers in Immunology. 2021; 12 . DOI: 10.3389/fimmu.2021.672346 - 33.
Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nature Reviews Immunology. 2006; 6 :823-835 - 34.
Fillatreau S, Manfroi B, Dörner T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nature Reviews Rheumatology. 2021; 17 :98-108 - 35.
Arnaboldi F, Opizzi E, Rasile M, Menegola E, Di Renzo F, Barajon I. An immunohistochemical study of TLR-4 and -7 expression during murine embryonic development: Respiratory apparatus and peripheral nervous system. Italian Journal of Anatomy and Embryology. 2018; 123 :6 - 36.
Balounová J, Vavrochová T, Benešová M, Ballek O, Kolář M, Filipp D. Toll-like receptors expressed on embryonic macrophages couple inflammatory signals to iron metabolism during early ontogenesis. European Journal of Immunology. 2014; 44 :1491-1502 - 37.
Aboussahoud WS, Smith H, Stevens A, Wangsaputra I, Hunter HR, Kimber SJ, et al. The expression and activity of toll-like receptors in the preimplantation human embryo suggest a new role for innate immunity. Human Reproduction. 2021; 36 :2661-2675 - 38.
Lathia JD, Okun E, Tang S-C, Griffioen K, Cheng A, Mughal MR, et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. The Journal of Neuroscience. 2008; 28 :13978-13984 - 39.
Hillman NH, Moss TJM, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, et al. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatric Research. 2008; 63 :388-393 - 40.
Benjelloun F, Quillay H, Cannou C, Marlin R, Madec Y, Fernandez H, et al. Activation of toll-like receptors differentially modulates inflammation in the human reproductive tract: Preliminary findings. Frontiers in Immunology. 4 Aug 2020; 11 :1655. DOI: 10.3389/fimmu.2020.01655 - 41.
Fazeli A, Bruce C, Anumba DO. Characterization of toll-like receptors in the female reproductive tract in humans. Human Reproduction. 2005; 20 :1372-1378 - 42.
Fujita Y, Mihara T, Okazaki T, Shitanaka M, Kushino R, Ikeda C, et al. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Human Reproduction. 2011; 26 :2799-2806 - 43.
Khanmohammadi S, Rezaei N. Role of toll-like receptors in the pathogenesis of COVID-19. Journal of Medical Virology. 2021; 93 :2735-2739 - 44.
Mantovani S, Oliviero B, Varchetta S, Renieri A, Mondelli MU. TLRs: Innate immune sentries against SARS-CoV-2 infection. International Journal of Molecular Sciences. 29 Apr 2023; 24 (9):8065. DOI: 10.3390/ijms24098065 - 45.
Kumar V. Understanding the complexities of SARS-CoV2 infection and its immunology: A road to immune-based therapeutics. International Immunopharmacology. 2020; 88 :106980 - 46.
Mukherjee S, Huda S, Sinha Babu SP. Toll-like receptor polymorphism in host immune response to infectious diseases: A review. Scandinavian Journal of Immunology. 2019; 90 :e12771 - 47.
Skevaki C, Pararas M, Kostelidou K, Tsakris A, Routsias JG. Single nucleotide polymorphisms of toll-like receptors and susceptibility to infectious diseases. Clinical and Experimental Immunology. 2015; 180 :165-177 - 48.
Kumar V. Going, toll-like receptors in skin inflammation and inflammatory diseases. EXCLI Journal. 2021; 20 :52-79 - 49.
Rocca WA. The burden of Parkinson's disease: A worldwide perspective. Lancet Neurology. 2018; 17 :928-929 - 50.
Dabi YT, Ajagbe AO, Degechisa ST. Toll-like receptors in pathogenesis of neurodegenerative diseases and their therapeutic potential. Immunity, Inflammation and Disease. 2023; 11 :e839 - 51.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010; 140 :918-934 - 52.
Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. The Journal of Clinical Investigation. 2017; 127 :3577-3587 - 53.
Zhang W, Xiao D, Mao Q , Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduction and Targeted Therapy. 2023; 8 :267 - 54.
Li L, Acioglu C, Heary RF, Elkabes S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain, Behavior, and Immunity. 2021; 91 :740-755 - 55.
Owens T. Toll-like receptors in neurodegeneration. Current Topics in Microbiology and Immunology. 2009; 336 :105-120 - 56.
Pascual M, Calvo-Rodriguez M, Núñez L, Villalobos C, Ureña J, Guerri C. Toll-like receptors in neuroinflammation, neurodegeneration, and alcohol-induced brain damage. IUBMB Life. 2021; 73 :900-915 - 57.
Cacace R, Zhou L, Hendrickx Van de Craen E, Buist A, Hoogmartens J, Sieben A, et al. Mutated toll-like receptor 9 increases Alzheimer’s disease risk by compromising innate immunity protection. Molecular Psychiatry. 11 Jul 2023. DOI: 10.1038/s41380-023-02166-0. [Online ahead of print] - 58.
Heckmann BL, Teubner BJW, Tummers B, Boada-Romero E, Harris L, Yang M, et al. LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell. 2019; 178 :536-551.e514 - 59.
Magné J, Green DR. LC3-associated endocytosis and the functions of Rubicon and ATG16L1. Science Advances. 2022; 8 :eabo5600 - 60.
Heckmann BL, Teubner BJW, Boada-Romero E, Tummers B, Guy C, Fitzgerald P, et al. Noncanonical function of an autophagy protein prevents spontaneous Alzheimer’s disease. Science Advances. 2020; 6 :eabb9036 - 61.
Scholtzova H, Do E, Dhakal S, Sun Y, Liu S, Mehta PD, et al. Innate immunity stimulation via toll-like receptor 9 ameliorates vascular amyloid pathology in Tg-SwDI mice with associated cognitive benefits. The Journal of Neuroscience. 2017; 37 :936-959 - 62.
Conte C, Ingrassia A, Breve J, Bol JJ, Timmermans-Huisman E, van Dam AM, et al. Toll-like receptor 4 is upregulated in Parkinson's disease patients and co-localizes with pSer129αSyn: A possible link with the pathology. Cells. 11 May 2023; 12 (10):1368. DOI: 10.3390/cells12101368 - 63.
Selles MC, Fortuna JTS, Santos LE. Immunomodulation via toll-like receptor 9: An adjunct therapy strategy against Alzheimer's disease? The Journal of Neuroscience. 2017; 37 :4864-4867 - 64.
Nilsson P, Loganathan K, Sekiguchi M, Matsuba Y, Hui K, Tsubuki S, et al. Aβ secretion and plaque formation depend on autophagy. Cell Reports. 2013; 5 :61-69 - 65.
Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. The EMBO Journal. 2008; 27 :1110-1121 - 66.
Delgado MA, Deretic V. Toll-like receptors in control of immunological autophagy. Cell Death and Differentiation. 2009; 16 :976-983 - 67.
Liu J, Ke P, Guo H, Gu J, Liu Y, Tian X, et al. Activation of TLR7-mediated autophagy increases epileptic susceptibility via reduced KIF5A-dependent GABAA receptor transport in a murine model. Experimental & Molecular Medicine. 2023; 55 :1159-1173 - 68.
Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: Mechanisms and therapeutic potential. Clinical Science (London, England). 2011; 121 :367-387 - 69.
Pampuscenko K, Morkuniene R, Krasauskas L, Smirnovas V, Brown GC, Borutaite V. Extracellular tau stimulates phagocytosis of living neurons by activated microglia via toll-like 4 receptor-NLRP3 inflammasome-caspase-1 signalling axis. Scientific Reports. 2023; 13 :10813 - 70.
Lei P, Ayton S, Finkelstein DI, Adlard PA, Masters CL, Bush AI. Tau protein: Relevance to Parkinson's disease. The International Journal of Biochemistry & Cell Biology. 2010; 42 :1775-1778 - 71.
Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, et al. The burden of neurological diseases in Europe: An analysis for the global burden of disease study 2017. The Lancet Public Health. 2020; 5 :e551-e567 - 72.
Zhou Y, Chen Y, Xu C, Zhang H, Lin C. TLR4 targeting as a promising therapeutic strategy for Alzheimer disease treatment. Frontiers in Neuroscience. 2020; 14 :602508 - 73.
Gambuzza ME, Sofo V, Salmeri FM, Soraci L, Marino S, Bramanti P. Toll-like receptors in Alzheimer's disease: A therapeutic perspective. CNS & Neurological Disorders Drug Targets. 2014; 13 :1542-1558 - 74.
Su Y, Wang D, Liu N, Yang J, Sun R, Zhang Z. Clostridium butyricum improves cognitive dysfunction in ICV-STZ-induced Alzheimer's disease mice via suppressing TLR4 signaling pathway through the gut-brain axis. PLoS One. 2023; 18 :e0286086 - 75.
Cassir N, Benamar S, La Scola B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clinical Microbiology and Infection. 2016; 22 :37-45 - 76.
Kumar V, Stewart JH IV. Immunometabolic reprogramming, another cancer hallmark. Frontiers in Immunology. 2023; 14 :1125874 - 77.
Kumar V. Inflammation research sails through the sea of immunology to reach immunometabolism. International Immunopharmacology. 2019; 73 :128-145 - 78.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420 :860-867 - 79.
Urban-Wojciuk Z, Khan MM, Oyler BL, Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A, et al. The role of TLRs in anti-cancer immunity and tumor rejection. Frontiers in Immunology. 2019; 10 :2388 - 80.
Gonzalez C, Williamson S, Gammon ST, Glazer S, Rhee JH, Piwnica-Worms D. TLR5 agonists enhance anti-tumor immunity and overcome resistance to immune checkpoint therapy. Communications Biology. 2023; 6 :31 - 81.
Keshavarz A, Pourbagheri-Sigaroodi A, Zafari P, Bagheri N, Ghaffari SH, Bashash D. Toll-like receptors (TLRs) in cancer; with an extensive focus on TLR agonists and antagonists. IUBMB Life. 2021; 73 :10-25 - 82.
So EY, Ouchi T. The application of toll like receptors for cancer therapy. International Journal of Biological Sciences. 2010; 6 :675-681 - 83.
Yang Y, Feng R, Wang YZ, Sun HW, Zou QM, Li HB. Toll-like receptors: Triggers of regulated cell death and promising targets for cancer therapy. Immunology Letters. 2020; 223 :1-9 - 84.
Kesler A, Agrawal DK, Thankam FG. Toll-like receptors and damage-associated molecular patterns in the pathogenesis of heart transplant rejection. Molecular and Cellular Biochemistry. 2022; 477 :2841-2850 - 85.
Hennessy EJ, Parker AE, O'Neill LAJ. Targeting toll-like receptors: Emerging therapeutics? Nature Reviews Drug Discovery. 2010; 9 :293-307 - 86.
Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in toll-like receptor targeting therapeutics. Medicinal Research Reviews. 2019; 39 :1053-1090 - 87.
Bzówka M, Bagrowska W, Góra A. Recent advances in studying toll-like receptors with the use of computational methods. Journal of Chemical Information and Modeling. 2023; 63 :3669-3687 - 88.
Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019; 574 :45-56 - 89.
Mushegian AA. Harnessing innate immunity to fight neurodegeneration. Science Signaling. 2017; 10 :eaam8102