Abstract
Human reproduction, a pas de deux, is dependent on the functional competence of both male and female reproductive systems. Male factor infertility accounts for about half of the causes of infertility and strictly affects about 7% of all men. While most cases are idiopathic, a smaller proportion can be adduced to a wide variety of causes generally classified as pre-testicular, testicular, and post-testicular. Extrinsic factors bordering on behaviour and habits which are generally modifiable, should be given due attention in the evaluation and initial management of male infertility. A range of investigations can be employed in the evaluation of male infertility, however, semen analysis, the least invasive and most cost effective, is prognostic but does not always guarantee fecundity as multiple interrelated factors have been implicated in male infertility. Treatment options though varied, aim at improving semen quality and assisted reproductive technique (ART) is offered in cases of severe male infertility. This chapter provides an overview of male factor infertility with a focus on investigation and contemporary management in a dynamic world. It further provides insights into advances in stem cell therapeutics and artificial intelligence.
Keywords
- male infertility
- aetiology
- management
- challenges
- contemporary practice
1. Introduction
Infertility refers to the inability of a couple to achieve conception after one year of regular unprotected sexual intercourse [1]. Reproduction on the other hand is important for the continued existence of the human species as a means of compensation for death or disease. Involuntary childlessness is a pervasive medical condition that has been found to adversely affect the psychosocial wellbeing of the affected couple [2]. Infertility has been associated with an increased economic burden on both patient and the healthcare institution [3] and this burden is exaggerated in many low-income countries where funding through health insurance is lacking, and this further worsens the outlook for the infertile couple [4].
Infertility is estimated to globally affect about 8–12% of couples with both parties contributing equally to its incidence [5]. In the United Kingdom, it has been estimated that one in seven couples experience challenges with conception with the male partner contributing up to half of the cases [6], however, male infertility is thought to strictly affect 7% of all men [7]. Impaired male reproductive health has been linked to a decreased general health status and severe male infertility has been associated with a higher risk of malignancy [8, 9].
Male factor infertility may result from several factors which include idiopathic, endocrine, environmental, anatomic, behavioural/lifestyle and iatrogenic factors. Idiopathic or unknown factors are the most prevalent and account for over half of the cases seen. Understanding the aetiology of male infertility is made easier by classifying them into pre-testicular, testicular, and post-testicular causes.
2. Epidemiology
A variety of health conditions can adversely affect male fertility and male reproductive disorders can be identified in about half of male partners of an infertile union. Male factor infertility evidenced by abnormal semen parameters is noted to affect about 7% of all men and its extreme form, azoospermia is found in 1% of the general population and in about 20% of patients attending a fertility clinic [10]. Evidence suggests that the health status of a male partner at the time of conception may affect the metabolic health and reproductive potential of the progeny [11].
Globally over the past five decades, sperm count has been reported to decline and a systematic review by Levine et al demonstrated a decline of between 50–60% between 1973 and 2011, further underscoring the rising contribution of male factor infertility [12]. Prognostic factors influencing the outcome of fertility management includes the duration of infertility, age of the female partner, disorders of semen production and the type of infertility which may be either primary or secondary. Despite advances in male reproductive health, progress in the management of male factor infertility has been limited and often restricted to assisted reproduction. Early diagnosis through prompt and thorough evaluation of the male reproductive system is critical in the successful management of male infertility. This chapter provides an overview of the aetiology and contemporary management strategies for male factor infertility especially in the context of advances in assisted reproductive technique.
3. Aetiology
The aetiology of male factor infertility has traditionally been classified as pre-testicular, testicular, and post-testicular factors and these factors have been viewed as congenital, acquired, or idiopathic. Idiopathic factors account for at least 50% of the causative factors of male infertility and a precise diagnosis is often lacking.
Congenital causes of male infertility include cryptorchidism (undescended testis), congenital absence of the vas deferens (CAVD), anorchia, genetic endocrinopathy, genetic abnormalities and Robertsonian translocations. Acquired factors on the other hand range from infectious morbidities to trauma. These include recurrent urogenital infections leading to urogenital tract obstructions, inflammatory conditions such as epididymitis and orchitis, testicular trauma, torsion, tumours, exposure to radiation, chemotherapy, and heat. Other acquired factors are groin surgeries, anti-sperm antibodies, systemic diseases such as liver cirrhosis, varicocele, and erectile dysfunction.
Behavioural factors which affect male fertility include obesity and dietary factors, smoking/vaping, alcohol ingestion, use of recreational drugs and exposure to environmental toxins. These extrinsic factors are often modifiable as they relate to physical activity, environmental exposure, diet, and body habitus. When appropriately controlled, therapy may be achieved and therefore should be the initial step in the management of male infertility. Advanced paternal age is an independent risk factor for poor quality semen and should be considered in the evaluation of male factor infertility especially in the elderly couple. An important extrinsic factor often experienced in rapidly industrialized nations is “stress” which may be physical or psychological. Stress is postulated to negatively affect male fertility due to the associated elevated corticosteroid which suppresses testosterone production and ultimately spermatogenesis [13, 14]. Reactive oxygen species which are by-products of oxygen can be detrimental to semen function as a preponderance of these free radicals over the antioxidant defence mechanism of the body may be injurious to sperm survival [15, 16].
Besides a detailed history and thorough physical examination, the diagnosis of male infertility relies majorly on conventional semen analysis. This is consequent on the fact that normal semen parameters (motility, morphology & count) have been associated with timely reproduction [17, 18]. Furthermore semen analysis is non-invasive and cost effective and it provides in a timely manner the evidence and extent of male contribution to infertility. The latest classification of semen disorders is based on the World Health Organization’s (WHO) reference values introduced in 2010 in which semen characteristic threshold for impairments were markedly lowered [19].
4. Investigations
Evaluating male factor infertility begins with a detailed medical history and a comprehensive physical examination. The history seeks to evaluate possible medical causes and risk factors, while the examination aims at providing holistic feedback on the systemic consequences of past exposures and evaluating the current health status of the male partner. During history taking, factors that may affect fertility should be actively sought after such as infectious diseases, genital trauma, groin surgery, diseases in childhood and puberty, exposure to environmental toxins and social/sexual habits. Examination should document body habitus along with the presence or absence of secondary sexual characteristics. A thorough examination of the testis should emphasize size, consistency, presence of masses and symmetry. Varicoceles should be excluded, while a rectal examination is performed to evaluate the prostate gland.
The following are useful investigations in the evaluation of male infertility.
Seminal fluid analysis: This is an inexpensive, non-invasive prognostic test of male fertility. Due to wide variability, usually 2 samples are collected 2 weeks apart following 2–5 days of abstinence. Ejaculation occurs preferably in the laboratory into a sterile container. However, for certain psychological reasons, this may be done in a more conducive location such as the patients’ home with the aid of the spouse and delivered to the laboratory within 60 minutes of production. Parameters evaluated include semen count, motility, morphology, pH, volume, viscosity, liquefaction time and presence of biochemical markers.
Hormone profile: This is very important in distinguishing between obstructive and non-obstructive azoospermia. Very commonly, evaluating follicle-stimulating hormone (FSH), luteinizing hormone (LH) and testosterone will suffice in establishing an endocrine basis for male infertility. More detailed studies are seldomly performed except when indicated.
Imaging studies: Ultrasonography is especially important in the assessment of testicular size and evaluation of testicular masses, obstruction, and absence of the vas deferens. It can also be used to evaluate testicular blood floor and reflux in cases of varicocele. The trans-rectal ultrasonography (TRUS) is usually performed in cases of suspected obstruction evidence by seminal volume. Ultrasonography provides an avenue for anatomical evaluation of the male external genitalia with the view to exclude congenital malformations.
Semen culture: This is not routinely performed but indicated in the presence of genital tract infections especially following sexually transmitted disease (STD). An increased white cell count in the presence of minimal ejaculate may suggest partial obstruction of the ejaculatory ducts. Spermatotoxic free radicals are increased in the presence of genital infections and may be responsible for diminishing male fertility.
Testicular biopsy: A useful diagnostic tool in men with suspected obstructive azoospermia. It is therapeutic during assisted reproductive technique where aspirated/biopsied tissue is teased out to obtain sperm cells which are injected directly into the oocyte via intracytoplasmic sperm injection (ICSI).
Sperm DNA fragmentation: is a complimentary test used to evaluate sperm functionality, however its diagnostic accuracy is limited because of lack of discriminatory power especially in predicting outcomes following assisted reproductive technique (ART). There are several tests used to evaluate sperm DNA fragmentation such as sperm chromatin dispersion test (SCD) and sperm chromatin structure assay (SCSA). Infertile men especially oligospermic, obese and alcoholics have been shown to have a higher percentage of fragmented DNA [20].
Genetic testing: Certain conditions require genetic testing in the management of male factor infertility, and it has been suggested that the incidence of genetic abnormalities is higher in infertile men requiring assisted reproductive technique. Routine tests recommended for severe semen abnormalities include Y chromosome microdeletion analysis, karyotyping and transmembrane conductance regulator (CFTR) mutation analysis. Fluorescence in situ hybridization technology (FISH) is employed in the direct genetic testing of spermatozoa especially for chromosomal aneuploidy.
5. Management
The management of male factor infertility can sometimes be enigmatic and must take into consideration the presence of concomitant female factors. Generally, management involves lifestyle modification and a combination of either medical or surgical management. Severe male factor infertility will necessitate the deployment of assisted reproductive technology (ART) and in certain instances may require the use of donor sperm especially in the presence of testicular failure. Lifestyle modification through counselling is important in the initial management of alcohol, substance abuse, hazardous occupational exposure, and obesity. A review of medications is quite important since many drugs used in the management of other systemic ailments can affect spermatogenesis.
5.1 Medical management
A variety of hormonal medications have been employed in the medical management of male factor infertility, however, evidence about their efficacy measured by actual pregnancy outcome is questionable [21]. The exception is in the clinical management of gonadotrophin deficiency where replacement with exogenous gonadotrophin is quite effective at enhancing sperm production and conception. In patients with hypogonadotropic hypogonadism, pulsatile gonadotrophin releasing hormone (GnRH) has been found useful and have been associated with improved spermatogenesis. Dopamine agonists such as bromocriptine, cabergoline have been found useful in the management of hyperprolactinaemia. Glucocorticoids on the other hand, have been employed in the successful management of sperm autoimmunity, however the risk of long-term high dose therapy is quite detrimental and early recourse to assisted reproductive technique is preferred. Furthermore, in certain patients with idiopathic oligospermia, tamoxifen, an antioestrogen has been found to increase the rate of natural conception [22]. Antioxidants have been demonstrated to decrease the DNA fragmentation induced by oxidative stress and multivitamin supplementation with vitamins C & E may improve conception [23].
5.2 Surgical management
Several surgical techniques have been employed in the management of male factor infertility with variable outcomes. Varicocelectomy is one of the most performed surgical techniques and it has been demonstrated to improve semen quality in about 44% of those treated [24]. Reconstructive surgical procedures include epididymovasostomy and vasovasostomy which are generally performed as microsurgical procedures. Indications for epididymovasostomy include congenital or acquired obstructions at the level of the epididymis. An important caveat prior to embarking on these procedures is ascertaining testicular function through a hormone profile or testicular biopsy. The development of antisperm antibodies is a limiting factor and this influences pregnancy outcomes in combination with other prognostic factors such as semen quality and age of the female partner.
Obstruction of the prostatic urethra is usually treated by transurethral incision, and this has been demonstrated to improve semen quality and natural conception [25]. Surgical sperm aspiration techniques such as testicular sperm aspiration (TESA), microsurgical epididymal sperm aspiration (MESA) and percutaneous epidydimal sperm aspiration (PESA) are combined with intracytoplasmic sperm injection (ICSI) during assisted reproductive technique in men with obstructive azoospermia. These procedures allow limited spermatozoa to be collected from the male reproductive organ and injected into the oocyte during in-vitro-fertilization (IVF).
5.3 Assisted reproductive technique (ART)
Advancement in the management of infertility has resulted in the deployment of ART which has significantly improved the ability of infertile couples to have their own biologic offspring. This can be achieved using intrauterine insemination (IUI), in-vitro-fertilization (IVF) and intracytoplasmic sperm injection (ICSI). In men with mild semen abnormalities, progressively motile spermatozoa are washed and inseminated during the mid-cycle into the uterine cavity following ovulation induction. However, in the presence of severe semen abnormalities, in-vitro-fertilization, and intracytoplasmic sperm injection (IVF/ICSI) are offered. In men with severe male factor infertility and non-obstructive azoospermia, clinical pregnancy rates following IVF/ICSI have been shown to be lower than men with normospermia, thus demonstrating the importance of careful morphological selection of spermatozoa during ART [26]. Testicular derived spermatozoa have lower amounts of sperm DNA fragmentation compared with ejaculated sperm [27] and it has been suggested albeit cautiously that testicular sperm extraction in combination with ICSI may be beneficial in non-azoospermic men with elevated sperm DNA Fragmentation. Sperm donation should be given consideration in cases of testicular failure.
6. Challenges in developing countries
Male infertility is often relegated to the background in many developing countries and management often focuses on female factors due to deeply rooted sociocultural beliefs and norms. This situation is further compounded by the inequity in access to health facilities and treatment of infertility which is largely uninsured and expensive. There however exist a high premium on childbearing and this inadvertently predisposes infertile couples to seeking alternative and unorthodox care which often result in delays that further worsens the outcome of care. Infertility particularly male factor increases conjugal mobility in a quest to confirm potency and possibly have an offspring. Investigation for male factor infertility is often fraught with resistance and very often only basic tests such as seminal fluid analysis, hormone profile and ultrasonography are readily available. Advanced tests are usually performed at specialized fertility centres which are very few and often concentrated in urban cities.
The cost of assisted conception services is prohibitive and has remained a rate limiting step in the access to advanced fertility care in many developing countries lacking effective health insurance coverage. Furthermore, there exist a preponderance of risk factors ranging from environmental pollution, exposures to occupational hazards, poorly treated sexually transmitted diseases and harmful traditional practices. The panacea for infertility care in developing countries will entail a paradigm shift in perception about aetiology and management through the provision of educational and information services. Efforts must be geared towards the elimination of harmful traditional practices which negatively influence the health seeking behaviour and sometimes pose a risk to compliance with orthodox care. There is also an urgent need to expand the health insurance system such that provision is made for the management of involuntary childlessness. Mitigating risk factors will include the control of environmental pollution and hazard while prioritizing the management and prevention of sexually transmitted diseases. Concerted effort is needed in developing countries to address the gap in access to care through better funding and incorporation of holistic care in the management of male infertility.
7. What does the future hold?
There has been a gradual inclination towards research into protein biomarkers of male infertility, however an important limitation is the lack of unique markers associated with specific medical conditions [28, 29]. It is expected that advancement in disease-targeted sequencing and epigenetic semen analysis will expand the scope of genetic testing and treatment of male infertility [30]. Stem cell therapeutics have witnessed significant improvement and induced pluripotent stem cells have been employed during in-vitro models to produce sperm [31]. It has been suggested that these induced pluripotent stem cells can be utilized with gene editing to correct genetic disorders and restore spermatogenesis in patients who have been exposed to either chemotherapy or radiotherapy [32]. Artificial Intelligence (AI) predictive algorithms have been developed to select men who will benefit from genetic testing and ART [33]. Though in its infancy, the application of AI in andrology and urology seems promising and may be the game changer in the near future [33, 34].
8. Conclusion
Male factor infertility is responsible for about half of the cases of infertility and a sound understanding of the aetiology and treatment options is important for the successful management of involuntary childlessness. In many cases, male infertility is amenable to treatment through lifestyle modifications, medical and surgical interventions and in severe cases, assisted reproductive techniques. Advances in assisted reproductive techniques has offered men who naturally would not be able to conceive the opportunity of having biologic progenies and where testicular failure has been demonstrated, gamete donation or adoption remains a viable option in well motivated couples. Advances in the development of protein biomarkers, stem cell therapeutics and artificial intelligence will further widen the spectrum of therapeutic opportunities available to infertile men (Figure 1).
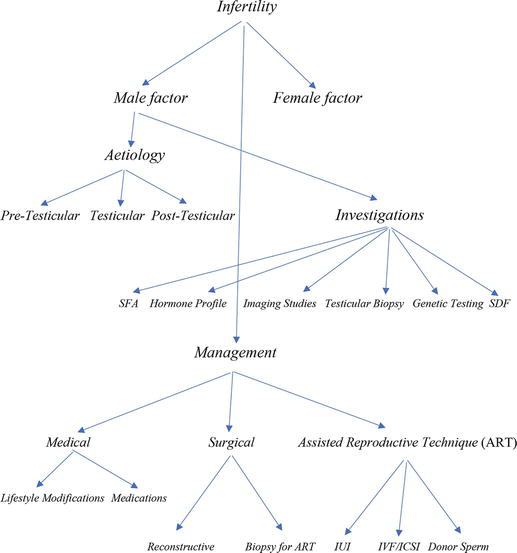
Figure 1.
Schematic representation of the management of male factor infertility. Key: SFA: seminal fluid analysis; SDF: sperm DNA fragmentation; IUI: intrauterine insemination; IVF/ICSI: in-vitro-fertilization/intracytoplasmic sperm injection.
References
- 1.
WHO. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. United Kingdom: Cambridge University Press; 2000 - 2.
Petersen GL, Blenstrup LT, Peterson BD, Knudsen LB, Schmidt L. Impact of childlessness on life and attitudes towards continuation of medically assisted reproduction and/or adoption. Human Fertility Cambridge. Jun 2015; 18 (2):121-127. DOI: 10.3109/14647273.2015.1006691 - 3.
Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: The hidden hardship of building a family. Fertility and Sterility. 2013; 99 (7):2025-2030. DOI: 10.1016/j.fertnstert.2013.01.145 - 4.
Obajimi GO, Kolade CO, Aladejare A. Is male factor infertility rising? Another side of the equation from an in vitro fertilization clinic in southwestern Nigeria. African Journal of Infertility Assistant Concept. 2021; 6 :10-13 - 5.
Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, et al. Male infertility. Lancet. 2021; 397 (10271):319-333. DOI: 10.1016/S0140-6736(20)32667-2 - 6.
Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reproductive Biology and Endocrinology. 2015; 13 :37. DOI: 10.1186/s12958-015-0032-1 - 7.
Krausz C. Male infertility: Pathogenesis and clinical diagnosis. Best Practice & Research. Clinical Endocrinology & Metabolism. 2011; 25 (2):271-285. DOI: 10.1016/j.beem.2010.08.006 - 8.
Salonia A, Matloob R, Gallina A, Abdollah F, Saccà A, Briganti A, et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. European Urology. 2009; 56 (6):1025-1031. DOI: 10.1016/j.eururo.2009.03.001 - 9.
Hanson BM, Eisenberg ML, Hotaling JM. Male infertility: A biomarker of individual and familial cancer risk. Fertility and Sterility. 2018; 109 (1):6-19. DOI: 10.1016/j.fertnstert.2017.11.005 - 10.
Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics (Sao Paulo). 2013; 68 (Suppl 1):27-34. DOI: 10.6061/clinics/2013(sup01)04 - 11.
Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertility and Sterility. 2017; 107 (4):848-859. DOI: 10.1016/j.fertnstert.2017.02.115 - 12.
Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: A systematic review and meta-regression analysis. Human Reproduction Update. 2017; 23 (6):646-659. DOI: 10.1093/humupd/dmx022 - 13.
Nargund VH. Effects of psychological stress on male fertility. Nature Reviews. Urology. 2015; 12 (7):373-382. DOI: 10.1038/nrurol.2015.112 - 14.
Arya ST, Dibb B. The experience of infertility treatment: The male perspective. Human Fertility (Cambridge, England). 2016; 19 (4):242-248. DOI: 10.1080/14647273.2016.1222083 - 15.
de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Human Reproduction. 1995; 10 (1):15-21 - 16.
Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian Journal of Experimental Biology. 2005; 43 (11):963-974 - 17.
Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES Jr, Agarwal A. Critical appraisal of World Health Organization’s new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012; 79 (1):16-22. DOI: 10.1016/j.urology.2011.08.003 - 18.
Agarwal A, Bragais FM, Sabanegh E. Assessing sperm function. The Urologic Clinics of North America. 2008; 35 (2):157-171. DOI: 10.1016/j.ucl.2008.01.012 - 19.
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press; 2010 - 20.
Ajayi AB, Afolabi BM, Ajayi VD, Oyetunji IO, Atiba A, Saanu S, et al. Evaluation of Sperm DNA Fragmentation amongst Infertile Black Africans. A Nigerian Study. Open Journal of Urology. 2018; 8 :297-316. DOI: 10.4236/oju.2018.811034 - 21.
Liu PY, Handelsman DJ. The present and future state of hormonal treatment for male infertility. Human Reproduction Update. 2003; 9 (1):9-23. DOI: 10.1093/humupd/dmg002 - 22.
Adamopoulos DA, Pappa A, Billa E, Nicopoulou S, Koukkou E, Michopoulos J. Effectiveness of combined tamoxifen citrate and testosterone undecanoate treatment in men with idiopathic oligozoospermia. Fertility and Sterility. 2003; 80 (4):914-920. DOI: 10.1016/s0015-0282(03)01123-3 - 23.
Makker K, Agarwal A, Sharma R. Oxidative stress and male infertility. The Indian Journal of Medical Research. 2009; 129 (4):357-367 - 24.
Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A. Weidner W; EAU Working Group on Male Infertility. EAU guidelines on male infertility. European Urology. 2005; 48 (5):703-711. DOI: 10.1016/j.eururo.2005.06.002 - 25.
Schroeder-Printzen I, Ludwig M, Köhn F, Weidner W. Surgical therapy in infertile men with ejaculatory duct obstruction: Technique and outcome of a standardized surgical approach. Human Reproduction. 2000; 15 (6):1364-1368. DOI: 10.1093/humrep/15.6.1364 - 26.
Lee SH, Song H, Park YS, Koong MK, Song IO, Jun JH. Poor sperm quality affects clinical outcomes of intracytoplasmic sperm injection in fresh and subsequent frozen-thawed cycles: Potential paternal effects on pregnancy outcomes. Fertility and Sterility. 2009; 91 (3):798-804. DOI: 10.1016/j.fertnstert.2007.12.061 - 27.
Esteves SC, Roque M, Bradley CK, Garrido N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: Systematic review and meta-analysis. Fertility and Sterility. 2017; 108 (3):456-467. DOI: 10.1016/j.fertnstert.2017.06.018 - 28.
Swain N, Samanta L, Agarwal A, Kumar S, Dixit A, Gopalan B, et al. Aberrant upregulation of compensatory redox molecular machines may contribute to sperm dysfunction in infertile men with unilateral varicocele: A proteomic insight. Antioxidants & Redox Signaling. 2020; 32 (8):504-521. DOI: 10.1089/ars.2019.7828 - 29.
Panner Selvam MK, Agarwal A, Pushparaj PN. A quantitative global proteomics approach to understanding the functional pathways dysregulated in the spermatozoa of asthenozoospermic testicular cancer patients. Andrology. 2019; 7 (4):454-462. DOI: 10.1111/andr.12620 - 30.
Thirumavalavan N, Gabrielsen JS, Lamb DJ. Where are we going with gene screening for male infertility? Fertility and Sterility. 2019; 111 (5):842-850. DOI: 10.1016/j.fertnstert.2019.03.036 - 31.
Nagamatsu G, Hayashi K. Stem cells, in vitro gametogenesis and male fertility. Reproduction. 2017;154 (6):F79-F91. DOI: 10.1530/REP-17-0510 - 32.
Fang F, Li Z, Zhao Q , Li H, Xiong C. Human induced pluripotent stem cells and male infertility: An overview of current progress and perspectives. Human Reproduction. 2018; 33 (2):188-195. DOI: 10.1093/humrep/dex369 - 33.
Chu KY, Nassau DE, Arora H, Lokeshwar SD, Madhusoodanan V, Ramasamy R. Artificial intelligence in reproductive urology. Current Urology Reports. 2019; 20 (9):52. DOI: 10.1007/s11934-019-0914-4 - 34.
Bentellis I, Guérin S, Khene ZE, Khavari R, Peyronnet B. Artificial intelligence in functional urology: How it may shape the future. Current Opinion in Urology. 2021; 31 (4):385-390. DOI: 10.1097/MOU.0000000000000888