Abstract
The Developmental Origin of Health and Disease (DOHaD) theory, in which the prenatal environment is involved in the development of diseases after birth, has been widely accepted. This theory is widely accepted, and the involvement of the prenatal environment in the development of adult diseases (lifestyle diseases) is almost certain. As an extension of the DOHaD theory, the Testicular Dysgenesis Syndrome (TDS) hypothesis, which focuses specifically on diseases of the male reproductive system, proposes that environmental changes during the embryonic period are involved in the development of a number of diseases of the male reproductive system, such as hypospadias, cryptorchidism, low sperm count, and infertility. A few experimental studies were performed; however, the results have been limited and have not addressed the pathogenic mechanism of TDS. We have conducted research using a mouse model of maternal nutritional deprivation. In this study, under/hyponutrition during fetal life impairs testosterone production in the fetal testis and causes a decrease in sperm count after growth. Further studies elucidated that this may be due to oxidative stress-induced germ cell apoptosis caused by fetal testosterone depletion. The molecular biological background to the DOHaD theory is epigenetic modification, but very few studies have focused on epigenetic modification in TDS, which shares the same background as the DOHaD phenomenon. We will further discuss the contribution of epigenomic modifications in the development of TDS.
Keywords
- developmental origin of health and diseases
- testicular dysgenesis syndrome
- maternal under/hyponutrition
- male infertility
- epigenomic modification
1. Introduction
The Developmental Origin of Health and Disease (DOHaD) theory that posits the prenatal environment is involved in the development of diseases after birth is widely accepted, and the involvement of the prenatal environment in the development of adult diseases has been established [1]. Furthermore, as an extension of the DOHaD theory, the Testicular Dysgenesis Syndrome (TDS) hypothesis, which particularly focuses on male reproductive system diseases, has been proposed by Professor Skakkebeak in Denmark [2, 3]. This hypothesis proposes that environmental changes during the embryonic period are involved in the development of a series of male reproductive system diseases, such as hypospadias, cryptorchidism, testicular cancer, decreased sperm count, and infertility. The pathogenesis of TDS is assumed to be reduced male hormone (testosterone) action due to testicular damage during the embryonic period [4].
2. Fetal growth restriction (FGR) and male reproductive system disorder
Nutrition and metabolism are essential factors when discussing the prenatal environment. Maternal undernutrition is one of the important causes of FGR [5]. It has been reported that FGR is strongly associated with mild phenotype disorder of sex development/differentiation including hypospadias [6, 7] and cryptorchidism [8, 9, 10]. Furthermore, a recent, large cohort study on the relationship between birth records and fertility has been reported from Denmark [11]. The study included more than 10,000 individuals born between 1984 and 1987, consisting of 5342 women and 5342 men. Of these, approximately 10% were born small for gestational age (SGA- a birth weight below the 10th percentile). This study found that there was a 55% increased risk for infertility in men born SGA compared with men born appropriate for gestational age (AGA), not in women. The following study from Sweden also showed that men born SGA or with low birth weight had a lower chance of becoming fathers than men born AGA or with normal birth weight [12]. In addition, an association between FGR and the development of TDS, which considers multiple male reproductive system diseases as a syndrome related to the fetal environment, has been reported by a human study [13]. Together, many epidemiological data from human studies have reported the significant relationship between FGR and wide-ranging male reproductive problems.
Here are several previous reports from basic studies using experimental animals. Maternal 50% food restriction during both gestation and lactation or lactation alone significantly reduced testicular growth in offspring, and also reduced circulating levels of FSH in rats [14]. In an experiment in which pregnant ewes were fed with 50% calory intake in early and late gestation, male lambs born from nutritionally restricted mothers showed a decrease in Sertoli cells in the testis at 10 months of age. Correspondingly, an excess response of FSH in the GnRH loading test was observed [15]. A study in piglets found that maternal calorie restriction during pregnancy reduced Sertoli cells, embryonic cells, and Leydig cells in male piglets born. Apoptotic cells were also found to be more in male piglets from calorie-restricted mothers [16]. Transcriptome analysis in the testes of male pigs revealed that maternal calorie restriction altered a group of genes involved in lipid metabolism, apoptosis, and cell proliferation. In rat offspring, maternal protein restriction during pregnancy reduces the testicular and epidydimal sperm count and affects fertility in the rat offspring [17, 18, 19].
Although these experiments provide scientific support for the association between maternal nutrition and the development of male reproductive problems after birth, basic data are still scarce, and the molecular biological background is in the process of being elucidated.
3. Importance of energy metabolism in various aspects of the testis
The testis is the organ responsible for spermatogenesis and the secretion of the major male hormone testosterone. Energy metabolism in the testis has been shown to be important for differentiation and development of testis, and maintenance of testicular function.
In mice, a key event in the early stages of testis differentiation is the activation of Sex-determining region Y (SRY) in pre-Sertoli cells in the gonadal ridges. At this time, testis-specific glycogen accumulates in the pre-sertoli cells. This serves to store an energy source for morphogenesis and hormone production during testis development [20]. Sertoli cell differentiation, a central event in testis formation, requires SRY expression and subsequent SRY-Box9 (SOX9) activation. Through glucose deprivation and metabolic rescue experiments in mice genital ridge cultures, it was demonstrated that an adequate supply of glucose was the most important environment for establishing SOX9 activation in testis differentiation [21]. Additionally, during the differentiation of fetal Leydig cells in mice, a number of genes involved in metabolic pathways, such as tricarboxylic acid cycle, glycolysis, and oxidative phosphorylation, are heavily expressed [22]. Turning to germ cells primordial germ cells (PGCs), the origin of germ cells are found to have very different energy metabolism from pluripotent stem cells (PSCs). Furthermore, for the differentiation of PSCs into PGCs, oxidative phosphorylation is essential [23]. Together, the unique energy metabolic system is important for establishing and maintaining PGC characteristics.
These results suggest that proper nutrition and metabolism play a crucial role in the growth and functioning of the testes. Therefore, it has been hypothesized that intrauterine malnutrition contributes to the emergence of “testicular dysgenesis syndrome (TDS),” which is primarily brought on by unfavorable environmental factors during fetal life and is linked to a number of reproductive abnormalities, such as hypospadias, cryptorchidism, and infertility (Figure 1).
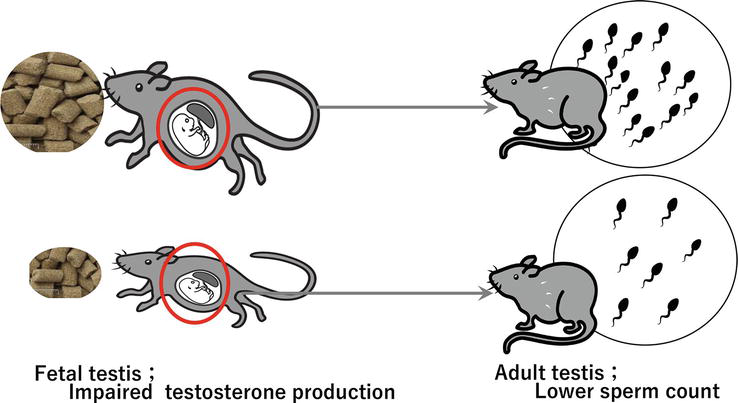
Figure 1.
Maternal caloric restriction resulted in decreased sperm counts after birth (Fujisawa et al. [
4. Intrauterine under/hyponutrition leads to male reproductive dysfunction
Previous studies have shown that maternal under/hyponutrition is thought to increase the chance of developing TDS in the human. However, the underlying mechanism(s) remain largely unknown despite some experimental studies. To clarify the underlying mechanism(s), we performed experimental studies using mice. We set two groups: control females (C-females) were given regular food ad libitum throughout the course of pregnancy while calorie-restricted females (R-females) received 50% of the C-females’ mean daily intake from 6.5 dpc. Then, we evaluated male reproductive results between 17.5-days post-coitum-old male mice delivered from C-females (C-fetuses) and those from R-females (R-fetuses) and between six-week-old male mice born to C-females (C-offspring) and those born to R-females (R-offspring) (Figure 2) [24].
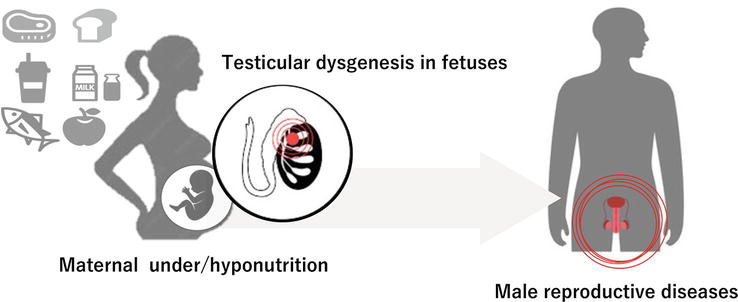
Figure 2.
Maternal under/hyponutrition and male reproductive diseases associated with testicular dysgenesis in fetuses.
The external genitalia of the R-fetuses were morphologically normal. However, the anogenital distance (AGD) index (AGDI) calculated by dividing the AGD by the cube root of body weight was significantly shorter in the male R-fetuses than in the male C-fetuses. This indicates reduced exposure to androgen during the fetal period. Intratesticular testosterone levels were significantly low in the R-fetuses compared with the C-fetuses, in association with significantly reduced expressions of steroidogenic genes including
Furthermore, sperm count was significantly lower in the R-offspring than in the C-offspring at 6 weeks of age while the testicular size and sperm motility were comparable between the two groups. In addition, the number of the R-offspring’s TUNEL-positive cells—which are apoptotic cells—was noticeably larger than the C-offspring’s. Moreover, the number of tubules containing TUNEL-positive cells was much higher in the R-offspring than in the C-offspring, and the percentage of TUNEL-positive cells per 100 tubules was obviously high in the R-offspring. The examined sperms must have been generated during the perinatal period, when it is expected that the intratesticular testosterone in R-fetuses is still low, taking into consideration the length of spermatogenesis and movement from the testis to the cauda epididymis [31]. Given that it has been reported that testosterone deprivation leads to germ cell apoptosis [32], a low testosterone environment during the fetal period is likely to be associated with lower sperm count induced by cell apoptosis.
Microarray analysis on the testis in offspring at 6 weeks of age revealed more than 1000 genes that showed significant variation. Next, we picked two genes that showed up-regulation and eight genes that showed down-regulation that were reportedly important for spermatogenic activity. The R-offspring showed considerably up-regulated expressions of
Of note, by microarray analysis at 6 weeks of age, the R-offspring showed lower expressions of
5. Epigenomic modifications as the biological basis for the TDS hypothesis
TDS can be considered part of the clinical spectrums of DOHaD theory. This theory proposes that epigenetic changes occurring during fetal and early neonatal period determine disease risk and health [51]. In fact, a number of epigenetic changes have been reported to occur during early life induced by nutritional conditions [52]. Thus, the pathophysiological background of TDS would contain epigenomic modification. Very few studies have focused on epigenomic modifications in TDS, which shares a common background with the DOHaD phenomenon. Recently the epigenome during gametogenesis is altered by factors such as nutritional environment and aging, and can cause multiple diseases.
For example, DNA methylation in sperm due to aging increases or decreases in specific genomic regions [53, 54]. Furthermore, the histone modification H3K4me3 in sperm acts as an apologetic sensor for folate deficiency and obesity [55] and H3K9me2 in spermatozoa is reduced by protein deficiency in [56]. Together, germline-specific epigenomic regulation mechanisms very likely link to metabolic status. Namely the “metabolic-epigenomic crosstalk”, in which intracellular metabolic changes lead to epigenomic changes, may function during gametogenesis. Furthermore, non-coding RNAs (ncRNAs), which regulate gene expressions and chromatin structure has been found to involve in the epigenetic program. Recently, environmental stressors such as environmental chemicals have been shown to induce TDS-like symptoms in the next generation through ncRNA-mediated epigenetic modifications in the germline of pups [57, 58]. These findings suggest that research focusing on the importance of epigenomic modification mechanisms as a pathogenic mechanism of TDS is expected to be developed in near future.
References
- 1.
Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004; 305 (5691):1733-1736. DOI: 10.1126/science.1095292 - 2.
Skakkebaek NE. Testicular dysgenesis syndrome. Hormone Research. 2003; 60 (Suppl. 3):49. DOI: 10.1159/000074499 - 3.
Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertility and Sterility. 2008; 89 (2 Suppl):e33-e38. DOI: 10.1016/j.fertnstert.2007.12.026 - 4.
Joensen UN, Jorgensen N, Rajpert-De Meyts E, Skakkebaek NE. Testicular dysgenesis syndrome and Leydig cell function. Basic and Clinical Pharmacology and Toxicology. 2008; 102 (2):155-161. DOI: 10.1111/j.1742-7843.2007.00197.x - 5.
Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biology of Reproduction. 2010; 83 (3):325-331. DOI: 10.1095/biolreprod.110.084517 - 6.
Nordenvall AS, Frisen L, Nordenstrom A, Lichtenstein P, Nordenskjold A. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: Incidence and risk factors. Journal of Urology. 2014; 191 (3):783-789. DOI: 10.1016/j.juro.2013.09.058 - 7.
Gatti JM, Kirsch AJ, Troyer WA, Perez-Brayfield MR, Smith EA, Scherz HC. Increased incidence of hypospadias in small-for-gestational age infants in a neonatal intensive-care unit. BJU International. 2001; 87 (6):548-550 - 8.
Jensen HI, Ammentorp J, Erlandsen M, Ording H. End-of-life practices in Danish ICUs: Development and validation of a questionnaire. BMC Anesthesiology. 2012; 12 :16. DOI: 10.1016/j.pedneo.2020.07.011 - 9.
Nemec SF, Nemec U, Brugger PC, Bettelheim D, Weber M, Graham JM Jr, et al. Male genital abnormalities in intrauterine growth restriction. Prenatal Diagnosis. 2012; 32 (5):427-431. DOI: 10.1002/pd.3831 - 10.
Barthold JS. Undescended testis: Current theories of etiology. Current Opinion in Urology. 2008; 18 (4):395-400. DOI: 10.1002/pd.3831 - 11.
Thorsted A, Lauridsen J, Hoyer B, Arendt LH, Bech B, Toft G, et al. Birth weight for gestational age and the risk of infertility: A Danish cohort study. Human Reproduction. 2020; 35 (1):195-202. DOI: 10.1093/humrep/dez232 - 12.
Liffner S, Bladh M, Nedstrand E, Hammar M, Martinez HR, Sydsjo G. Men born small for gestational age or with low birth weight do not improve their rate of reproduction over time: A Swedish population-based study. Fertility and Sterility. 2021; 116 (3):721-730. DOI: 10.1016/j.fertnstert.2021.05.078 - 13.
Xing JS, Bai ZM. Is testicular dysgenesis syndrome a genetic, endocrine, or environmental disease, or an unexplained reproductive disorder? Life Sciences. 2018; 194 :120-129. DOI: 10.1016/j.lfs.2017.11.039 - 14.
Leonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biolpgy of Reproduction. 2003; 68 (2):390-400. DOI: 10.1095/biolreprod.102.003269 - 15.
Kotsampasi B, Balaskas C, Papadomichelakis G, Chadio SE. Reduced Sertoli cell number and altered pituitary responsiveness in male lambs undernourished in utero. Animal Reproductive Science. 2009; 114 (1-3):135-147. DOI: 10.1016/j.anireprosci.2008.08.017 - 16.
Lin Y, Xu XY, Wu D, Lin H, Fang ZF, Feng B, et al. Maternal energy insufficiency affects testicular development of the offspring in a swine model. Scientific Reports. 2019; 9 (1):14533. DOI: 10.1016/j.anireprosci.2008.08.017 - 17.
Toledo FC, Perobelli JE, Pedrosa FP, Anselmo-Franci JA, Kempinas WD. In utero protein restriction causes growth delay and alters sperm parameters in adult male rats. Reproductive Bioligt and Endocrinology. 2011; 9 :94. DOI: 10.1186/1477-7827-9-94 - 18.
Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. Journal of Physiology. 2006; 571 (Pt 1):221-230. DOI: 10.1113/jphysiol.2005.100313 - 19.
Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. Journal of Physiology. 2005; 563 (Pt 1):275-284. DOI: 10.1113/jphysiol.2004.078543 - 20.
Matoba S, Kanai Y, Kidokoro T, Kanai-Azuma M, Kawakami H, Hayashi Y, et al. A novel Sry-downstream cellular event which preserves the readily available energy source of glycogen in mouse sex differentiation. Journal of Cell Sciences. 2005; 118 (Pt 7):1449-1459. DOI: 10.1242/jcs.01738 - 21.
Matoba S, Hiramatsu R, Kanai-Azuma M, Tsunekawa N, Harikae K, Kawakami H, et al. Establishment of testis-specific SOX9 activation requires high-glucose metabolism in mouse sex differentiation. Developmental Biology. 2008; 324 (1):76-87. DOI: 10.1016/j.ydbio.2008.09.004 - 22.
Inoue M, Shima Y, Miyabayashi K, Tokunaga K, Sato T, Baba T, et al. Isolation and characterization of fFetal Leydig progenitor cells of male mice. Endocrinology. 2016; 157 (3):1222-1233. DOI: 10.1210/en.2015-1773 - 23.
Hayashi Y, Otsuka K, Ebina M, Igarashi K, Takehara A, Matsumoto M, et al. Distinct requirements for energy metabolism in mouse primordial germ cells and their reprogramming to embryonic germ cells. Proceedings of the National Academy of Sciences of the United States of America. 2017; 114 (31):8289-8294. DOI: 10.1073/pnas.1620915114 - 24.
Fujisawa Y, Ono H, Konno A, Yao I, Itoh H, Baba T, et al. Intrauterine hyponutrition reduces fetal testosterone production and postnatal sperm count in the mouse. Journal of Endocrogy Society. 2022; 6 (4):bvac022. DOI: 10.1210/jendso/bvac022 - 25.
Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sexual Development. 2008; 2 (4-5):200-209. DOI: 10.1159/000152036 - 26.
Baba T, Otake H, Inoue M, Sato T, Ishihara Y, Moon JY, et al. Ad4BP/SF-1 regulates cholesterol synthesis to boost the production of steroids. Communications Biology. 2018; 1 :18. DOI: 10.1038/s42003-018-0020-z - 27.
Baba T, Otake H, Sato T, Miyabayashi K, Shishido Y, Wang CY, et al. Glycolytic genes are targets of the nuclear receptor Ad4BP/SF-1. Nature Communiations. 2014; 5 :3634. DOI: 10.1038/ncomms4634 - 28.
Li B, Baba T, Miyabayashi K, Sato T, Shima Y, Ichinose T, et al. Role of Ad4-binding protein/steroidogenic factor 1 in regulating NADPH production in adrenocortical Y-1 cells. Endocrine Journal. 2017; 64 (3):315-324. DOI: 10.1507/endocrj.EJ16-0467 - 29.
Vining B, Ming Z, Bagheri-Fam S, Harley V. Diverse regulation but conserved function: SOX9 in vertebrate sex determination. Genes (Basel). 2021; 12 (4):486, 1-23. DOI: 10.3390/genes12040486 - 30.
Teixeira J, Maheswaran S, Donahoe PK. Mullerian inhibiting substance: An instructive developmental hormone with diagnostic and possible therapeutic applications. Endocrine Reveiws. 2001; 22 (5):657-674. DOI: 10.1210/edrv.22.5.0445 - 31.
McCarrey JR. Toward a more precise and informative nomenclature describing fetal and neonatal male germ cells in rodents. Biology of Reproduction. 2013; 89 (2):47. DOI: 10.1095/biolreprod.113.110502 - 32.
Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010; 107 (35):15565-15570. DOI: 10.1073/pnas.1002178107 - 33.
Huang Z, Rivas B, Agoulnik AI. NOTCH1 gain of function in germ cells causes failure of spermatogenesis in male mice. PLoS One. 2013; 8 (7):e71213. DOI: 10.1371/journal.pone.0071213 - 34.
Dumasia K, Kumar A, Deshpande S, Sonawane S, Balasinor NH. Differential roles of estrogen receptors, ESR1 and ESR2, in adult rat spermatogenesis. Molecular and Cellular Endocrinology. 2016; 428 :89-100. DOI: 10.1016/j.mce.2016.03.024 - 35.
Ohyama K, Ohta M, Hosaka YZ, Tanabe Y, Ohyama T, Yamano Y. Expression of anti-Mullerian hormone and its type II receptor in germ cells of maturing rat testis. Endocrine Journal. 2015; 62 (11):997-1006. DOI: 10.1507/endocrj.EJ15-0370 - 36.
Mullen RD, Ontiveros AE, Moses MM, Behringer RR. AMH and AMHR2 mutations: A spectrum of reproductive phenotypes across vertebrate species. Developmental Biology. 2019; 455 (1):1-9. DOI: 10.1016/j.ydbio.2019.07.006 - 37.
Teng YN, Lin YM, Lin YH, Tsao SY, Hsu CC, Lin SJ, et al. Association of a single-nucleotide polymorphism of the deleted-in-azoospermia-like gene with susceptibility to spermatogenic failure. Journal of Clinical Endocrinol Metab. 2002; 87 (11):5258-5264. DOI: 10.1210/jc.2002-020016 - 38.
Schrans-Stassen BH, Saunders PT, Cooke HJ, de Rooij DG. Nature of the spermatogenic arrest in Dazl −/− mice. Biology of Reproduction. 2001; 65 (3):771-776. DOI: 10.1095/biolreprod65.3.771 - 39.
Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, et al. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nature Cell Biology. 2011; 13 (5):599-610. DOI: 10.1038/ncb2213 - 40.
Lalli E, Sassone-Corsi P. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Molecular Endocrinology. 2003; 17 (8):1445-1453. DOI: 10.1210/me.2003-0159 - 41.
Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nature Genetics. 1998; 20 (4):353-357. DOI: 10.1038/3822 - 42.
Pointis G, Fiorini C, Defamie N, Segretain D. Gap junctional communication in the male reproductive system. Biochimica et Biophysica Acta. 2005; 1719 (1-2):102-116. DOI: 10.1016/j.bbamem.2005.09.017 - 43.
Roscoe WA, Barr KJ, Mhawi AA, Pomerantz DK, Kidder GM. Failure of spermatogenesis in mice lacking connexin43. Biology of Reproduction. 2001; 65 (3):829-838. DOI: 10.1095/biolreprod65.3.829 - 44.
Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008; 105 (39):14976-14980. DOI: 10.1073/pnas.0807297105 - 45.
Barakat B, O'Connor AE, Gold E, de Kretser DM, Loveland KL. Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction. 2008; 136 (3):345-359. DOI: 10.1530/REP-08-0140 - 46.
Itman C, Bielanowicz A, Goh H, Lee Q , Fulcher AJ, Moody SC, et al. Murine inhibin alpha-subunit Haploinsufficiency causes transient abnormalities in Prepubertal testis development followed by adult testicular decline. Endocrinology. 2015; 156 (6):2254-2268. DOI: 10.1210/en.2014-1555 - 47.
Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: Protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxidants and Redox Signaling. 2004; 6 (2):289-300. DOI: 10.1089/152308604322899350 - 48.
Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxidants and Redox Signaling. 2011; 15 (7):1957-1997. DOI: 10.1089/ars.2010.3586 - 49.
Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003; 424 (6948):561-565. DOI: 10.1038/nature01819 - 50.
Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends in Biochemical Sciences. 2015; 40 (8):435-445. DOI: 10.1016/j.tibs.2015.05.001 - 51.
Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Americal Journal of Clinical Nutrition. 2000; 71 (5 Suppl):1344S-1352S. DOI: 10.1093/ajcn/71.5.1344s - 52.
Jimenez-Chillaron JC, Diaz R, Martinez D, Pentinat T, Ramon-Krauel M, Ribo S, et al. The role of nutrition on epigenetic modifications and their implications on health. Biochimie. 2012; 94 (11):2242-2263. DOI: 10.1016/j.biochi.2012.06.012 - 53.
Potabattula R, Zacchini F, Ptak GE, Dittrich M, Muller T, El Hajj N, et al. Increasing methylation of sperm rDNA and other repetitive elements in the aging male mammalian germline. Aging Cell. 2020; 19 (8):e13181 - 54.
Yoshizaki K, Kimura R, Kobayashi H, Oki S, Kikkawa T, Mai L, et al. Paternal age affects offspring via an epigenetic mechanism involving REST/NRSF. EMBO Reports. 2021; 22 (2):e51524. DOI: 10.1016/j.biochi.2012.06.012 - 55.
Lismer A, Dumeaux V, Lafleur C, Lambrot R, Brind'Amour J, Lorincz MC, et al. Histone H3 lysine 4 trimethylation in sperm is transmitted to the embryo and associated with diet-induced phenotypes in the offspring. Developmental Cell. 2021; 56 (5):671-686. DOI: 10.1016/j.devcel.2021.01.014 - 56.
Yoshida K, Maekawa T, Ly NH, Fujita SI, Muratani M, Ando M, et al. ATF7-dependent epigenetic changes are required for the intergenerational effect of a paternal low-protein diet. Molecular Cell. 2020; 78 (3):445-458 e446. DOI: 10.1016/j.molcel.2020.02.028 - 57.
Stenz L, Escoffier J, Rahban R, Nef S, Paoloni-Giacobino A. Testicular dysgenesis syndrome and long-lasting epigenetic silencing of mouse sperm genes involved in the reproductive system after prenatal exposure to DEHP. PLoS One. 2017; 2017 :13. DOI: 10.1371/journal.pone.0170441 - 58.
Larriba E, Del Mazo J. Role of non-coding RNAs in the transgenerational epigenetic transmission of the effects of reprotoxicants. International Journal of Molecular Sciences. 2016; 17 (4):452, 1-13. DOI: 10.3390/ijms17040452