Summary of complexation of Schiff base lanthanide complexes (O: Success, X: Failure).
Abstract
[Introductory chapter does not have abstract] Introductory Chapter: Electrophile and Lewis Acid Shunsuke Aoki, Asaki Ishizuka, Daisuke Nakane, Takashiro Akitsu*, Department of Chemistry, Faculty of Science, Tokyo University of Science, Tokyo, Japan, akitsu2@rs.tus.ac.jp Introduction Synthesis of metal complexes often involves electrophilic or nucleophilic reactions of organic ligands and acid/base (catalytic) reactions. Furthermore, the formation of coordination bonds between ligands and metal ions essentially involves the process of association between Lewis bases and Lewis acids (Scheme 1). As a concrete example of the concept of this book, this chapter outlines the conditions for a new synthetic method targeting metal complexes.
Keywords
- Lewis acid
- Schiff base
- coordination compound
1. Introduction
Synthesis of metal complexes often involves electrophilic or nucleophilic reactions of organic ligands and acid/base (catalytic) reactions. Furthermore, the formation of coordination bonds between ligands and metal ions essentially involves the process of association between Lewis bases and Lewis acids (Figure 1). As a concrete example of the concept of this book, this chapter outlines the conditions for a new synthetic method targeting metal complexes.
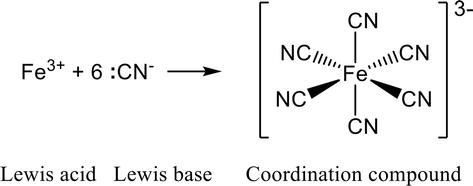
Figure 1.
Formation of coordination compounds from Lewis acid and Lewis base.
Microfluidic device may be a tool for precise and systematic control of energy propagation in minute solutions [1, 2]. The solution in the channel becomes a Hagen–Poiseuille flow with a parabolic streamline distribution due to friction with the inner wall of the microchannel, causing collisions of molecules inside; the solution is mixed at a high speed at the same time as the molecular motion in the solution is activated, and a reaction field can be constructed in which the temperature of the solution is instantaneously switched. Inertial force and viscous force are related, and the smaller the container size, the shorter the solution mixing time. Such system may also be effective for weak intermolecular interactions recently.
We have reported the conventional synthesis of azo-salen type manganese(II) complexes in three steps [3]. Accurate pH control and adjustment of the reaction temperature are indispensable in this synthesis, and the reaction takes about 4 h. Synthetic experiments showed that both the conventional method and the microfluidic device method succeeded in obtaining azo compounds. This result proves that it is possible to synthesize azo compounds under mild pH control using a microfluidic device. In addition, by using a microfluidic device, the chemical reaction was promoted, and the reaction proceeded even with low-concentration hydrochloric acid. In addition, temperature control was unnecessary in microfluidic devices because reactions occur at interfaces. In steps II and III, the microfluidic device was operated at room temperature, and the conventional method was performed at 40°C. In addition, the conventional method required a reaction time of 4 h, but the microfluidic reaction was completed in less than 1 sec. Furthermore, the synthesis by the conventional method had to be performed in a nitrogen atmosphere, but it was possible to perform it in an air atmosphere by using microfluidics.
On the other hand, a reaction system fuses and dissociates “droplets” flowing through a microfluidic device with a nano-scale level solvent and reacts them [4, 5]. Microfluidic devices are tools that can precisely and systematically control energy propagation in microfluidic solutions. We would like to create a reaction system that fuses and dissociates droplets flowing through a microfluidic device and reacts and separates them in a nanoscale solvent. To demonstrate the usefulness of microfluidic devices/droplet synthesis, it is necessary to synthesize them.
In our laboratory, organic solvents are used to prepare and form single crystals of rare earth complexes of Schiff base. Since crystals are formed by volatilizing the organic solvent in the solution, there is a risk of leakage. Indeed, the results of environmental measurement in our laboratory may potentially suggest both fire and health hazards. When organic solvents volatilize to fill a room of laboratory, the vapor is harmful to human health.
In this context, information or knowledge of “interfacial organic solvents” used in preparation (two layers in test tubes) and crystallization (mutually dissolving solvents and solutes, vapor diffusion method) will be important or useful for experimental design of microfluid preparation devices in the near future. From the viewpoint of such analogy, in this chapter, we will present chemistry using interfacial organic solvents taken from our conventional experiments.
Herein, we present several examples that are difficult to synthesize in conventional ways. In order to demonstrate the usefulness of microfluidic/droplet synthesis, search for examples that cannot (or are difficult to) be synthesized in a beaker work (normal scale preparations) should be carried out. Comparing examples of metal complexes synthesized and crystallized at the interface of two solvents, we will study the accumulation of difficult conditions (so-called ‘negative data’) for synthesis by changing metals and substituents of salen-type lanthanide complexes that can be synthesized and crystallized depending on the metals.
2. Results
At first, preparations of metal complexes using two solutions systems are mentioned below.
These two-solvent cyano-bridged manganese salen complexes can be easily synthesized at the solution interface [6, 7]. However, if the substituents (X, Y) in aldehyde precursors and synthesis method are changed even slightly from the previously reported methods, conventional synthesis becomes difficult (Figure 2). Porous coordination polymers composed of metal ions and cyano groups exhibit magnetism, charge transfer, proton conductivity, etc. derived from metal ions. There are some expected advantages as follows: It is easy to design and synthesize. Previous studies with large spaces at the outer methoxy groups showed that the redox potential of salen complexes shifted due to substituent effects. Since there is a large space at the 3,3′-positions, it is possible to introduce a substituent to further construct the reaction space.
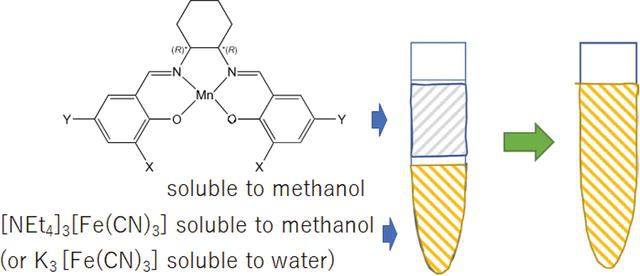
Figure 2.
Conventional preparation of cyano-bridged manganese salen complexes.
By slight modification of preparation conditions, many trials resulted in failure unfortunately, which are as follows:
The pH adjustment (metal source is chloride and triethylamine, and no combination of pH > 7 is possible) was not appropriate. In addition, we were concerned about whether the deprotonation of phenol is possible and whether the form of manganese(II) ion exists (heating/stirring). (What are the stable species in the E vs. pH diagram?) What is the nature of the hydrolysis of the salt (MnCl2) of a weak base (Mn (OH)2) and a strong acid (HCl)? If no crystals precipitate, the concentration of the solution may be too low. The pH adjustment (metal source is chloride and triethylamine and impossible to combine pH > 7) was not proper. In addition, whether the deprotonation of phenol is possible and what is the mode of existence of manganese(II) ions (Figure 3)?
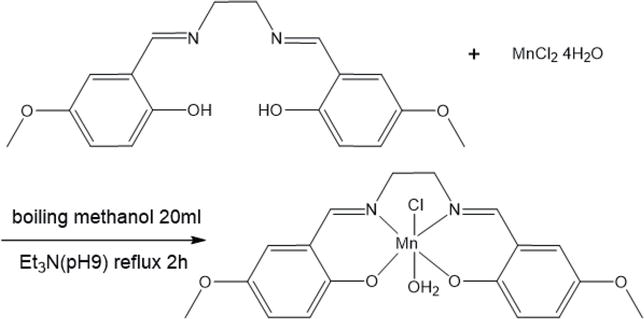
Figure 3.
Failure due to pH adjustment and redox of manganese(II) ion.
What is the nature of the hydrolysis of the salt (MnCl2) of a weak base (Mn (OH)2) and a strong acid (HCl)? From the beginning of ligand synthesis using manganese acetate, it should not make sense to add a counter anion (ClO4−). Here, methanol was used as the solvent and heated to 40°C. If manganese(II) is not oxidized to manganese(III), hexacyanoferrate(III) acid (anion) will not bind either. In rare cases, manganese(III) was generated during stirring (nitrate ions contained in the crystals were confirmed).
From the beginning of the ligand synthesis using manganese(II) acetate, even if counter anion (ClO4−) is added, it is meaningless. Here, the solvent used was methanol, which was heated to 40°C. If manganese(II) is not oxidized to manganese(III), hexacyanoferrate(III) acid (anion) also does not bind (Figure 4). In rare cases, manganese(III) was formed during stirring (which was confirmed nitrate ions included in the crystal).
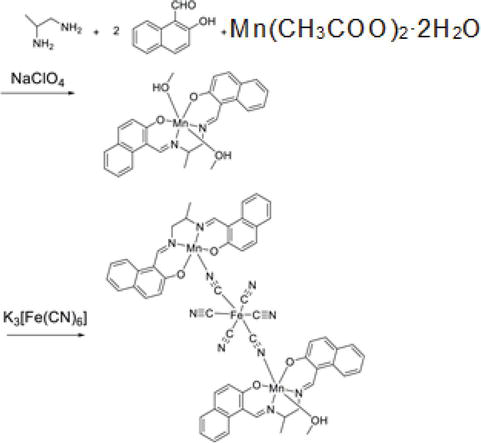
Figure 4.
Failure due to ClO4− ion.
Potassium hexacyanoferrate(III) is soluble in water and in methanol, but it is not necessary. Nitrogen does not oxidize or reduce iron(III) for cations that are soluble in methanol under a nitrogen atmosphere. Synthetic method for obtaining manganese(III) complexes, in one pot, ClO4− (−1 valence, acid) for isolation of salen-type manganese(III) complexes is added at the same time. Manganese(III) acetate alone is weakly acidic with acetate ions, and the added strong acid prevents deprotonation of phenol and inhibits coordination to manganese(III) ions. Furthermore, the ClO4− anion is more crystalline than the counterion and hexacyanoferrate(III) acid, which precipitates good ionic crystals. Therefore, it is difficult to form a cyano-bridged complex by such modified methods in contrast to established methods (Figure 5).
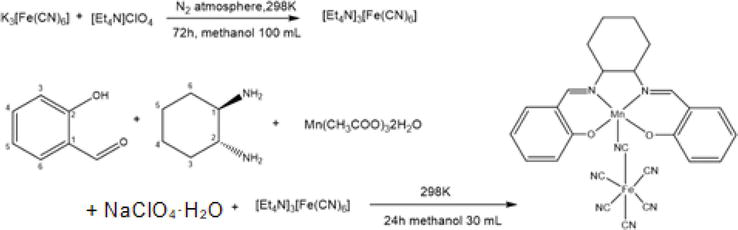
Figure 5.
Established methods of this preparation.
As for Schiff base lanthanide complexes, by the way, we have reported successful synthesis and crystal structure as well as crystallization of salen-type Schiff base mononucluear samarium(III) (or terbium(III)) complexes. These complexes were suitably prepared in methanol solutions by the conventional method [8]. Synthesis consisted of azo synthesis by diazo coupling with vanillin and aniline using the existing method, followed by Schiff base condensation with diphenylethylenediamine to synthesize ligands. The azo-free version was obtained by direct Schiff base condensation of
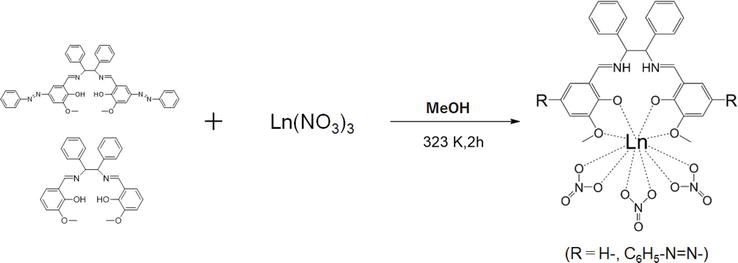
Figure 6.
Preparation of Schiff base lanthanide complexes. Hydrates and bases were not presented for clarity.
However, the formation of metal complexes was dependent on ligands or lanthanide metals in this series of complexes, which may be slightly different because of preparation conditions. In the presence of lanthanide(III) metal sources in methanol solution, only some ions were able to form salen-type complexes with two ligands. In the case of the cerium(III) complex, f-f transitions were not observed both with and without azo groups; in the neodymium(III) complex, both f-f transitions were observed; and in the dysrosium(III) complex, f-f transitions were observed only without azo-groups. The results were able to be divided it into 3 patterns (Table 1). This is thought to be caused by differences in the ability of lanthanide(III) ions to form complexes with the same ligand, which are known in solvent extraction systems and differences in the hydrophobicity of azobenzene groups.
| La | Ce | Pr | Nd | Sm | Gd | Dy | Ho | Er | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azo free | × | × | ○ | ○ | ○ | × | ○ | ○ | × | ○ | ○ | × |
| With azo | × | × | × | ○ | × | × | × | × | × | ○ | ○ | × |
Table 1.
Investigation of poor solvents to test the vapor diffusion method was also carried out using diethyl ether, hexane, and toluene corresponding to azo-free dysrosium(III) complex. Toluene is useless because the substance inside turns into oil. When methanol and diethyl ether were used, grainy crystals appeared, but they did not appear to be single crystals (Figure 7).

Figure 7.
Poor crystals.
3. Summary and perspective
To demonstrate the utility of microfluidic/droplet synthesis (two solvent systems), I’m looking for examples that cannot (or are difficult to) be synthesized in a beaker work. In particular, we will scrutinize examples of metal complexes that are synthesized and crystallized at the interface of two solvents, which are positioned as models of droplet synthesis in green chemistry, and organize viewpoints that should be considered. At the same time, some general knowledge about solvents is presented. In this review, we present the process of accumulating negative data for the synthesis of cyano-bridged iron and Schiff base salen-type manganese complexes and a series of salen-type lanthanide complexes. Schiff base ligand that can be synthesized and crystallized by metals and substituents (from hydrates) by changing various conditions, namely, solutions, will be discussed.
Microdroplet synthesis is expected to enable preparation under mild conditions in a short period of time. (These failure examples may be good examples for searching compounds and reaction conditions.) Slight modifications of the two solution reactions can be difficult to prepare and may be attractive targets for microdroplet methods. “Nanoscale thermodynamics” has not yet been established, but there may be useful hints in the field of basic chemistry.
Acknowledgments
This work was partly supported by a grant-in-aid for scientific research (A) KAKENHI (20H00336).
References
- 1.
Yoon DH, Jamshaid A, Ito J, Nakahara A, Tanaka D, Akitsu T, et al. Active microdroplet merging by hydrodynamic flow control using a pneumatic actuator-assisted pillar structure. Lab on a Chip. 2014; 14 :3050-3055 - 2.
Tanaka D, Kawakubo W, Tsuda E, Mitsumoto Y, Yoon DH, Sekiguchi T, et al. Microfluidic synthesis of chiral salen Mn(ii) and Co(ii) complexes containing lysozyme. RSC Advances. 2016; 6 :81862-81868 - 3.
Tanaka D, Sawai S, Yoon DH, Sekiguchi T, Akitsu T, Shoji S. Synthesis of an azo-Mn(ii) complex with mild pH control using a microfluidic device. RSC Advances. 2017; 7 :39576-39582 - 4.
Tanaka D, Sawai S, Hattori S, Nozaki Y, Yoon DH, Fujita H, et al. Microdroplet synthesis of azo compounds with simple microfluidics-based pH control. RSC Advances. 2020; 10 :38900-38905 - 5.
Hattori S, Tang C, Tanaka D, Yoon DH, Nozaki Y, Fujita H, et al. Development of microdroplet generation method for organic solvents used in chemical synthesis. Molecules. 2020; 25 :5360 - 6.
Akitsu T, Takeuchi Y, Einaga Y. 4-phenyldiazenyl-2-[(R)-(1-phenylethyl)iminomethyl]phenol. Acta Crystallographica Section E. 2005; 61 :m502-m505 - 7.
Akitsu T, Einaga Y. Asian Chemistry Letters. 2006; 10 :103-112 - 8.
Okumura Y, Takiguchi Y, Nakane D, Akitsu T. Acta Crystallographica Section E. 2021; 77 :579-582