Abstract
Arsenic is the biggest threat to all living organisms across the world. It is typically present in very minute amounts in rock, soil, air, and water, but these levels are rising as a result of both natural and man-made activity. Exposure to arsenic increases the risk of developing liver, lung, kidney, and bladder malignancies as well as vascular illnesses such as stroke, ischemic heart disease, and peripheral vascular disease. Arsenic generates oxidative stress, which disrupts the redox balance. In fact, in plants arsenic gets accumulated in different parts of plants upon exposure to either contaminated soil or water, causing hazardous effects on the plant. Therefore, this chapter is aimed to understand the effect of arsenic exposure on the growth and development of the plant as a whole.
Keywords
- arsenic
- plant health
- human health
- toxicity
- arsenic metabolism
1. Introduction
Arsenic is a ubiquitous metalloid found all across the earth’s crust and is considered to be highly toxic to all living organisms [1, 2]. The arsenic concentration of 10 μg/l in drinking water is considered to be safe as per the recommendation of the World Health Organization and the U.S. Environmental Protection Agency (EPA) [3]. But many countries like China, Chile, Argentina, Vietnam, Canada, Laos, Mexico, Ghana, many parts of the United States, Bangladesh, and India have high concentrations of arsenic in their groundwater [3, 4, 5, 6, 7]. This elevated concentration of arsenic in the groundwater level is caused by both anthropogenic activities as well as natural sources. The International Agency of Research on Cancer (IARC) classified arsenic as a class I human carcinogen and also ranks 1st on the US Agency for Toxic Substances and Disease Registry (ATSDR) Priority List of Hazardous Substances (https://www.atsdr.cdc.gov/spl/index.html) [8]. Arsenic exposure can cause various hazardous effects in human beings causing diseases like diabetes, hypertension, cardiovascular disease, gastrointestinal, renal, and neurological disorders, skin keratosis, and cancer [6, 9, 10]. In Bangladesh, where arsenic contamination at the groundwater level is extremely elevated, incidences of “black foot disease” is reported to be very high [11]. Arsenic even enters the food chain of human beings through the food chain via contaminated crops. There are different ways by which arsenic gets into a farming system that includes natural geochemical processes, irrigation using arsenic-contaminated water, and the use of pesticides containing arsenic [12, 13]. Arsenic can induce toxicity in plants as characterized by decreased germination; decreased plant biomass and chlorophyll content [14].
Thus, this chapter is aimed to understand the mechanism by which arsenic gets accumulated in the plants and thereby causing toxicity at the cellular level, and eventually degrading the well-being of the exposed plants.
2. Types of arsenic
In the water and soil ecosystems, arsenic can appear in three primary forms: inorganic, organic, and arsine gas (−3 oxidative state). It also has three main valence states: arsenic element (0), arsenite (trivalent +3), and arsenate (pentavalent +5) [1]. Both organic (monomethyl arsenic) and inorganic (arsenite) trivalent arsenic compounds are often viewed as being more hazardous than pentavalent ones. Compared to As V, As III is 60 times more poisonous [10]. The chemical compounds arsenate As(V), arsenite As(III), monomethyl arsenic acid (MMAC), dimethyl arsenic acid (DDMA), trimethylarsine (TMA), arsenocholine (AsC), arsenobetaine (AsB), and arsenosugars can all be found in the environment (Figure 1) [15]. Environmental versions include methyl arsenic acid, dimethyl arsenic acid, arsine, etc., and also consist of arsenious acids (H3AsO3, H3AsO32−), arsenic acids (H3AsO4, H3AsO4−, H3AsO42−), arsenates and arsenates [16]. The Marine invertebrates such as lobsters, brown algae, seaweed, fish, and seawater bivalves are the principal sources of the organic arsenic species (MMA, DMA, AsC, and AsB). As(V) and As(III) are the predominant arsenic species in inorganic terrestrial settings, however small amounts of organic arsenic species have been discovered as a result of microbial activity or the application of organic herbicides and insecticides [15]. Arsenate As(V) dominates in the soil solution under oxidizing conditions, whereas As(III) dominates under mildly reducing conditions. In contrast to organic arsenic, inorganic arsenic is poisonous. Most people believe that inorganic arsenic is more hazardous than organic arsenic [17].
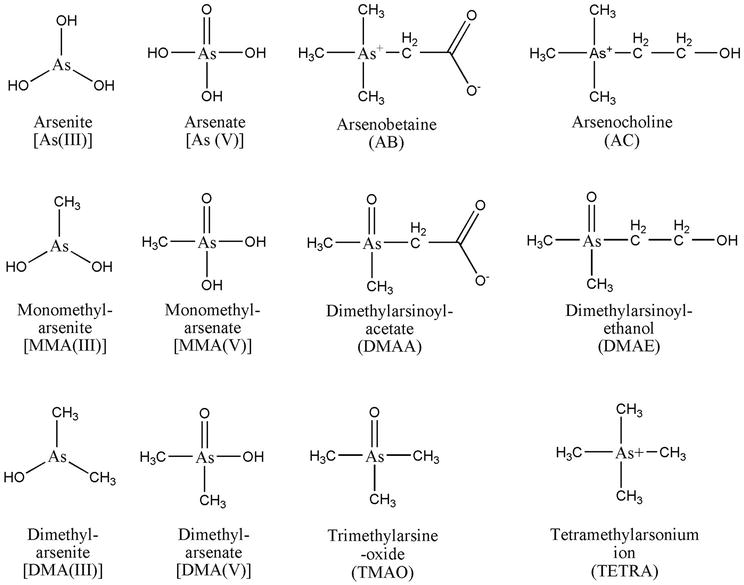
Figure 1.
Chemical structure of As species detected in terrestrial plants [
Arsine gas is the form of arsenic that is the most dangerous. While concentrations over 25 ppm are reported to be fatal in under an hour of exposure and concentrations over 250 ppm are stated to be instantly fatal, concentrations above 10 ppm are said to be fatal upon inhalation. Arsine gas is colorless, does not irritate tissues, and has a hardly perceptible odor. In terrestrial ecosystems, arsenic can be found in four oxidation states (III, 0, +III, and +V). Under aerobic soil conditions and anaerobic circumstances (submerged soils), respectively, arsenate (AsV) and arsenite (AsIII) are the prevalent forms [16]. The interconversion of these two arsenic forms (arsenate and arsenite) is highly influenced by both biotic (microorganisms) and abiotic (changes in redox potential and pH) variables [18, 19].
3. Sources of arsenic exposure
3.1 Natural sources
3.1.1 Geologic sources
The geological features of the area are frequently linked to the presence of arsenic in groundwater. For example, in Bangladesh and West Bengal, the source of arsenic in groundwater is linked to the presence of arsenic-bearing minerals in the Ganges delta deposits [20].
3.1.2 Contaminated soil
Natural processes like the weathering of rocks and minerals can cause soil to become contaminated with arsenic. But in some places, human endeavors like mining have markedly raised the amount of arsenic in the soil [21].
3.2 Anthropogenic sources
3.2.1 Industrial activities
Industrial processes including mining, smelting, and the use of pesticides are examples of anthropogenic sources of arsenic. For instance, major arsenic poisoning of soil and water has been related to the use of arsenic in pesticides [24].
3.2.2 Food
Due to the usage of arsenic-containing pesticides or polluted soil, arsenic may be found in food. Due to irrigation with arsenic-contaminated water, it has been discovered that rice in some locations contains high levels of arsenic [25].
3.2.3 Wood preservatives
Up until the early 2000s, arsenic was often utilized in wood preservatives, resulting in significant arsenic pollution of the soil and water around treated wood sites [26].
4. Arsenic-induced toxicity in human health
Clinical symptom development differs depending on whether arsenic exposure was acute or persistent. However, clinical signs of acute exposure to arsenic emerge considerably more quickly than those of chronic exposure, which take a longer time to manifest. Organ damage and possible death are possible in acute arsenic intoxication. On the other hand, malignant tumors may develop as a result of the deformity of the extremities brought on by long-term exposure to arsenic [23, 27]. Ingestion and inhalation are the two most common ways to be exposed to arsenic, and these are also the ways that cause health issues most commonly. Dermal exposure can also result in sickness, but it is less likely to do so than ingesting or inhaling arsenic. Based on how arsenic affects different organs such as the skin, brain, heart, pancreas, lungs, liver, and kidney, arsenic-induced toxicity in humans can be studied. A detailed study of it is discussed below.
4.1 Skin
Skin conditions are the early signs of arsenic intoxication. The consequences of arsenic exposure on health have been the subject of numerous researches. For instance, a study carried out in Bangladesh revealed that drinking water contaminated with arsenic over time was linked to an increased risk of skin lesions and skin cancer [2, 21, 28].
4.2 Heart
An elevated risk of cardiovascular disease was linked to long-term exposure to arsenic in drinking water, according to a systematic review and meta-analysis of epidemiological data [2, 6, 29]. An increase in the number of deaths from aneurysms, arteriosclerosis, and other related diseases was observed in another study carried out in the USA in locations where the drinking water contained high arsenic contents >20 g/l. [30]. Wang et al. [31] discovered that Taiwanese communities living in areas with arsenic-polluted wells (>0.35 mg/l) had an elevated incidence of illness in the blood vessels.
4.3 Pancreas
A study by Hassan et al. discovered a direct correlation between eating rice that has been exposed to arsenic contamination and developing diabetes. For the two billion people who live in Asia, rice is the main food source. According to studies, rice has high levels of inorganic arsenic, which can damage pancreatic beta cells and interfere with the body’s ability to regulate glucose levels [32].
4.4 Brain and the nervous system
The central nervous system is the primary target organ for the harmful effects of heavy metals like arsenic [27]. A case–control study involving 57 people who had arsenic-induced skin lesions and had long-term exposure to arsenic-contaminated drinking water was conducted in China. According to the study, exposed individuals experienced a variety of neurological problems, including aberrant distal feeling, decreased temperature and pressure perception, and functional lesions of the vegetative nerves, such as hypohidrosis and adiaphoresis [33]. In a recent study conducted in West Bengal, India, patients exposed to drinking groundwater tainted with arsenic experienced peripheral neuropathy as their main neurological consequence [34].
4.5 Lungs
Both prenatal and childhood exposure to arsenic through drinking water was associated with both long-term lung function and non-malignant lung illnesses in humans, according to Dauphiné et al. [35]. Respiratory problems such as persistent cough, laryngitis, bronchitis, and rhinitis are frequently caused by inhaling arsenic dust or fumes while mining or milling ores [36]. Workers exposed predominantly to arsenic trioxide dust have been found to have an elevated incidence of lung cancer [37, 38].
4.6 Liver
Following repeated exposures, there is a proportionally higher accumulation of arsenic in the liver, which makes it more harmful to the liver [10]. Some of the early clinical signs of liver illness brought on by exposure to arsenic include esophageal varices bleeding, ascites, jaundice, or liver enlargement. Hepatic lesions may develop in the later stages of severe toxicity coupled with other problems such as cirrhosis with a high likelihood of liver failure, non-cirrhotic portal fibrosis, and hepatic fibrosis [39].
4.7 Kidney
Renal injury (acute tubular necrosis) caused by arsenic toxicity is one of the clinical symptoms, which is followed by hypogea, high serum creatinine levels, blood urea nitrogen, and proteinuria [40].
5. Arsenic exposure to animals and humans through the food chain
Millions of people are exposed to arsenic through food, air, water, and soil, leading to adverse long-term health consequences. It is important to monitor the food chain as humans are the top consumers, making it a challenging task. Arsenic enters the ecosystem through several processes, including geogenic phenomena (thermal regime, volcanic activities, weathering, and leaching), anthropogenic activities (urbanization, industrial establishments, groundwater extraction for irrigation, extensive use of pesticides and fertilizers), and biogenic (induced by plants, animals, microorganisms, and aquatic biota) phenomena. Due to its significant industrial importance, the use of As is unavoidable as it is employed in the production of semiconductors, explosives, munitions, paints, cosmetics, insecticides, herbicides, and fertilizers, among other things [41]. Food security is threatened and human health is further jeopardized by arsenic entering the food chain and affecting crop quality and production. Chronic exposure to arsenic can seriously deteriorate health across the food pyramid. In addition to overall As concentration, bioavailability also plays a significant role in the accumulation of arsenic from one trophic level to the next. Since humans may also absorb arsenic via tainted rice, vegetables, milk, and meat, “plant-human” and “plant–animal-human” could be additional possible food chain routes for arsenic buildup.
Arsenic may enter the body through a number of different routes, including drinking and cooking water, crops and vegetables grown in areas with high arsenic levels, and animal products (meat, milk, and eggs) [42]. It has been determined that two key pathways for its entry into the food chain are through the consumption of contaminated crops and drinking water. Due to its high water solubility and propensity for bioaccumulation in several environmental matrices, arsenic becomes exceedingly hazardous even at low exposure levels. Only the bioavailable form of arsenic enters the body directly after being consumed in tainted food or water, interfering with many metabolic processes. The process by which plants absorb arsenic changes according to chemical speciation. As(V) has reportedly been shown to enter plant cells through inorganic Phosphate (Pi) channels. However, because their chemical structures are similar, plants may absorb As(III) through a variety of intrinsic proteins that resemble nodulin-26 and silicon transporters. Whether in the root or shoot tissue, plants normally store the majority of arsenic in vacuolar compartments. Animals can absorb As from a variety of sources, but they mostly do so from the soil matrix. They are exposed to elevated levels of arsenic, and continuous consumption of such crops is associated with a number of health risks [43].
One of the worst environmental catastrophes in the previous century was chronic arsenic poisoning brought on by drinking water. In India, groundwater arsenic contamination was first reported in West Bengal in 1983. With a concentration of up to 4000 g/l, the Ganges-Brahmaputra-Meghna plain is the most severely arsenic-polluted place in the world. Besides drinking, rice cooked using contaminated groundwater contains more arsenic than uncooked rice due to the chelation of As by rice grain and evaporation of water while cooking, which concentrates the metalloid in the remaining water [42].
In addition to drinking water, vegetables, and other crops cultivated in groundwater contaminated with arsenic can significantly increase the daily consumption of arsenic in human meals. Agriculture is the primary industry in West Bengal’s heavily arsenic-affected districts, therefore over the past few decades, unrestricted groundwater pumping for irrigation has been a common procedure. A substantial quantity of arsenic is accumulated in West Bengal by the crops and vegetables grown in soil contaminated with arsenic, and this arsenic eventually makes its way into the food chain people. The arsenic toxicity is disseminated through the fruits, vegetables, and grains that are grown on soil that contains arsenic. In some countries, it has been found that rice has a 10-fold greater As content than other cereal grains [43]. In comparison to wheat (0.1) and barley (0.2), rice has a higher arsenic translocation factor (TF) of 0.8. Elements that affect the amount of arsenic in rice are how the grain is processed, the kind of rice farm, the area where it is grown, the irrigation system used, and the way of cooking.
Food crops including cereals and vegetables have been considered key conduits for arsenic exposure in humans due to plants’ intrinsic capacity to collect minerals from the soil [41]. Vegetables can be exposed to As due to either natural or anthropogenic activitiesIt is well known that irrigation water from polluted groundwater resources may introduce arsenic into the food chain. Vegetable kind, soil type, irrigation water quality, proximity region, human pressure, and geogenic contamination are some of the variables affecting arsenic contamination in vegetables [44]. Arsenic levels in vegetables were found to be above both national and international standards in research from the hamlet of Samta in West Bengal, India. Tube well water used for irrigation that had 0.24 mg/l of arsenic was linked to the contamination in plants. Compared to its abundance in roots and other below-ground plant organs, arsenic is found in relatively lower concentrations in grains, seeds, lentils, and fruits. Additionally, root vegetables’ exterior root skin contains more arsenic than the actual root itself does.
Arsenic enters the food chain through arsenic-contaminated water and feed for cattle. Cattle feeding practices, food, water, and environmental contaminants all affect the amount of arsenic in the milk they produce High arsenic concentration in raw milk was reported from cows grazing near lava ground in Turkey. In Pakistan, elevated arsenic concentration was found in milk samples of different milch animals due to contaminated drinking water (geogenic arsenic). This is likely due to the contaminated drinking water given to cattle [41]. The content of As in the scalp hair of children of three age groups was high, with goat and sheep milk being significantly higher than cows and camel, suggesting that the main exposure of As is not only due to milk [45].
Commercial chicken meat is a common source of animal protein and is consumed globally. Despite being nutritious, the quality of poultry meat may be compromised by the presence of harmful metals caused by a variety of human activities. The use of organo-arsenic pharmaceuticals as animal feed and antiparasitic treatments encourages the transmission of arsenic in cattle and poultry products. Roxarsone is linked to elevated arsenic levels in chicken tissues [41]. High arsenic levels were reported in poultry meat in Bangladesh due to drinking contaminated groundwater [46].
Arsenic is also abundant in seafood at concentrations as high as several hundred micrograms per gram [47]. Organic arsenic, including arsenosugars, arsenobetaine, and arsenolipids, is mostly found in seafood. The most prevalent arsenic substances in fish and seaweed are arsenobetaine and arsenosugars. Arsenic poisoning is more prevalent among coastal populations whose diets are mostly composed of seafood. Arsenic poisoning is more common among coastal populations whose diets are mostly composed of seafood, with higher levels of arsenic in the blood and urine, baby cord blood, and breast milk [48].
6. Arsenic metabolism in plants
Most plants are dangerously affected by arsenic exposure at lower or even greater doses. Exposure to arsenic can interfere with metabolic processes and inhibit plant development [49]. When examining the effects of arsenic on plant cellular metabolism, it’s important to take into account the various arsenic species that are present in soils, their capacity to enter plant cells, their potential to change from one arsenic species to another, and the different arsenic transport pathways that are present within the plant. Cell types exposed to high concentrations of specific arsenic species can be used to discriminate between hyperaccumulators and non-accumulators. While non-accumulators prefer to reserve arsenic in root cells and have much lower quantities of arsenic in shoot cells, hyperaccumulators have abnormally high concentrations of arsenic in the cells of aerial tissues compared to the root [50]. The metabolism of arsenic has a significant impact on how dangerous it is [51]. According to research done by Martinez-Castillo et al. [52] As interacts with a variety of metabolic processes, causing physiological and morphological abnormalities that affect plant development (poor nutrient absorption) and productivity (reduced seed germination), while also inhibiting root growth and cell death.
The first step in the detoxification of arsenate is the reduction of arsenate to arsenite after arsenic has entered plant root cells [18, 53]. According to a report, several enzymes from various systems exhibit AsV reductase activity [18, 50]. They include glycogen phosphorylase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), polynucleotide phosphorylase, purine nucleoside phosphorylase (PNP), and mitochondrial F1Fo ATP synthase. As V is first reduced to As III to start As a metabolism, this reduction can happen both enzymatically and without the use of an enzyme. As V is converted to As III through a non-enzymatic process in which two molecules of reduced glutathione (GSH) are oxidized to produce oxidized GSH, which is then converted back to two GSH molecules with the aid of GSH reductase. But the reduction of arsenate is a very slow process. Arsenate reductase directly converts As V to As III in enzymatic processes. According to a study, arsenic primarily enters plants in an inorganic form, As(III) or As(V), through transporter proteins. This process is likely controlled by a gradient of arsenic concentration between plant cells and growth media [54]. AsV is quickly converted into AsIII, the more hazardous of the two forms, once it penetrates plant cells [55]. Once present in the soil, the two inorganic forms of arsenic, arsenate (AsV) and arsenite (AsIII) are promptly absorbed by the plant roots’ cells. According to reports, plants are extremely capable of converting AsV and MMA(V) that their roots have ingested into AsIII and MMAIII, respectively. When MMA(III) was completely absent from the medium, it was found that MMAV was transformed into MMA(III) within the roots (Figure 2) [51]. The reduction of a trivalent state and oxidative methylation to a pentavalent state are the two main steps in the metabolism of arsenic in plants. The plant ACR2 gene, a homolog of the yeast arsenate reductase, was thought to cause As(V) reduction in plant cells. HAC1 (for High As Content 1) or ATQ1 (for Arsenate Tolerance QTL1), a new As(V) reductase, has recently been found in Arabidopsis. The ability of the protein from the rhodanese-like family to reduce As(V) to As(III) HAC1/ATQ1 both in vitro and in planta has been described [56].
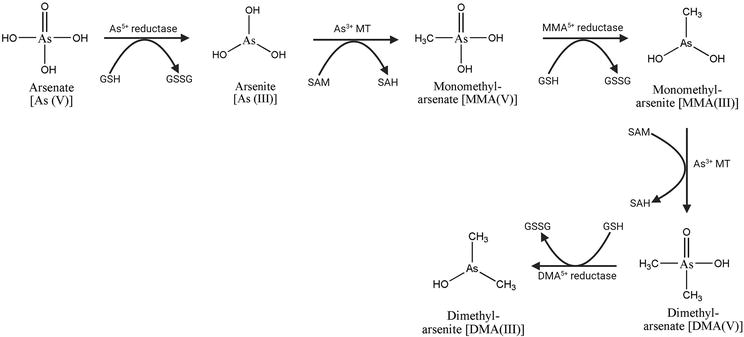
Figure 2.
Pathways for arsenic methylation in plant [
Compared to trivalent arsenicals, pentavalent arsenicals are less hazardous [17]. Any type of arsenic that is absorbed by plants causes the cellular conversion of inorganic arsenic to organic species. The enzyme arsenate reductase speeds up the process of conversion of AsV to AsIII in the plant cell after arsenic absorption. AsIII, on the other hand, destroys the functionality of proteins by creating a molecule that contains their thiol groups. Nevertheless, GSH in vitro facilitates this reduction non-enzymatically [52].
Arsenic-induced phytotoxicity has negative effects on plant growth, development, and metabolism, which lower plant output [1, 57]. Even though the absorption mechanisms for arsenite and arsenate are different, arsenite was successfully transported as arsenate across the plasmalemma [58].
Inorganic arsenic only occasionally travels through the majority of plants. From the roots to the stems to the leaves to the grain, there was a dramatic fall in the concentration of As. AsV is most likely to be carried by Pi transporters, although AsIII was found to be transferred across membranes by silicon transport proteins such as OsLsi1 and OsLsi2 [50].
7. Bioaccumulation of arsenic in plants
In soils, arsenic may be present in a wide range of chemical forms, such as exchangeable, easily soluble (slightly bound to carbonate and particle surface), poorly crystalline, amorphous, and crystalline Fe and Mn oxide, Fe sulfide, and organic-matter-bound phases, as well as incorporated in crystal structures [59]. The amount of arsenic that accumulates in plants is dependent on many factors the physicochemical characteristics of the soil, which have a detrimental effect on plant growth, and nutrients that restrict plant growth and the bioavailability of arsenic [60]. Several parts of plants exposed to arsenic-contaminated soil and water have been found to contain arsenic.
Arsenic can penetrate terrestrial and aquatic ecosystems naturally or as a result of anthropogenic activity. These ecosystems have a route in the roots that permit plants to take up arsenic along with nutrients. Depending upon the variety of plants (whether As hyperaccumulator/non-hyperaccumulator or arsenic tolerant/susceptible), the mechanisms of arsenic accumulation, absorption, or toxicity response may be different. A large amount of As flow occurs through the xylem or phloem and may be transported to different parts of plants including the stem, leaf, reproductive portions, and even seeds after inflowing the root epidermis and moving through the apoplastic and symplastic pathways. Arsenic accumulation in leaves and roots is directly proportional to the concentrations of AsV and AsIII, except at the peak of AsIII concentration, probably due to AsIII extrusion [61]. However, certain plants, known as hyperaccumulating plants, can metabolize high concentrations of arsenic without experiencing any physiological damage, while others can get damaged from even small doses of arsenic exposure.
Arsenic concentration in plants varies considerably depending on the type of plant, the nature of the soil, and the concentration of arsenic in the irrigation water [62]. It has been revealed that roots are more accountable in terms of arsenic accumulation. It was reported the main arsenic species in aerobic soils is arsenate [18]. The solubility of arsenic is highly affected by the soil properties Regardless of the overall As content of the soil, soil properties such as pH, organic carbon, texture, and minerals, apart from the arsenic content of the soil [60, 63]. Arsenic uptake by plants is dependent mainly on the source, pedology, species, and age. Even though the quantity of absorption by plants differs based on several conditions, it was evident that soil level is directly proportional to the concentrations of inorganic arsenic, both Arsenate As(V) and arsenite As(III), which highly prevail in plant tissue, however, a trace amount of organic arsenic species (<1%) has been found in the shoots. It was revealed that the vegetables with juicy content attain the maximum concentrations of arsenic, while the fruits with slight juicy content have a diminished concentration of arsenic [64].
Arsenic contamination is the most important problem worldwide associated with either groundwater or irrigation of rice cultivated in affected areas [9]. Rice is the most important crop for providing essential nourishment to 50% of the world’s population. However, rice has the propensity to accumulate heavy metals to a greater extent, especially arsenic, thus, having a hazardous effect on human health [65, 66]. In comparison to other cereal crops that are used as staples, rice is faraway efficient in absorbing arsenic into the grains. The bioavailability of arsenic in soil is soaring as because rice is normally produced under submerged, flooded conditions. Since some arsenic species are phytotoxic, arsenic may hurt the volume of rice formed overall. The sequence of the toxicities of the various arsenic species is AsH3 > As(III) > As(V) > MMAA (monomethyl arsenic acid) > DMAA (dimethyl arsenic acid) [62]. The main crucial form of arsenic species available in paddy fields is arsenite, but a small amount of arsenate, MMAA, and DMAA is also found [60]. The ingress of MMA(V) and DMA(V) in
In reducing conditions, arsenite As(III) is the principal As species, such as in flooded paddy soils. The bioavailability of arsenic in rice plants is elevated by moving the arsenite into the soil solution by flooding the paddy fields and arsenite may be rapidly absorbed by the roots of the plants from the surrounding environment. In the roots of rice as well as of other aquatic plants whose roots flourish in anaerobic or semi-anaerobic conditions, the absorption of arsenite is particularly very important [18]. According to a recent study, the glycerol-transporting channel facilitates the uptake of undissociated (III) (pKa 9.2) in
The Chinese brake (Pterisvittata L.) fern can accumulate up to 23 g of arsenic per kilogram owing to its growth on arsenic-contaminated soil [70], while the concentrations of As in other varieties of terrestrial plant shoots are tremendously low. This plant has immense capacity to transfer arsenic to its above-ground biomass (up to 90% of the total As intake), in addition to absorbing a considerable quantity of As from the soil (up to 2.3% of dry plant weight).
Comparatively speaking to the As chemical form prevalent in soil solution, rice (O. sativa, L.) absorbs arsenic. Most of the arsenic is present as As when the soil redox potential falls below 0 mV. (III). Both As(III) and As(V) are present in more oxidizing circumstances. The photo availability of As in the water-soluble fraction is influenced by its chemical speciation. The arsenic content [As(III)] in the plant is increased with rising arsenic concentrations in solutions and rising levels of soluble arsenic [67].
Several arsenic hyperaccumulators have been reported, including
8. Consequences of arsenic exposure in plants
Some harmful exposures come from naturally existing compounds, like the arsenic frequently present in drinking water, therefore environmental health problems are not just restricted to toxic waste sites and poisoning incidents. Arsenic contamination of soil can impair a plant’s ability to function normally, resulting in stunted growth and subpar agricultural output [54]. Arsenic can still have an effect on several biological systems for years or even decades after exposure levels have diminished. Arsenic generates oxidative stress, which disrupts the redox system [72]. The world’s two main sources of arsenic pollution are groundwater and the irrigation of rice farmed in polluted areas. When arsenic levels in irrigation water or soil are elevated, it may hinder normal plant growth by causing symptoms including lower biomass in root and shoot wilting and necrosis of leaf blades, decreased leaf area and photosynthesis, and decreased fruit and grain output [62]. Several artificial arsenic compounds were utilized in agriculture as efficient pests, parasites, and weed control tools, and they slowly accumulated in the soil. Moreover, certain additional morphological processes may cause the quantity of arsenic in the environment to grow, thus increasing human exposure to arsenic [73]. ROS may be produced as a result of arsenate toxicity, which then damages membranes. Lipid peroxidation may cause structural cell membrane damage and accelerate ionic exosmosis [17]. Arsenic frequently has the effect of shortening the root and shoot length. Because plant roots are the initial point of contact for these lethal arsenic species in the nutritional media, the growth of root length was noticeably hindered when the arsenic content rose. It is well known that modest concentrations of arsenicals can alter the phosphorylation states of signal transduction proteins, resulting in the start of gene transcription. Arsenic disrupts phosphorylation via activating specific phosphatases, thiol-dependent phosphatases, or phosphotransferase activities. The expression of genes for reactive oxygen-scavenging enzymes in maize rises with arsenic exposure [74].
Arsenic sensitivity varies greatly among different plant species. Arsenic accumulates to varied degrees in crops that receive irrigation water poisoned with arsenic, depending on the species and variety of the crop. Photosynthesis is among the most crucial physiological traits of plants, along with other metabolic processes. Research has shown that arsenic’s impact on photosynthesis can reduce plants’ capacity to produce food [75]. Arsenic’s mobility and bioavailability may be influenced by the amount and kind of organic matter in the soil [60]. Plants exhibit morphological and physio-biochemical aberrations as a result of arsenic toxicity. It has been asserted that arsenic prevents root growth and expansion since the root is the organ that is most frequently exposed to the poison. Arsenic may also harm plants by resulting in stunted roots, withered leaves, a decline in the pigment required for photosynthetic activity, yellowing of the leaves, and a drop in chlorophyll, which can affect plant metabolism. However, because some of the defense mechanisms have not yet developed, seed germination and the early phases of seedling growth are more vulnerable to metal pollution and are therefore important considerations in the assessment of toxicity [76]. Arsenic binds to enzymes and proteins that harm cell biochemistry in addition to interfering with physiological activities such as photosynthesis, respiration, and transpiration in plants. It can lessen a plant’s capacity for reproduction, obstructing the photosynthetic process, which lowers plant growth and output. While the entire plant in Arum is found to contain a significant concentration of arsenic [62]. Frequently, natural soil-grown plants contain little or no arsenic (3.6 mg/kg).
The root membranes of tomato plants (Lycopersicumesculentum) were shown to be destroyed at 10 mg/l of arsenic in an experiment conducted by Barrachina et al. [79]. Exposure to arsenic significantly decreased key plant development indicators, with the largest decreases occurring at 76.8% for leaf fresh weight and 79.6% for tomato fruit output, respectively. Smith et al. evaluated the body of research and found that whereas maize and radish have a value of 100 mg/kg, rice, beans, and oats only have a value of 20 mg/kg. Arsenic is translocated to the grain and shoots in lentils, where it can significantly impede physiological development by delaying or preventing the creation of biomass and hindering plant reproduction. Stress also affects lipid peroxidation, electrolyte leakage, H2O2 accumulation, root oxidizability, and antioxidant enzyme activity in a substantial way [80]. When soil arsenic concentrations increase, so does the amount of arsenic that plants deposit. Terrestrial plants, such as legume crops, however, have a higher concentration of arsenic in the shoot-to-root region as compared to emergent plants [81], leading to physiological disorders in plants.
9. Conclusion
The toxicity of arsenic in numerous crops is a severe problem for human health. As exposure often deregulates several cellular functions, including epigenetic control, DNA repair, apoptosis resistance, and regular gene expression. Targeting these signaling pathways may offer a therapeutic method or alternative for the treatment and prevention of malignancies linked to chronic arsenic exposure because of their importance in the progression of carcinogenesis. When plants are exposed to arsenic, it induces reactive oxygen species (ROS), which put the plants under oxidative stress, eventually causing damage not only at the cellular level but also at the genetic level. Thus, the need of the hour is to develop an effective and cost-effective method to combat the accumulation of arsenic in different parts of the plants.
References
- 1.
Singh R, Singh S, Parihar P, Singh VP, Prasad SM. Arsenic contamination, consequences, and remediation techniques: a review. Ecotoxicology and Environmental Safety. 2015; 112 :247-270 - 2.
Barbhuiya SN, Barhoi D, Giri S. Application of nanotechnology for heavy metals remediation from contaminated water. In: Shukla SK, Kumar S, Madhav S, Mishra SK, editors. Metals in Water. Massachusetts, USA: Elsevier; 2023. pp. 369-386 - 3.
Hettick BE, Canas-Carrell JE, French AD, Klein DM. Arsenic: a review of the element’s toxicity, plant interactions, and potential methods of remediation. Journal of Agricultural and Food Chemistry. 2015; 63 (32):7097-7107 - 4.
Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. The Lancet. 2010; 376 (9737):252-258 - 5.
Basu A, Saha D, Saha R, Ghosh T, Saha B. A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Research on Chemical Intermediates. 2014; 40 :447-485 - 6.
Nath Barbhuiya S, Barhoi D, Giri A, Giri S. Arsenic and smokeless tobacco exposure induces DNA damage and oxidative stress in reproductive organs of female Swiss albino mice. Journal of Environmental Science and Health, Part C. 2020; 38 (4):384-408 - 7.
Barbhuiya SN, Barhoi D, Giri S. Impact of Arsenic on Reproductive Health. In: Otsuki T, editor. Environmental Health. London, UK: IntechOpen; 2021. p. 89-102 - 8.
Mondal S, Mukherjee S, Chaudhuri K, Kabir SN, Kumar Mukhopadhyay P. Prevention of arsenic-mediated reproductive toxicity in adult female rats by high protein diet. Pharmaceutical Biology. 2013; 51 (11):1363-1371 - 9.
Das S, Upadhaya P, Barhoi D, Nath Barbhuiya S, Langthasa P, Giri S. GCMS analysis of sadagura (smokeless tobacco), its enhanced genomic instability causing potential due to arsenic co-exposure, and vitamin-C supplementation as a possible remedial measure: a study involving multiple model test systems. Drug and Chemical Toxicology. 2022; 45 (1):185-196 - 10.
Ratnaike RN. Acute and chronic arsenic toxicity. Postgraduate Medical Journal. 2003; 79 (933):391-396 - 11.
Martinez VD, Becker-Santos DD, Vucic EA, Lam S, Lam WL. Induction of human squamous cell-type carcinomas by arsenic. Journal of Skin Cancer. 2011. pp. 1-9 - 12.
Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environmental Science & Technology. 2009; 43 (5):1612-1617 - 13.
Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry. 2002; 17 (5):517-568 - 14.
Beniwal R, Yadav R, Ramakrishna W. Multifarious effects of arsenic on plants and strategies for mitigation. Agriculture. 2023; 13 (2):401 - 15.
Quaghebeur M, Rengel Z. Arsenic speciation governs arsenic uptake and transport in terrestrial plants. Microchimica Acta. 2005; 151 :141-152 - 16.
Mohan D, Pittman CU Jr. Arsenic removal from water/wastewater using adsorbents—a critical review. Journal of Hazardous Materials. 2007; 142 (1-2):1-53 - 17.
Geng CN, Zhu YG, Hu Y, Williams P, Meharg AA. Arsenate causes differential acute toxicity to two P-deprived genotypes of rice seedlings (Oryzasativa L.). Plant and Soil. 2006; 279 :297-306 - 18.
Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytologist. 2009; 181 (4):777-794 - 19.
Nearing MM, Koch I, Reimer KJ. Complementary arsenic speciation methods: A review. Spetrochimica Acta Part B: Atomic Spectroscopy. 2014; 99 :150-162 - 20.
Gaus I, Kinniburgh DG, Talbot JC, Webster R. Geostatistical analysis of arsenic concentration in groundwater in Bangladesh using disjunctive kriging. Environmental Geology. 2003; 44 :939-948 - 21.
Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bulletin of the World Health Osrganization. 2000; 78 (9):1093-1103 - 22.
Ravenscroft P, Brammer H, Richards K. Arsenic in North America and Europe. In: Ravenscroft P, Brammer H, Richards K, editors. Arsenic Pollution: a Global Synthesis. New Jersey, USA: Wiley; 2009. pp. 387-454 - 23.
Das S, Langthasa P, Barhoi D, Upadhaya P, Giri S. Effect of nutritional status on arsenic and smokeless tobacco induced genotoxicity, sperm abnormality and oxidative stress in mice in vivo. Environmental and Molecular Mutagenesis. 2018; 59 (5):386-400 - 24.
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, et al. Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environmental Science & Technology. 2006; 40 (16):4903-4908 - 25.
Meharg AA, Zhao FJ, Meharg AA, Zhao FJ. Arsenic in Rice Grain. Netherlands: Springer; 2012 - 26.
Agency for Toxic Substances and Disease Registry (ATSDR). (2007). Toxicological profile for wood creosote, coal tar creosote, coal tar, coal tar pitch, and coal tar pitch volatiles. U.S. Department of Health and Human Services, Public Health Service - 27.
Abdul KS, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PM. Arsenic and human health effects: a review. Environmental Toxicology and Pharmacology. 2015; 40 (3):828-846 - 28.
Kumar A, Ali M, Kumar R, Kumar M, Sagar P, Pandey RK, et al. Arsenic exposure in Indo Gangetic plains of Bihar causing increased cancer risk. Scientific Reports. 2021; 11 :2376 - 29.
Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Annals of Internal Medicine. 2013; 159 (10):649-659 - 30.
Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Archives of Environmental Health: An International Journal. 1994; 49 (5):418-427 - 31.
Wang SL, Chiou JM, Chen CJ, Tseng CH, Chou WL, Wang CC, et al. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environmental Health Perspectives. 2003; 111 (2):155-159 - 32.
Hassan FI, Niaz K, Khan F, Maqbool F, Abdollahi M. The relation between rice consumption, arsenic contamination, and prevalence of diabetes in South Asia. EXCLI Journal. 2017; 16 :1132 - 33.
Singh N, Kumar D, Sahu AP. Arsenic in the environment: effects on human health and possible prevention. Journal of Environmental Biology. 2007; 28 (2):359 - 34.
Mukherjee SC, Rahman MM, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, et al. Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal, India. Journal of Environmental Science and Health, Part A. 2003; 38 (1):165-183 - 35.
Dauphiné DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. International Archives of Occupational and Environmental Health. 2011; 84 :591-600 - 36.
Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A review of arsenic poisoning and its effects on human health. Critical Reviews in Environmental Science and Technology. 1999; 29 (3):281-313 - 37.
Järup L, Pershagen G, Wall S. Cumulative arsenic exposure and lung cancer in smelter workers: a dose-response study. American Journal of Industrial Medicine. 1989; 15 (1):31-41 - 38.
Welch K, Higgins I, Oh M, Burchfiel C. Arsenic exposure, smoking, and respiratory cancer in copper smelter workers. Archives of Environmental Health: An International Journal. 1982; 37 (6):325-335 - 39.
Kapaj S, Peterson H, Liber K, Bhattacharya P. Human health effects from chronic arsenic poisoning–a review. Journal of Environmental Science and Health, Part A. 2006; 41 (10):2399-2428 - 40.
Sasaki A, Oshima Y, Fujimura A. An approach to elucidate potential mechanism of renal toxicity of arsenic trioxide. Experimental Hematology. 2007; 35 (2):252-262 - 41.
Singh S, Yadav R, Sharma S. Singh AN. Arsenic contamination in the food chain: a threat to food security and human health. Journal of Applied Biology & Biotechnology. 2023; 10 (20):1-10 - 42.
Sarkar SD, Swain PR, Manna SK, Samanta S, Majhi P, Bera AK, et al. Arsenic contamination in food chain-a menace to food safety, human nutrition and health. Journal of Environmental Biology. 2022; 43 (3):339-349 - 43.
Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. The International Journal of Biochemistry & Cell Biology. 2009; 41 (8-9):1665-1677 - 44.
Haque MM, Niloy NM, Khirul MA, Alam MF, Tareq SM. Appraisal of probabilistic human health risks of heavy metals in vegetables from industrial, non-industrial and arsenic contaminated areas of Bangladesh. Heliyon. 2021; 7 (2):e06309 - 45.
Kazi TG, Brahman KD, Afridi HI, Arain MB, Talpur FN, Akhtar A. The effects of arsenic contaminated drinking water of livestock on its total levels in milk samples of different cattle: risk assessment in children. Chemosphere. 2016; 165 :427-433 - 46.
Mottalib MA, Zilani G, Suman TI, Ahmed T, Islam S. Assessment of trace metals in consumer chickens in Bangladesh. Journal of Health and Pollution. 2018; 8 (20):181208 - 47.
Le XC, Lu X, Li XF. Peer reviewed: arsenic speciation. Analytical Chemistry. 2004; 1 :26-33 - 48.
Bentley K, Soebandrio A. Arsenic and mercury concentrations in marine fish sourced from local fishermen and fish markets in mine-impacted communities in Ratatotok Sub-district, North Sulawesi, Indonesia. Marine Pollution Bulletin. 2017; 120 (1-2):75-81 - 49.
Tripathi RD, Tripathi P, Dwivedi S, Dubey S, Chatterjee S, Chakrabarty D, et al. Arsenomics: omics of arsenic metabolism in plants. Frontiers in Physiology. 2012; 3 :275 - 50.
Finnegan PM, Chen W. Arsenic toxicity: the effects on plant metabolism. Frontiers in Physiology. 2012; 3 :182 - 51.
Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicology Letters. 2002; 133 (1):1-6 - 52.
Martínez-Castillo JI, Saldaña-Robles A, Ozuna C. Arsenic stress in plants: A metabolomic perspective. Plant. Stress. 2022; 3 :100055 - 53.
Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE. Reduction and coordination of arsenic in Indian mustard. Plant Physiology. 2000; 122 (4):1171-1178 - 54.
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, et al. Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. International Journal of Environmental Research and Public Health. 2018; 15 (1):59 - 55.
Kumar K, Gupta D, Mosa KA, Ramamoorthy K, Sharma P. Arsenic transport, metabolism, and possible mitigation strategies in plants. In: Srivastava S, Srivastava AK, Suprasanna P, editors. Plant- Metal Interactions. Swizerland AG: Springer Nature; 2019:141-168 - 56.
Zhang J, Hamza A, Xie Z, Hussain S, Brestic M, Tahir MA, et al. Arsenic transport and interaction with plant metabolism: Clues for improving agricultural productivity and food safety. Environmental Pollution. 2021; 290 :117987 - 57.
Garg N, Singla P. Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environmental Chemistry Letters. 2011; 9 :303-321 - 58.
Meharg AA, Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytologist. 2002; 154 (1):29-43 - 59.
Farooq MA, Islam F, Ali B, Najeeb U, Mao B, Gill RA, et al. Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environmental and Experimental Botany. 2016; 132 :42-52 - 60.
Anawar HM, García-Sánchez A, Zabed HM. Biogeochemical cycling of arsenic in soil–plant continuum: perspectives for phytoremediation. In: Gupta DK, Corpas FJ, Palma JM, editors. Heavy Metal Stress in Plants. Swizerland AG: Springer Nature; 2013:203-224 - 61.
Gusman GS, Oliveira JA, Farnese FS, Cambraia J. Arsenate and arsenite: the toxic effects on photosynthesis and growth of lettuce plants. Acta Physiologiae Plantarum. 2013; 35 :1201-1209 - 62.
Huq SI, Joardar JC, Parvin S, Correll R, Naidu R. Arsenic contamination in food-chain: transfer of arsenic into food materials through groundwater irrigation. Journal of Health, Population, and Nutrition. 2006; 24 (3):305 - 63.
Fitz WJ, Wenzel WW. Arsenic transformations in the soil–rhizosphere–plant system: fundamentals and potential application to phytoremediation. Journal of Biotechnology. 2002; 99 (3):259-278 - 64.
Mishra BK, Dubey CS, Shukla DP, Bhattacharya P, Usham AL. Concentration of arsenic by selected vegetables cultivated in the Yamuna flood plains (YFP) of Delhi, India. Environmental Earth Sciences. 2014; 72 :3281-3291 - 65.
Wu X, Hu J, Wu F, Zhang X, Wang B, Yang Y, et al. Application of TiO2 nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryzasativa L.): a mechanistic study. Journal of Hazardous Materials. 2021; 405 :124047 - 66.
Mawia AM, Hui S, Zhou L, Li H, Tabassum J, Lai C, et al. Inorganic arsenic toxicity and alleviation strategies in rice. Journal of Hazardous Materials. 2021; 408 :124751 - 67.
Marin AR, Masscheleyn PH, Patrick WH. Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant and Soil. 1993; 152 :245-253 - 68.
Xie ZM, Huang CY. Control of arsenic toxicity in rice plants grown on an arsenic-polluted paddy soil. Communications in Soil Science and Plant Analysis. 1998; 29 (15-16):2471-2477 - 69.
Marin AR, Masscheleyn PH, Patrick WH. The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant and Soil. 1992; 139 :175-183 - 70.
Ma LQ , Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED. A fern that hyperaccumulates arsenic. Nature. 2001; 409 (6820):579 - 71.
Ding D, Li W, Song G, Qi H, Liu J, Tang J. Identification of QTLs for arsenic accumulation in maize (Zea mays L.) using a RIL population. PLoS One. 2011; 6 (10):e25646 - 72.
Gupta A, Dubey P, Kumar M, Roy A, Sharma D, Khan MM, et al. Consequences of arsenic contamination on plants and mycoremediation-mediated arsenic stress tolerance for sustainable agriculture. Plants. 2022; 11 (23):3220 - 73.
Roy P, Saha A. Metabolism and toxicity of arsenic: a human carcinogen. Current Science. 2002; 82 (1):38-45 - 74.
Mylona PV, Polidoros AN, Scandalios JG. Modulation of antioxidant responses by arsenic in maize. Free Radical Biology and Medicine. 1998; 25 (4-5):576-585 - 75.
Zemanová V, Pavlíková D, Hnilička F, Pavlík M. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteriscretica and Spinaciaoleracea. Plants. 2021; 10 (10):2009 - 76.
Liu X, Zhang S, Shan X, Zhu YG. Toxicity of arsenate and arsenite on germination, seedling growth and amyl olytic activity of wheat. Chemosphere. 2005; 61 (2):293-301 - 77.
Abedin MJ, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J. Arsenic accumulation and metabolism in rice (Oryzasativa L.). Environmental Science & Technology. 2002; 36 (5):962-968 - 78.
Abedin MJ, Meharg AA. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryzasativa L.). Plant and Soil. 2002; 243 :57-66 - 79.
Barrachina AC, Carbonell FB, Beneyto JM. Arsenic uptake, distribution, and accumulation in tomato plants: effect of arsenite on plant growth and yield. Journal of Plant Nutrition. 1995; 18 (6):1237-1250 - 80.
Smith ER, Naidu R, Alston AM. Arsenic in the soil environment. In: Sparks DL, editor. Advances in Agronomy. Massachusetts, USA: Academic Press; 1998; 64 :149-189 - 81.
Alam MZ, Hoque MA, Ahammed GJ, McGee R, Carpenter-Boggs L. Arsenic accumulation in lentil (Lens culinaris) genotypes and risk associated with the consumption of grains. Scientific Reports. 2019; 9 (1):9431