Abstract
The lumbar foraminoplasty is a novel surgical option for appropriately indicated patients, and high success rates have been reported in the literature. Complications and failures are often associated with patient indications or technical variables, and the goal of this chapter is to assist surgeons in understanding these factors.
Keywords
- lumbar foraminoplasty
- foraminoplasty
- radiculopathy
- stenosis
- foraminal stenosis
- foraminotomy lumbar degenerative disc disease
- lumbar disc herniation
- herniated disc
1. Introduction
Spine surgeons are confronted with several surgical approaches when managing lumbar stenosis and radicular pains which have not responded to conservative treatment options. There are several conventional approaches to addressing foraminal stenosis, each facing specific challenges in the operative setting. Often in order to perform a sufficient foraminal decompression surgeons must balance the resection of a considerable amount of the overlying facet joints, with the need to preserve and maximize their bony architecture in order to ensure postoperative spinal stability. In fact residual foraminal stenosis has been shown to be a leading cause of failed back surgery syndrome [1]. Moreover, there are other potential long term drawbacks with the standard decompression ranging from the development of instability, postlaminectomy syndrome, neuropathic pain, residual disc, persistent pain, and the degeneration of adjacent segments [1, 2, 3]. During a standard foraminotomy instruments can only reach a specified distance within the neuroforamina, and this often leads to an incomplete decompression Video 1a, https://drive.google.com/file/d/1b9zjYYhP_EsOkEB_Qmo-HsWe-p037TvS/view. In efforts to reach far lateral pathology standard instruments could potentially excise more of the facet joint than required, which could inadvertently lead to long term segmental instability. Ahuja et al. have demonstrated that a resection of 30% of the facet joint led to an increase in mediolateral spinal mobility [4, 5]. The same study found that an excision of 45% of the facet joints resulted in an increase of segmental instability in both anteroposterior and mediolateral planes, which was compounded with bilateral resections [4, 5].
In patients with symptomatic lumbar neuroforaminal stenosis, there has been a growing enthusiasm towards definitive management in the form of a foraminoplasty with a microblade shaver [6, 7, 8, 9, 10]. In essence the lumbar foraminoplasty is a reshaping and restructuring of the neuroforaminal arch using a flexible microblade shaver (Spinal Elements, Carlsbad, California) Figures 1 and 2 and Video 1b, https://drive.google.com/file/d/1VnbEUGuI0eyiYCglgFkSAxwF9MKH1UNV/view; https://drive.google.com/file/d/1474vZSCOC3gRnhcICipt1fYkH8o_OH6p/view; https://drive.google.com/file/d/1nremJQLLeceyb15qJLozVvOtAVyvKksz/view and Video 1c, https://drive.google.com/file/d/1sFq3vwLhxOoUXF1QV3qJwE3sG_HAfFvQ/view. This is performed in conjunction with neuromonitoring and fluoroscopy, which maximizes neural safety while providing live onscreen visualization as the neural arch is expanded and remodeled. Foraminoplasty with a microblade shaver provides the unique opportunity to perform a full foraminal decompression along the entire length and width of the superior articular process. Lauryssen et al. compared traditional decompression techniques with the microblade shaver in order to discover any iatrogenic insults which could later lead towards the development of segmental instability [9]. Using quantitative image CT scan analysis they determined the microblade shaver excised less laminar bone, less bone from the central canal, and less bone from the structural pars compared with traditional decompression methods. Moreover they found the foraminoplasty decompressed and reshaped the foramina in an anteroposterior plane, as opposed to the traditional medial to lateral plane, while simultaneously increasing the neuro foraminal volume. Compared to traditional instrumentation the microblade shaver was also found to have resected less facet width, facet cross sectional and facet surface area from both nondiseased and stenotic spines [9, 10]. This procedure has been demonstrated to be a safe and effective means of treating single or multiple level lumbar neuroforaminal stenosis by several studies as well as from the United States Food and Drug Administration [6, 8, 9, 10].
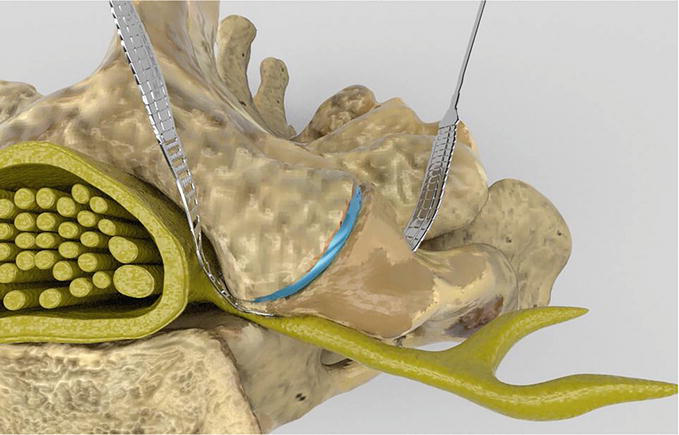
Figure 1.
The lumbar foramina prior to reciprocations with neuroforaminal stenosis and the microblade shaver in place.
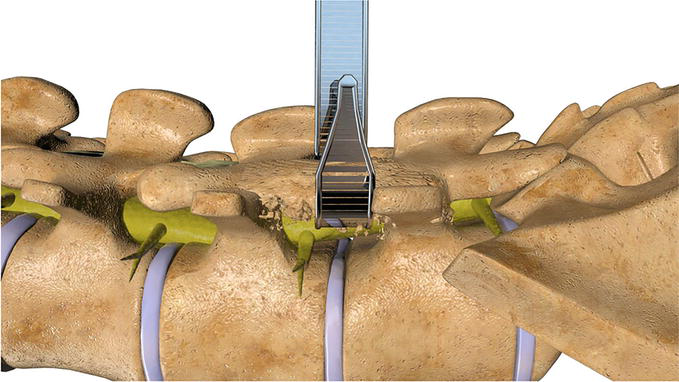
Figure 2.
The foraminoplasty involves a foraminal decompression through a remodeling of the neuroforaminal arch with a thin microblade shaver.
1.1 Facet joint biomechanics and degeneration
Morphologically the spinal facets are paired diarthrodial joints with opposing articular cartilaginous surfaces that provide a low friction environment which are designed to guide and constrain motion while dispersing loads [11, 12, 13, 14]. Anatomical studies have demonstrated that contact is not uniform across the lumbar facet articular surfaces [15, 16, 17, 18]. Specifically the regions being contacted vary in accordance to the given loading scenario and activity. This in turn influences the overall architecture and creates unique distortions along the surface of the individual facet joints [15, 19]. In addition to mitigating compressive loads along the spine, spinal facet joints also provide torsional constraint, and impedance to shear. The orientation of the facet bony articular pillars serve to resist vertebral translation. Peripherally the capsular ligaments and synovial lining help limit joint distraction. Ultimately spinal degeneration fundamentally leads to an alteration along the avascular layer of hyaline cartilage and the development of osteoarthritis. Initially this is marked by the development of microtears along the superficial cartilaginous surface, which leads to fibrillation along the periphery and the evolution of ulcers spanning the superficial and transitional zones. Exposure of the deep cartilaginous zone threatens the underlying subchondral bone and in direct response there is a development of cysts, osteophytic bone spurs and a calcification of the facet joint capsule [20, 21, 22]. The ensuing ligamentum flavum and facet joint bony hypertrophy gradually begins to compromise the neuroforaminal volume, leading to varying degrees of stenosis.
2. Methodology
2.1 Indications
Surgeons interpret the severity of neuroforaminal stenosis through the lens of their own training, expertise, and experience. For surgeons addressing the foramina begins with developing an awareness of the locus of the pathology and then deploying various tactics either percutaneous, or open to decompress the foraminal obstruction. While indications for foraminoplasty and foraminal decompression are always in a state of flux certain considerations can be made to this point.
Foraminoplasty Inclusion Criteria:
Lumbar foraminal pathology at one or more levels (from L1 to S1) requiring surgical treatment and involving intractable radiculopathy, and/or back pain.
Lumbar disc pathology at one or more levels (from L1 to S1) requiring surgical treatment and involving intractable radiculopathy, and/or back pain.
Herniated disc and/or osteophyte formation that is producing symptomatic nerve root and/or thecal sac compression. The pathology correlates directly with documented findings on patient history and exam (e.g., back pain with concordant leg pain, functional deficit and/or neurological deficit), and the requirement for surgical treatment is confirmed by imaging studies (e.g., MRI, CT, x-rays, etc.).
Subadjacent facet cyst pathology at one or more levels (from L1 to S1) requiring surgical treatment and involving intractable radiculopathy, and/or back pain.
Must be at least 18 years of age and be skeletally mature at the time of surgery;
Advanced degeneration and/or enlargement of the facet joints on the back of the spine.
2.2 Contraindications
Often with lumbar foraminal pathology a simultaneous fusion based implant may be warranted, so for the purpose of this chapter we will set our focus on contraindications specific to the foraminoplasty.
Advanced abnormal changes such as bony collapse at the proposed surgery level.
Pars defect and/or fracture such that foraminoplasty can lead to further instability
An active systemic infection or infection at the surgical site.
An unnatural shape (e.g. hyperkyphosis deformity, hyperlordosis deformity) of the back.
Documented or diagnosed lumbar instability relative to adjacent segments at either level, defined by dynamic (flexion/extension) radiographs showing:
Sagittal plane translation >3.5 mm
Previously diagnosed with osteopenia or osteomalacia.
Previously diagnosed with diagnosis of osteoporosis
If the level of bone mineral density is a T score of −1.5 or lower
Presence of spinal metastases and/or primary tumors along the spinal column.
Overt or active bacterial infection, either local or systemic.
Chronic or acute renal failure or prior history of renal disease.
Received drugs or therapies that may interfere with bone metabolism within 2 weeks prior to the planned date of spinal surgery (e.g., chemotherapy, radiation, steroids or methotrexate), excluding routine perioperative anti-inflammatory drugs.
History of an endocrine or metabolic disorder known to affect osteogenesis (e.g., Paget’s Disease, renal osteodystrophy, Ehlers-Danlos Syndrome, or osteogenesis imperfecta).
A condition that requires postoperative medications that could interfere with postoperative healing/stability such as steroids, chemotherapy, or radiation. (This does not include low-dose aspirin for prophylactic anticoagulation and routine perioperative anti-inflammatory drugs).
A history of heterotopic ossification
Abnormal anatomy which could necessitate supplemental procedures i.e. Conjoined nerve root, Tarlov Cysts
History of a prior failed or attempted foraminoplasty at the proposed arthroplasty level.
3. Implants
3.1 Microblade shaver implants
The disposable microblade shaver comes in a variety of widths (5.5 mm, 7.5 mm, 10 mm, 12 mm) which can allow for a customized decompression of the given neuroforamina Figure 3 and Video 2, https://drive.google.com/file/d/1BJVSFnid_oXpfKfXyy06VLNRHvoGskZC/view?usp=sharing. Along the entire length of the microblade is a flattened bezeled surface designed to run safely against the ventral aspect of the neuroforamina inclusive of any neurovascular structures. Each stainless steel shaver has a narrow distal tapered section, to allow for a gradual foraminal entry. The mid aspect of the microblade contains a chamfered surface with both bone and ligament cutting teeth designed to manually grind away any deterrent pathology. While performing sequential reciprocations the transition from smooth to barbed cutting teeth is tangible and as such allows the surgeon to confine the working zone to a distinct intended area.
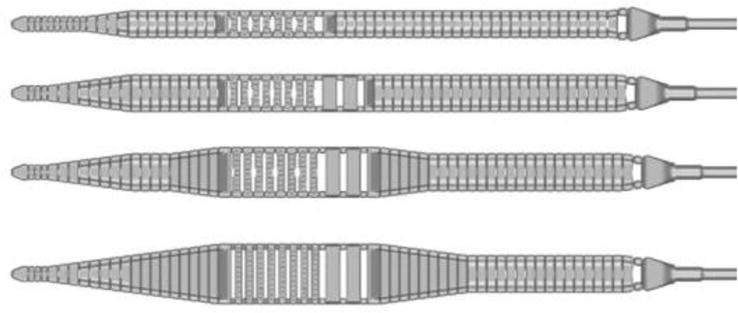
Figure 3.
The disposable microblade shaver comes in a variety of widths (5.5, 7.5, 10, and 12 mm) which can allow for a customized decompression of the given neuroforamina.
4. Surgical rationale and decision making
4.1 Implant placement and rationale
The sequence of implantation should be considered well in advance in order to limit unnecessary complications. Based on the imaging, pathology, and the patient’s symptoms the decision should be made to either proceed with a laminotomy or a full laminectomy. It should be noted that a foraminoplasty with a microblade shaver provides the unique opportunity where through one laminotomy aperture the surgeon can address the exiting and traversing nerve roots of both the ipsilateral and contralateral sides Figure 4 and Video 3, https://drive.google.com/file/d/1a7EkR4T-y4xpJ9o979evwACiGhxMJWRW/view?usp=sharing. However, if used in conjunction with a full laminectomy then the microblade shaver should be sequentially placed ipsilaterally within each individual neuroforamina to perform each foraminoplasty.
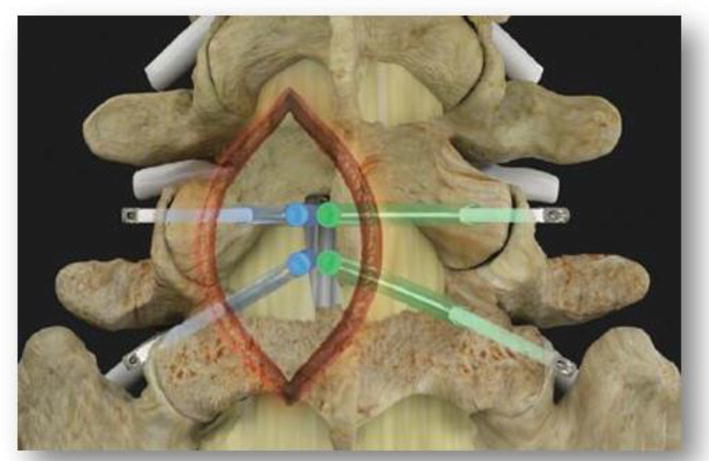
Figure 4.
It should be noted that a foraminoplasty with a microblade shaver provides the unique opportunity where through one laminotomy aperture the surgeon can address the exiting and traversing nerve roots of both the ipsilateral and contralateral sides.
5. Surgical technique and pearls
5.1 Patient positioning
The patient should be positioned prone on the operative frame, with the abdomen slightly elevated off the bed to allow for decreased intra abdominal and epidural venous pressure. The shoulders and arms should be abducted 90 degrees and raised overhead to allow for intraoperative fluoroscopic visualization. Once all bony prominences are padded, a wider than normal surgical prep and drape should be performed to allow sufficient room for potential guidewire egress Figure 5.
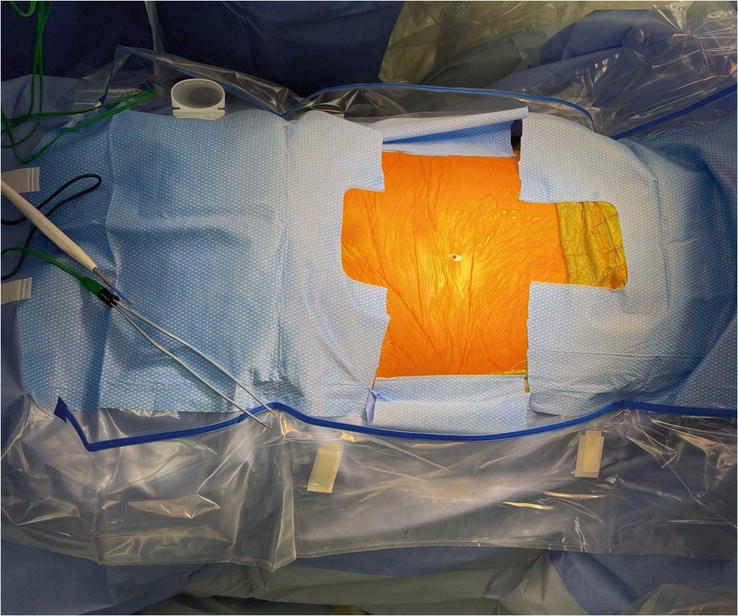
Figure 5.
A wider than normal surgical prep and drape should be performed to allow sufficient room for potential guidewire egress. Here the surgical drape is cut and folded backwards. Later in the procedure this will allow for sufficient room to visualize the guidewire as it exits the skin laterally.
5.2 Surgical approach and foraminoplasty
5.2.1 Sequence of implantation
A standard laminotomy or laminectomy is performed to provide initial access to the neuroforamina. If a microdiscectomy is needed at the index level, it should be performed prior to foraminoplasty as the procedure will result in bleeding bony surfaces which may later serve to obstruct visualization. Prior to implant insertion in the laminotomy, it is first necessary to confirm adequate clearance for the intended shaver. This can be ascertained by placing a Woodson dental within the laminotomy defect and confirming a 360 degree clearance around the perpendicular post of the instrument handle Figure 6 and Video 4, https://drive.google.com/file/d/1f6_55OhfWI0GSbYsELtEniVgTmmzMP6r/view?usp=sharing. If there are still aspects of the hemilaminae abutting the post of the instrument then, prior to microblade insertion, a wider laminotomy should first be performed. Once sufficient clearance has been obtained the ipsilateral or contralateral probe is placed through the laminotomy and into the neuroforamina. During entry the probe should make direct contact against the bone, as if scraping the undersurface of the facet joints as it is being inserted into the neuroforamina. This maneuver provides tactile confirmation the probe was kept in a dorsal plane upon foraminal entry which provides an initial safeguard against any potential ventral nerve entrapment. Fluoroscopic imaging confirms exact placement of the probe within the neuroforamina at the intended position. Placement of the probe within the inferior third of the neuroforamina is recommended to avoid the more cephalad neural elements, while maximizing decompression of the superior articular process. A guidewire is placed through the hollow probe and exits the skins laterally. The hollow probe is subsequently removed leaving the guidewire in place, which will serve to establish the eventual trajectory of the microblade shaver. Next the guidewire is connected to the neurocheck device which will be used to localize the position of the nerve within the foramina. The banded neurocheck is a radiopaque flexible flat 2 sided channel bipolar array with an active central region bordered by proximal and distal markers visible under fluoroscopy. Once in the neuroforamina the banded neurocheck device resembles a parabola under fluoroscopy. Its ideal position is with the proximal marker at the nadir of the curve, thus ensuring the active region is securely centered within the neuroforamina Figure 7. Once in position the neurocheck device is designed to provide distinct dorsal and ventral measurements in order to help pinpoint the exact location of the nerve. These readings are then compared in relation to each other in order to confirm the neural elements lie safely in a plane ventral to the shaver’s intended path. The microblade should only be inserted once the dorsal neurocheck reading measures a difference of 3 mA (milliamperes) greater than the corresponding ventral measurement. If the reading is equal to or less than 3 mA then the guidewire should be completely removed and the entire process should be repeated in a new position within the foramina to ensure neurovascular safety.
Figure 6.
Prior to insertion of the microblade shaver, sufficient clearance can be confirmed by first placing a Woodson dental probe within the laminotomy defect inside the neuroforamina. If there is a 360 degree clearance around the perpendicular post of the instrument handle then there will be sufficient clearance for the microblade shaver.
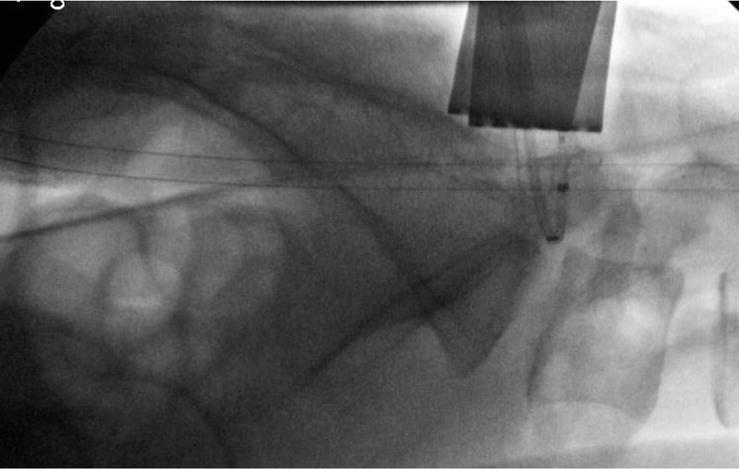
Figure 7.
Once in the neuroforamina the neurocheck device bends into a parabolic shape and is visible under fluoroscopy. The ideal neurocheck position is with the proximal marker at the bottom of the parabola’s curve, as this ensures the active bipolar region is securely centered within the neuroforamina. Dorsal and ventral readings are obtained and compared with each other. If the dorsal reading is 3 mA (milliamperes) greater than the ventral reading then this confirms the neural elements are ventral to the intended path of the microblade shaver. The reference guideline of 3 mA is based on data compiled from the initial successful treatment of over 8000 neuroforamina. Fluoroscopic imaging can be used to determine the expected width of the microblade shaver. The neurocheck device is 4.1 mm wide and its size can be applied towards determining which size microblade shaver (5, 7.5, 10, and 12 mm) can be inserted.
5.2.2 Measuring intraoperative width
Fluoroscopic imaging can be used to determine the expected width of the microblade shaver. The neurocheck device is 4.1 mm wide and can be easily visualized within the neuroforamina on the lateral fluoroscopic image Figure 7. The width of the microblade shaver is selected based on which size can sufficiently reach and therefore decompress the most cephalad tip of the superior articular process. Therefore an extrapolation of neurocheck’s size can be applied towards determining which size microblade shaver (5 mm, 7.5 mm, 10 mm and 12 mm) the neuroforamina can most comfortably accommodate.
5.2.3 Microblade placement
The neurocheck device is removed and the guidewire is then attached to the microblade shaver, which is slowly introduced into the neuroforamina. As the device is guided into position within the neuroforamina attention is placed towards any potential neural activity or irritation. A pre reciprocation fluoroscopic image is obtained and saved for later comparison. The microblade shaver is then pulled dorsally against the neuroforamina, and if there is no neural activity, gentle reciprocations are performed. During consecutive oscillations a tactile feeling of where the cutting teeth are centered within the device can help control bony resection and ultimately limit blood loss.
5.2.4 Measuring the amount of facet resection
During the foraminoplasty the goal is to maximize the overall neuroforaminal volume by maximizing the ligament and bony resection while in essence recreating the neuroforaminal arch. The neuroforaminal arch spans the inferior third of the neuroforamina and is comprised of the curvature formed by the superior border of the pedicle and the ventral cortical limb of the superior articular process. The neuroforaminal arch is biomechanically designed to tolerate multiaxial, translational, compressive and torsional forces Figure 8. Bony outgrowth in the form of facet hypertrophy can inadvertently compress the neuroforamina and lead to differing grades of neuroforaminal stenosis. In order to limit bony resection, prior to reciprocations the original contour of the neuroforaminal arch can be estimated, and the foraminoplasty should conclude as the shaver nears this boundary Figures 9–11. The pre reciprocation fluoroscopic image is saved and compared to imaging obtained from sequential reciprocations until the neuroforaminal arch, and therefore a sufficient volume of the neuroforamina, has been restored Video 5, https://drive.google.com/file/d/1k8wFSAWeuPBwc4ArN8YsqrFgVPRZbRpK/view and Video 1b, https://drive.google.com/file/d/1VnbEUGuI0eyiYCglgFkSAxwF9MKH1UNV/view; https://drive.google.com/file/d/1474vZSCOC3gRnhcICipt1fYkH8o_OH6p/view; https://drive.google.com/file/d/1nremJQLLeceyb15qJLozVvOtAVyvKksz/view.
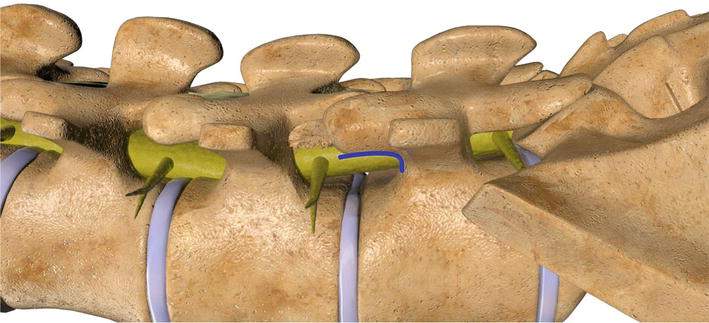
Figure 8.
The neuroforaminal arch spans the inferior third of the neuroforamina and is comprised of the curvature formed by the superior border of the pedicle and the ventral cortical limb of the superior articular process. The neuroforaminal arch is biomechanically designed to tolerate multiaxial, translational, compressive and torsional forces.
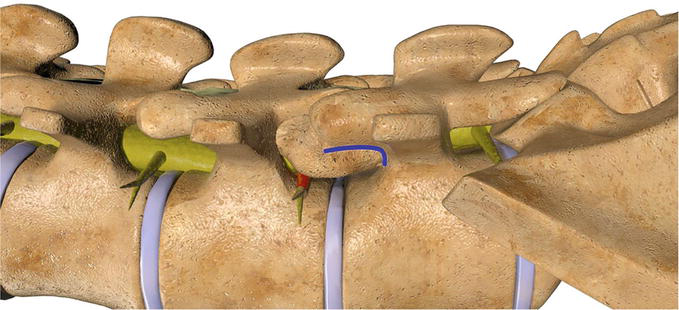
Figure 9.
In order to limit excessive bony resection, prior to reciprocations the original contour of the neuroforaminal arch can be estimated.
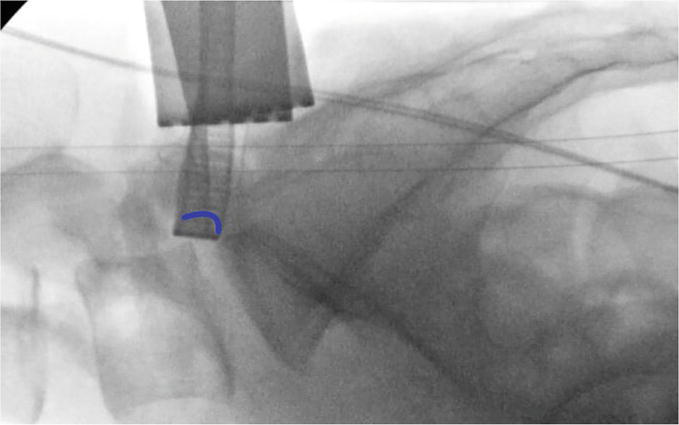
Figure 10.
Prior to any reciprocations an initial image is saved and will be used to compare against with sequential images. Mentally the surgeon makes an approximation of the neuroforaminal arch (Blue curved line) on the fluroscopic image.
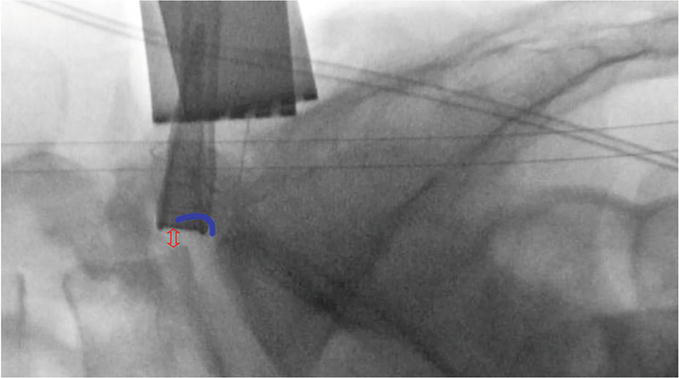
Figure 11.
The pre reciprocation fluoroscopic image is compared to sequential images until the neuroforaminal arch and therefore a sufficient volume of the neuroforamina has been restored (Double headed red arrow). Several images should be obtained throughout the surgery and the foraminoplasty should conclude as the shaver nears this boundary. If the shaver touches the arched boundary line then an inadvertent facetectomy could be performed.
5.2.5 Complications
As the microblade shaver has been incorporated into mainstream clinical practices, the literature remains somewhat limited in regards to its long term results, efficacy and complications. Overall the incidence of neural and vascular complications compare favorably to the rates seen with standard means of decompression [23, 24]. The incidence of surgery-related complications in the initial 59 patients treated was found to be 5.1% [6]. Dickinson et al. described a 3.4% incidence of nerve complications ranging from transient “paresthetic” foot pain (n = 59, 1.7%), worsening of sciatic pain, and a postoperative weakness of the decompressed nerve (n = 59, 1.7%) [7]. When poor neurocheck signals were obtained the flexible shaver system was abandoned in favor of a traditional decompression [6]. A recent series evaluated 31 consecutive patients with a mean age of 54.6 years and the procedure was performed with a neural mapping SentioMMG system. Buraimoh et al. detailed a 9.7% rate of transient numbness postoperatively, with 3.2% complaining of transient hypersensitivity pain and no postoperative motor deficits [25]. The composite nerve complication rate was 12.9%.With widespread use reports of complications have ranged from dural tears, post-surgical hematomas, infections, neural complications, paresthesias, foot drop, and fractures of the articular process [26, 27].
5.3 Case study
61 year old female presents with lumbar complaints which are 80% bilateral leg pain and 20% low back pain. The radiculopathy is rated 6 to 8 out of 10. The radicular pains are more severe in the right leg. The patient has tried and failed a course of conservative measures inclusive of transforaminal epidural injections, physical therapy, bracing, acupuncture, and Nonsteroidal anti inflammatories. Lumbar MRI demonstrated a considerable amount of Central stenosis, Right and Left sided Foraminal Stenosis Figures 12–14. Her diagnosis for neuroforaminal encroachment leading to radiculopathy was confirmed by three separate L4–5 transforaminal epidural steroid injections performed a minimum of 2 months apart. L4–5 central decompression and foraminoplasty was performed bilaterally through L4–5 laminotomies without complication Figures 15 and 16. Postoperatively the patient noted a resolution of both back pain and radiculopathy and returned to regular activities including work within 3 months. She remained asymptomatic at her 1 year postoperative appointment.
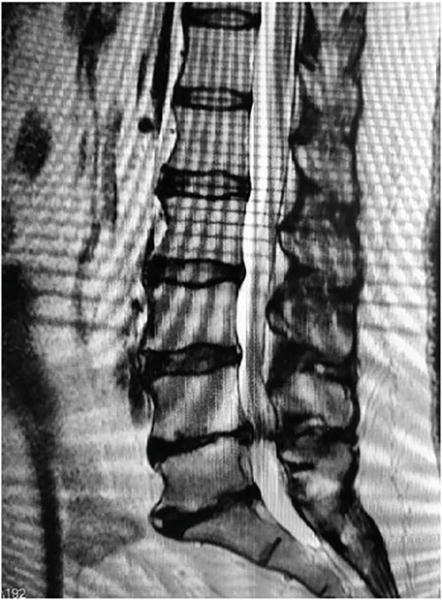
Figure 12.
Preoperative Lumbar Sagittal MRI Demonstrating some decreased height and signal intensity at the L4-L5 and L5-S1Disc concomitant disc herniation.
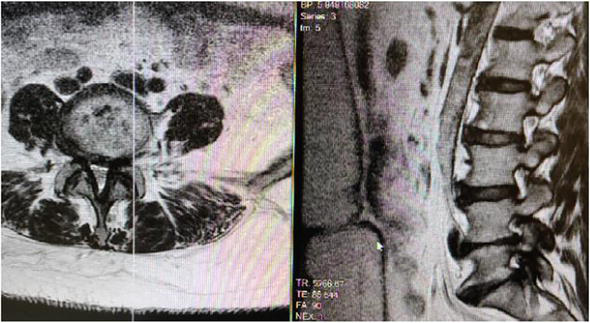
Figure 13.
Left Parasagittal Lumbar MRI of Lumbar 45 demonstrates Severe neuroforaminal stenosis and central stenosis as a result of ligamentum flavum hypertrophy and facet joint hypertrophy.
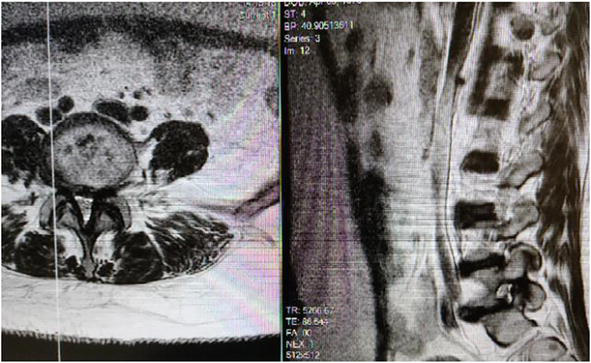
Figure 14.
Right Parasagittal Lumbar MRI of Lumbar 45 demonstrates Severe neuroforaminal stenosis and central stenosis as a result of ligamentum flavum hypertrophy and facet joint hypertrophy.
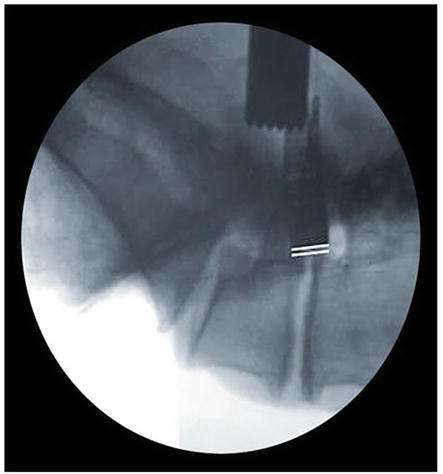
Figure 15.
PreReciprocation Lateral Fluoroscopy: The microblade shaver is slowly inserted into the neuroforamina and a lateral radiograph is obtained. The baseline anteroposterior foraminal depth is delineated by the white reference lines depicting the anterior aspect of the microblade shaver in relation to the posterior margin of the vertebral body. Note the decreased neuroforaminal volume present.
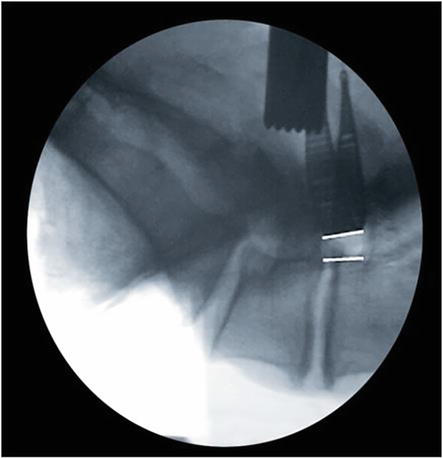
Figure 16.
PostReciprocation Lateral Fluoroscopy: The foraminoplasty is performed under live fluoroscopy or with sequential lateral imaging. There is a notable increase in the anteroposterior neuroforaminal depth and volume.
6. Case study
51 year old male presents with lumbar complaints which are 70% Right leg pain and 30% low back pain. The radiculopathy and back pains are both rated 8 out of 10. The patient has tried and failed a course of conservative measures inclusive of physical therapy, bracing, acupuncture, and Nonsteroidal anti inflammatories. Lumbar MRI demonstrated a considerable amount of Right sided Foraminal Stenosis and a large disc herniation Figures 17–19. His diagnosis for right sided neuroforaminal encroachment leading to radiculopathy was confirmed by three separate transforaminal epidural steroid injections performed a minimum of 3 months apart. First a microdiscectomy was performed at L5-S1. Then a foraminoplasty was performed on the right side through the same laminotomy without complication Figures 20 and 21. Postoperatively the patient noted a resolution of both back pain and radiculopathy and returned to regular activities including work within 6 weeks. He remained asymptomatic at his 1 year postoperative appointment and the final flexion and extension plain films demonstrated no segmental instability.

Figure 17.
Sagittal MRI of L5S1 demonstrating decreased signal intensity with a large extruded fragment lodged posterior to the sacral one vertebral body.
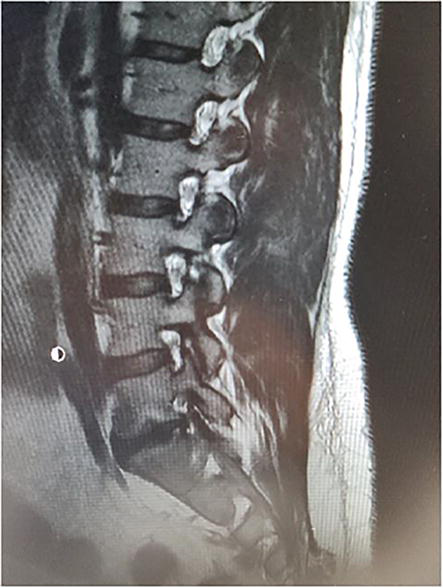
Figure 18.
Right Parasagittal Lumbar MRI demonstrates Severe Lumbar 5-S1 neuroforaminal stenosis.
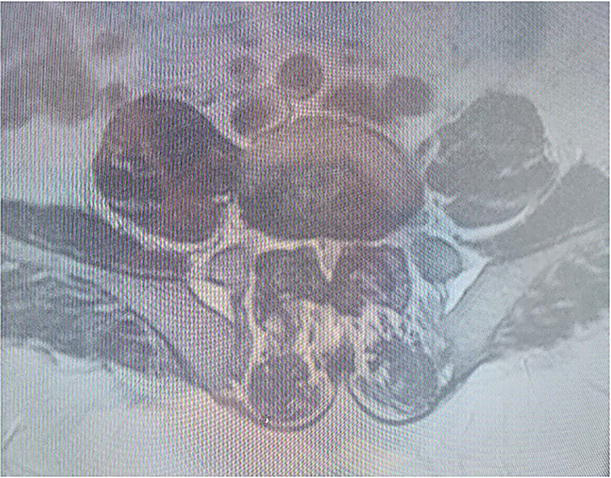
Figure 19.
Axial MRI demonstrating central stenosis and severe facet joint hypertrophy causing neuroforaminal stenosis along the lateral recess with extension into the foraminal zone.
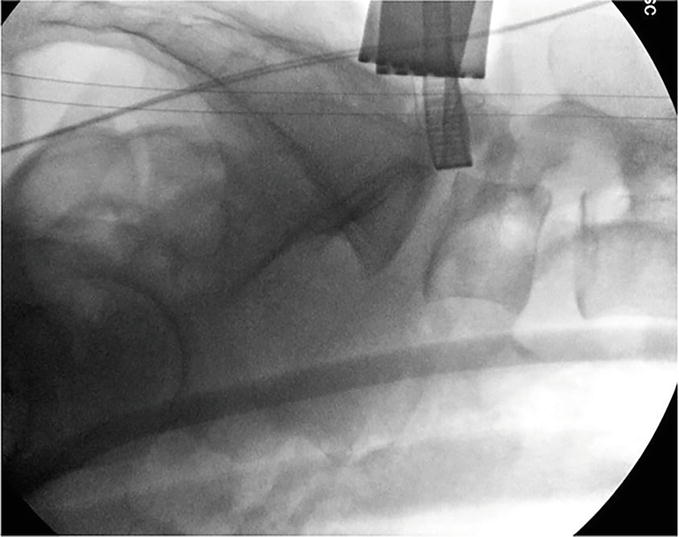
Figure 20.
PreReciprocation Lateral Fluoroscopy: Following the microdiscectomy, the microblade shaver was slowly inserted into the neuroforamina and a lateral radiograph is obtained. The baseline anteroposterior foraminal depth is delineated by the anterior aspect of the microblade shaver in relation to the posterior margin of the vertebral body. Note the decreased neuroforaminal volume present.
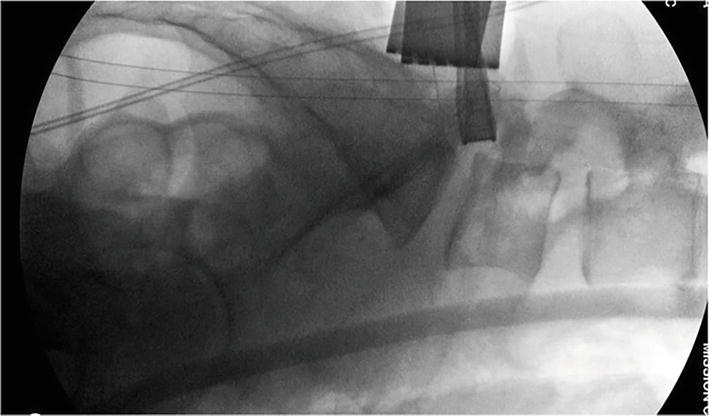
Figure 21.
PostReciprocation Lateral Fluoroscopy: The foraminoplasty is performed under live fluoroscopy or with sequential lateral imaging. There is a notable increase in the anteroposterior neuroforaminal depth and volume.
7. Conclusions
There has been an association of decreased lower back pains in the majority of patients who have undergone the microblade foraminoplasty and this may represent important areas for further study [25, 28, 29, 30]. The statistically significant improvement in VAS and ODI scores postoperatively may be attributed towards the removal of painful bony architecture, or an incidental disruption of the medial branch fibers of the posterior ramus which can occur during the foraminoplasty.
In clinical practice the progression of radiculopathy from foraminal stenosis is of great concern. With properly indicated patients, meticulous preoperative planning, and sound surgical technique, lumbar foraminoplasty offers an excellent surgical option for many patients with symptomatic foraminal stenosis. Foraminoplasty performed in conjunction with fluoroscopy provides an effective means of definitively controlling the extent of the foraminal decompression while directly visualizing the foraminoplasty as it occurs under live fluoroscopy. Moreover the decompression can be performed in concert with neuromonitoring in order to maximize neural safety while maintaining the overall strength and integrity of the newly reconstructed neuroforaminal arch.
Conflict of interest
The author declares no current conflict of interest. From 2011 to 2013 Dr. Pazmiño worked as a surgeon clinical instructor for Baxano.
References
- 1.
Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O’Neill C. Failed back surgery: Etiology and diagnostic evaluation. The Spine Journal. 2003; 3 (5):400-403. DOI: 10.1016/s1529-9430(03)00122-0 - 2.
Carroll SE, Wiesel SW. Neurologic complications and lumbar laminectomy. A standardized approach to the multiply-operated lumbar spine. Clinical Orthopaedics and Related Research. 1992; 284 :14-23 - 3.
McLain RF, Bell GR, Kalfas I, Tetzlaff JE, Yoon HJ. Complications associated with lumbar laminectomy: A comparison of spinal versus general anesthesia. Spine. 2004; 29 (22):2542-2547. DOI: 10.1097/01.brs.0000144834.43115.38 - 4.
Ahuja S, Moideen AN, Dudhniwala AG, Karatsis E, Papadakis L, Varitis E. Lumbar stability following graded unilateral and bilateral facetectomy: A finite element model study. Clinical Biomechanics. 2020; 75 :105011. DOI: 10.1016/j.clinbiomech.2020.105011 - 5.
Ahuja S, Dudhniwala AG, Tsouknidas A, Sarrigiannidis S, Michailidis N. Lumbar stability following graded unilateral and bilateral graded facetectomy - A finite element model study. The Spine Journal. 2016; 16 :S46-S47. DOI: 10.1016/j.spinee.2016.01.030 - 6.
Dickinson LD, Phelps J, Summa CD, Vanichkachorn JS, Jeshuran WR, Randall JB, et al. Facet-sparing decompression with a minimally invasive flexible microblade shaver. Journal of Spinal Disorders & Techniques. 2013; 26 :427-436. DOI: 10.1097/bsd.0b013e318290fc62 - 7.
Dickinson LD, Phelps J, Summa CD, Vanichkachorn JS, Jeshuran WR, Randall JB, et al. Facet-sparing decompression with a minimally invasive flexible microblade shaver: A prospective operative analysis. Journal of Spinal Disorders & Techniques. 2013; 26 (8):427-436 - 8.
Lauryssen C. Technical advances in minimally invasive surgery: Direct decompression for lumbar spinal stenosis. Spine. 2010; 35 (26 Suppl):S287-S293 - 9.
Lauryssen C, Berven S, Mimran R, Summa C, Sheinberg M, Miller LE, et al. Facet-sparing lumbar decompression with a minimally invasive flexible MicroBlade Shaver® versus traditional decompression: Quantitative radiographic assessment. Clinical Interventions in Aging. 2012; 7 :257-266 - 10.
Block J, Miller L, Berven M, Summa, et al. Facet-sparing lumbar decompression with a minimally invasive flexible MicroBlade Shaver® versus traditional decompression: Quantitative radiographic assessment. Clinical Interventions in Aging. 2012; 7 :257. DOI: 10.2147/cia.s32536 - 11.
Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: An update. Current Opinion in Rheumatology. 2006; 18 :147-156. DOI: 10.1097/01.bor.0000209426.84775.f8 - 12.
Côté P, Cassidy JD, Carroll L, Frank JW, Bombardier C. A systematic review of the prognosis of acute whiplash and a new conceptual framework to synthesize the literature. Spine. 2001; 26 (19):E445-E458. DOI: 10.1097/00007632-200110010-00020 - 13.
White AA. Clinical biomechanics of cervical spine implants. Spine. 1989; 14 (10):1040-1045. DOI: 10.1097/00007632-198910000-00002 - 14.
Adams MA, Hutton WC. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. Journal of Bone and Joint Surgery. British Volume (London). 1980; 62 (3):358-362. DOI: 10.1302/0301-620X.62B3.6447702 - 15.
Dunlop RB, Adams MA, Hutton WC. Disc space narrowing and the lumbar facet joints. Journal of Bone and Joint Surgery. British Volume (London). 1984; 66 (5):706-710. DOI: 10.1302/0301-620X.66B5.6501365 - 16.
Zhu QA, Park YB, Sjovold SG, Niosi CA, Wilson DC, Cripton PA, et al. Can extra-articular strains be used to measure facet contact forces in the lumbar spine? An in-vitro biomechanical study. Proceedings of the Institution of Mechanical Engineers. Part H. 2008; 222 (2):171-184. DOI: 10.1243/09544119JEIM290 - 17.
Wilson DC, Niosi CA, Zhu QA, Oxland TR, Wilson DR. Accuracy and repeatability of a new method for measuring facet loads in the lumbar spine. Journal of Biomechanics. 2006; 39 (2):348-353. DOI: 10.1016/j.jbiomech.2004.12.011 - 18.
Kim K, Lee S-K, Kim YH. The biomechanical effects of variation in the maximum forces exerted by trunk muscles on the joint forces and moments in the lumbar spine: A finite element analysis. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2010; 224 :1165-1174. DOI: 10.1243/09544119jeim765 - 19.
Rousseau M-A, Bradford DS, Bertagnoli R, Hu SS, Lotz JC. Disc arthroplasty design influences intervertebral kinematics and facet forces. The Spine Journal. 2006; 6 (3):258-266. DOI: 10.1016/j.spinee.2005.07.004 - 20.
Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Archives of Biochemistry and Biophysics. 1995; 322 (1):87-96. DOI: 10.1006/abbi.1995.1439 - 21.
Whiteside RA, Jakob RP, Wyss UP, Mainil-Varlet P. Impact loading of articular cartilage during transplantation of osteochondral autograft. The Journal of Bone and Joint Surgery. 2005; 87 (9):1285-1291. DOI: 10.1302/0301-620X.87B9.15710. Erratum in: Journal of Bone and Joint Surgery. British Volume (London) 2005;87(11):1583 - 22.
Morel V, Merçay A, Quinn TM. Prestrain decreases cartilage susceptibility to injury by ramp compression in vitro. Osteoarthritis and Cartilage. 2005; 13 :964-970. DOI: 10.1016/j.joca.2005.06.016 - 23.
Desai A, Ball PA, Bekelis K, Lurie J, Mirza SK, Tosteson TD, et al. SPORT: Does incidental durotomy affect long-term outcomes in cases of spinal stenosis? Neurosurgery. 2011; 69 (1):38-44 - 24.
Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine. 2010; 35 (14):1329-1338 - 25.
Buraimoh M, Ansok C, Pawloski J, Jung EK, Bartol S. Facet sparing foraminal decompression using the flexible shaver foraminotomy system: Nerve safety, pain relief, and patient satisfaction. International Journal of Spine Surgery. 2018; 12 (2):92-97 - 26.
Pazmino PR. Conversations and Lectures Baxano Faculty Meeting. California: Napa; 2013 - 27.
Pazmino PR, Verma RB. Conversations With Rohit Binod Verma. New York: NY; 2018 - 28.
Pazmino PR, Lauryssen CL. Lifestyle Outcomes Analysis After Minimally Invasive Microscopic Decompression for Lumbar Spinal Stenosis. Orlando, Florida: Presented at The Annual Meeting of The AANS/CNS Section on Disorders of the Spine and Peripheral Nerves; 2008 - 29.
Pazmino Lauryssen CL. Lifestyle Outcomes Analysis After Minimally Invasive Microscopic Decompression For Lumbar Spinal Stenosis. London: Presented at sAS 9 Spine Arthroplasty Society Annual Meeting, England; 2009 - 30.
Pazmino PR. IoFlex Baxano Neuroforaminal Decompression Following Traumatic Radiculopathy. Paris France: Annual Meeting of The World Congress on Minimally Invasive Spine Surgery and Techniques Association; 2014