Abstract
Polyaniline (PANI) is one of the oldest, yet most profound conducting polymer discovered. It’s ease of synthesis, high conductivity, and environmental stability in the doped state makes it very attractive for a variety of potential applications. However, its insolubility and lack of redox stability has hindered many commercial applications. Consequently, many researchers have sought to overcome PANI’s deficiencies in many ways including the development of PANI derivatives. This chapter will discuss the synthesis, properties, and applications of PANI derivatives. We will discuss three types of PANI derivatives—substitution on the benzene ring, substitution on the nitrogen atom, and fused ring cores. The properties of the PANI-derivatives will be compared to pristine PANI. Finally, we will emphasize the applications that arise from these derivatives and how they compare to PANI.
Keywords
- Buchwald/Hartwig
- polyaniline derivatives
- conductive polymer
- redox polymer
- electroactive polymers
1. Introduction
Electronic devices based on inorganic conductors and semiconductors (e.g., Au, Cu, Pt, and Si) often require numerous etching and lithographic development steps during fabrication leading to longer processing times, costly equipment development, and higher priced devices [1]. In the middle of the twentieth century, these limitations spurred on extensive engineering and research efforts focused on optimizing fabrication methodologies; however, despite significant improvements, these devices are still limited by the physical properties of the inorganic materials they employ. Serendipitously, the discovery of
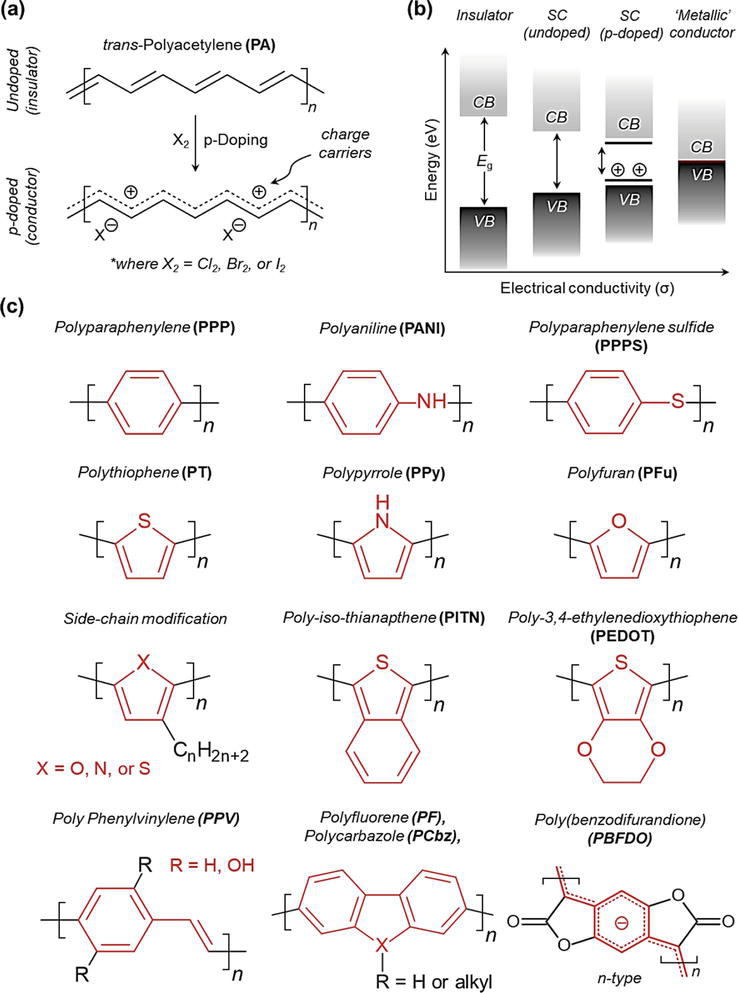
Figure 1.
(a) Illustration of the p-doping effect on PA, (b) various band gaps (Eg) of different material types and, (c) representative CPs and their chemical structures.
The discovery of the properties of CPs has paved the way for creating new devices and novel applications where the benefits of plastics (i.e., scalability, processability, low-cost, etc.) and retention of high electrical conductivity are desired [10]. In this regard, solution and melt processable polymers could offer a facile approach to applying CP coatings and fabricating devices on large dimensional scales [11]. Furthermore, unlike their inorganic counterparts, CPs can potentially be processed using more modern solution-based processing techniques (e.g., inkjet printing), which could dramatically reduce the complexity, fabrication time, and cost when used to develop integrated electronic devices. Nevertheless, early development of CPs has lacked focus on developing truly “processable CPs” which has hindered their implementation to date.
A non-comprehensive list containing some classical CPs is shown in Figure 1c. Among them, polyaniline (PANI), polypyrrole (PPy), polythiophene (PT), and (poly-(3,4 ethylene dioxythiophene) (PEDOT) have been studied and employed to the greatest extent to date. Interestingly, although not fully appreciated at the time, the conductive nature of PANI was first observed more than 150 years ago after being prepared electrochemically from an acidic solution of aniline monomer [4, 6, 12]. Since then, PANI has become one of the most extensively studied CPs, especially over the last 20 years [12]. This interest in PANI is in large part due to its superior properties that impart high electrical conductivity, redox and ion-exchange properties, and high operational stability (i.e., is stable under a variety of environmental, thermal, and chemical conditions) (Figure 2) [13]. In addition, PANI’s straightforward synthesis, tunable electrical conductivity, potential for chemical functionalization, and low synthetic cost make it an economically viable option compared to other CPs [8, 13, 14, 15, 16, 17, 18]. PANI is therefore being investigated for use in various disciplines, including sensors [19], energy storage devices [20], corrosion inhibitors [21], solar cells [22], and electromagnetic shielding materials [23].
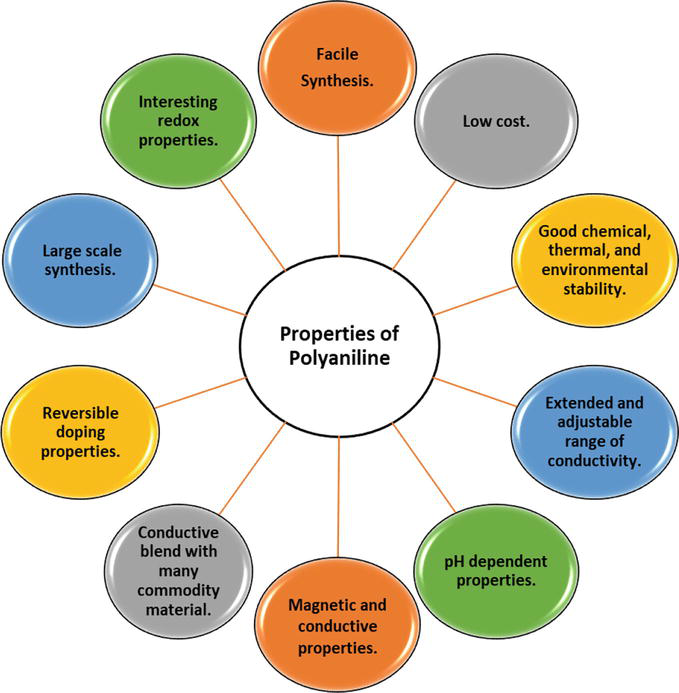
Figure 2.
Properties of polyaniline.
Doping of CPs can dramatically improve their electrical conductivity by many orders of magnitude and is required to obtain highly conductive materials. Doping usually occurs through an oxidation or reduction process and the polymers are considered p- and n-type, respectively [1, 7]. The oxidative/reductive doping process results in the alteration of the electronic configuration of the polymers making them unstable under environmental conditions. Another form of doping is protonic or acid doping. Unlike the oxidative/reductive doping, protonic doping (acid) does not alter the polymer’s electron configuration; consequently, the polymer is environmentally stable in the doped state. It was shown that polyaniline could be doped with acid thus maintaining the same number of π-electrons in the backbone. Inorganic acids such as hydrochloric (HCl), sulfuric (H2SO4), nitric acid (HNO3) and chloric acid (HClO3) have traditionally been utilized as dopants in conductive PANI production. Polystyrene sulphonic acid (PSS), para-toluene sulfonic acid (
MacDiarmid proposed three forms for the structure of PANI based on the oxidation states [1, 25]. The leucoemeraldine base (LB) (colorless) is the completely reduced state of PANI, the emeraldine base (EB) (blue) is half-reduced and half-oxidized, and the pernigraniline base (PB) (blue/violet) is the fully oxidized form [1, 26, 27]. The leucoemeraldine base form has only benzenoid rings, the emeraldine base form has both benzenoid and quinonoid rings, while the pernigraniline base form has only the quinoid rings [18]. Upon doping, the emeraldine base is converted to the emeraldine salt (ES) (green) and becomes conducting. The transformation of the emeraldine base form of PANI from an insulating state to the conductive emeraldine salt form is due to the formation of polaronic carriers, i.e., the protonated species in the emeraldine state. The protonation occurs on the imine N rather than the amine N because imines are more basic than amines [24, 28, 29]. Consequently, protonic doping of the ES form produces a bipolaronic species (protonation of both imine Ns) that undergoes an internal relaxation to form the highly conductive polarons [29, 30]. Polaron separations results in charge flow. Due to its excellent stability at ambient temperature and the fact that after doping with acid, the resulting emeraldine salt form of polyaniline is highly electrically conductive, EB is regarded as the most practical form of polyaniline. Unfortunately, even after being doped with an acid, leucoemeraldine and pernigraniline still have low conductivity [1]. Figure 3 shows the different oxidation states of PANI before and after protonic doping.
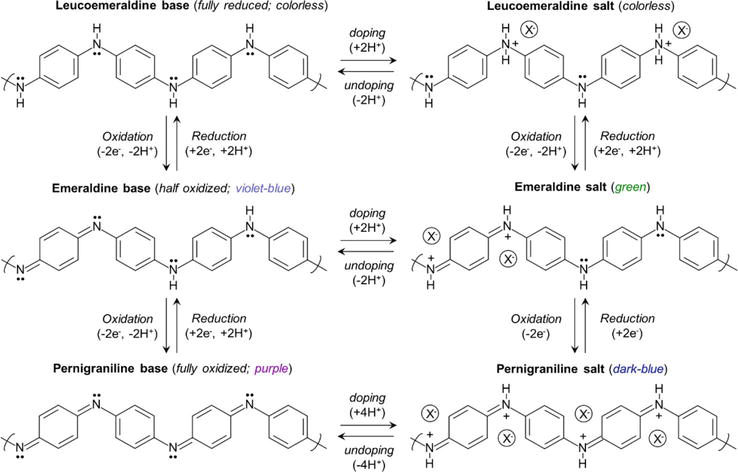
Figure 3.
Oxidation states and doping of various forms of polyaniline.
Despite the excellent properties and potential applications of PANI, it has some disadvantages that prevent its wide-spread applications. Actually PANI has been used in limited large scale commercial applications [31]. Of the major challenges to commercializing PANI, poor long term electrochemical stability and manufacturing costs due to low solubility in most solvents making processing difficult, are the two major issues [32]. The most successful improvements in PANI’s processability have been achieved by using suitable dopants to increase the solubility and modifying the monomers to incorporate solubilizing alkyl chains. The stability of PANI is improved by forming composite materials [33, 34, 35, 36]. In this chapter, we will focus on modifications of PANI (PANI derivatives) that address the limitations of poor solubility and electrochemical instability. The synthesis, characterization, and applications of these derivatives are the major focus here.
1.1 Synthesis of polyaniline
The three primary methods for producing PANI and its derivatives are chemical (i.e., oxidative polymerization), electrochemical (i.e., anodization), and more recently, palladium-catalyzed C-N cross-coupling (i.e., Buchwald-Hartwig reaction). Historically, chemical oxidative polymerization and electrochemical polymerization were the main methods of producing PANI due to their simplicity and availability of the monomers. Chemical oxidative polymerization involves an acidic aqueous media (pH < 2) with strong oxidants (e.g., potassium dichromate, hydrogen peroxide, ferric chloride, ceric nitrate, sulfate, and ammonium persulfate (APS)) in order to create the electrically conductive emeraldine salt form of PANI (Figure 4) [37, 38]. Unfortunately, the oxidative polymerization conditions produce PANI and PANI-analogs with variation in their structures and properties due to the many side reactions taking place simultaneously. While the overall mechanism is likely quite complex, a simplified mechanism for aniline polymerization in acidic media (pH < 2) is typically agreed to involve the initial formation of aniline radical cations (Figure 5a) that can undergo para-coupling forming a dimer (4ADA), due to the ortho/para (
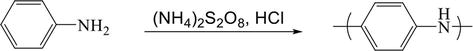
Figure 4.
Oxidative polymerization of aniline in acidic medium.
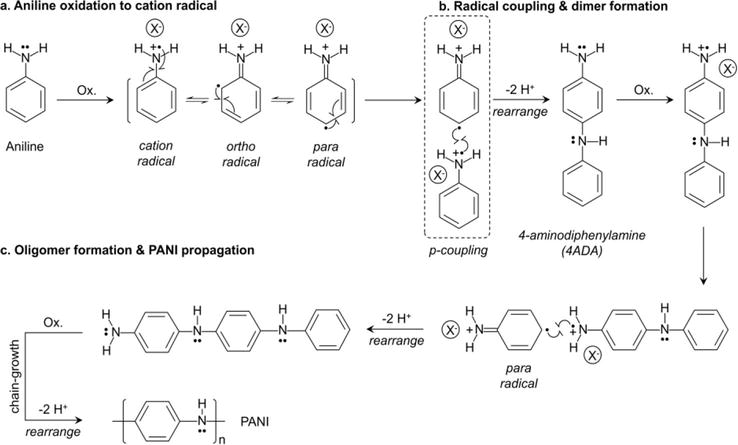
Figure 5.
Mechanism of polymerization for polyaniline. Adapted from Refs. [
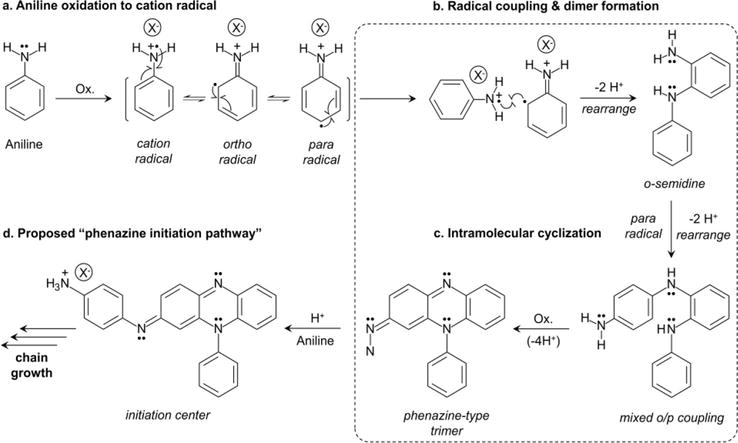
Figure 6.
Proposed alternative initiation pathway for polyaniline polymerization. Adapted from Ref. [
Similar to the chemical-based oxidative polymerization route, electrochemical polymerization also involves the oxidation of aniline monomers, and its mechanism closely resembles that of oxidative polymerization (Figure 5) [39, 40]. Likewise, low pH (pH < 2) is preferred to achieve an electrically conductive PANI film. However, this process utilizes an applied potential at the electrode surface (e.g., using potentiostatic, galvanostatic, or cyclic voltammetry techniques) as opposed to an oxidizer in the bulk solution. In this way, the oxidation of aniline monomers to cation radicals occurs only at the electrode surface. These radicals then undergo coupling with one another to form dimers and ultimately polymers that becomes sufficiently large that it is no longer soluble in the electrolyte. Subsequently, the polymer forms a film at the electrode surface. The process can be terminated by removing the bias or exhausting the monomer [13, 39, 40]. This process typically produces insoluble and very high molecular weight PANI films with a fair amount of regio-irregularity (i.e., cross-linking).
Due to the presence of side reactions for both the chemical and electrochemical polymerization methods and the difficulty of obtaining a soluble PANI product from either methods, a new approach has recently been employed. This approach is based on the palladium-catalyzed C-N cross-coupling reaction developed by Buchwald and Hartwig that utilizes a Pd-catalyst, amine, aryl- halide, and hindered base in a suitable solvent [44, 45, 46]. The Buchwald/Hartwig reaction mechanism (Figure 7a) involves an oxidative insertion of the Pd(0) species I into an aryl halide substrate forming a Pd(II) species II. The increased acidity of the coordinated amine (III) at this point then allows for deprotonation with a hindered base (e.g., HN(SIMe3)2) and formation of a palladium-amido complex IV. Reductive elimination regenerates the Pd(0) catalyst I and produces an arylated-amine product. Significant improvements in ligand architecture (Figure 7b) and overall catalyst optimization have led to higher efficiency and broader application of the C-N coupling reaction. Consequently, coupling between numerous hetero(aryl) (pseudo)halides and amines at low temperature and low catalyst loading (ppm) is enabled. Buchwald and co-workers utilized this reaction to perform an efficient synthesis of a tert-butyloxycarbonyl (BOC) protected PANI (Figure 8) [47]. The BOC group, which can be removed during processing, was bulky enough to render the polymer soluble in common organic solvents. The Meyer group incorporated ortho-substituted repeat units into the backbone of PANI chain using the Buchwald/Hartwig reaction [48]. Due to the stepwise mechanism of the polymerization, alternative co-polymers can be produced. The
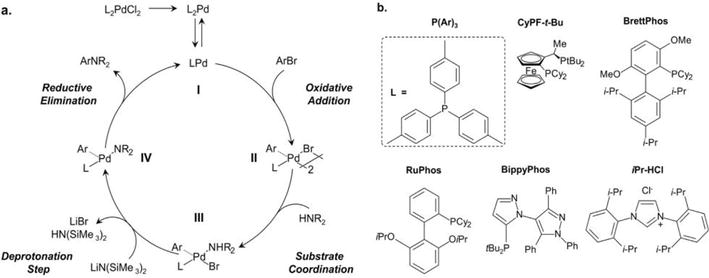
Figure 7.
Mechanism of the Buchwald-Hartwig palladium catalyzed C-N cross-coupling reaction and several optimized ligands.
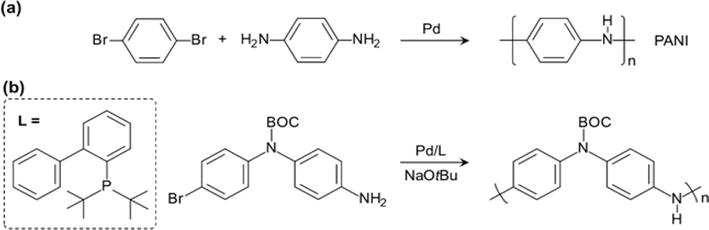
Figure 8.
PANI and a derivative by Buchwald/Hartwig reaction.
2. Polyaniline derivatives
Although conductive polymers, particularly PANI, have numerous distinct benefits and possibilities as previously stated, PANI also has significant downsides. The fundamental disadvantage of PANI’s conductive ES form is its low solubility in common organic solvents due to its stiff backbone, which makes it difficult to process. This insolubility hampers the practical application of PANI. Over the last few years, a lot has been done to make it easier to process PANI [11, 26, 52, 53]. Another, less studied problem of PANI is its electrochemical instability, which is mainly due to the defects in the polymer during the synthesis that resulted from the inherent instability of the radical species produced during the reaction [54, 55, 56]. The main approach to address the solubility, processability, and other aspects of PANI is to prepare PANI derivatives, which is discussed in this chapter. PANI derivatives can be broken down into three groups; (i) substitution on the benzene ring, (ii) substitution on the amino nitrogen, and (iii) fused ring systems (Figure 9) [26, 57].
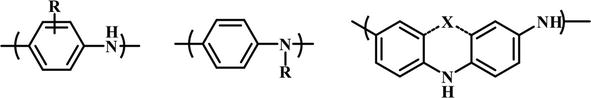
Figure 9.
Three types of PANI derivatives.
2.1 PANI derivatives with benzene ring substitution
Traditionally, increasing PANI’s solubility was obtained by including substituents on the benzene rings, which weakens the chain’s stiffness and interchain interaction [26, 58, 59, 60, 61]. When substituents such as alkyl, alkoxy, and amino groups are attached to the aromatic rings, the steric barrier between the polymeric chains of the polymer increases, reducing the intermolecular force and increasing the dissolution of the polymer (Figure 10). For example, poly(2,3-dimethylaniline) (P(2,3-DMA)) was synthesized by Ma et al. using oxidative polymerization and was shown to have improved anticorrosion, electrochemical, and crystal properties compared to PANI [62, 63]. Unfortunately, no information was given by the authors pertaining to the solubility, conductivity, or electrochemical stability of the pristine polymer. Patil et al. prepared poly(o-anisidine) (POA) as coatings on copper by the electrochemical polymerization method [64]. Interestingly, the polymer film contained a mixed phase of both the pernigraniline base (PB) and emeraldine salt (ES) form of POA according to the optical absorption spectroscopy data. This polymer also lowered the corrosion rate of copper metal ~100 times more compared to the uncoated copper metal. However, due to the nature of the synthetic method that formed the film on the copper surface, there is no information about the solubility of the polymer. Sathyanarayana et al. prepared an ortho substituted poly ethoxy aniline (POEA) with excellent solubility and corrosion inhibition of iron in the presence of acid chloride [65]. A co-polymer, poly(o−/m-toluidine-co-o-nitroaniline), prepared by emulsion polymerization under oxidative conditions was also reported by the same authors. Interestingly, while the homopolymer of
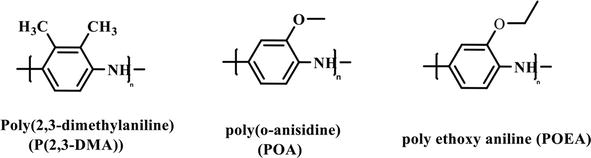
Figure 10.
Examples aryl substituted PANI derivatives.
To achieve water solubility of PANI, derivatives were prepared with water solubilizing hydrophilic groups such as a sulfonate [67, 68], carboxylate [69, 70], phosphonate, boronate [71], and hydroxyl groups) to the aromatic backbone (Figure 11). These groups preform dual function, i.e., they provide water solubility and self-doping of the polymer. Sulfonic acid ring-substituted polyaniline (SPAN), first reported by Epstein and coworkers, was obtained by the sulfonation of the emeraldine base with fuming sulfuric acid [68, 72]. The transport, magnetic, and electrochemical properties of SPAN was later determined through a collaboration with the MacDiarmid group that provided insight to the effect of the sulfonate group on the solubility, doping mechanism, and charge transport of SPAN [73]. The polymer was shown to be self-doping as there was no pH effect on the conductivity for pH < 7.5. Interestingly, the polymer can be casted into a film from aqueous alkaline solvents (0.1 M NH4OH) forming the blue-violet solution, which when casted onto a substrate and dried, reverts to the shiny green ES form that is highly conductive (0.1 S/cm).
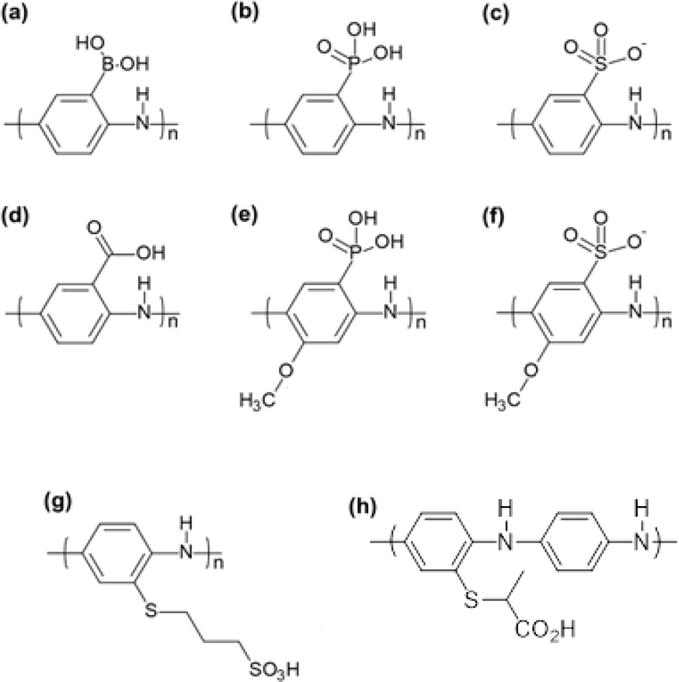
Figure 11.
Hydrophilic self-doped substituted derivatives of PANI.
Carboxylate substituted PANI were synthesized from anthranilic acid (
Some other interesting PANI derivative structures containing fused aryl and benzoquinone rings at the 2,3-positions of the PANI aryl group, have also been prepared. Poly(1-naphthylamine) [74, 75], poly(1-amino anthracene) [76, 77, 78], and copolymers from aminoquinoline(AQ) and anisidine (AS) [79] were prepared by both oxidative polymerization and electrochemical polymerization. The polymers have PANI-like features. They exist in several different oxidation states with conductivities ranging from 0.055–0.083 S/cm for the naphthalene and anthracene analogs. The conductivity was much less for the AQ/AS copolymer. PANI derivatives containing benzoquinone units fused to the 2,3-positions on the aniline moiety were also developed and their electrochemical properties with respect to cathode electrodes were studied. Some examples are shown in Figure 12. Poly(1,5-diaminoanthraquinone) [poly(DAAQ)] were prepared from 1,5-diaminoanthraquinone (DAAQ) by electrochemical polymerization and the conductivity values at different potential was measured [80]. It was shown that the conductivity values of the polymer ranged from 0.3 to 2.0 S/cm when the voltage range from −2.0 to 0.8 V, which are much higher than PANI at the lower potential. Poly(5-amino-1,4-naphthoquinone) (PANQ), reported by Pham et al., was prepared by electrochemical polymerization [81, 82]. The films had properties similar to PANI and the structure contained one quinone group per ANQ moiety. The polymer had a conductivity of 10−1 S/cm. Poly(5-amino-1,4-dyhydroxy anthraquinone) (PADAQ) was synthesized by oxidative polymerization for cathode material and was determined to behave like PANI [83]. Likewise, poly(1-aminoanthraquinone) (PAAQ) films were prepared by the electrochemical polymerization and tested as efficient cathode material [84]. These polymers were studied for energy storage properties, which is discussed later.
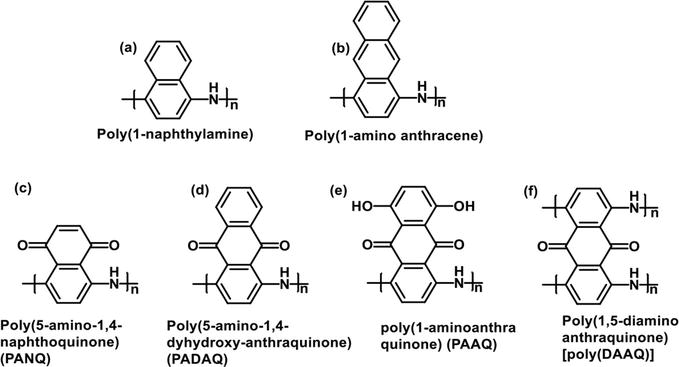
Figure 12.
Fused-aryl substituted PANI derivatives.
2.2 Substituted derivatives of PANI on amino nitrogen atom
Another approach for increasing the solubility of PANI is to prepare N-substituted PANI derivatives (Figure 13) [85, 86]. While N-substitution has been demonstrated to have little effect on the synthesis of the polymers, it has significant effect on the properties of the final polymer due to the steric component of the substituent. The substitutions ranged from phenyl, substituted phenyl, to substituted alkyl chains. PANI N-substituted derivatives have improved solubility and processability; however, the final electronic properties are usually altered owing to the addition of the substituent on the N atom. One of the first studies of N-substituted PANI was reported by Chen and co-workers who prepared a series of N-alkylated PANI, where the alkyl chains ranged from butyl (C4) to the hexadecyl (C16) [86]. The solubility of the PANI derivatives in common organic solvents (tetrahydrofuran (THF), dichloromethane (DCM), and chloroform (CHCl3)) increased with the size of the alkyl chain, especially above C-6. Lowering the polarity and stiffness of the polymer chains through integration of the flexible alkyl substituent is responsible for the improved solubility. On the other hand, N-alkylated EB become insoluble in highly polar solvents like N-methyl-2-pyrrolidone (NMP) and dimethyl sulfoxide (DMSO) due to lack the amine hydrogen necessary for hydrogen bonding. Importantly, the polymer existed in the EB form and can be effectively doped to produced soluble ES. The conductivity ranged from 10−4 to 10−2 S/cm with the C-6 alkyl chain having the highest conductivity. Other examples are poly-(aniline-co-N-(4-sulfophenyl)aniline) (PAPSA) [87] and its homo polymer, poly-(N-(4-sulfophenyl)aniline) [88], which were synthesized from aniline and sodium diphenylamine-4-sulfonate by the chemical oxidative polymerization. The polymer is soluble in aqueous basic media. While the conductivity is 102–106 times higher than other N-alkylsulfonate substituted PANI [89], it is 3 orders of magnitude lower than PANI. In general, N-substitution significantly lowers the conductivity of the polymer.
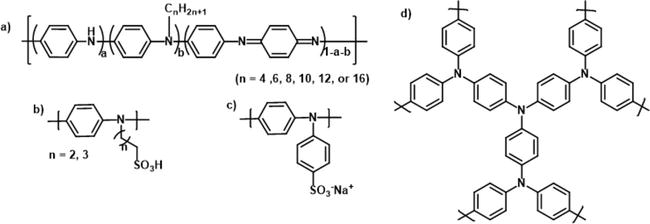
Figure 13.
N-substituted PANI derivatives.
2.3 Fused-ring PANI derivatives
Fused-ring PANI derivatives have been prepared and studied since the 1960s [90]. These rigid ladder-type PANI derivatives are based on phenazine, phenoxazine, phenothiazine, and their co-polymers (Figure 14). The PANI derivatives maintain the PANI backbone with π-extended structures, which leads to mostly insoluble polymers [91, 92]. Fors and coworkers prepared PANI derivative poly(N-methylphenothiazine dimethylphenylenediamine) (PT-DMPD), from N-methylphenothiazine and dimethylphenylenediamine (Figure 14a), and poly(N-methylphenothiazine benzidine) (PT-BZ) from N-methylphenothiazine and tetramethylbenzidine using Buchwald/Hartwig reaction as redox active materials [93, 94]. Our group also synthesized PANI derivatives containing phenoxazine, carbazole, and phenothiazine heterocycles by the Buchwald/Hartwig cross coupling reaction to study their processability and electrochemical stability (Figure 14b–d) [95, 96, 97]. Unlike classical PANI, these polymers were electrochemically quite stable over 100 CV cycles without noticeable degradation while maintaining processibility by attaching long alkyl chain pendant. Overall, these PANI-analogs offer a unique insight into alternative ways of tuning PANI’s structural, chemical, and physical properties. The electrical conductivities of the undoped (pristine) structures ranges from 10−5 to 10−6 S/cm and increase 2–4 orders of magnitude upon doping. More recent work has described additional side chain modification (Figure 14b and d), which can impart greater solubility for processable polymers. Orlov et al. describe the chemical oxidative polymerization of 2,5-dianiline-3,6-dichloro-1,4-benzoquinone (DADCB) to give a PANI derivative (Poly-DADCB) containing an N-substituted 5-aniline-3,6-dichloro-1,4-benzoquinone [98]. It was discovered that Poly-DADCB forms at a faster rate that PANI. However, the electrical conductivity was on the same order of magnitude as PANI.
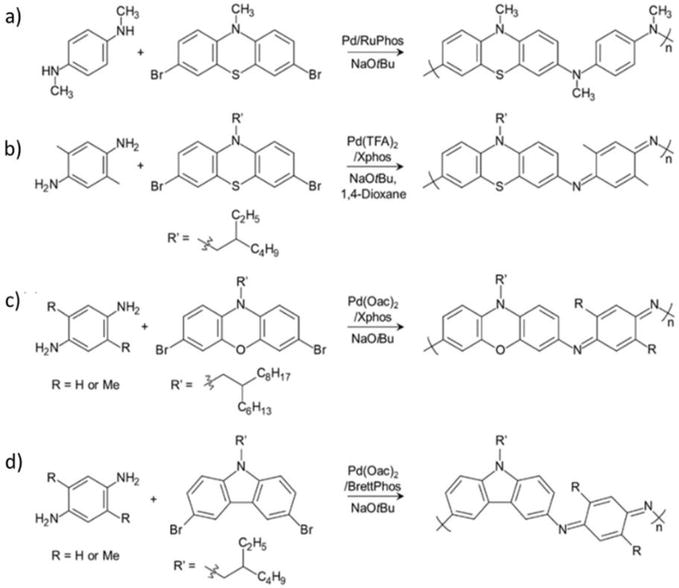
Figure 14.
Fused ring PANI derivatives.
3. Applications of PANI derivatives
PANI has been explored for many different types of applications such as biological sensors, anti-static wrappings, and electronic devices. Unfortunately, many of these applications are limited by its insolubility and electrochemical instability. However, PANI derivatives have been successful in achieving adequate solubility and electrochemical stability that they have become preferred to PANI in these applications. This section focuses on the applications of PANI derivatives in a variety of fields.
3.1 Antimicrobial activity
PANI and its derivatives have been shown to have antimicrobial properties where the mode of action is different for each derivative. For example, Andriianova et al. demonstrated the substituents effect of PANI derivatives on bactericidal properties of the polymer [99]. It was shown that PANI derivatives with substituents on the benzene ring, regardless of the bulkiness of the substituents, (POT, POA, PPA, PMA, and PClMA) had a negative or no effect on antibacterial activity compared to PANI. Alternatively, N-substituted PANI derivatives (PNClA and PNMA) increased the antibacterial properties against gram-positive (
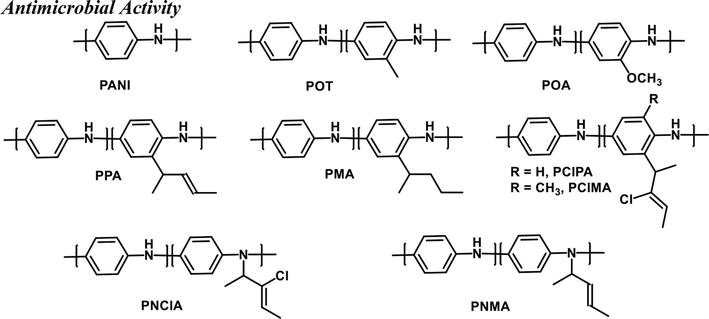
Figure 15.
Structures of PANI-derivatives with antimicrobial activity. Structures drawn from Ref. [
PANI derivatives also showed bacteriostatic properties towards the microorganisms. Here it was determined that the mode of bactericidal activity is due to the positive charges on PANI and its derivatives that disrupt the nature of the bacteria’s cell membrane that ultimately leads to cell lysis. In another example, PANI and poly(3-aminobenzoic acid) (P3ABA) (Figure 16a and b) were investigated as antimicrobial agents and found that the mechanism of action is different for each polymer. While both polymers adversely affected the growth of bacteria, the mechanism of antimicrobial activity for PANI is believed to be a result of the production of hydrogen peroxide (H2O2) that leads to hydroxyl radicals. On the other hand, the antimicrobial activity of P3ABA is due to the disruption of the metabolic and respiratory machinery of the bacteria by targeting the ATP synthase, thus causing acid stress [100]. PANI-based materials have also been investigated in tissue engineering applications, which was recently reviewed [102]. Benzyl-substituted PANI (BP), bromine-benzyl-disubstituted PANI (BBP), (Figure 16c) and PANI nanoparticles were tested against both Gram-negative E
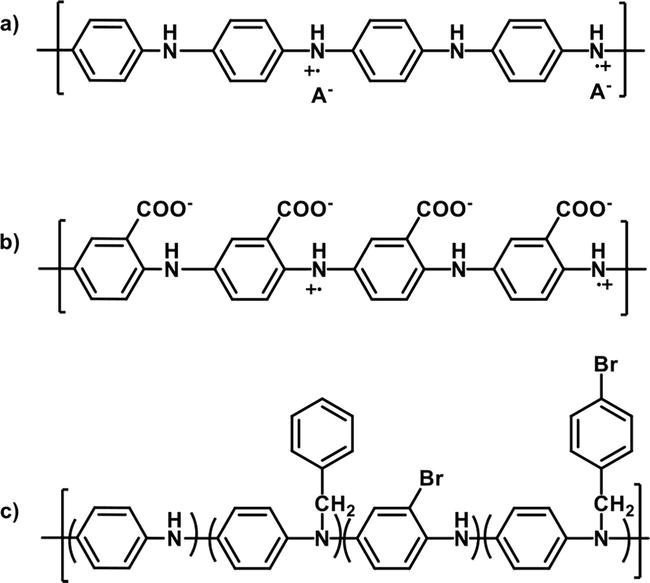
Figure 16.
(a) PANI (b) P3ABA, and (c) BBP. Structures drawn from Refs. [
3.2 Bio- and chemo-sensors
3.2.1 Biosensors
PANI and its derivatives are explored as biosensors. PANI derivative with Au/Pd composites were prepared and analyzed as biosensors for cancer biomarkers [103]. Specifically, the polymers, poly(N-methyl-
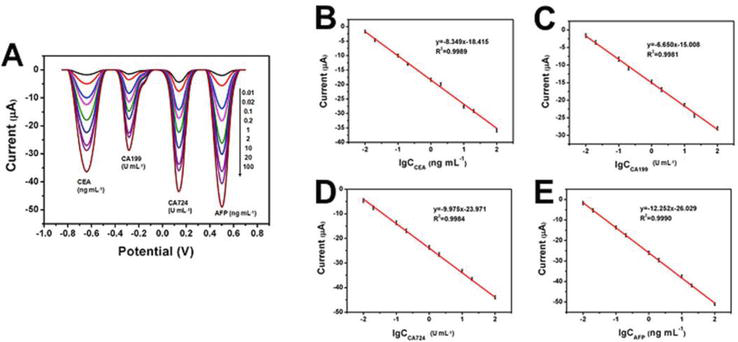
Figure 17.
SWV responses (A) and calibration curves for different concentration of CEA (B), CA199 (C), CA724 (D), and AFP (E) in PBS (pH 5.5) with 1.5 mM H2O2. Reproduced from Ref. [
3.2.2 Chemosensors
PANI has also been widely explored as chemosensors due to its high conductivity and electrochromic properties. However, there are only few examples of PANI derivatives in sensor applications. PANI derivatives containing
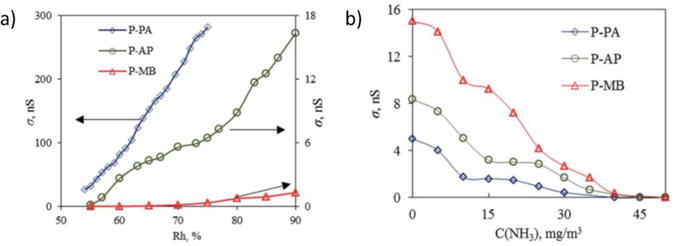
Figure 18.
Humidity and Gas sensors. (a) Plots of the conductivity of P-PA, P-AP and P-MB films on the air humidity. (b) Plots of the conductivity of P-PA, P-AP and P-MB films on the concentration of ammonia vapor. Reproduced from Ref. [
3.3 Electronic devices: electrochromic glass, solar cells, and LEDs
PANI is well known for its electrochromic properties as the polymer changes color reversibly with an electric field [107]. These materials are usually desirable for smart glass. A similar behavior is also demonstrated for its derivatives. For example, poly(2-methoxyaniline) and its copolymer, poly(2-methoxyaniline-co-3-aminobenzene sulphonic acid), were shown to have electrochromic behavior in a display device by Saharan et al. [108]. The response time was determined to be about 6 s going between the oxidized and reduced states (Figure 19). Additionally, the cycling behavior of the device was deemed to be stable. A PANI derivative containing phenoxazine unit was shown by Almtiri et al. to undergo similar color changes based on the oxidation state of the polymer [96]. Between 0 and 0.4 V, the polymer is light yellow, which changes to dark green between 0.4 and 0.6 V. Further oxidation renders the film deep blue at voltages greater than 0.6 V (Figure 20). The cycling stability was measured by cyclic voltammetry (CV) and was shown to be very stable over 100 cycles. Electrochromic conducting copolymers from aniline and different feed ratio of 4,4′-diaminodiphenyl sulfone (DDS) were deposited on ITO glass plates for spectrochemical analysis [109]. The copolymers displayed multiple colors similar to PANI when the applied potential was switched between −0.2 and 1.2 V, where the color changed from yellow to green and then blue. Upon changing the voltage from −0.2 to 0.0 V, a neutral yellow color formed. Further oxidation from 0.1 to 0.8 V, resulted in the conductive green color known to PANI and its derivatives. From 1.0 to 1.2 V, the polymer formed the blue color known to the fully oxidized pernigraniline form.
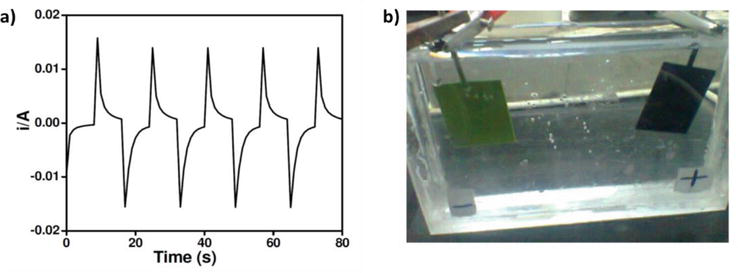
Figure 19.
Chronoamperometry of poly(OMA) and electrochromic devices of poly(OMA). Reproduced from Ref. [
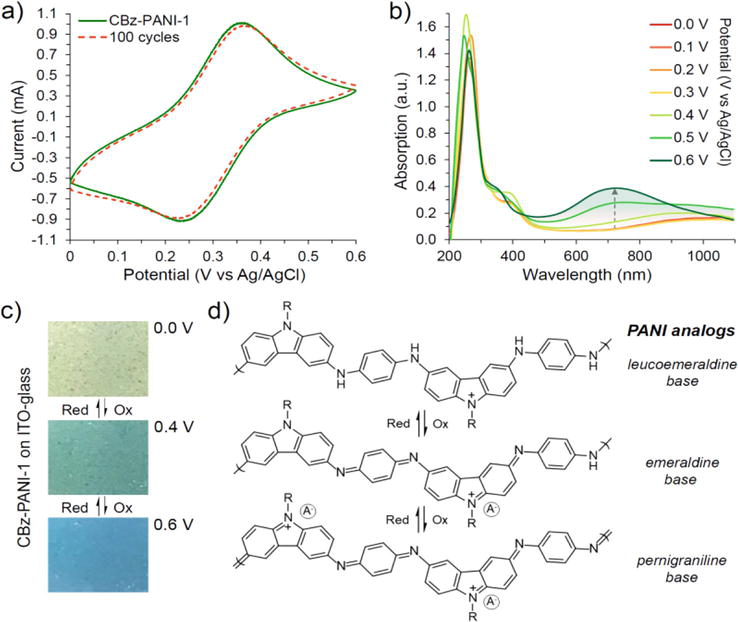
Figure 20.
Electrochemical and electrochromic behavior of carbazole PANI. Reprinted with permission from Ref. [
PANI and its derivatives are utilized as hole transport layers in Perovskite solar cells (PSC). For example, poly(
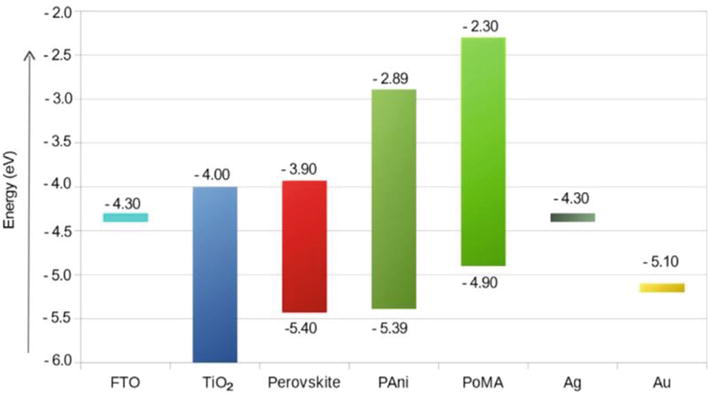
Figure 21.
Energy diagram of the different materials used in perovskite solar cells assembled in this work. Reprinted from Ref. [
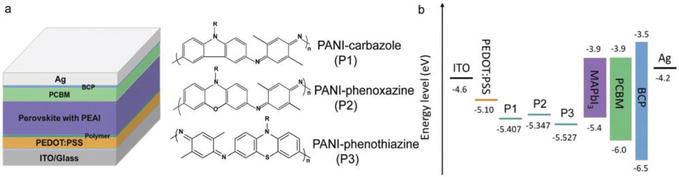
Figure 22.
(a) MAPbI3-based PSC device configuration and the chemical structure of three polymers. (b) Energy level alignment of the polymers in the MAPbI3-based PSC devices. Reproduced from Refs. [
3.4 Anti-corrosion and anti-fouling
PANI and its derivatives have been widely explored as anti-corrosion materials [111]. Trimethylsilyl-substituted polyaniline (PSiAn) was synthesized and used as anticorrosion materials [112]. The fabricated filler was highly hydrophobic due to the trimethylsilyl group. It was incorporated into the epoxy matrix to provide a low porous material with high barrier to aggressive corrosive substances. Anticorrosion coatings prepared from poly (aniline-co-2-ethylaniline) (PEA) micro/nanostructures displayed good anticorrosion performance with corrosion protection efficiency of 87.29% [113]. The hydrophobic property (CA = 145°), low conductivity, and low porosity was attributed to the anticorrosion performance of the composite material. Chloro- and bromo-substituted PANI derivatives have been shown to have excellent anti-corrosion and anti-fouling properties. Aluminum alloy 3105 (AA3105) was coated with poly(2-chloroaniline) (PClAni) by electropolymerization and the polymer was investigated for its anticorrosion properties. Potentiodynamic polarization technique and electrochemical impedance spectroscopy data showed that the metal coated with PClAni had outstanding performance against corrosion versus the untreated metal when subjected to 3.5% NaCl solution [114]. A similar result was obtained with bromo-substituted PANI derivatives (Br-PANI). De-doped Br-PANI in epoxy resin with varying amounts of Br substituents (EBP coatings) were prepared and investigated for anti-fouling and anti-corrosion properties by the accelerated immersion test, electrochemical impedance spectroscopy (EIS), XPS, antibacterial test, and field test [115]. It was shown that the polymers possessed excellent anticorrosion properties after immersion in 12.0 wt% NaCl solution at 95°C for 100 days. Furthermore, the EBP coatings also demonstrated better antibacterial and antifouling performance when compared to pure epoxy coating or de-doped PANI composite coatings. Poly(1-naphthylamine) (PNA) composites with polyvinyl alcohol (PVA) and polyvinylchloride (PVC) have also shown both anticorrosion and antifouling properties. PNA/PVA coatings have shown good corrosion protective efficiency and resistance in acid, alkaline, and saline media [116]. The same polymer composite also demonstrated antibacterial activity against
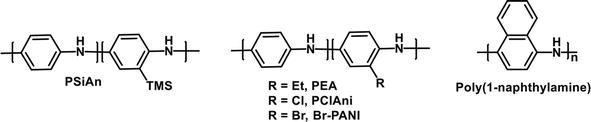
Figure 23.
Structures of anti-corrosion/anti-fouling PANI derivatives.
3.5 Energy storage devices: supercapacitors and batteries
Supercapacitors are energy storage devices that are characteristically high in energy and power density, as evidenced by a more extended battery life and fast charge/discharge. They are the future of portable consumer electronics that have excellent operational lifetimes. Supercapacitor energy storage devices have 10 times more operational lifetimes than lithium-ion batteries. Some studies have focused on enhancing the capacity of the materials and PANI derivatives are among the materials being studied for increased capacitance. PANI derivative, poly(DAAQ), was investigated as an electrochemical capacitor. The constructed symmetrical (poly(DAAQ)/poly(DAAQ)) electrochemical capacitor was shown to exhibit high specific energy (25–46 Wh/kg) and high specific power (10,200–30,500 W/kg) at discharge rates from 30 to 90 C [80]. Our group also developed supercapacitor from PANI derivative containing the carbazole unit. Cbz-PANI was used to construct electrodes for a supercapacitor device that showed a maximum areal capacitance of 64.8 mF cm−2 and a specific capacitance of 319 F g−1 at a current density of 0.2 mA cm−2 in a symmetrical device (Figure 24) [96]. Moreover, the electrode showed excellent cyclic stability (≈ 83% of capacitance retention) over 1000 CV cycles and a 91% capacitance retention after 1000 cycles of charge and discharge in a device.
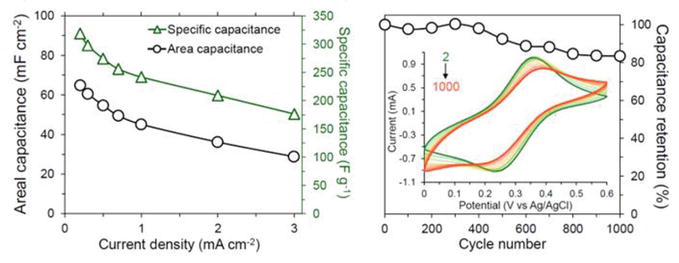
Figure 24.
Areal capacitance, specific capacitance, and capacitance retention of Cbz-PANI-1/AU@PET. Reprinted with permission from Ref. [
On the other hand, batteries provide high energy densities that can be delivered over time. Poly(5-amino-1,4-dyhydroxy anthraquinone) (PADAQ) was used as the cathode material in a lithium ion battery [83]. The initial discharge capacity was 101mAhg−1 at the current density of 400 mAhg−1 with the cutoff voltage of 1.5–3.7 V. At a high current density of 1400 mAhg−1, the capacity of the polymer was 95 mAhg−1. Poly(5-amino-1,4-naphthoquinone) (PANQ) recorded an experimental charge storage capacity of 220–290 Ah/kg in nonaqueous electrolytes. The specific energy of the battery is estimated to be about 100 Wh/kg with a 1 h discharge rate in a thin-layer cell where LiC6 is the negative electrode and a PANQ as the positive electrode [81]. The mean redox potential was determined to be about 2.6 V more positive than Li/Li+ couple, which makes it a potential positive electrode for lithium metal or lithium-ion batteries. The PANI derivatives, PT-DMPD and PT-BZ, developed by Fors and coworkers, were investigated as cathode materials in Lithium-ion battery (LiB) technology [93]. Li-Coin cells devices with PT-DMPD or PT-BZ as the cathode delivered discharge capacities of 128 mAh/g and 97 mAh/g, which are 82% and 76% of the theoretical capacity for PT-DMPD and PT-BZ, respectively. However, the Coulombic efficiency (35% for PT-DMPD and 44% for PT-BZ) were relatively low. Additionally, the cycling performances were also poor with the discharge capacities for PT-DMPD and PT-BZ after 50 cycles being 82 mAh/g (64% retention) and 64 mAh/g (66% retention), respectively. To improve the performance of the polymers, Pt-DMPD was copolymerized with a second phenothiazine unit that was crosslinked to another one through a N-atom. The amount of cross-linked monomer was varied. Improved Coulombic efficiencies (150 mAh/g) at very positive operating voltages (2.8–4.3 V vs. Li+/Li) were obtained, which yielded high energy densities. Also, a greater retention capacity (82%) was observed at ultrafast discharge rates (120 C) for the crosslinked co-polymers.
4. Conclusion
To conclude, PANI derivatives are being widely developed in an attempted to address the limitations of PANI, which are insolubility and electrochemical instability. This short summary outlined both older but includes many new developments of PANI derivatives that are able to sufficiently overcome PANI’s challenges and in some cases outperform PANI in several applications. Interestingly, with the developments of new synthetic approach by the Buchwald/Hartwig reaction to synthesize PANI derivatives, many new structures can be achieved that are not possible with the previous synthetic methods of oxidative and electrochemical polymerization. Consequently, it serves to continue developing new PANI derivatives by expanding the substrate scopes to continue to move the field forward in improving the current applications and merge into newer fields.
Acknowledgments
The authors are grateful for the financial support from the National Science Foundation for an award (CHE-1945503).
References
- 1.
MacDiarmid AG. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angewandte Chemie International Edition. 2001; 40 (14):2581-2590 - 2.
Basescu N, Liu Z-X, Moses D, Heeger AJ, Naarmann HD, Theophilou N. High electrical conductivity in doped polyacetylene. Nature. 1987; 327 :403-405 - 3.
Shirakawa H, Louis EJ, MacDiarmid AG, Chiang CK, Heeger AJ. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH). Journal of the Chemical Society, Chemical Communications. 1977; 16 :578-580 - 4.
Swager TM. 50th anniversary perspective: Conducting/semiconducting conjugated polymers. A personal perspective on the past and the future. Macromolecules. 2017; 50 (13):4867-4886 - 5.
Guo X, Facchetti A. The journey of conducting polymers from discovery to application. Nature Materials. 2020; 19 (9):922-928 - 6.
Rasmussen SC. Conjugated and conducting organic polymers: The first 150 years. ChemPlusChem. 2020; 85 (7):1412-1429 - 7.
Tang H, Liang Y, Liu C, Hu Z, Deng Y, Guo H, et al. A solution-processed n-type conducting polymer with ultrahigh conductivity. Nature. 2022; 611 (7935):271-277 - 8.
Baker CO, Huang X, Nelson W, Kaner RB. Polyaniline nanofibers: Broadening applications for conducting polymers. Chemical Society Reviews. 2017; 46 (5):1510-1525 - 9.
Sinha S, Bhadra S, Khastgir D. Effect of dopant type on the properties of polyaniline. Journal of Applied Polymer Science. 2009; 112 (5):3135-3140 - 10.
Yadav A, Kumar H. Polyaniline plastic nanocomposite as multi-functional nanomaterial. ChemistrySelect. 2022; 7 (29):e202201475 - 11.
Cao Y, Smith P, Heeger AJ. Counter-ion induced processibility of conducting polyaniline and of conducting polyblends of polyaniline in bulk polymers. Synthetic Metals. 1992; 48 (1):91-97 - 12.
Rasmussen SC. The early history of polyaniline: Discovery and origins. Substantia. 2017; 1 (2):99-109 - 13.
Majeed AH, Mohammed LA, Hammoodi OG, Sehgal S, Alheety MA, Saxena KK, et al. A review on polyaniline: Synthesis, properties, nanocomposites, and electrochemical applications. International Journal of Polymer Science. 2022; 2022 :9047554 - 14.
Das TK, Prusty S. Review on conducting polymers and their applications. Polymer-Plastics Technology and Engineering. 2012; 51 (14):1487-1500 - 15.
Taka T. Humidity dependency of electrical conductivity of doped polyaniline. Synthetic Metals. 1993; 57 (2):5014-5019 - 16.
Jin J, Wang Q , Haque MA. Length-scale effects on electrical and thermal transport in polyaniline thin films. Organic Electronics. 2010; 11 (1):29-35 - 17.
Campos M, Bulhões LOS, Lindino CA. Gas-sensitive characteristics of metal/semiconductor polymer Schottky device. Sensors and Actuators A: Physical. 2000; 87 (1):67-71 - 18.
Beygisangchin M, Abdul Rashid S, Shafie S, Sadrolhosseini AR, Lim HN. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers. 2021; 13 (12):2003 - 19.
Mustafin AG, Latypova LR, Andriianova AN, Mullagaliev IN, Salikhov SM, Salikhov RB, et al. Polymerization of new aniline derivatives: Synthesis, characterization and application as sensors. RSC Advances. 2021; 11 (34):21006-21016 - 20.
Eftekhari A, Li L, Yang Y. Polyaniline supercapacitors. Journal of Power Sources. 2017; 347 :86-107 - 21.
Rangel-Olivares FR, Arce-Estrada EM, Cabrera-Sierra R. Synthesis and characterization of polyaniline-based polymer nanocomposites as anti-corrosion coatings. Coatings. 2021; 11 (6):653 - 22.
Zeng M, Zhu W, Luo J, Song N, Li Y, Chen Z, et al. Highly efficient nonfullerene organic solar cells with a self-doped water-soluble neutral polyaniline as hole transport layer. Solar RRL. 2021; 5 (3):2000625 - 23.
Gupta TK, Singh BP, Mathur RB, Dhakate SR. Multi-walled carbon nanotube–graphene–polyaniline multiphase nanocomposite with superior electromagnetic shielding effectiveness. Nanoscale. 2014; 6 (2):842-851 - 24.
Neoh KG, Pun MY, Kang ET, Tan KL. Polyaniline treated with organic acids: Doping characteristics and stability. Synthetic Metals. 1995; 73 (3):209-215 - 25.
MacDiarmid AG, Epstein AJ. Polyanilines: A novel class of conducting polymers. Faraday Discussions of the Chemical Society. 1989; 88 (0):317-332 - 26.
Liao G, Li Q , Xu Z. The chemical modification of polyaniline with enhanced properties: A review. Progress in Organic Coatings. 2019; 126 :35-43 - 27.
Malhotra B, Dhand C, Lakshminarayanan R, Dwivedi N, Mishra S, Solanki P, et al. Polyaniline-based biosensors. Nanobiosensors in Disease Diagnosis. 2015; 4 :25 - 28.
MacDiarmid AG, Epstein AJ. The concept of secondary doping as applied to polyaniline. Synthetic Metals. 1994; 65 (2):103-116 - 29.
Kuzmany H, Sariciftci NS, Neugebauer H, Neckel A. Evidence for two separate doping mechanisms in the polyaniline system. Physical Review Letters. 1988; 60 (3):212-215 - 30.
Wang L, Jing X, Wang F. On the iodine-doping of polyaniline and poly-ortho-methylaniline. Synthetic Metals. 1991; 41 (1):739-744 - 31.
Huang WS, Angelopoulos M, White JR, Park JM. Metallization of printed circuit boards using conducting polyaniline. Molecular Crystals and Liquid Crystals Incorporating Nonlinear Optics. 1990; 189 (1):227-235 - 32.
Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S. Applications of conducting polymers and their issues in biomedical engineering. Journal of the Royal Society Interface. 2010; 7 (suppl_5):S559-SS79 - 33.
Bhadra S, Chattopadhyay S, Singha NK, Khastgir D. Improvement of conductivity of electrochemically synthesized polyaniline. Journal of Applied Polymer Science. 2008; 108 (1):57-64 - 34.
Bhadra S, Singha NK, Khastgir D. Polyaniline by new miniemulsion polymerization and the effect of reducing agent on conductivity. Synthetic Metals. 2006; 156 (16):1148-1154 - 35.
Bhadra S, Singha NK, Khastgir D. Effect of aromatic substitution in aniline on the properties of polyaniline. European Polymer Journal. 2008; 44 (6):1763-1770 - 36.
Bednarczyk K, Matysiak W, Tański T, Janeczek H, Schab-Balcerzak E, Libera M. Effect of polyaniline content and protonating dopants on electroconductive composites. Scientific Reports. 2021; 11 (1):7487 - 37.
Sapurina IY, Shishov MA. Oxidative polymerization of aniline: Molecular synthesis of polyaniline and the formation of supramolecular structures. In: Ailton De Souza G, editor. New Polymers for Special Applications. Rijeka: IntechOpen; 2012. p. 9 - 38.
Tang S-J, Wang A-T, Lin S-Y, Huang K-Y, Yang C-C, Yeh J-M, et al. Polymerization of aniline under various concentrations of APS and HCl. Polymer Journal. 2011; 43 (8):667-675 - 39.
Sapurina I, Stejskal J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polymer International. 2008; 57 (12):1295-1325 - 40.
Ćirić-Marjanović G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synthetic Metals. 2013; 177 :1-47 - 41.
Yasuda A, Shimidzu T. Chemical and electrochemical analyses of polyaniline prepared with FeCl3. Synthetic Metals. 1993; 61 (3):239-245 - 42.
Mezhuev YO, Korshak YV. Theory of chain growth in chemical oxidative polymerization of aniline derivatives. Synthetic Metals. 2020; 267 :116445 - 43.
Gospodinova N, Terlemezyan L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Progress in Polymer Science. 1998; 23 (8):1443-1484 - 44.
Wolfe JP, Wagaw S, Marcoux J-F, Buchwald SL. Rational development of practical catalysts for aromatic carbon−nitrogen bond formation. Accounts of Chemical Research. 1998; 31 (12):805-818 - 45.
Hartwig JF. Transition metal catalyzed synthesis of arylamines and aryl ethers from aryl halides and triflates: Scope and mechanism. Angewandte Chemie International Edition. 1998; 37 (15):2046-2067 - 46.
Yang BH, Buchwald SL. Palladium-catalyzed amination of aryl halides and sulfonates. Journal of Organometallic Chemistry. 1999; 576 (1):125-146 - 47.
Zhang X-X, Sadighi JP, Mackewitz TW, Buchwald SL. Efficient synthesis of well-defined, high molecular weight, and processible polyanilines under mild conditions via palladium-catalyzed amination. Journal of the American Chemical Society. 2000; 122 (31):7606-7607 - 48.
Ward RE, Meyer TY. O,p-Polyaniline: A new form of a classic conducting polymer. Macromolecules. 2003; 36 (12):4368-4373 - 49.
Spetseris N, Ward RE, Meyer TY. Linear and hyperbranched m-polyaniline: Synthesis of polymers for the study of magnetism in organic systems. Macromolecules. 1998; 31 (9):3158-3161 - 50.
Ito A, Ota K-i, Tanaka K, Yamabe T, Yoshizawa K. n-alkyl group-substituted poly(m-aniline)s: Syntheses and magnetic properties. Macromolecules. 1995; 28 (16):5618-5625 - 51.
Goodson FE, Hartwig JF. Regiodefined poly(N-arylaniline)s and donor−acceptor copolymers via palladium-mediated amination chemistry. Macromolecules. 1998; 31 (5):1700-1703 - 52.
Casado N, Hernández G, Sardon H, Mecerreyes D. Current trends in redox polymers for energy and medicine. Progress in Polymer Science. 2016; 52 :107-135 - 53.
Geng Y, Jing X, Wang F. Solution properties of doped polyaniline. Journal of Macromolecular Science, Part B. 1997; 36 (1):125-135 - 54.
Rannou P, Nechtschein M, Travers JP, Berner D, Woher A, Djurado D. Ageing of PANI: Chemical, structural and transport consequences. Synthetic Metals. 1999; 101 (1):734-737 - 55.
Marmisollé WA, Inés Florit M, Posadas D. Electrochemically induced ageing of polyaniline. An electrochemical impedance spectroscopy study. Journal of Electroanalytical Chemistry. 2012; 673 :65-71 - 56.
Kobayashi T, Yoneyama H, Tamura H. Polyaniline film-coated electrodes as electrochromic display devices. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 1984; 161 (2):419-423 - 57.
Liao G. The chemical modification of polyaniline with enhanced properties: A review. Progress in Organic Coatings. 2018; 126 :35-43 - 58.
Lindfors T, Ivaska A. pH sensitivity of polyaniline and its substituted derivatives. Journal of Electroanalytical Chemistry. 2002; 531 (1):43-52 - 59.
Park YW, Moon JS, Bak MK, Jin JI. Electrical properties of polyaniline and substituted polyaniline derivatives. Synthetic Metals. 1989; 29 (1):389-394 - 60.
Athawale AA, Kulkarni MV. Polyaniline and its substituted derivatives as sensor for aliphatic alcohols. Sensors and Actuators B: Chemical. 2000; 67 (1):173-177 - 61.
D’Aprano G, Leclerc M, Zotti G, Schiavon G. Synthesis and characterization of polyaniline derivatives: Poly(2-alkoxyanilines) and poly(2,5-dialkoxyanilines). Chemistry of Materials. 1995; 7 (1):33-42 - 62.
Ma L, Huang C-Q , Gan M-Y. Synthesis and anticorrosion properties of poly(2,3-dimethylaniline) doped with phosphoric acid. Journal of Applied Polymer Science. 2013; 127 (5):3699-3704 - 63.
Ma L, Wei S-J, Gan M-Y, Chen F-F, Tian N, Zeng J. Rapid emuision polymerization of poly(2,3-dimethylaniline) initiated by APS/Fe2+ composite oxidants and its performances. Acta Polymerica Sinica. 2013; 8 :1033-1038 - 64.
Patil S, Sainkar SR, Patil PP. Poly(o-anisidine) coatings on copper: Synthesis, characterization and evaluation of corrosion protection performance. Applied Surface Science. 2004; 225 (1):204-216 - 65.
Sathiyanarayanan S, Dhawan SK, Trivedi DC, Balakrishnan K. Soluble conducting poly ethoxy aniline as an inhibitor for iron in HCl. Corrosion Science. 1992; 33 (12):1831-1841 - 66.
Savitha P, Sathyanarayana DN. Synthesis and characterization of soluble conducting poly(o−/m-toluidine-co-o-nitroaniline). Synthetic Metals. 2004; 145 (2):113-118 - 67.
Vicentini DS, Salvatierra RV, Zarbin AJG, Dutra LG, Sá MM. Synthesis and characterization of carboxyl-substituted polyanilines doped with halogenated acids: Combining conductivity with solubility. Journal of the Brazilian Chemical Society. 2014; 25 :1939-1947 - 68.
Yue J, Epstein AJ. Synthesis of self-doped conducting polyaniline. Journal of the American Chemical Society. 1990; 112 (7):2800-2801 - 69.
Milakin KA, Morávková Z, Taboubi O, Acharya U, Pop-Georgievski O, Bober P. Facile preparation of water-dispersible carboxylated polyaniline. Synthetic Metals. 2023; 293 :117249 - 70.
Karyakin AA, Strakhova AK, Yatsimirsky AK. Self-doped polyanilines electrochemically active in neutral and basic aqueous solutions: Electropolymerization of substituted anilines. Journal of Electroanalytical Chemistry. 1994; 371 (1):259-265 - 71.
Komkova MA, Valeev RG, Kolyagin YG, Andreev EA, Beltukov AN, Nikitina VN, et al. Solid-state survey of boronate-substituted polyaniline: On the mechanism of conductivity, electroactivity, and interactions with polyols. Materials Today Chemistry. 2022; 26 :101070 - 72.
Yue J, Epstein AJ. XPS study of self-doped conducting polyaniline and parent systems. Macromolecules. 1991; 24 (15):4441-4445 - 73.
Yue J, Wang ZH, Cromack KR, Epstein AJ, MacDiarmid AG. Effect of sulfonic acid group on polyaniline backbone. Journal of the American Chemical Society. 1991; 113 (7):2665-2671 - 74.
Saidu FK, Joseph A, Varghese EV, Thomas GV. Characterization and electrochemical studies on poly(1-naphthylamine)-graphene oxide nanocomposites prepared by in situ chemical oxidative polymerization. Journal of Solid State Electrochemistry. 2019; 23 (10):2897-2906 - 75.
Jadoun S, Verma A, Ashraf SM, Riaz U. A short review on the synthesis, characterization, and application studies of poly(1-naphthylamine): A seldom explored polyaniline derivative. Colloid and Polymer Science. 2017; 295 (9):1443-1453 - 76.
Faria RC, Bulhões LOS. Synthesis and electrochemical response of poly-(1-aminoanthracene) films. Electrochimica Acta. 1999; 44 (10):1597-1605 - 77.
Faria RC, Bulhões LOS. Hydrogen ion selective electrode based on poly(1-aminoanthracene) film. Analytica Chimica Acta. 1998; 377 (1):21-27 - 78.
Gou P, Kraut ND, Feigel IM, Bai H, Morgan GJ, Chen Y, et al. Carbon nanotube Chemiresistor for wireless pH sensing. Scientific Reports. 2014; 4 (1):4468 - 79.
Li X-G, Huang M-R, Hua Y-M, Zhu M-F, Chen Q. Facile synthesis of oxidative copolymers from aminoquinoline and anisidine. Polymer. 2004; 45 (14):4693-4704 - 80.
Naoi K, Suematsu S, Manago A. Electrochemistry of poly(1,5-diaminoanthraquinone) and its application in electrochemical capacitor materials. Journal of The Electrochemical Society. 2000; 147 (2):420 - 81.
Häringer D, Novák P, Haas O, Piro B, Pham MC. Poly(5-amino-1,4-naphthoquinone), a novel lithium-inserting electroactive polymer with high specific charge. Journal of The Electrochemical Society. 1999; 146 (7):2393 - 82.
Pham MC, Piro B, Bazzaoui EA, Hedayatullah M, Lacroix J-C, Novák P, et al. Anodic oxidation of 5-amino-1,4-naphthoquinone (ANQ) and synthesis of a conducting polymer (PANQ). Synthetic Metals. 1998; 92 (3):197-205 - 83.
Zhao L, Wang W, Wang A, Yuan K, Chen S, Yang Y. A novel polyquinone cathode material for rechargeable lithium batteries. Journal of Power Sources. 2013; 233 :23-27 - 84.
Ismail KM, Khalifa ZM, Azzem MA, Badawy WA. Electrochemical preparation and characterization of poly(1-amino-9,10-anthraquinone) films. Electrochimica Acta. 2002; 47 (12):1867-1873 - 85.
Yano J, Ota Y, Kitani A. Electrochemical preparation of conductive poly(N-alkylaniline)s with long N-alkyl chains using appropriate dopant anions and organic solvents. Materials Letters. 2004; 58 (12):1934-1937 - 86.
Hwang G-W, Wu K-Y, Hua M-Y, Lee H-T, Chen S-A. Structures and properties of the soluble polyanilines, N-alkylated emeraldine bases. Synthetic Metals. 1998; 92 (1):39-46 - 87.
Nguyen MT, Kasai P, Miller JL, Diaz AF. Synthesis and properties of novel water-soluble conducting polyaniline copolymers. Macromolecules. 1994; 27 (13):3625-3631 - 88.
DeArmitt C, Armes SP, Winter J, Uribe FA, Gottesfeld S, Mombourquette C. A novel N-substituted polyaniline derivative. Polymer. 1993; 34 (1):158-162 - 89.
Hany P, Geniès EM, Santier C. Polyanilines with covalently bonded alkyl sulfonates as doping agent. Synthesis and properties. Synthetic Metals. 1989; 31 (3):369-378 - 90.
Stille JK, Mainen EL. Thermally stable ladder polyquinoxalines. Macromolecules. 1968; 1 (1):36-42 - 91.
Kim O-K. Electrical conductivity of heteroaromatic ladder polymers. 3. Phenothiazine and the structurally related ladder polymers. Journal of Polymer Science: Polymer Letters Edition. 1985; 23 (3):137-139 - 92.
Wu J, Rui X, Long G, Chen W, Yan Q , Zhang Q. Pushing up lithium storage through nanostructured Polyazaacene analogues as anode. Angewandte Chemie International Edition. 2015; 54 (25):7354-7358 - 93.
Peterson BM, Ren D, Shen L, Wu Y-CM, Ulgut B, Coates GW, et al. Phenothiazine-based polymer cathode materials with ultrahigh power densities for lithium ion batteries. ACS Applied Energy Materials. 2018; 1 (8):3560-3564 - 94.
Peterson BM, Shen L, Lopez GJ, Gannett CN, Ren D, Abruña HD, et al. Elucidation of the electrochemical behavior of phenothiazine-based polyaromatic amines. Tetrahedron. 2019; 75 (32):4244-4249 - 95.
Almtiri M, Dowell TJ, Chu I, Wipf DO, Scott CN. Phenoxazine-containing polyaniline derivatives with improved electrochemical stability and processability. ACS Applied Polymer Materials. 2021; 3 (6):2988-2997 - 96.
Almtiri M, Dowell TJ, Giri H, Wipf DO, Scott CN. Electrochemically stable carbazole-derived polyaniline for Pseudocapacitors. ACS Applied Polymer Materials. 2022; 4 (5):3088-3097 - 97.
Qi Y, Almtiri M, Giri H, Jha S, Ma G, Shaik AK, et al. Evaluation of the passivation effects of PEDOT: PSS on inverted perovskite solar cells. Advanced Energy Materials; n/a (n/a):2202713 - 98.
Orlov AV, Kiseleva SG, Karpacheva GP, Muratov DG. Peculiarities of oxidative polymerization of Diarylaminodichlorobenzoquinones. Polymers. 2021; 13 (21):3657 - 99.
Andriianova AN, Latypova LR, Vasilova LY, Kiseleva SV, Zorin VV, Abdrakhmanov IB, et al. Antibacterial properties of polyaniline derivatives. Journal of Applied Polymer Science. 2021; 138 (47):51397 - 100.
Robertson J, Gizdavic-Nikolaidis M, Nieuwoudt MK, Swift S. The antimicrobial action of polyaniline involves production of oxidative stress while functionalisation of polyaniline introduces additional mechanisms. PeerJ. 2018; 6 :e5135 - 101.
Quan X, Wang J, Souleyman T, Cai W, Zhao S, Wang Z. Antibacterial and antifouling performance of bisphenol-a/poly(ethylene glycol) binary epoxy coatings containing bromine-benzyl-disubstituted polyaniline. Progress in Organic Coatings. 2018; 124 :61-70 - 102.
Rai R, Roether JA, Boccaccini AR. Polyaniline based polymers in tissue engineering applications: A review. Progress in Biomedical Engineering. 2022; 4 (4):042004 - 103.
Wang L, Feng F, Ma Z. Novel electrochemical redox-active species: One-step synthesis of polyaniline derivative-Au/Pd and its application for multiplexed immunoassay. Scientific Reports. 2015; 5 (1):16855 - 104.
Borole DD, Kapadi UR, Mahulikar PP, Hundiwale DG. Glucose oxidase electrodes of polyaniline, poly(o-anisidine) and their co-polymer as a biosensor: A comparative study. Journal of Materials Science. 2007; 42 (13):4947-4953 - 105.
Lu Q , Qian J, Xu F, He G, Liu Y, Xia J. Synthesis of 2D fluorescent polyaniline derivatives as multifunctional fluorescent chemosensor. Journal of Polymer Science. 2023; 61 (3):211-222 - 106.
Qian J, Zhang Y, Liu X, Xia J. Carbazole and fluorene polyaniline derivatives: Synthesis, properties and application as multiple stimuli-responsive fluorescent chemosensor. Talanta. 2019; 204 :592-601 - 107.
Prakash R, Santhanam KSV. Electrochromic window based on polyaniline. Journal of Solid State Electrochemistry. 1998; 2 (2):123-125 - 108.
Saharan R, Kaur A, Dhawan SK. Synthesis and characterization of poly(o -methoxy aniline) and its copolymer for electrochromic device energy applications. Indian Journal of Pure & Applied Physics. 2015; 53 :316-319 - 109.
Manisankar P, Vedhi C, Selvanathan G, Somasundaram RM. Electrochemical and electrochromic behavior of novel poly(aniline-co-4,4′-diaminodiphenyl sulfone). Chemistry of Materials. 2005; 17 (7):1722-1727 - 110.
Marques AS, Szostak R, Marchezi PE, Nogueira AF. Perovskite solar cells based on polyaniline derivatives as hole transport materials. Journal of Physics: Energy. 2019; 1 (1):015004 - 111.
Brusic V, Angelopoulos M, Graham T. Use of polyaniline and its derivatives in corrosion protection of copper and silver. Journal of The Electrochemical Society. 1997; 144 (2):436 - 112.
Zhou Z, Xiao X, Wang W, Wei S, Wang Y. Enhanced hydrophobicity and barrier property of anticorrosive coatings with silicified polyaniline filler. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2022; 645 :128848 - 113.
Xing C, Song X, Zhang Z, Jiang X, Yu L. Anticorrosion coatings from poly (aniline-co-2-Ethylaniline) micro/nanostructures. Journal of Ocean University of China. 2019; 18 (6):1371-1381 - 114.
Jafari Y, Shabani-Nooshabadi M, Ghoreishi SM. Poly(2-chloroaniline) Electropolymerization coatings on Aluminum alloy 3105 and evaluating their corrosion protection performance. Transactions of the Indian Institute of Metals. 2014; 67 (4):511-520 - 115.
Cai W, Wang J, Quan X, Zhao S, Wang Z. Antifouling and anticorrosion properties of one-pot synthesized dedoped bromo-substituted polyaniline and its composite coatings. Surface and Coatings Technology. 2018; 334 :7-18 - 116.
Ahmad S, Ashraf SM, Riaz U, Zafar S. Development of novel waterborne poly(1-naphthylamine)/poly(vinylalcohol)–resorcinol formaldehyde-cured corrosion resistant composite coatings. Progress in Organic Coatings. 2008; 62 (1):32-39 - 117.
Riaz U, Khan S, Islam MN, Ahmad S, Ashraf SM. Evaluation of antibacterial activity of nanostructured poly(1-naphthylamine) and its composites. Journal of Biomaterials Science. Polymer Edition. 2008; 19 (11):1535-1546