Few electrochemical properties of PANI based nanocomposites as electrode materials for supercapacitor applications.
Abstract
The long lasting intrinsic conducting polymers (ICPs) including polyaniline (PANI), polypyrole (PPy), Polyindole (PIn), Poly (methyl methacrylate) (PMMA), Polythiophene (PT), poly (3,4-ethylene dioxythiophene) (PEDOT) have been recognized for their significant benefits in optoelectronic devices. In the last few decades, polyaniline has gained recognition over metals, owing its low cost, flexibility, and high conductivity, as well as the ease with which it may be produced using chemical or electrochemical processes. Due to its high electrical conductivity, light weight, ease of fabrication, and excellent environmental stability, PANI has an extensive range of applications, including batteries, sensors, supercapacitors, waste water treatment and organic electronic devices. It also has the potential for chemical and electrochemical synthesis. Polyaniline has promising potential in many optoelectronic applications as well as in supercapacitors. In this chapter, the basic historical background, different synthesis mechanism about conducting polymer polyaniline is discussed in details. Polyaniline has great potential application such as in sensors, supercapacitor and optoelectronic devices etc. due to its ability of ease of synthesis by various methods. Polyaniline based nanocomposites with different metals, metal oxide, metal sulfides, and carbon nanomaterials, graphene, carbon nanotubes (CNTs) etc. are described in this section in detail.
Keywords
- polyaniline
- protonation
- chemical oxidative polymerization
- OLEDs
- supercapacitors
1. Introduction
Since ancient times researchers have been trying to develop new smart materials which have unique amalgamations of properties. In modern times, conducting polymer-based nanocomposites and nano blending technologies were extensively utilized in various fields such as supercapacitors, solar cells, sensors, and different types of electronic device applications. Conducting polymer becomes promising candidates due to their unique properties such as transparency, lightweight, low cost, flexibility, optical, electrical, and dielectric over the inorganic semiconductors [1]. Uses of inorganic semiconductors are replaced by conducting polymers all over the technologies. Intrinsic conducting polymers are divided into three types according to the conduction mechanism: П conjugated, redox, and ionic conducting polymers [2]. In П conjugated systems, the electron is carriers to conduct electricity through the backbone skeleton of the polymer. Thus, these electrons are called delocalization of electrons. The redox polymers contain immobilized redox or electroactive centers. These electroactive centers are not connected to each other. Charge (electrons) are transferred in this system via a hopping mechanism from one active center to the other. To increase the conductivity, systems need to have large numbers of redox-active centers. In the case of ionic polymers contain ions. This system conducts electricity due to the movements of ions in the polymer chain [3]. The conducting polymers’ electrical conductivity, mechanical stability, and optical properties were enhanced multiple times by doping or mixing inorganic semiconductors as filler elements. Recently, inorganic-based polymeric nanocomposites have been extensively used in various fields [2, 3].
Hideki Shirakawa, Alan Heeger, and Alan Mac Diarmid discovered the simplest conducting polymer in 1977 and got the Nobel Prize in 2000 [4]. After that, a new novel conducting polymer, polymerization mechanism, and electron transport mechanism gradually developed in front of researchers. Polyacetylene was the first synthesized conducting polymer; after that, several conducting polymers were developed, such as polythiophene, PEDOT: PSS, polypyrrole, polyaniline, and polycarbazole. Among these conducting polymers, polyaniline is the most reported conducting polymer in electronic devices like sensors, solar cells, supercapacitors, anticorrosion materials, and optoelectronic device applications [5, 6]. Henry Letheby first developed aniline black or PANI by using HNO3 as an oxidant agent via the oxidative chemical polymerization method, which is insulating in nature. In 1997 Mac Diarmid introduced another derivative (green emeraldine salt), the aniline family, which conducts electricity. He introduced three different derivative aniline families one is colorless reduced leucoemeraldine base (LEB), the second is blue/green half oxidized emeraldine base/salt (EB), and the third one is blue/violet fully oxidized pernigraniline base (PAB). Emeraldine salt is extensively used out of these three derivatives because it has outstanding optical, electrical, and anticorrosive properties [7]. Optical band gap energy (Eg = 1.20 eV–3.2 eV) and electrical conductivity (σele = 10–8 s/cm–10 s/cm) depend on the synthesis method. The refractive index of polyaniline is 1.31–1.36. The polyaniline exhibited a different type of nanostructure (such as tower-like, nanoflower-like, leaf-like, rod-like, and sea urchin-like morphologies) depending on the synthesis techniques [8]. Different type of microstructure has been shown in Figure 1.
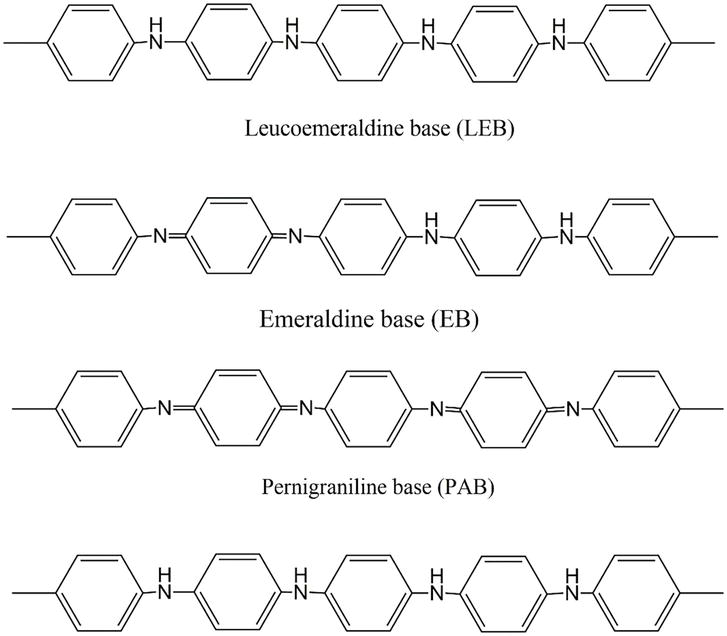
Figure 1.
Different derivatives of PANI.
In this book chapter, we have discussed the basic historical background of conducting polymers, and the main focus of our work is details about conducting polymer polyaniline. Polyaniline based nanocomposites with different metals, metal oxide, metal sulfides, and carbon nanomaterials, graphene, carbon nanotubes (CNTs), reduced graphene oxide (rGO) etc. are described in this section. Different types of synthesis methods and polymerization mechanisms were broadly discussed. In the last section, we discussed the application of polyaniline, specifically in supercapacitor and optoelectronic device applications.
2. Synthesis of polyaniline (PANI)
PANI is one of the promising and electrically conductive polymers owing its conversion ability between base and salt form. Access of oxidation and reduction and ease of synthesis made polyaniline more popular that attracts great deal of research. The most widely used synthesis method for preparing polyaniline is chemically oxidative polymerization technique using aniline monomer in acidic medium. The various method can be used for the synthesis of polyaniline are following:
Chemically oxidative polymerization
Electrochemical polymerization
Vapor-phase polymerization
Photochemically initiated polymerization
2.1 Chemically oxidative polymerization (COP)
For preparing monomers to polymer such as aniline to polyaniline, pyrrole to polypyrrole etc. chemical oxidative polymerization plays most prominent role. It is a cheap method for preparing large quantities of polymers with less time. In this method, chemical oxidizing agent initiates the polymerization process. The monomer compounds exhibit high electron donating properties. Chemical oxidative polymerization has been done with the help of oxidizing agents such as ammonium persulfate (APS), potassium dichromate, hydrogen peroxide etc. Ammonium persulfate (NH4)2S2O8 is a strong oxidizing agent which has been used to prepare polyaniline (PANI) from aniline monomer in our research work as it highly soluble in water. APS helps in generating radical cation sites in monomer and initiating the polymerization process. The prepared monomer solution is kept within aqueous acidic medium at a low temperature. This reaction is later allowed on a constant stirring for 5–8 hours in order to obtain the polymer precipitate (Figure 2) [9, 10].
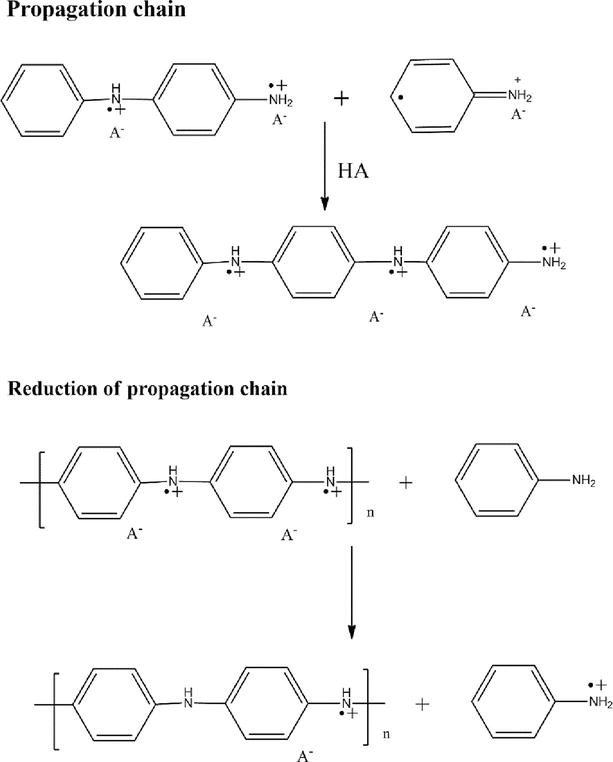
Figure 2.
Chemically oxidative polymerization of PANI.
2.2 Electrochemical polymerization (ECP)
For the preparation of thin film polyaniline with a huge surface area electrochemical process plays a vital role. Electrochemical method is quite similar to electrodeposition method used for metals. Electrochemical method provides homogenous polymer deposition over the electrode which contains a power source, an electrode and an electrolyte solution. Electrochemical polymerization of aniline is carried out in a strong acidic electrolyte such as acetonitrile which helps in the formation of anilinium radical cation by aniline oxidation on the electrode (Figure 3) [11].
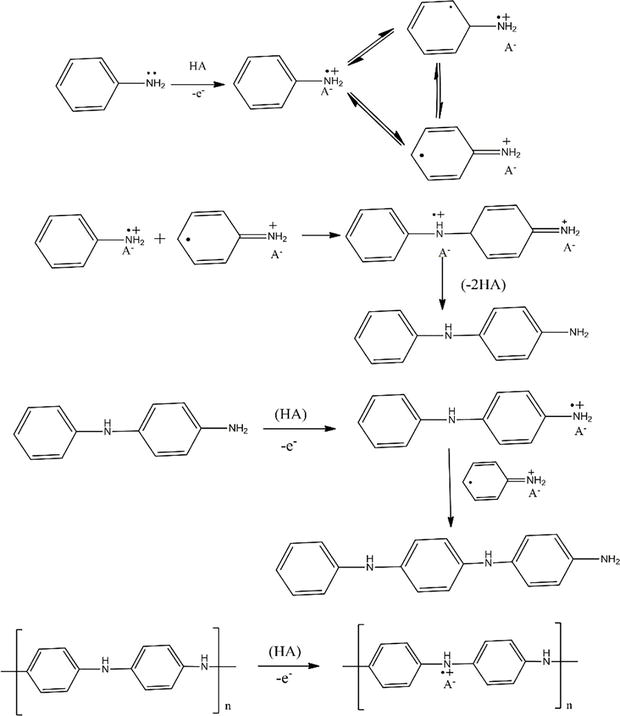
Figure 3.
Electrochemical polymerization of PANI.
2.3 Vapor phase polymerization (VPP)
Vapor phase polymerization is used to prepare polyaniline from aniline monomer by introducing to an oxidant coated substrate in vapor form. At the oxidant vapor interface, the polymerization takes place. Vapor phase polymerization (VPP) can be either chemical vapor phase polymerization (CVPP) or electrochemical vapor phase polymerization EVPP). CVPP is a solvent free process use to get highly uniform conductive polyaniline which allows formation of PANI layers of any thickness on an insulating substrate. In VPP monomers are applied as vapor rather than solution or liquid which restricts the particle agglomeration. Intrinsic conjugated polymers formed via VPP have high electrical conductivity and they are agglomeration free [12, 13]. VPP provides polymers of very high purity with excellent conductivity and scope of synthesis at the nanoscale range (Figure 4) [14].
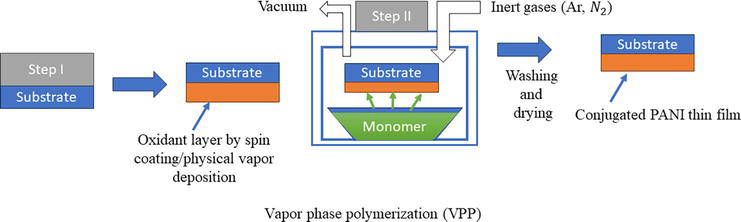
Figure 4.
Vapor phase polymerization method.
2.4 Photochemically initiated polymerization
In this method light (photon) is used to initiate free radical polymerization. This technique consumes less energy and higher productivity at lower reaction temperature compared to other conventional polymerization techniques. In this method, the photochemical initiation is achieved by subjecting suitable photo initiators to ultra-violet radiations. The formation of PANI using photopolymerization requires an external source of gamma rays, microwaves, UV rays and X-rays. PANI can be synthesized using bilayer films containing [Ru(bipy)3]2+ as a primer and methylviologen (MV2+) as an oxidizer polyaniline through irradiated with visible electromagnetic light. The process of electron transfer between [Ru(bipy)3]2+ and (MV2+) is responsible for the oxidation of aniline and formation of PANI [15]. The photochemical polymerization is similar to the free radical polymerization but initiation via light.
The different morphologies of PANI can be obtained using different synthesis method as shown in Figure 5
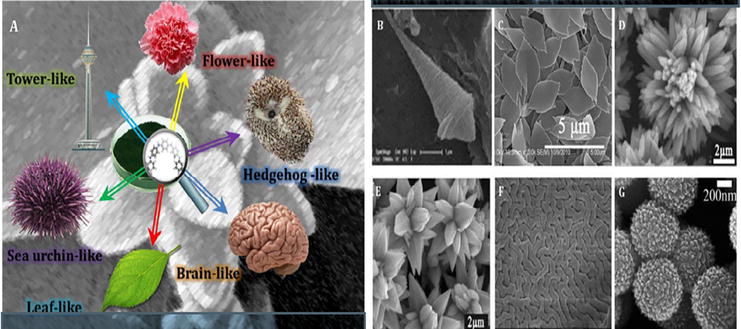
Figure 5.
(a) Various nanostructures/microstructures of PANI; SEM image of (b) tower/spindle-like PANI, (c) leaf-like PANI, (d) hedgehoglike PANI, (e) flower-like PANI, (f) brain-like PANI, and (g) sea urchin-like PANI [
3. Different methods for synthesis of nanocomposites
Synthesis of nanocomposites involves the combination of two or more materials at the nanoscale to create new materials with enhanced properties. There are various methods for synthesizing nanocomposites, and here are some commonly used techniques:
In-situ polymerization: This method involves the formation of nanoparticles within a polymer matrix during the polymerization process. Monomers and nanoparticles are combined, and polymerization occurs simultaneously, resulting in a nanocomposite material.
Solution mixing: In this method, nanoparticles and a polymer are separately dissolved in a common solvent and then mixed together. The solvent is subsequently removed, resulting in the formation of a nanocomposite.
Melt mixing: This technique involves the direct blending of nanoparticles and a polymer in the molten state. The mixture is then cooled and solidified, leading to the formation of a nanocomposite material.
Electrospinning: Electrospinning is a versatile method used to fabricate nanofibers. It involves the application of an electric field to a polymer solution or melt, resulting in the formation of ultrafine fibers. Nanoparticles can be incorporated into the polymer solution or melt prior to electrospinning to create nanocomposite fibers.
Layer-by-layer assembly: This technique involves the sequential deposition of alternating layers of nanoparticles and polymers. Electrostatic interactions or other forces are utilized to form the multilayered structure, resulting in the creation of nanocomposite films or coatings.
Sol-gel method: The sol-gel process involves the transformation of a sol (a dispersion of nanoparticles in a liquid) into a gel (a three-dimensional network of interconnected nanoparticles) by hydrolysis and condensation reactions. By controlling the reaction conditions, nanocomposite materials can be obtained.
Chemical vapor deposition (CVD): CVD is a technique used to deposit thin films of materials onto a substrate. In the case of nanocomposites, nanoparticles are introduced into the gas phase and allowed to react and deposit onto the substrate surface, resulting in the formation of a nanocomposite film.
Template synthesis: This method involves using a template or scaffold to guide the growth of nanoparticles or polymers. The template can be a porous material or a sacrificial structure that is subsequently removed, leaving behind a nanocomposite material with a specific structure.
These are just a few examples of the methods used for synthesizing nanocomposites. The choice of method depends on factors such as the desired properties of the nanocomposite, the nature of the materials involved, and the intended application.
4. Polyaniline-based nanocomposites
Polyaniline (PANI) is a conducting polymer with unique electrical, optical, and chemical properties. When combined with nanomaterials, such as nanoparticles or nanofibers, PANI-based nanocomposites exhibit enhanced properties and a wide range of potential applications. Polyaniline-based nanocomposites can be synthesized through various methods, including in situ polymerization, solution blending, electrochemical deposition, and template-assisted synthesis. The choice of synthesis method depends on the desired properties and application requirements. PANI can be combined with different types of nanomaterials, including metal nanoparticles (e.g., gold, silver), metal oxides (e.g., titanium dioxide, zinc oxide), carbon-based materials (e.g., carbon nanotubes, graphene), and organic/inorganic nanofibers. These nanomaterials serve as fillers or reinforcements, imparting unique properties to the nanocomposite. Enhanced electrical conductivity: PANI itself is a conductive polymer, but the addition of nanomaterials further enhances the electrical conductivity of the nanocomposite. This property makes PANI-based nanocomposites suitable for applications such as sensors, actuators, electrostatic discharge materials, and energy storage devices. Improved mechanical properties: The incorporation of nanomaterials in PANI matrices improves the mechanical strength and toughness of the nanocomposite. Nanofillers act as reinforcing agents, reducing the brittleness of PANI and enhancing its resistance to mechanical stress. Enhanced thermal stability: PANI exhibits limited thermal stability at high temperatures. However, the addition of nanomaterials can improve the thermal stability of PANI-based nanocomposites, allowing their use in applications that require higher operating temperatures. Tailored optical properties: PANI-based nanocomposites can exhibit tunable optical properties, including changes in color, absorbance, and emission, depending on the nanomaterials incorporated. These properties make them suitable for applications such as optoelectronics, light-emitting devices, and sensors. Polyaniline-based nanocomposites find applications in various fields, including energy storage and conversion (batteries, supercapacitors, solar cells), electronics (printed circuit boards, conductive coatings), sensors and actuators, corrosion protection, electromagnetic shielding, and biomedical applications (drug delivery, tissue engineering). Despite their promising properties, the development of PANI-based nanocomposites faces challenges related to achieving uniform dispersion of nanofillers, controlling the size and morphology of nanoparticles, and maintaining stability over time. Additionally, the scale-up of synthesis methods and the cost-effectiveness of production are areas that require further research and development. Polyaniline-based nanocomposites offer exciting opportunities for advancing materials science and developing innovative technologies. Continued research and exploration of these materials will likely lead to further improvements and new applications in the future. In past decades, the incorporation of inorganic filler into organic conducting polymer forming a composites or nanocomposites matrix attracted huge attention. The combination of inorganic provides better performance, stability and electrical conductivity to polymer matrix and has application in sensors, actuators, OLEDs, solar cells, supercapacitors and so on. Many publications have been reported for the synthesis of PANI with metal oxides, metal, sulfides, graphene and other inorganic materials.
4.1 PANI-based binary/ternary nanocomposites
Polyaniline nanocomposites can be considered of as a material composed of a PANI matrix and one component, including semiconductors, metal nanoparticles, organic compounds, inorganic compounds, biological and natural compounds, to modify the surface morphologies and enhanced the polymer stability, optical and electrical properties. Similarly, in case of ternary nanocomposites surface interaction enhanced modify the electronic structure [16]. In Figure 6 shows the PANI based nanocomposites revealed from the literature survey.
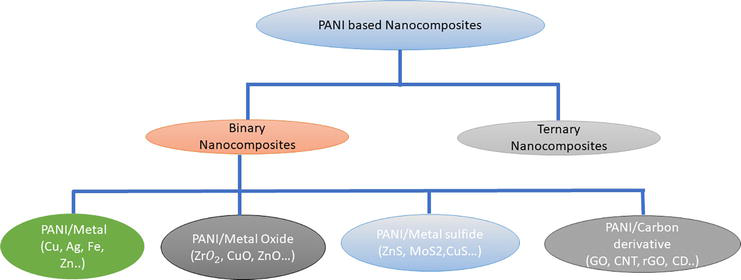
Figure 6.
PANI based nanocomposites.
PANI-based binary nanocomposites: Researchers synthesized and developed new electrode materials by refining different parameters in response to the rising demand for the development of high-performance supercapacitors.
4.2 Applications of polyaniline
Polyaniline exhibited in three different oxidation states such as Leucomeraldine (LM), pernigraline (PN) and emeraldine (EM). Emeraldine salt is a conducting nature polymer. Due to different surface morphology (such as nanofiber, nanoflowers, nano- leaf) of polyaniline shows distinct physiochemical properties. Large specific surface area, easily dissolved in alcohol, ketone and other organic solvent of polyaniline can improve the reactivity in the application of supercapacitor, sensor and water pollutant free applications. Due to semiconducting nature, excellent optical, electrical properties and higher thermal stability polyaniline has extensive application in the field of optoelectronic device (like organic light emitting diode (OLED), organic solar cell). In Figure 6 shows the various applications of PANI based nanocomposites. Engineering is the current focus of medicine, and advancements in this field need for new intellectual technologies. Devices that compensate for nerve weakening and advance neuroscience are needed by neuroscientists. Biocompatibility conductive scaffolding has high bio-counterfeit qualities, and it has been used to treat organ problems. Moreover, PANI applications in delivery systems have drawn a lot of interest; as a consequence, novel delivery structures, such electro-drug delivery systems, are being investigated. PANI has been successfully used as an anti-corrosion barrier with positive outcomes [17]. Figure 7 showed the application of the PANI in different field.
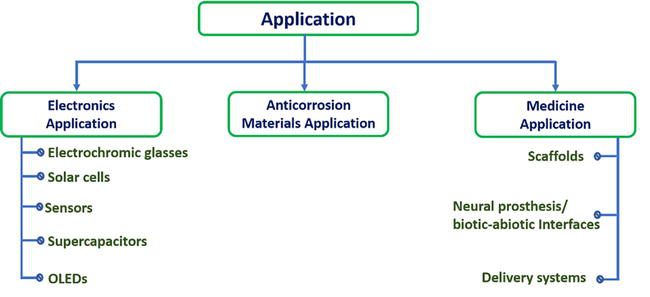
Figure 7.
Various applications of PANI based nanocomposites.
4.2.1 Supercapacitor applications
Supercapacitor is one of the highly anticipated energy storage technologies having numerous advantages over its most common counterpart i.e., rechargeable battery. It possessed quick energy supply, long life-span and environment compatibility. These properties suggest this technology as the energy storage for modern era [18, 19].
Conventional carbon material based supercapcitor (electric double layer capacitor) suffer from low specific capacitance and energy density [20, 21]. Therefore, recently researchers have turn towards conducting polymer based supercapcitor (pseudocapacitor) which provides enhanced specific capacitance due to presence of numerous redox active sites. Zhang et al. synthesized PANI via chemical oxidative polymerization. The resultant polymeric electrode exhibited specific capacitance of 330 Fg−1 with capacitance retention of 75% [22]. Similarly, Khdary et al. synthesized mesoporous PANI which passed much improved specific capacitance (532 Fg−1) and cyclic stability (85% after 1000 cycles) [23]. The mesoporous nature of the material proved to be beneficial for charge mobility and increase its charge accumulation ability. However, PANI in its pristine form is not usually recommended for supercapacitor due to lack of stability [24]. The stability material can be improved by introducing different filler materials such as carbon materials, metal oxides, metal sulfides, metal organic frameworks (MOFs) etc. For instant, Shafi et al. synthesized ternary composite of LaMnO3/RGO/PANI having excellent cyclic stability and energy density [25]. The polymeric material possessed specific capacitance of 802 Fg−1 at current density of 1 Ag−1. Further the asymmetric supercapcitor (ASC) employing LaMnO3/RGO/PANI as positive electrode and RGO as negative electrode showed highly improved cyclic behavior with capacitance retention of 117% even after 100 k cycles as represented in Figure 8. Table 1 summarized electrochemical properties of some important PANI based electrode materials for supercapacitor applications.
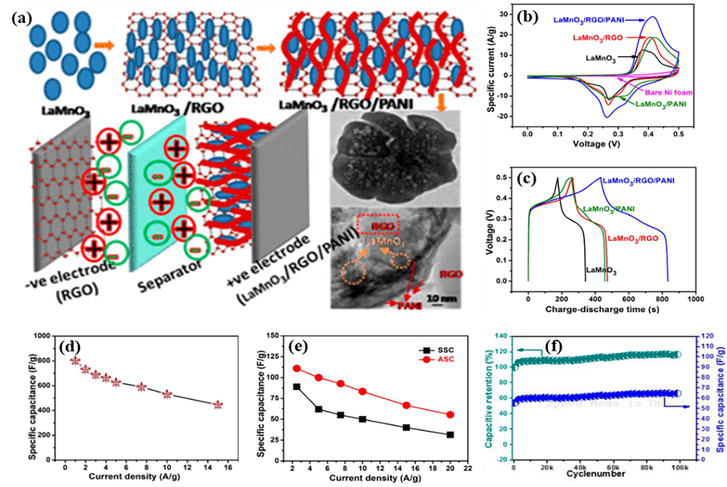
Figure 8.
(a) Schematic representation of LaMnO3/RGO/PANI composite and its asymmetric supercapacitor; (b) CV curves for different electrode materials; (c) GCD curves for different electrode materials; (d) variation of specific capacitance of LaMnO3/RGO/PANI electrode with current density; (e) variation of specific capacitance of symmetric and asymmetric supercapacitor devices with current density; and (f) cyclic stability of ASC up to 100 k cycles. Adapted with permission from [
| Sl. No. | Nanocomposite | Current density (Ag−1) | Specific capacitance (Fg−1) | Capacity retention | Ref. |
|---|---|---|---|---|---|
| 1. | PANI | 1 | 330 | 75% after 1000 cycles | [22] |
| 2. | Mesoporous PANI | 1.5 | 532 | 85% after 1000 cycles | [23] |
| 3. | PANI/GO/Cu | 1 | 558 | 90% after 1000 cycles | [26] |
| 4. | PANI/RGO/LaMnO3 | 1 | 802 | 117% after 100 k cycles | [25] |
| 5. | PANI/Graphene/MnO2 | — | 395 | 92% after 1200 cycles | [27] |
| 6. | PANI-Ag/ZnO | 0.8 | 635 | 96% | [28] |
| 7. | PANI/graphene/manganese ferrite | 0.2 | 454 | 76.4% after 5000 cycles | [29] |
Table 1.
4.2.2 Organic solar cell applications
Inexpensive, high-performance film solar cells based on an electrolyte and a semiconductor are known as dye-sensitive solar cells (DSSCs) [30, 31]. They typically include a counter electrode, which is often composed of platinum, a redox electrode, and a color-sensitive titanium dioxide electrode. Pt, which is replaced with carbon-based materials, is the component in DSSCs that costs the highest. Because of its simple synthesis, cheap cost, and high conductivity, PANI is used in DSSC. Due to complicated electrocatalytic rooting activity at I3- and a lower charge transfer ratio of oxidation reactions, microporous PANI electrodes seem better than Pt electrodes [32]. The catalytic activity advances, the absorbency of PANI rises at a certain surface area, and the effectiveness of trapping liquid electrolytes for DSSC rises. In order to produce counter electrodes, PANI is electrolyzed in FTO glass by a number of counteractions, such as SO42-, BF4-, CL-, ClO4-, and p-toluene sulfonate [TsO-]. As a result, PANI-SO4 offers the greatest amount of porous medium while requiring the least amount of charge transfer resistance and the greatest amount of reduction current [33]. The electrical area of PANI and total conductivity are increased by PANI polymerization on the graphene surface [34]. Hence, an anti-electrode in DSSC or other electrocatalytic activity is improved by this strategy.
The absorbance range of the active layer material of organic solar cell should be in visible range of electromagnetic spectrum for the better efficiency of the device. The device with PSSA-g-PANI exhibits about 4% PCE, which is 20% higher than that of the device with PEDOT: PSS due to unique high transparency in the UV-vis region (especially 450–650 nm) and high conductivity of PSSA-g-PANI, when the blend of P3HT and PCBM is used as the active layer of PSCs as shown in Figure 9.
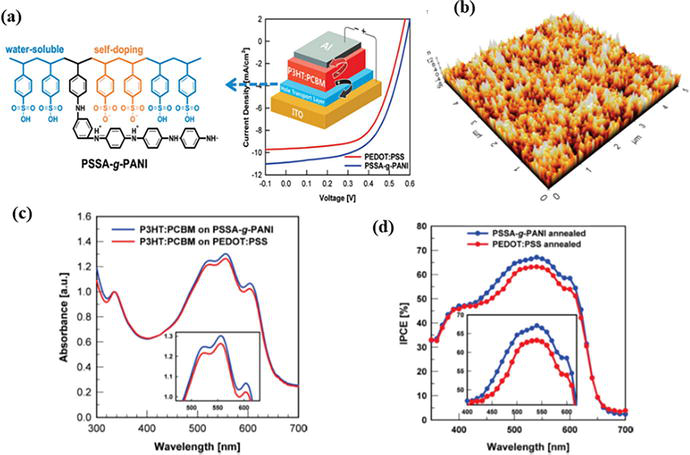
Figure 9.
(a) Device structure of solar cell. (b) AFM of the PSSA-g-PANI layer, (c) absorbance spectra of the device and (d) efficiency of the device.
4.2.3 Organic light emitting diode (OLED) application
OLED is a device that produces light as a result of the induction of an electric current or electric field are known as electroluminescence devices. When a suitable voltage is applied, they can serve as a source for organic light-emitting diodes (OLEDs) with a p-n connection diode. As a result, photon energy can be discharged by combining electrons with the device’s electron holes [35]. With the semiconductor energy bandgap, a vivid color will be produced. The organic LED (OLED) is a perforated injection film made of conductive polymer. OLED performance is improved by using PANI-poly (styrene sulfonate; PPS). Comparing PANI-PPS to commercial PEDOTPPS, more complex performance is obtained, and hole injection increases. PANI-PPS, in contrast, performs with the highest efficiency and lowest voltage given the high conductivity, medium clarity, and roughness. In comparison to traditional PEDOT-PPS, PPS with a conductive polymer, such as its PANI copolymer solution, offers easier doping, solubility, and film quality for thematic monitors/screens [36]. To create a perforation injection film in two-layer electroluminescence with an orange electroluminescence display in comparison to a single-layer electroluminescence device, PANI self-doping based on aniline and (aminobenzene sulfonic acid) is assembled in its ITO glass [37]. Similar to ZnO/PANI nanowire (type n/p), this organic-mineral ray-emitting diode is produced by the recombination of the electron boundary in the conduction band and the holes over a broad light spectrum [38]. The less the separation between the heterostructure of hole transport layer and emissive layer the greater will be the efficiency of the device due to the more transport of holes from HTL to EL in the device. Similarly, the less the separation between the heterostructure of electron transport layer and emissive layer the greater will be the efficiency of the device due to the more transport of electrons from ETL to EL in the device (Figure 10).
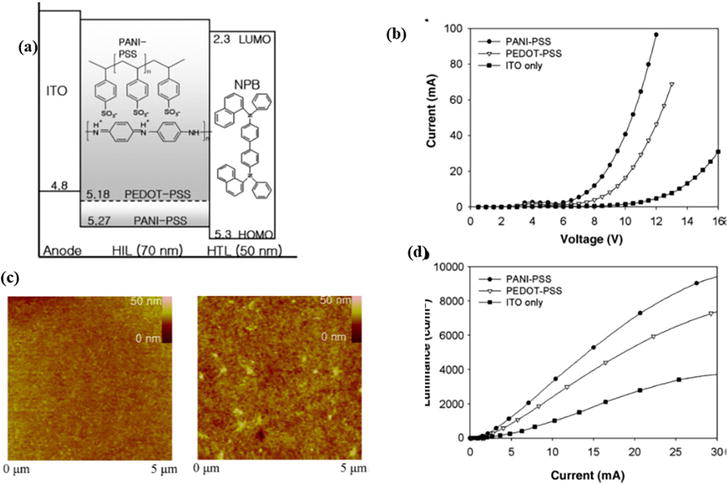
Figure 10.
(a) energy level diagram of heteromolecular (b) Current versus voltage curve of OLED device (c) AFM surface structure of layer and (d) luminescence versus current curve of device, Image reused with permission from Ref. [
4.2.4 Waste-water treatment applications
Application of PANI based binary nanocomposites as absorbents in waste water treatment: Polyaniline nanocomposites have been employed as adsorbents to remove various impurities from effluent in recent years. Due to their excellent interaction with the functional groups of PANI nanocomposites, further research has been conducted on the adsorption of organic pigments and ions of heavy metals. In the study of the association between PANI nanocomposites and impurities, various adsorption parameters including pH, incubation time, adsorbent dose, temperature, adsorbent nature, and pollutant concentration are investigated. The different PANI based nanocomposite employed as an absorbent of pollutants are listed in Table 2. To confirm the potential of PANI nanocomposites as adsorbents for water purification, the efficacy and adsorption capability of nanocomposites can be estimated based on these parameters.
| Adsorbent | Pollutant | Langmuir maximum capacity (qmax) in mg/g | pH | Conc. (mg/L) | Adsorbent dose (mg) | Ref. |
|---|---|---|---|---|---|---|
| PANI/PVP | Mn (II) | 50.30 | 7 | 100 | 250 | [39] |
| PANI/Fe | CR | 99.6 | 7 | 100 | 1000 | [40] |
| PANI/zeolite | Cr (VI) | — | 2 | 50 | 200 | [41] |
| PANI/ZnFe2O4 | Rhodamine BRHB | 1000 | 2 | 10 | 500 | [42] |
| PANI/PAN | Cr (VI) | 67.3 | 2 | 5 | 10 | [43] |
Table 2.
Contains the PANI based nanocomposite employed as an absorbent of pollutants.
5. Conclusions
Polyaniline (PANI) is a conducting polymer with a wide range of applications due to its unique electrical, mechanical, and chemical properties. PANI can be synthesized by various methods, including chemical oxidation, electrochemical polymerization, and template-assisted synthesis. Polyaniline exists in different oxidation states: emeraldine base (EB), emeraldine salt (ES), and pernigraniline (PN). The EB form is most widely studied and has both conductive and insulating properties. The doping of polyaniline with acids or other dopants enhances its electrical conductivity and other properties. Polyaniline exhibits good thermal stability, high mechanical strength, and excellent environmental stability. Polyaniline has wide range of applications such as in conductive coating material for corrosion protection of metals, anti-static coatings, and electromagnetic shielding. Polyaniline has shown promise in applications such as supercapacitors and rechargeable batteries due to its high specific capacitance and good cycling stability. Polyaniline-based sensors are used for gas sensing (e.g., detection of ammonia, carbon monoxide), humidity sensing, and biosensing applications. Polyaniline-based actuators can convert electrical energy into mechanical motion, making them suitable for applications in robotics and microfluidics. Polyaniline can be employed in organic field-effect transistors (OFETs), organic light-emitting diodes (OLEDs), and other organic electronic devices. Also, polyaniline-based adsorbents have been explored for the removal of various pollutants from water, including heavy metals and organic contaminants. Advancements in the design of polyaniline-based nanocomposites and hybrid materials are expected to further enhance its performance in various fields. The development of scalable and cost-effective synthesis methods is crucial to facilitate the commercialization of polyaniline-based technologies. It’s worth noting that the field of polyaniline research is vast, and ongoing discoveries may expand the range of applications and understanding of its properties.
Acknowledgments
The author sincerely than IIT (ISM) Dhanbad for all assistance and support.
References
- 1.
Bhadra S, Khastgir D, Singha NK, Lee JH. Progress in preparation, processing and applications of polyaniline. Progress in Polymer Science. 2009; 34 :783-810. DOI: 10.1016/j.progpolymsci.2009.04.003 - 2.
Lu H, Li X, Lei Q. Conjugated conductive polymer materials and its applications: A mini-review. Frontiers in Chemistry. 2021; 9 . DOI: 10.3389/fchem.2021.732132 - 3.
Bouarissa A, Gueddim A, Bouarissa N, Djellali S. Band structure and optical properties of polyaniline polymer material. Polymer Bulletin. 2018; 75 :3023-3033. DOI: 10.1007/s00289-017-2189-6 - 4.
Shirakawa H, McDiarmid A, Heeger A. Focus article: Twenty-five years of conducting polymers. Chemical Communications. 2003; 1 :1-4. DOI: 10.1039/b210718j - 5.
Bauri J, Choudhary RB, Mandal G. Recent advances in efficient emissive materials-based OLED applications: A review. Journal of Materials Science. 2021; 56 :18837-18866. DOI: 10.1007/s10853-021-06503-y - 6.
Kumar R, Ansari MO, Parveen N, Oves M, Barakat MA, Alshahri A, et al. Facile route to a conducting ternary polyaniline@TiO 2/GN nanocomposite for environmentally benign applications: Photocatalytic degradation of pollutants and biological activity. RSC Advances. 2016; 6 :111308-111317. DOI: 10.1039/c6ra24079h - 7.
Babel V, Hiran BL. A review on polyaniline composites: Synthesis, characterization, and applications. Polymer Composites. 2021; 42 :3142-3157. DOI: 10.1002/pc.26048 - 8.
Zarrintaj P, Ahmadi Z, Vahabi H, Ducos F, Reza Saeb M, Mozafari M. Polyaniline in retrospect and prospect. Materials Today: Proceedings. 2018; 5 :15852-15860. DOI: 10.1016/j.matpr.2018.05.084 - 9.
Mandal G, Choudhary RB. MnO2 integrated emeraldine polyaniline (PANI-MnO2) nanocomposites with inflated opto-electricaltraitsas ETLs for OLED applications. Materials Science in Semiconductor Processing. 2022; 151 :107000. DOI: 10.1016/j.mssp.2022.107000 - 10.
Sai S, Kumar A. Synthesis and morphological study of polyaniline. European Journal of Molecular and Clinical Medicine. 2020; 07 :2020 - 11.
Wang B, Tang J, Wang F. Electrochemical polymerization of aniline. Synthetic Metals. 1987; 18 :323-328. DOI: 10.1016/0379-6779(87)90899-X - 12.
Lawal AT, Wallace GG. Vapour phase polymerisation of conducting and non-conducting polymers: A review. Talanta. 2014; 119 :133-143. DOI: 10.1016/j.talanta.2013.10.023 - 13.
Winther-Jensen B, West K. Vapor-phase polymerization of 3,4-Ethylenedioxythiophene: A route to highly conducting polymer surface layers. Macromolecules. 2004; 37 :4538-4543. DOI: 10.1021/ma049864l - 14.
Majeed AH, Mohammed LA, Hammoodi OG, Sehgal S, Alheety MA, Saxena KK, et al. A review on polyaniline: Synthesis, properties, nanocomposites, and electrochemical applications. International Journal of Polymer Science. 2022; 2022 . DOI: 10.1155/2022/9047554 - 15.
Lee JH, Prud’homme RK, Aksay IA. Cure depth in photopolymerization: Experiments and theory. Journal of Materials Research. 2001; 16 :3536-3544. DOI: 10.1557/JMR.2001.0485 - 16.
Maponya TC, Hato MJ, Somo TR, Ramohlola KE, Makhafola MD, Monama GR, et al. Polyaniline-based nanocomposites for environmental remediation. In: Trace Met. Environ.—New Approaches Recent Adv. London, U.K.: IntechOpen; 2021. DOI: 10.5772/intechopen.82384 - 17.
Zarrintaj P, Vahabi H, Saeb MR, Mozafari M. Application of polyaniline and its derivatives. In: Fundam. Emerg. Appl. Polyaniline. Amsterdam, Netherlands: Elsevier; 2019. pp. 259-272. DOI: 10.1016/B978-0-12-817915-4.00014-2 - 18.
Ansari S, Bilash R, Gupta A. Nanoflower copper sulphide intercalated reduced graphene oxide integrated polypyrrole nano matrix as robust symmetric supercapacitor electrode material. Journal of Energy Storage. 2023; 59 :106446. DOI: 10.1016/j.est.2022.106446 - 19.
Vangari M, Pryor T, Jiang L. Supercapacitors: Review of materials and fabrication methods. Journal of Energy Engineering. 2013; 139 :72-79. DOI: 10.1061/(asce)ey.1943-7897.0000102 - 20.
Snook GA, Kao P, Best AS. Conducting-polymer-based supercapacitor devices and electrodes. Journal of Power Sources. 2011; 196 :1-12. DOI: 10.1016/j.jpowsour.2010.06.084 - 21.
Choudhary RB, Ansari S, Purty B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review. Journal of Energy Storage. 2020; 29 :101302. DOI: 10.1016/j.est.2020.101302 - 22.
Zhang X, Ji L, Zhang S, Yang W. Synthesis of a novel polyaniline-intercalated layered manganese oxide nanocomposite as electrode material for electrochemical capacitor. Journal of Power Sources. 2007; 173 :1017-1023. DOI: 10.1016/j.jpowsour.2007.08.083 - 23.
Khdary NH, Abdesalam ME, Enany GEL. Mesoporous polyaniline films for high performance supercapacitors. Journal of The Electrochemical Society. 2014; 161 :63-68. DOI: 10.1149/2.0441409jes - 24.
Wang H, Lin J, Shen ZX. Polyaniline (PANi) based electrode materials for energy storage and conversion. Journal of Science: Advanced Materials and Devices. 2016; 1 :225-255. DOI: 10.1016/j.jsamd.2016.08.001 - 25.
Shafi PM, Ganesh V, Bose AC. LaMnO 3/RGO/PANI ternary nanocomposites for supercapacitor electrode application and their outstanding performance in all-solid-state asymmetrical device design. ACS Applied Energy Materials. 2018; 1 :2802-2812. DOI: 10.1021/acsaem.8b00459 - 26.
Ma Y, Zhao D, Chen Y, Huang J, Zhang Z, Zhang X, et al. A novel core-shell polyaniline/graphene oxide/copper nanocomposite for high performance and low-cost supercapacitors. Chemical Papers. 2019; 73 :119-129. DOI: 10.1007/s11696-018-0556-x - 27.
Mu B, Zhang W, Shao S, Wang A. Glycol assisted synthesis of graphene–MnO2–polyaniline ternary composites for high performance supercapacitor electrodes. Physical Chemistry Chemical Physics. 2014; 16 :7872. DOI: 10.1039/c4cp00280f - 28.
Purty B, Choudhary RB, Kandulna R, Singh R. Remarkable enhancement in electrochemical capacitance value of Ag-ZnO/PANI composite for supercapacitor application. AIP Conference Proceedings. 2019; 2115 :030588. DOI: 10.1063/1.5113427 - 29.
Xiong P, Hu C, Fan Y, Zhang W, Zhu J, Wang X. Ternary manganese ferrite/graphene/polyaniline nanostructure with enhanced electrochemical capacitance performance. Journal of Power Sources. 2014; 266 :384-392. DOI: 10.1016/j.jpowsour.2014.05.048 - 30.
Hosseinnezhad M, Gharanjig K, Moradian S, Saeb MR. In quest of power conversion efficiency in nature-inspired dye-sensitized solar cells: Individual, co-sensitized or tandem configuration? Energy. 2017; 134 :864-870. DOI: 10.1016/j.energy.2017.06.045 - 31.
Hosseinnezhad M, Saeb MR, Garshasbi S, Mohammadi Y. Realization of manufacturing dye-sensitized solar cells with possible maximum power conversion efficiency and durability. Solar Energy. 2017; 149 :314-322. DOI: 10.1016/j.solener.2016.11.011 - 32.
Liu B, Huo L, Si R, Liu J, Zhang J. A general method for constructing two-dimensional layered mesoporous mono- and binary-transition-metal nitride/graphene as an ultra-efficient support to enhance its catalytic activity and durability for electrocatalytic application. ACS Applied Materials & Interfaces. 2016; 8 :18770-18787. DOI: 10.1021/acsami.6b03747 - 33.
Zhang D, Ryu K, Liu X, Polikarpov E, Ly J, Tompson ME, et al. Transparent, conductive, and flexible carbon nanotube films and their application in organic light-emitting diodes. Nano Letters. 2006; 6 :1880-1886. DOI: 10.1021/nl0608543 - 34.
Wang G, Xing W, Zhuo S. The production of polyaniline/graphene hybrids for use as a counter electrode in dye-sensitized solar cells. Electrochimica Acta. 2012; 66 :151-157. DOI: 10.1016/j.electacta.2012.01.088 - 35.
Jang J, Ha J, Kim K. Organic light-emitting diode with polyaniline-poly(styrene sulfonate) as a hole injection layer. Thin Solid Films. 2008; 516 :3152-3156. DOI: 10.1016/j.tsf.2007.08.088 - 36.
Huh DH, Chae M, Bae WJ, Jo WH, Lee T-W. A soluble self-doped conducting polyaniline graft copolymer as a hole injection layer in polymer light-emitting diodes. Polymer (Guildf). 2007; 48 :7236-7240. DOI: 10.1016/j.polymer.2007.09.046 - 37.
Yang C-H, Chih Y-K. Molecular assembled self-doped polyaniline interlayer for application in polymer light-emitting diode. The Journal of Physical Chemistry. B. 2006; 110 :19412-19417. DOI: 10.1021/jp0612174 - 38.
Liu YY, Wang XY, Cao Y, Chen XD, Xie SF, Zheng XJ, et al. A flexible blue light-emitting diode based on ZnO nanowire/polyaniline heterojunctions. Journal of Nanomaterials. 2013; 2013 :1-4. DOI: 10.1155/2013/870254 - 39.
Hallajiqomi M, Eisazadeh H. Adsorption of manganese ion using polyaniline and it’s nanocomposite: Kinetics and isotherm studies. Journal of Industrial and Engineering Chemistry. 2017; 55 :191-197. DOI: 10.1016/j.jiec.2017.06.045 - 40.
Bhaumik M, McCrindle RI, Maity A. Enhanced adsorptive degradation of Congo red in aqueous solutions using polyaniline/Fe0 composite nanofibers. Chemical Engineering Journal. 2015; 260 :716-729. DOI: 10.1016/j.cej.2014.09.014 - 41.
Shyaa AA, Hasan OA, Abbas AM. Synthesis and characterization of polyaniline/zeolite nanocomposite for the removal of chromium(VI) from aqueous solution. Journal of Saudi Chemical Society. 2015; 19 :101-107. DOI: 10.1016/j.jscs.2012.01.001 - 42.
Rachna K, Agarwal A, Singh N. Preparation and characterization of zinc ferrite—Polyaniline nanocomposite for removal of rhodamine B dye from aqueous solution. Environmental Nanotechnology, Monitoring & Management. 2018; 9 :154-163. DOI: 10.1016/j.enmm.2018.03.001 - 43.
Ren J, Huang X, Wang N, Lu K, Zhang X, Li W, et al. Preparation of polyaniline-coated polyacrylonitrile fiber mats and their application to Cr(VI) removal. Synthetic Metals. 2016; 222 :255-266. DOI: 10.1016/j.synthmet.2016.10.027