Lower extremity artery diseases—Fontaine and Rutherford’s classifications.
Abstract
Recently, there has been significant progress in finalizing devices for lower extremity artery disease. Especially in the region of the superficial femoral artery, it is possible to benefit from drug technology. It is necessary to select a device that is appropriate for the lesion, lesion length, and patient background. On the other hand, there are still issues in the treatment of chronic limb ischemia and below-the-knee arteries. In the first place, the pathologies of “above the knee” and “below the knee” are different, and the purpose of treatment is also different. Access sites for treatment have also become smaller and more diverse with the development of peripheral devices.
Keywords
- LEAD
- EVT
- DES
- covered stent
- DCB
- BTK
- BTA
1. Introduction
More than any other class of human disease, diseases of the arterial system are responsible for morbidity and mortality. Malformations of the vasculature are the underlying cause of clinical disease, which may present as either a narrowing or complete obstruction of the vessel lumen or as a progressive or sudden worsening.
Peripheral arterial disease is classified into carotid, vertebral, subclavian, renal, iliac, upper and lower extremity arteries, and treatment methods have been established for each region in recent years. Previously, peripheral arterial disease was referred to as arteriosclerosis obliterans, but since symptoms can appear without obstruction and can be severe, the term “lower extremity arterial disease (LEAD)” has come to be used generically. The number of LEAD is increasing due to the progression of atherosclerotic lesions. The increase in LEAD has led to the development of a wide range of treatment methods and the emergence of a variety of new treatment devices. Advances in technology have significantly improved patency rates, benefiting many patients.
2. Epidemiology and risk factors
2.1 Epidemiology
LEAD is an atherosclerotic disease that affects approximately 200 million people worldwide, with symptoms beginning at age 50 or older, and the incidence increasing rapidly after age 65. The incidence of LEAD increases dramatically after the age of 65, and the incidence rate exceeds 20% in people over the age of 80 [1]. LEAD tends to be higher in high-income populations and lower in low- to middle-income populations due to arteriosclerotic disease. However, a gender-specific analysis shows that LEAD is more prevalent among men in high-income populations and among women in low- to middle-income populations. There is now a growing consensus that the most reliable source of epidemiological data for assessing LEAD prevalence is ankle-brachial index (ABI) measurements (Figure 1). In a German study, about 18% of the patients were below 0.9, but only about 10% of them actually had claudication symptoms, and other reports have put the rate at 20–30% [2, 3, 4]. There were also racial differences, with blacks having higher incidence rates than non-Hispanic whites and blacks, and among all races, blacks of both male and female had higher rates of ABI < 0.9. Hispanic, Indian, and non-Hispanic whites were all comparable.
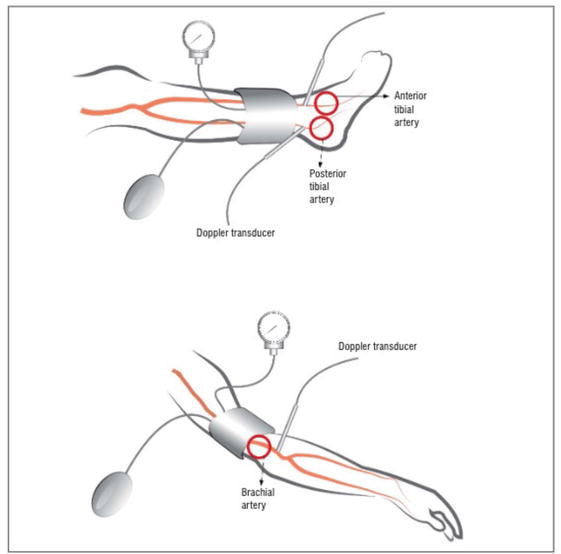
Figure 1.
Principles of correct measurement of the ankle brachial index.
2.2 Risk factor
The main cause of LEAD is atherosclerosis. Risk factors for LEAD include all factors that contribute to the development of atherosclerosis.
2.2.1 Age
Susceptibility to developing atherosclerosis increases with age. Symptoms typically appear after the age of 40 [5].
2.2.2 Gender
All other factors being equal, men are much more likely than women to be affected by atherosclerosis and its consequences. Myocardial infarction and other complications of atherosclerosis are uncommon in premenopausal women, unless they have a predisposition through diabetes, hyperlipidemia, or severe hypertension. After the menopause, however, the incidence of atherosclerosis-related diseases increases due to hormone changes. This is probably due to a decrease in natural estrogen levels. The incidence of myocardial infarction becomes the same for men and women by the seventh to eighth decade of life [5].
2.2.3 Genetics
There is a well-established familial predisposition to atherosclerosis and ischemic heart disease, which is most likely polygenic in nature. Most commonly, the genetic predisposition relates to a familial clustering of other risk factors, such as high blood pressure or diabetes, while less commonly it relates to a well-defined inherited genetic abnormality in lipoprotein metabolism that results in excessively elevated blood lipid levels, such as familial hypercholesterolemia.
2.2.4 Hyperlipidemia
Hyperlipidemia is an important risk factor for atherosclerosis. Hypercholesterolemia is the focus of most of the evidence. Even in the absence of other risk factors, elevated serum cholesterol alone is sufficient to promote lesion development. Low-density lipoprotein (LDL) cholesterol, which plays an important physiological role as a vehicle for transporting cholesterol to peripheral tissues, is the component of total serum cholesterol associated with increased risk. HDL, on the other hand, is thought to mobilize cholesterol from developing and existing arteriosclerosis and transport it to the liver to be excreted into the bile. High HDL levels are associated with a lower risk. As a result, there is a great deal of interest in dietary, pharmacological and behavioral methods to reduce LDL levels and increase HDL levels. Regular exercise and moderate alcohol consumption both increase HDL levels, while obesity and smoking decrease it. Statins lower circulating cholesterol indirectly by inhibiting HMG-CoA reductase, a key enzyme in cholesterol biosynthesis in the liver [6, 7, 8].
2.2.5 Hypertension
High blood pressure is a significant risk factor for atherosclerosis at all ages. Men aged 45–62 years with blood pressure above 169/95 mm Hg have more than five times the risk of ischemic heart disease as those with blood pressure 140/90 mm Hg or lower. Both systolic and diastolic levels increase risk. Antihypertensive therapy reduces the incidence of diseases related to atherosclerosis, in particular stroke and ischemic heart disease. High blood pressure was the strongest indicator of all acute forms of PAD, including acute limb ischemia, chronic limb-threatening ischemia (CLTI), and their respective treatment outcomes [9].
2.2.6 Cigarette smoking
A particularly strong risk factor for LEAD [9]. Its contribution to the total risk in the population has been estimated to be around 44%. Notably, the association between LEAD and smoking persists even after smoking cessation, although it is significantly reduced more than 10 years after quitting [10].
2.2.7 Diabetes mellitus
Similar to smoking, diabetes is a strong risk factor for LEAD. The longer a person has diabetes, the higher the rate of LEAD. It also increases the risk of amputation of the lower extremity, as well as the risk of wound infection. Wound infections after amputation are also a risk for sepsis and often require additional amputations [11].
2.2.8 Other risk factors
Inflammation plays an important pathophysiological role in atherosclerotic disease. Several markers of inflammation, including high-sensitivity C-reactive protein, fibrinogen, and interleukin-6, are associated with increased risk of LEAD incidence, progression, and complications. Some autoimmune and inflammatory disorders, such as systemic lupus erythematosus and rheumatoid arthritis, also confer an increased risk of LEAD. Homocysteine, a non-protein amino acid, has also been shown to provide weak additive prognostic information in addition to the standard lipid measures. Several genetic factors have been identified as potential risk factors for atherosclerosis, though the evidence for their clinical significance remains weak [12].
3. Diagnostic approach
A comprehensive personal and family medical history should be taken and reviewed. Family history of coronary artery disease (CAD), cerebrovascular disease, aortic aneurysm, and LEAD should be reviewed. A thorough assessment of cardiovascular risk factors and co-morbidities, as well as a review of symptoms in relation to different vascular territories, should also be part of the clinical history. Systematic assessment of lifestyle, diet, walking, and physical activity is needed. Levels of physical activity should be assessed, and questionnaires and measures of functional status can provide reasonably accurate outcome measures. These can be useful in determining the level of impairment and in selecting appropriate care.
4. Diagnostic methods for LEAD
4.1 Ankle-brachial index (ABI)
ABI is a non-invasive tool that is useful in diagnosing and monitoring PAD. It is also a strong marker of generalized atherosclerosis and of the risk of cardiovascular disease. An ABI of 1.40 represents atherosclerosis and is associated with increased risk of cardiovascular events and mortality. It is more common in older patients and in particular in patients with diabetes or those with chronic kidney disease (CKD). When added to a risk score, the ABI can be used to improve the estimate of risk in one third and one fifth of women and men at “low risk”, respectively. It is a valid method for the assessment of cardiovascular risk in a variety of ethnic groups, independent of the risk factors. In contrast to the coronary calcium score and the measurement of the intima-media thickness of the carotid arteries, the ABI is inexpensive and relatively quick to perform. It is important that the test is performed by trained staff. In addition to assessing general cardiovascular risk, ABI measurement can identify a patient’s risk for events in the lower extremities, which requires close attention and education for the prevention of foot wounds [13].
4.2 Duplex ultrasounds (DUS)
For both screening and diagnostic purposes, duplex ultrasound (DUS) is often the first step in the vascular examination. To detect and localize vascular lesions and quantify their extent and severity using velocity criteria, DUS includes B-mode echography, pulsed wave, continuous wave, color, and power Doppler modalities (Figure 2). More recent techniques, such as flow imaging or live three-dimensional (3D) echography, as well as the use of ultrasonic contrast agents, are further improving the capabilities of DUS, although their use is still limited. DUS can be used to detect subclinical arterial disease, which is important for the assessment of CV risk. DUS is also effective for endovascular treatment because it can draw images in real time. However, the skill of the examiner greatly affects image rendition, making training important.
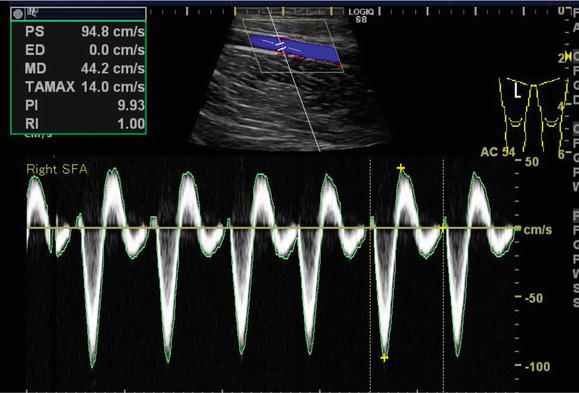
Figure 2.
Imaging of duplex ultrasound−courtesy of author.
4.3 Computed tomography angiography (CTA)
Multidetector computed tomography angiography (MDCTA) boasts a succinct examination duration while minimizing motion and respiration artifacts during the imaging of vessels and organs (Figure 3). The advantages of CT angiography (CTA) include its non-invasive acquisition, wide availability, high resolution, and ability to reformat images in three dimensions. Similar to digital subtraction angiography (DSA) and magnetic resonance angiography (MRA), CTA provides a complete “roadmap” of the vasculature, which is critical for determining interventional strategies, including the location and seriousness of the lesion, as well as the upstream and downstream status [14]. However, limitations of CTA include lack of functional and hemodynamic data, radiation exposure, and use of iodinated contrast, which should be limited in patients with chronic kidney disease (CKD) and used cautiously in allergic patients. Nephrotoxicity can be reduced by minimizing contrast agent volume and ensuring adequate hydration prior to and following imaging. The efficacy of acetylcysteine in limiting nephrotoxicity is uncertain. Recent studies have proposed that statins or sodium bicarbonate may prevent contrast agent-induced nephrotoxicity.
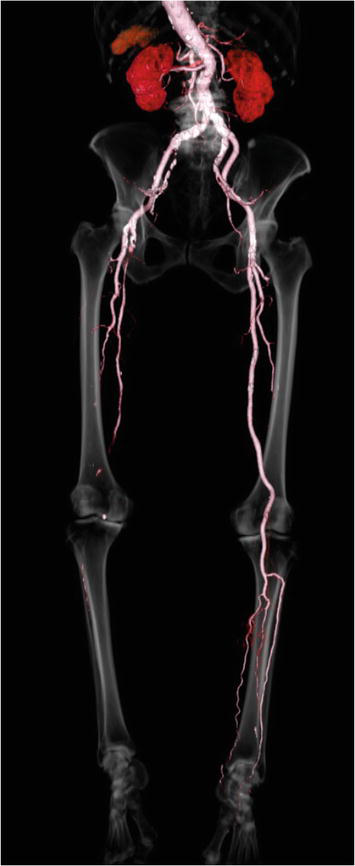
Figure 3.
Computed tomography angiography scan−courtesy of author.
4.4 Magnetic resonance angiography (MRA)
Magnetic resonance angiography (MRA) is a non-invasive, operator-independent imaging modality akin to CTA. High-fidelity three-dimensional (3D) reconstructions may be generated with high sensitivity and specificity of 86% and 93%, respectively, in comparison with conventional angiography. An advantage of this technique is the utilization of non-ionic contrast agents, such as gadolinium, which are considerably less nephrotoxic and 6–8 times less allergenic (1%). However, notable limitations of MRA include the prolonged examination duration, the potential for claustrophobia, contraindications in patients with pacemakers or other metallic implants, and the significant impact of motion artifacts. MRA scans may also be acquired without the administration of contrast agents, but this is associated with a decreased visualization of lesions and an increased number of artifacts (Figure 4).
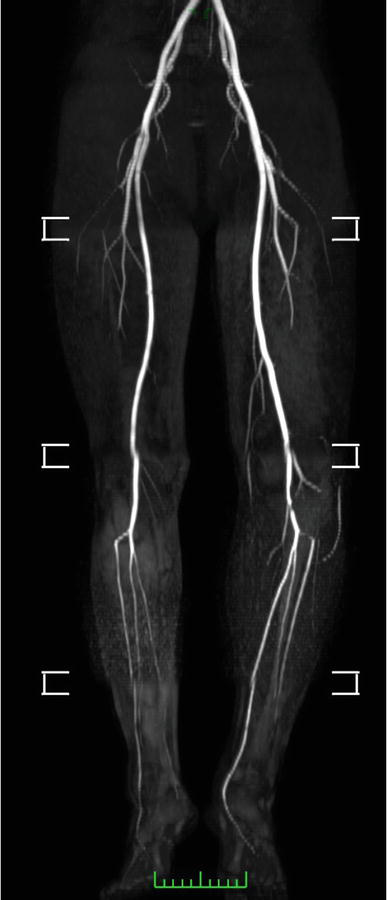
Figure 4.
Magnetic resonance angiography scan−courtesy of author.
4.5 Digital subtraction angiography (DSA)
Digital subtraction angiography (DSA) has long been considered the gold standard in vascular imaging (Figure 5). But its use has decreased because of its invasive nature and the possibility of serious, possibly lethal, complications. The incidence of iatrogenic complications such as hematoma, pseudoaneurysm, arteriovenous fistula, arterial thrombosis, and contrast media-related complications has been reported to be 0.7% with a mortality rate of 0.16% [5]. Despite these disadvantages, DSA remains a preferred imaging technique because it provides excellent resolution, particularly in small caliber arteries. Furthermore, it allows visualization of the collateral circulation and measurement of pressure gradients to assess the hemodynamic significance of the stenoses being studied.
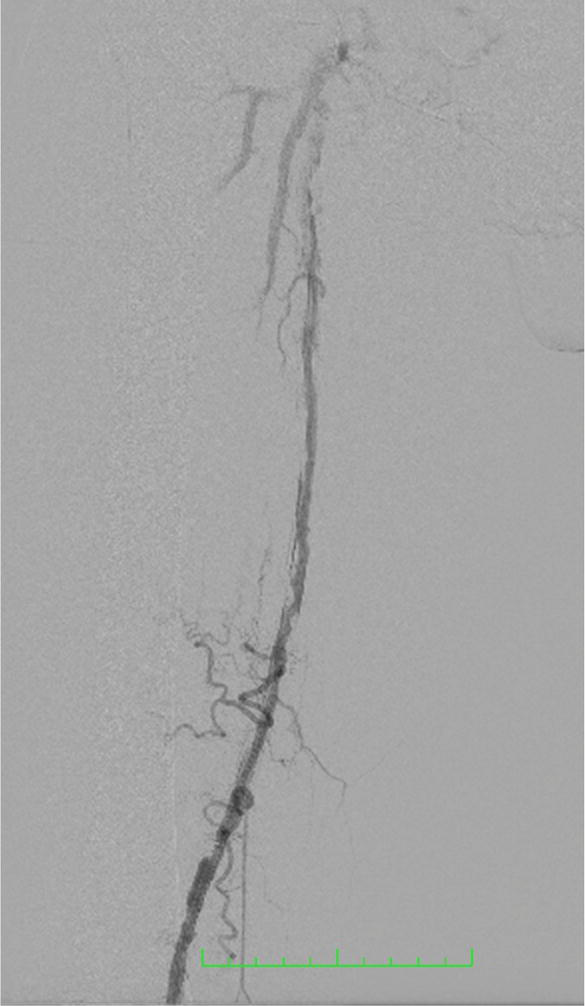
Figure 5.
Digital subtraction angiography−courtesy of author.
The advantage of subtractive angiography is that it combines the diagnostic evaluation with the therapeutic intervention. In patients with intermittent claudication, it should only be performed as part of a single interventional procedure.
5. Treatment approach
5.1 Smoking cessation
A body of research attests to the advantages of smoking cessation in reducing cardiovascular events and mortality, particularly in patients with cerebrovascular disease and peripheral arterial disease [15]. The 2016 European Society of Cardiology (ESC) guidelines on cardiovascular disease prevention extensively address the management and support of smoking cessation. Additionally, exposure to secondhand smoke should be evaluated and mitigated.
5.2 Lipid-lowering drugs
The same guidelines for lipid-lowering treatment apply to all individuals with PAD, including those undergoing medical therapy, those preparing for surgical or endovascular procedures, and those undergoing secondary prevention. Lipid-lowering treatment is essential for all patients with PAD because it extends life, reduces the risk of cardiovascular and cerebrovascular events, and decreases the need for endovascular revascularization and the incidence of amputation. Lipid profile analysis is recommended for every patient with PAD and should be repeated at least annually to assess achievement of LDL-C targets. Assessments at one-two months intervals are recommended if treatment needs to be modified and/or target levels are not achieved. LDL-C targets should be considered when evaluating the results of lipid-lowering treatment; once achieved, secondary therapeutic goals may be pursued by adding appropriate omega-3 fatty acids and/or fibrates to non-pharmacological management.
5.3 Antithrombotic therapy in lower extremity artery disease
Antiplatelet therapy is indicated in patients with any atherosclerotic lesion, including LEAD, where intermittent claudication is an indication for antiplatelet therapy. Both surgical and endovascular therapy require pre- and postoperative antiplatelet or anticoagulant therapy. Similar to the prevention of ischemic heart disease, ischemic events in the lower extremities are treated with similar pharmacologic therapies. Although guidelines describe the management of antiplatelet or anticoagulant therapy, a recent Voyager PAD publication demonstrated that anticoagulant therapy has no inferiority over aspirin alone. Depending on the patient’s condition, either single or dual antiplatelet therapy should be selected, and the patient should be treated according to guidelines, depending on the therapeutic situation.
5.4 Endovascular therapy (EVT)
Advances in endovascular therapy (EVT) have been remarkable, especially in devices. In the iliac artery region, bare metal stents are commonly implanted and have good long-term results. However, stent grafts are often used in highly calcified lesions or in areas with long occluded lesions, and results are comparable to bare metal stents [16]. Surgery is the first choice for occlusion or stenosis of the common femoral artery. EVT is recommended only for patients at high surgical risk, but the results are inferior to surgery [1, 17]. In the femoral below-the-knee artery region, the primary patency of drug-eluting stent was significantly higher (88%) than that of drug-coated stent in a non-inferiority trial between drug-eluting stent and drug-coated stent due to the advancement of drug technology [18]. The JET STREAM® and Diamond back 360® are debulking devices that directly ablate plaques and calcified lesions, while the intra- and intra-eluting stents are debulking devices that directly ablate plaques and calcified lesions. Shock wave intravascular lithotripsy is a device that directly ablates plaques and calcified lesions, while Shock wave intravascular lithotripsy can fully dilate calcified vessels with balloon dilation by applying shock waves from the balloon. In addition, covered stents and interwoven stents are also available, and both work well in TASC C and D lesions, with good patency rates.
In the area below the knee and ankle joint, balloon angioplasty is the main treatment, but coronary stent implantation may be effective in some cases. In CLTI, the amputation avoidance rate was 93% at 3 years with stenting of 10 cm [19]. In addition, below the knee artery and ankle joint, asymptomatic patients should not be treated, and intervention should be considered if the patient has a Rutherford classification (Table 1) of 4 or higher.
| Fontaine classification | Rutherford’s classification | |||
|---|---|---|---|---|
| Grade | Symptoms | Grade | Category | Symptoms |
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate or severe claudication | I | 2 | Moderate claudication |
| I | 3 | Severe claudication | ||
| III | Ischemic rest pain | II | 4 | Ischemic rest pain |
| IV | Ulceration or gangrene | III | 5 | Minor tissue loss |
| III | 6 | Major tissue loss | ||
Table 1.
The use of imaging modalities such as intravascular ultrasound (IVUS) and optical frequency domain imaging (OFDI) for peripheral vascular therapy is effective (Figures 6 and 7). Although angiography is generally used for treatment, IVUS allows detailed measurement of the reference vessel diameter and selection of a suitable stent or balloon size [20]. Compared to IVUS, OFDI provides more detailed observation of vessel characteristics, but unlike IVUS, it requires the removal of blood cells during imaging, making it difficult to position the device implantation site in real time.
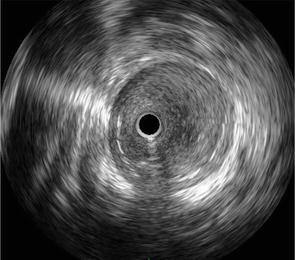
Figure 6.
Image of IVUS-courtesy of the author.
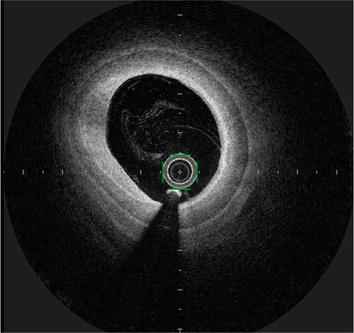
Figure 7.
Image of OFDI-courtesy of author.
The primary goal of endovascular or surgical intervention for LEAD is symptomatic improvement or wound healing.
6. Pathogenesis of LEAD
A primary factor in initiating the development of atherosclerotic lesions in the arterial system is vascular endothelial dysfunction. It is a complex and multifactorial process that results from natural changes in the function of the endothelium due to aging, as well as from adverse environmental factors and factors related to the metabolic status of the system. While there is no escaping the fact that the development of atherosclerosis is inevitable, it can be slowed down by appropriate lifestyle, diet, and medication choices. Increasing the antioxidant potential of endothelial cells is believed to delay aging, although the results of clinical studies are often contradictory. Elevated arginase I activity in endothelial cells accelerates their senescence by uncoupling endothelial nitric oxide (NO) synthase subunits with concomitant oxidative stress and inflammatory response. These processes were inhibited in the presence of N-acetylcysteine (NAC), a substrate of glutathione synthesis. Maintaining adequate endothelial NO production and its availability within the vascular bed is an important approach to slowing the development of atherosclerotic lesions. Another approach is to supplement with L-arginine, but also with L-citrulline, to slow down the aging of the endothelium, to inhibit oxidative stress, and to increase the production of NO. Administration of endothelial cell growth factors to stimulate new vessel formation or gene transfer to stimulate systemic production of these substances is another approach to treating or correcting atherosclerotic lesions. The applicability of autologous stem cells or endothelial progenitor cells for in vivo vascular reconstruction is also being investigated. Preliminary clinical trial results suggest the efficacy of these novel therapies. Preliminary clinical trial results indicate the effectiveness of these novel therapies in reducing pain and accelerating ulcer healing. In addition to these studies, research is underway to create biocompatible substrates that will facilitate the implantation and growth of stem cells to more rapidly initiate the formation of new blood vessels.
7. Microcirculation
The microcirculation constitutes the terminal network of the systemic circulation and encompasses micro vessels with diameters less than 20 μm, including arterioles, post-capillary venules, capillaries, and their associated cellular components. It represents the ultimate destination of the cardiovascular system, where the transfer of oxygen from the red blood cells in the capillaries to the parenchymal cells occurs, providing the necessary energy to sustain their functional activity. The microcirculation is also involved in the regulation of solute exchange between the intravascular and interstitial spaces, and facilitates the transport of hormones, nutrients, and immune cells to the tissue cells. As it is in direct contact with the parenchymal cells, the proper function of the microcirculation is vital for maintaining the viability of the organs and supporting their functions [21].
Endovascular therapy procedures aimed at reestablishing arterial perfusion to the targeted limb, such as percutaneous transluminal angioplasty, can significantly enhance transcutaneous oxygen tension in the revascularized distal limb. Patients who underwent amputation post-angioplasty exhibited no changes in transcutaneous oxygen tension, while those who displayed a progressive increase in the following weeks exhibited 100% limb salvage, implying that successful preservation of limb function necessitates the delivery of blood flow to the at-risk distal tissue. Microvascular dysfunction is characterized by a disturbance in the regulation of blood flow and vascular tone, leading to a decline in the delivery of oxygen to tissues, heightened oxidative stress, and a reduction in capillary density. Decreased microvascular density in calf muscle more accurately predicts lower limb functionality than the extent of atherosclerotic disease. The decline in microvascular density may serve as a common mechanism for limb dysfunction in various disease conditions that restrict leg function. The reduction of microvascular density in the skeletal muscle of the lower extremity also significantly contributes to the decline in exercise tolerance observed in patients with congestive heart failure [22].
In the coronary artery, coronary blood flow reserve (CFR) is an indication of the ability of the coronary circulation to deliver a maximal hyperemic blood flow and is indicative of impaired coronary microvascular function. Some patients with acute myocardial infarction (AMI) fail to achieve adequate tissue perfusion despite adequate epicardial blood flow after coronary intervention. Impaired CFR has been associated with microvascular injury resulting in increased morbidity and mortality [23]. A similar attempt was made in LEAD, where Fukunaga et al. performed a physiological assessment in all the patients before and after EVT. The subjects were Rutherford classification 5 patients with ulceration or gangrene in the territory of the infrapopliteal artery. Physiological measurements were performed in the infrapopliteal artery region to measure vascular flow reverse (VFR). The wound-healed group had significantly higher VFR after EVT, while the wound-healed group had significantly higher VFR after EVT. Skin perfusion pressure (SPP) is currently considered useful in the evaluation of microcirculation [24], and SPP > 40 mmHg is associated with wound healing. However, there was no difference in mean SPP values between the two groups, and patients in the nonhealing group could not dilate their microvascular resistant arteries during hyperemia [25].
Studies have posited the value of the angiosome concept in the context of bypass surgery and endovascular procedures aimed at limb salvage (Figure 8) [26, 27]. The angiosomes of the foot and ankle are demarcated by the three main supplying arteries, namely the anterior tibial artery, posterior tibial artery, and peroneal artery. The hypothesis of direct blood flow to the ulcer or gangrene being influenced by the angiosome concept was analyzed.
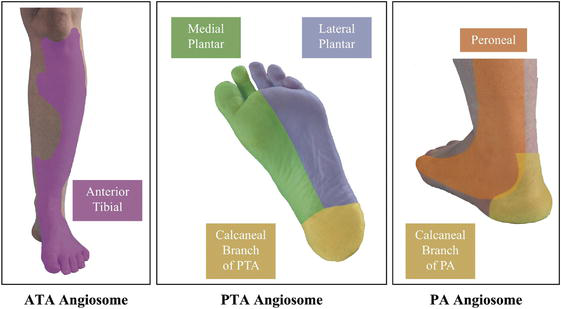
Figure 8.
Image of angiosome.
The microcirculatory status was assessed by angiography, utilizing the wound blush method, following endovascular therapy. A positive result was manifested by either the blushing of the tissue surrounding the wound or the opacification of vessels around the wound by contrast. The presence of a wound blush following endovascular procedures is indicative of an increase in skin perfusion pressure, which is in turn associated with a higher likelihood of limb salvage. The utilization of wound blush as an angiographic endpoint during endovascular procedures may be a novel predictor of limb preservation outcomes in patients with critical limb ischemia [28].
8. Conclusions
The primary goals of LEAD treatment are wound healing and amputation prevention. Vascular surgeons and endovascular interventionalists have focused on improving blood flow to the ischemic limb and have developed and refined a variety of treatment modalities. However, in rare cases, improving blood flow does not improve tissue perfusion, which is exacerbated by the intervention. In these cases, microcirculation, which is not allowed in DSA, must be considered. As mentioned above, blood vessels have endothelial function and are involved in a variety of chemical mediators and vasa vasorum, and in LEAD patients, inadequate angiogenesis and collateralization may be a mechanism that increases limb ischemia and leads to dysfunction. This may be a mechanism for causing functional impairment [29].
Microcirculation evaluation is not stated in the guidelines, but it clearly contributes to cutaneous wound healing and plays an important role. There is a need for further research.
References
- 1.
Aboyans V, Ricco JB, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). European Heart Journal. 2018; 39 (9):763-816. DOI: 10.1093/eurheartj/ehx095 - 2.
Diehm C, Schuster A, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: Cross-sectional study. Atherosclerosis. 2004; 172 (1):95-105. DOI: 10.1016/S0021-9150(03)00204-1 - 3.
Fowkes FGR, Aboyans V, et al. Peripheral artery disease: Epidemiology and global perspectives. Nature Reviews Cardiology. 2017; 14 (3):156-170. DOI: 10.1038/nrcardio.2016.179 - 4.
Jawień A, Filipiak KJ, et al. Polskie wytyczne postępowania w chorobie tętnic kończyn dolnych (LEAD) na podstawie wytycznych ESVS/ESC 2017 - stanowisko ekspertów polskiego towarzystwa chirurgii naczyniowej, polskiego towarzystwa nadciśnienia tętniczego oraz sekcji farmakoterapii sercowo-naczyniowej polskiego towarzystwa kardiologicznego. Acta Angiologica. 2019; 25 (4):1-53. DOI: 10.5603/AA.2019.0015 - 5.
Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). European Journal of Vascular Endovascular Surgery. 2007; 35 (1):S1-S75. DOI: 10.1016/j.ejvs.2006.09.024 - 6.
Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993; 88 (3):837-845. DOI: 10.1161/01.cir.88.3.837 - 7.
Meijer WT, Grobbee DE, Hunink MG, et al. Determinants of peripheral arterial disease in the elderly: The Rotterdam study. Archieves in Internal Medicine. 2000; 160 (19):2934-2938. DOI: 10.1001/archinte.160.19.2934 - 8.
Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for pe ripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of American College of Cardiology. 2006; 48 (6):1190-1197. DOI: 10.1016/j.jacc.2006.05.049 - 9.
Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circulatory Research. 2015; 116 (9):1509-1526. DOI: 10.1161/CIRCRESAHA.116.303849 - 10.
Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012; 308 (16):1660-1667. DOI: 10.1001/jama.2012.13415 - 11.
Jude EB, Oyibo SO, Chalmers N, et al. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care. 2001; 24 (8):1433-1437. DOI: 10.2337/diacare.24.8.1433 - 12.
Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001; 285 (19):2481-2485. DOI: 10.1001/jama.285.19.2481 - 13.
Rose S. Noninvasive vascular laboratory for evaluation of peripheral arterial occlusive disease: Part I —Clinical applications: Chronic, usually atherosclerotic, lower extremity ischemia. Journal of Vascular and Interventional Radiology. 2000; 11 (10):1257-1275. DOI: 10.1016/ s1051-0443(07)61300-1 - 14.
Ouwendijk R, Kock MC, van Dijk LC, et al. Vessel wall calcifications at multi-detector row CT angiography in patients with peripheral arterial disease: Effect on clinical utility and clinical predictors. Radiology. 2006; 241 (2):603-608. DOI: 10.1148/radiol.2412050781 - 15.
Lim SS, Vos T, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380 :2224-2260 - 16.
Piazza M, Squizzato F, et al. Editor’s choice—Outcomes of self expanding PTFE covered stent versus bare metal stent for chronic iliac artery occlusion in matched cohorts using propensity score modelling. European Journal of Vascular and Endovascular Surgery. 2017; 54 (2):177-185. DOI: 10.1016/j.ejvs.2017.03.019 - 17.
Nakama T et al. 1-year outcomes of thromboendarterectomy vs endovascular therapy for common femoral artery lesions. JACC: Cardiovascular Interventions. 2022; 15 (14):1453-1463. DOI: 10.1016/j.jcin.2022.03.010 - 18.
Gray WA, Keirse K, et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): A randomised, non-inferiority trial. The Lancet. 2018; 392 (10157):1541-1551. DOI: 10.1016/S0140-6736(18)32262-1 - 19.
Giaquinta A, Vincenzo A, et al. Everolimus-eluting stent for patients with critical limb ischemia and Infrapopliteal arterial occlusive disease. Vascular and Endovascular Surgery. 2017; 51 (2):60-66. DOI: 10.1177/1538574416689429 - 20.
Iida O, Takahara M, et al. Vessel diameter evaluated by intravascular ultrasound versus angiography. Journal of Endovascular Therapy. 2022; 29 (3):343-349. DOI: 10.1177/15266028211047946 - 21.
Guven G, Hilty MP, Ince C. Microcirculation: Physiology, pathophysiology, and clinical application. Blood Purification. 2020; 49 (1-2):143-150. DOI: 10.1159/000503775 - 22.
Martens RJH et al. Microvascular endothelial dysfunction is associated with albuminuria: The Maastricht study. Journal of Hypertension. 2018; 36 (5):1178-1187. DOI: 10.1097/HJH.0000000000001674 - 23.
Taqueti VR, di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. Journal of the American College of Cardiology. 2018; 72 (21):2625-2641. DOI: 10.1016/j.jacc.2018.09.042 - 24.
Castronuovo JJ, Adera HM, et al. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. Journal of Vascular Surgery. 1997; 26 (4):629-637. DOI: 10.1016/S0741-5214(97)70062-4 - 25.
Fukunaga M, Fujii K, et al. Vascular flow reserve immediately after infrapopliteal intervention as a predictor of wound healing in patients with foot tissue loss. Circulation: Cardiovascular Interventions. 2015; 8 (6). DOI: 10.1161/CIRCINTERVENTIONS.115.002412 - 26.
Attinger CE, Evans KK, Bulan E, et al. Angiosomes of the foot and ankle and clinical implications for limb salvage: Reconstruction, incisions, and revascularization. Plastic Reconstructive Surgery. 2006; 117 :261S-293S - 27.
Iida O, Soga Y, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. Journal of Vascular Surgery. 2012; 55 (2):363-370. DOI: 10.1016/j.jvs.2011.08.014 - 28.
Utsunomiya M, Nakamura M, et al. Impact of wound blush as an angiographic end point of endovascular therapy for patients with critical limb ischemia. Journal of Vascular Surgery. 2012; 55 (1):113-121. DOI: 10.1016/j.jvs.2011.08.001 - 29.
Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circulation Journal. 2017; 81 (3):281-289. DOI: 10.1253/circj.CJ-16-1286