The transfer functions for amine gas sweetening process.
Abstract
Model Predictive Control (MPC) is a widely used method that has numerous applications in process industries. In the MPC group of controllers, a clear process model is used directly for controlling, predicting future plant behavior, and calculating corrective control action required to maintain the output at the desired set point value. Most chemical processes exhibit inherent nonlinearities due to interactions among processes, disturbance, and set-point changes. MPC variants based on nonlinear process models have been a proven stiff control technique for process control along with improved handling of constraints, abnormal dynamics, and time delays. In addition to that, MPC is better in handling the nonlinearity and time-varying characteristics during run time by modifying model. The control of multi-input and multi-output reactive-separation process is difficult due to nonlinearity associated with the process and interactions of vapor-liquid equilibrium with chemical reactions. In order to obtain optimal performance, energy conservation, and cost-effectiveness in reactive-separation process, the application of optimal control technique is inevitable. This chapter addresses application of MPC and its benefits in reactive separation techniques, particularly in natural gas sweetening process. The recent application in MPC and its proven results for the above-mentioned reactive separation processes are discussed here.
Keywords
- reactive absorption
- conventional controller
- MPC
- performance specifications
- natural gas sweetening process
1. Introduction
Process Integration (PI) deals with the improvement of innovative equipment and techniques that fetches profound advancements in the field of chemical process and manufacturing industries. This technique facilitates to achieve reduction in the volume of equipment, consumption of energy, and decreasing waste that could result in safer, cheaper, and sustainable technologies. Reactive separation is a process that takes place of combination of reaction and separation in a single equipment unit (multifunction reactors). The reaction and separation combinedly take place in equipment without having any interdependence among them. The primary application of integrated processes has been focusing on the conservation of resources, prevention of pollution, and energy management. Process integration can be categorized into mass integration and energy integration [1].
Process integration and optimization are part of process systems engineering (PSE). Advanced process integration and optimization methods have been developed in the recent past. The integration has broad applications in chemical and other allied industries. Those applications in industries require best operating conditions that minimize the investment, environmental impact, operating cost, destruction of exergy.
Reactive separation processes are integrated processes that have a combination of reaction and separation into a single unit for simultaneous production and removal of products. It involves energy integration, mass integration, and optimization, which involves enhanced reaction rate, reducing energy, and reducing solvent need. Reactive distillation, reactive absorption, reactive adsorption, and reactive extraction are the well-known techniques involved in the reactive separation process. Owing to strong interactions of chemical reactions, heat, and mass transfer, the reactive separation process behaves in a complicated fashion. The reactive separation process is encouraging process integration technologies (PI) [2].
1.1 The need for operational control of reactive separation processes
The regular process operation and control of the reactive separation process are coupled with the regulation of variables. Every reactive separation process requires a tight control system to achieve product specifications despite a lack of information on design, material property changes, equipment degradation, and external disturbances. The deviation in process variables is compensated by deviations of actuated variables.
It is desired to isolate the disturbance at localized equipment/part of the process to avoid propagation of disturbances to other processes by using the measurement of the process and its decisive property. The integration of processes such as reactive separation demands counterbalancing effects of reaction kinetics and mass transfer. The efficiency of reactive separation process depends on the implementation of right processing parameters. When the buffer for control is removed, the deviations in process variables occur by losing the measurement information.
Variations in process variables are available for controlling the process. The case of merging of reaction and separation in a single column enables the information of external flows, temperature, and concentration along with the internal temperature. The process information and manipulated variables get reduced but the control task becomes difficult. The manipulation of variables is concerned with degrees of freedom (DOF). The constricted operation degrees of freedom are likely to have an influencing effect on other process variables and independent control loops become insufficient to handle the process. Integrated processes exhibit immoderate critical process parameters as compared to the separated process that exhibits maximum concentration or conversion within the operating region. Thus, the immoderate process parameters change the sign of process gains and manifest the design of the controller more difficult.
Even though the integrated processes have the advantages of minimal equipment and minimal energy consumption, it is confronted by process control challenges. These challenges could be overcome by the implementation of complex and better-tuned regular control structures or nonlinear model-based controllers. As shown in recent studies on reactive separation processes. The industrial case studies and experiences show that process nonlinearity makes the control problems difficult and deviations result in loss of process efficiency. Better results are obtained by implementing model predictive control as compared to conventional proportional-integral-derivative (PID) controller. The model predictive control can handle the process interactions, disturbance rejection, and set-point tracking suitable for stability and robust performance in presence of process noise [3, 4].
2. Model predictive control (MPC)
Model predictive control utilizes a process model to predict future behavior while satisfying constraints in the process. Predictive technique calculates deviations from the reference. The algorithm works on repeated optimization of mathematical model of the process. The system model is used for calculating future system behavior that determines the optimal trajectory of manipulated variable (Figure 1) [5].
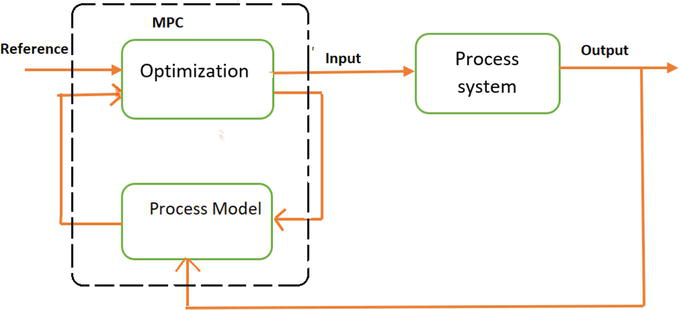
Figure 1.
A MPC-based control loop.
The cost index is minimized by MPC minimizes by tracking the error between reference and output
MPC has a diverse range of techniques encompassing dynamic matrix control(DMC), generalized predictive control (GPC), and receding horizon control (RHC). These techniques are based on their control sequence implementations and constraint and model formulation. MPCs are widely implemented in Petroleum refineries and petrochemical plants.
As MPC technique is a proven control technique that can handle the state and input constraints, interaction, and measured disturbances. Modern process control techniques rely on the application of dynamic models. Intensive processes exhibit specific and dynamic characteristics. The intensified processes are directed toward optimization and model-based control [6].
This chapter explains recent advancements happened in MPC applicable to reactive separation processes and presents a case study on the implementation of Linear MPC in the natural gas sweetening process.
2.1 Significant role of MPC and its previous proven benefits in reactive separation processes
The demerits associated with conventional PID controller invoke the need for advanced controllers such as model predictive controller, non-linear model predictive controller (NLMPC). In case of reactive distillation which has a multiplicity of steady states, vapor-liquid equilibrium interaction, and a higher degree of nonlinearity, demand application of Nonlinear model predictive control and multi-parametric MPC. The employment of NMPC in batch esterification process has been considered to be suitable where ethanol and acetic acid are used as reactants to produce ethyl acetate and water. The application of nonlinear-based MPC produced stringent control of the system. The NMPC algorithm was useful for the input for providing the desired amount of distillate [7].
In case of an ideal reactive distillation column that has two ideal reactants and two products, the performance of MPC was analyzed for various scenarios. It was observed that MPC performed well in set point tracking and regulatory control. In addition to that, MPC presented a smooth variation in manipulated variables that yielded minimized energy requirement for control [8].
Optimal control based on design configuration was attained inspite of the presence of range of measured disturbances [4]. In this study, MTBE distillation column used in the study had time invariant design variables such as column diameter, catalyst distribution, number of trays, feed tray and time-variant design variables such as feed flow rate, molar flow rate, liquid methanol feed flow rate.
Application of MPC strategies for reactive distillation column used for benzene hydrogenation that was utilized in gPROMS [9]. In the study, the performance of Several MPCs was analyzed for different sets of manipulated and controlled variables analyzed by Vishal Mahindrakar and Juergen Hahn by keeping the remaining variable in feedback control. It was observed that all MPCs exhibited better responses than the PI feedback control scheme. In the case of feed benzene concentration disturbances, Single input-Single output (SISO) performed better than Multi input- Multi output (MIMO). MPC inputs with significant disturbances exhibited a reduction in an upset condition.
The implementation of ANN also quite useful in process applications. The capability of ANN to predict future behavior is quite useful to model various complicated physics-based processes. The computational cost is also less in the case of ANN model. For example in the case of a reactor where the kinetics information is not available, data-based modeling is quite useful. MPC coupled with the ANN model can estimate the behavior of nonlinear system accurately under operating variable change and disturbance. In the case of application of a neural network model predictive control (NNMPC) was developed for depropanizer column, the NNMPC strategy predicted the output correctly which was confirmed by the low MSE index. This meant the simulation and predicted data were very small [10].
Najim and Ruiz [11] investigated the concentration of CO2 in a gas mixture reduced to a specified value using Diethanolamine (DEA) solvent. Long-range adaptive control was achieved by using model parameters developed from the least square algorithm. The primary aim of the study was to achieve horizon-control policy by attaining minimization of quadratic criterion function composed of the input and output tracking errors in feedback. Desired performance was accomplished by the implementation of the adaptive control strategy. High sensitive absorption column efficiency was attained in this study [11].
Optimal control of amine plant using non-dominated sorting genetic algorithm-II was investigated by Behroozsarand and Shafiei [12]. In this study, a multi-objective genetic algorithm concept in concurrence with PID controller was adopted to control amine plant. The tuning of the PID controller was executed through the non-dominated sorting genetic algorithm (NSGA-II). The result showed that NSGA-II-based tuning provided optimal control of plant.
3. Control of reactive separation process: a case study with natural gas sweetening absorption process
Natural gas is one of the promising energy resources that emit fewer carbon-di-oxide (CO2) emissions as compared to other fossil fuels such as coal and oil. The contribution of natural gas to the world energy basket is significant. The consumption of natural gas has been on positive growth. It is used as a fuel in power plants, feedstock for production of ammonia & urea, fuel in furnaces and heating applications, for heating spaces and water for cooking, non-polluting fuel, and the raw material for variety of chemical products. Various forms of NG, such as LNG, RLNG, and CNG, are used in market. The natural gas processing units deal with the production of sales specified natural gas requirement by upgrading sub-quality natural gas from reservoir.
The natural gas from the reservoir contains significant quantities of impurities such as carbon-di-oxide (CO2), hydrogen sulfide (H2S), mercaptans, etc. These contaminants produce various problems as follows: In the presence of water, these contaminants can form acids that corrode equipment and pipelines. The presence of CO2 in natural gas reduces the calorific value of gas. Hence, the contaminants CO2 and H2S removal is essential to meet market specifications and environmental compliances. A typical consumer gas should have H2S content of less than 4 ppmv (parts per million by volume) and CO2 content of 1% by volume. Figure 2 represents the scope of control applications in natural gas sweetening process.
Figure 2.
Scope of process control application in natural gas sweetening.
3.1 Reactive absorption process description
The natural gas sweetening process is a reactive absorption process where a liquid solvent methyldiethanolamine (MDEA), is used to contact the gaseous phase to capture the contaminants/acid gases (H2S and CO2). The process is carried out at a counter-current absorber through which the gas flows upwards and liquid solvent flows downwards. Generally, the acid gases H2S and CO2 are captured by solvent through column. The tray in the tower provides an essential contact area between gas and liquid. The residence time of reaction is governed by the flow rates of streams. Removal of any acid gases by amine washing results in the liberation of heat (heat of absorption). The diagram of acid gas absorber is given in Figure 3 [13].
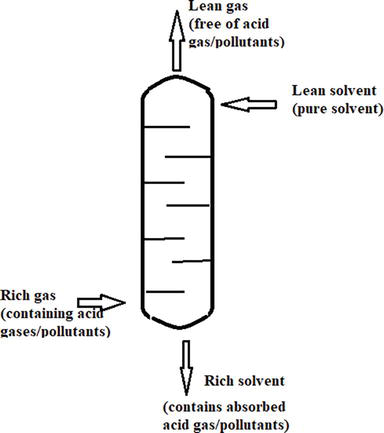
Figure 3.
A typical reactive absorption column.
3.2 Identification of natural gas sweetening absorption process
The sensitivity studies reveals that input and output variables are involved in the amine absorption process. The transfer function of the amine absorber was developed by introducing step changes on input variables. Step responses of variables (y1 and y2) have been obtained using the reaction curve technique [14].
The acid gas removal efficiency can be influenced by the flow rate of solvent. The fluctuations in the flow rate of natural gas occur because of production disturbances at upstream level and acid gas content also is changed naturally depending on the reservoir conditions. The concentration of H2S content in the outlet is influenced by MDEA flow. MDEA flow rate influences the dynamics of H2S and CO2 concentration in the absorber.
The typical controlled variables in the reactive absorption process are concentration and throughput of sweet gas/lean gas compositions are controlled variables. Flow rate and concentration of solvent are manipulated variables. The disturbances are feed flow rate and mole fractions in the feed gas. The control strategies for the reactive absorption process have one of the following techniques: Proportional, Integral, Derivative (PID) (or)model-based (Model predictive control) (or) intelligence heuristics. The following figure presents input and output variables involved in the natural gas sweetening process (Figures 4 and 5).
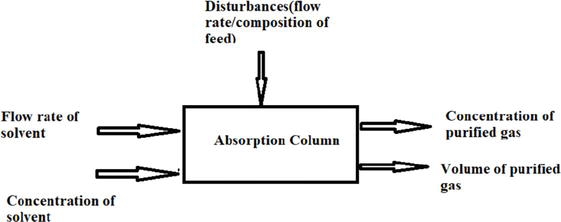
Figure 4.
Reactive absorption process input and output variables.
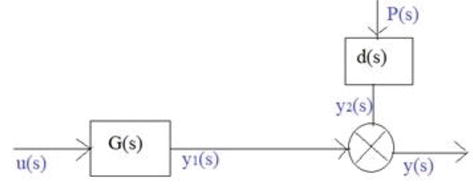
Figure 5.
Representation of amine absorber in terms of input-output variables.
The absorber output H2S and CO2 content can be expressed according to (Arteaga & Contreras 1998) [15].
u(s) = manipulated variables/input variables.
y(s) = output (H2S and CO2 contents in moles or ppm).
G(s) = input–output transfer function y(1).
P(s) = disturbance variable natural gas input flow.
D(s) = disturbance output y1(s) transfer function.
Y1(s) = y1(s) for P(s) = constant,
Y2(s) = y2(s) for U(s) = constant,
3.3 Control of natural gas sweetening process
The control strategy for the natural gas sweetening process depends on avoiding any undesirable effects during plant operations and obtaining satisfactory performance. The frequent changes in natural gas load disturb the process as well as the feed fluctuations cause transient behavior. The control system should ensure plant to run at steady conditions to achieve desirable outputs with optimal inputs though the presence of significant uncertainty about plant behavior and disturbances. The removal of H2S is emphasized in natural gas sweetening process and control strategy is devised based on the H2S removal. An appropriately designed controller does ensure the solvent flow concentration and handling fluctuations in load disturbance (Table 1) [12].
| S. no. | Description | Transfer function of FOPDT model |
|---|---|---|
| 1 | Transfer function relating the flow rate of solvent to concentration of acid gases (G11) | |
| 2 | Transfer function relating flow rate of solvent to throughput of sweet gas (G12) | |
| 3 | Transfer function relating the concentration of solvent to throughput of sweet gas (G22) | |
| 4 | Transfer function of the concentration of MDEA solvent to concentration of sweet gas (G21) | |
| 5 | Transfer function to flow rate of natural gas to throughput of sweet gas (d12) |
Table 1.
A typical natural gas sweetening absorption column system has following controls: a flow controller performs the action of regulating the water makeup by monitoring the water amount going out of the top of the stripper; pressure controller, acts on the gas stream exiting the top of the columns; a flow controller regulates the water/solvent recycle stream from the stripper to the absorber; a level controller is employed to govern the bottom holdup of the absorber [16].
In this chapter, an IMC-PID controller and model predictive controller are used for controlling the absorption column. The performance of controllers is presented under various operating scenarios such as solvent flow rate fluctuations, solvent concentration fluctuations, as well as disturbance in gas flow rate. The dynamics of the process are studied under the inducement of a step change of ±10% to solvent flow rate, solvent concentration, and natural gas flow rate.
The manipulated variables are the MDEA(solvent) solution flow rate and MDEA solution concentration. The disturbances are the natural gas feed flow rate and the compositions of acid gases in incoming feed. The design parameters are the number of trays in the absorption column and the number of trays in the regeneration column. The H2S removal rate, energy efficiency, and stability of plant are affected by above factors. Sweet gas compositions in the exit and throughput of sweet gas coming out of absorption tower are controlled variables.
The important variable in sweetening process is outlet H2S concentration. The removal efficiency of H2S is used to measure the performance of the control system. The removal efficiency is defined as the ratio of inlet to outlet H2S concentration. A good control system guarantees the removal of H2S by more than 90%
The control loops for the sweetening of natural gas are either PID, model-based, or intelligent heuristics. On account of their simplicity, credibility, and implementation PID controller has wide applications in industry.
Two different control strategies have been proposed for the control of natural gas sweetening columns. A 2 × 2 First Order Plus Dead Time (FOPDT) model is used for control purposes. The models are obtained through linearization around nominal operating conditions.
The PID control scheme and MPC control scheme have been investigated under flexible operating conditions concerning regulatory requirements and load changes (disturbance) in the presence of economical, environmental, and operational constraints. The control strategies performances are evaluated based on their efficiency in acid gas capturing capability, that is, the ability to reach the set point quickly without error.
3.3.1 Proportional integral derivative controller (PID) design
PID controllers are used to control the process with time delay The PID controllers are widely adopted due to their simple structure and easy implementation and maintenance. PID controller is applied when noisy signals are not observed and the dynamic response is essential. The requirement of sweetening process is to Maintain the H2S concentration less than 2.5 moles/m3 and limiting the absorption of CO2 subject to the restriction of control limit. The control objective of natural gas sweetening process is to maintain the H2S concentration actual value (y1(t)) close to desired value (y*)
The control problem of the sweetening process can be described as follows. The control action is subject to variation in the domain [u1minimum,u1maximum] & [u2minimum,u2 maximum] & where the domain represents input manipulated variable flow rate of solvent and concentration of solvent are limited.
The general PID has the following structure
where Kc is the proportional gain, τI is the integral time, and τd is the derivative time. PID controller for absorber is designed by considering the following methodology from the literature.
The operation of the process at an unstable steady state leads to open-loop unstable systems. Due to the presence of unstable poles, the unstable process is difficult to reach stability. Because of the presence of negative or positive zeros, These systems exhibit overshoot or inverse response. The instability can be overcome by incorporating the model of the system in the controller design. This equivalent design has the robustness and it is quite promising for stabilizing higher-order systems too. A PID controller for pure integrating plus a time delay system was proposed by [17]. The controller used in the method exhibited robust performance.
The industrial time delay process transfer function model possesses one pole at origin because of their nonself regulating nature. Various methods have been proposed to tune PID controllers such as the Internal Model Control (IMC) method, direct synthesis method, empirical method, two degrees of freedom method, stability analysis method, and optimization method [18].
3.3.2 PID controller synthesis
A decentralized control system with a decoupling matrix can be designed by combining a diagonal controller Kd(s) with a block compensator D(s) and the variable u1 is manipulated by the controller in case of 2 × 2 system. This type of controller configuration provides n completely independent processes or minimal interaction processes. The purpose of decoupling is to impose calculations that cancel the existing process interactions that will provide single-loop independent control [19].
The individual loop controllers are placed diagonally in the design of decentralized control. Even though the system still exhibits interactions, the interactions are minimized. Though various types of decoupling methods are available, linear decoupling has been adopted in this work. In this, the decoupling matrices have
Figure 6.
The control loop diagram for the natural gas sweetening process.
The following elements are obtained for a 2 × 2 system
The decoupling matrix can be implemented by steady-state decoupling technique. To obtain SISO response, the transfer function of the closed loop is obtained after eliminating interactions in the decentralized structure. The transfer function of closed loop is written as follows: The controller matrix is:
3.3.3 MPC controller synthesis for amine sweetening process
The model predictive control for amine sweetening process is represented in state space form as follows:
where
x(k + 1) is the state vector at instant “k + 1”
x (k)is the state vector at instant “k”
u(k) is the input vector at instant “k”
Y(k) is the output vector at instant “k”
Δu(k) is the change in input state vector “k”
The state space matrices of gas sweetening are given by:
Input delays (seconds): [6 3]
The cost function used with MPC, which penalizes the tracking error as well as the change in manipulated variable is defined in
This cost function is minimized to obtain optimal values of inputs as
4. Results and discussions
The section discusses the implementation of PID and MPC control for the natural gas sweetening process and compares the performance of PID and MPC in natural gas sweetening applications.
The effects of manipulating variables such as MDEA solution flow rate and MDEA concentration variation on output variables are presented. The specification of the sweet gas concentration in the outlet gas is fixed by operational goals and must be kept within 0.5% of its set point at a steady state.
4.1 PID controller responses
A decentralized or multi-loop controller of GC11 and GC22 as components has been designed and implemented for the natural gas sweetening process. The closed loop response of acid gases (H2S and CO2) concentration to change or adjustment of MDEA flow rate is shown in Figure 7a and b. The closed-loop response of the concentration on account of the adjustment of MDEA concentration is shown in Figure.
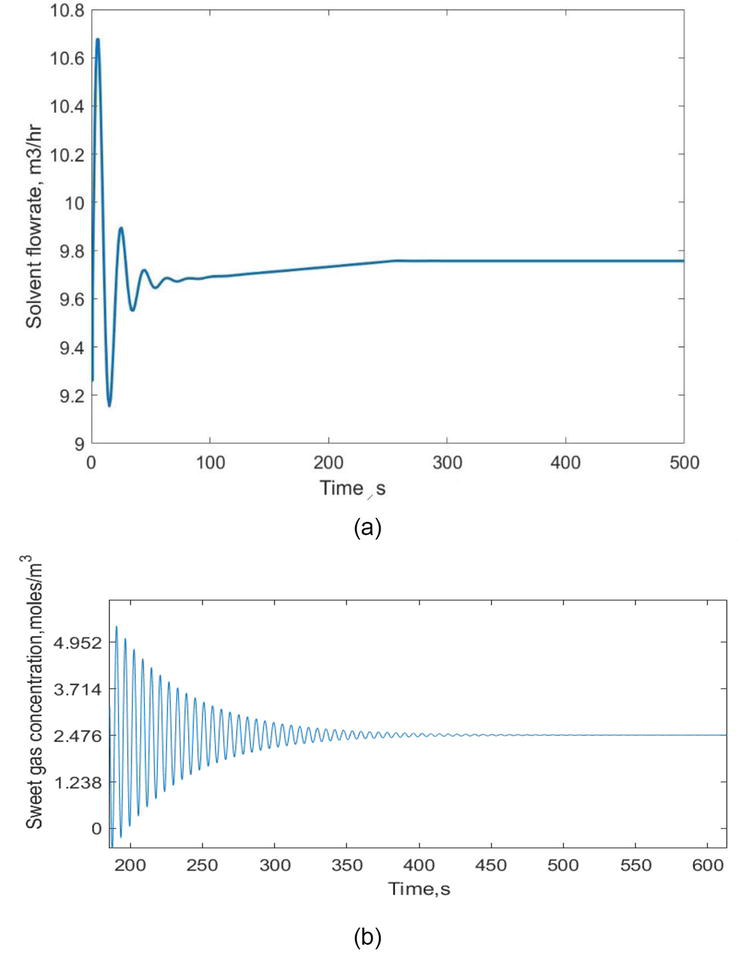
Figure 7.
(a) The solvent flow rate (manipulated variable) response and (b) the sweet gas concentration response using PID controller.
4.1.1 PID controller response (y11)
The acid gas concentration with respect to time using PID controller was obtained and given in Figure 6. The response of change in concentration with respect to a longer time span is given in Figure 7a, to visualize the changes occur in shorter time span Figure 7b can be seen. It is observed from Figure 7b, the concentration change in acid gases is initially oscillatory. The oscillations are gradually vanished with respect to time and reaches the steady value of 2.476 moles/m3 of acid gas after a period of 500 s.
4.1.2 PID controller response (y22)
In Figure 8a and b, the solvent concentration response and the controller’s action on throughput of sweet gas have been given respectively. The throughput of sweet gas has evolved very slowly and takes a longer time duration to achieve steady values. The response has reached the steady value of 8.13 m3/hr. after approximately 2.77 hours.
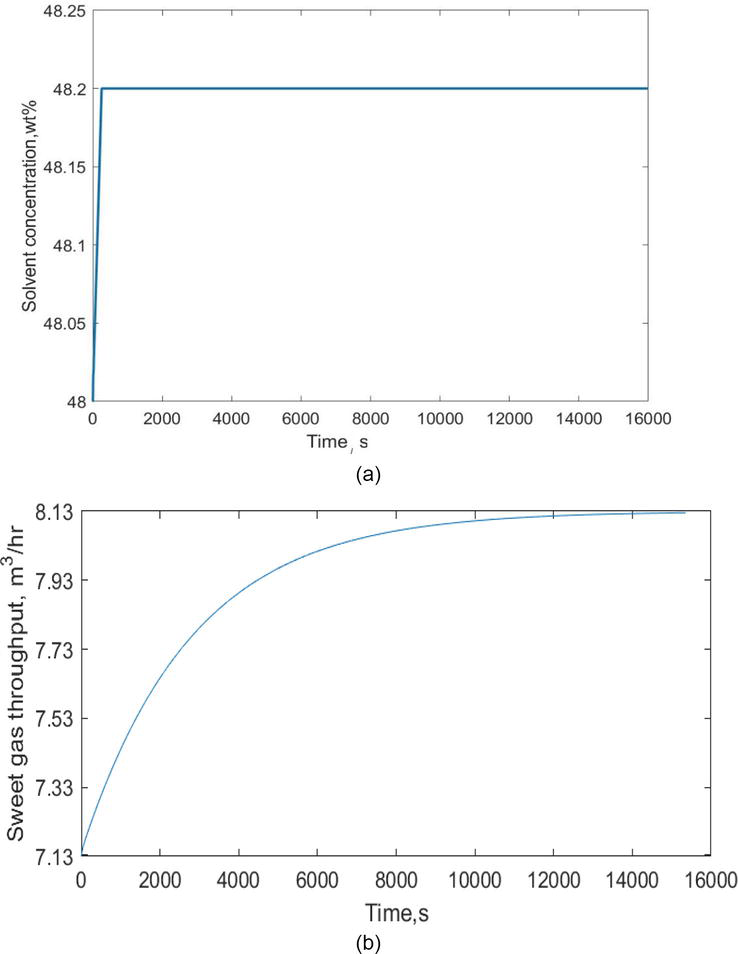
Figure 8.
(a) The solvent concentration(manipulated variable) response (b) The response of sweet gas throughput using PID controller.
4.2 Model predictive control implementation
A model predictive control has been designed for natural gas sweetening absorber by considering scenarios of plant operations. The MPC controller has been assessed based on the capability of disturbance rejection and tracking about setpoint. The removal of acid gases (primarily focus on H2S removal followed by CO2) rate depends on the qualitative concentration of the amine solution.
The basic purpose of control strategy is to maintain acid gas concentration at 2.5 moles/m3. The acid gas concentration is measured as output variable y1 and throughput is measured as y2. The following section discusses the various scenarios of plant operations controlled by MPC and its performance assessment in the handling of the plant under changes in input variables.
4.2.1 Setpoint tracking of the controller during closed loop response
The control objective is to maintain the output variables at the setpoint. The controlled variable is the concentration of acid gases (H2S and CO2). The scenario (conditions under normal plant operation) was simulated for constant solvent flow rate and constant solvent concentration.
It is observed from Figure 9b the MPC controller ensures the concentration of H2S and CO2 to reach the desired set point. In the case of throughput, sweet gas going out of absorption tower has been increased. The steady-state values have been attained after 35 minutes.
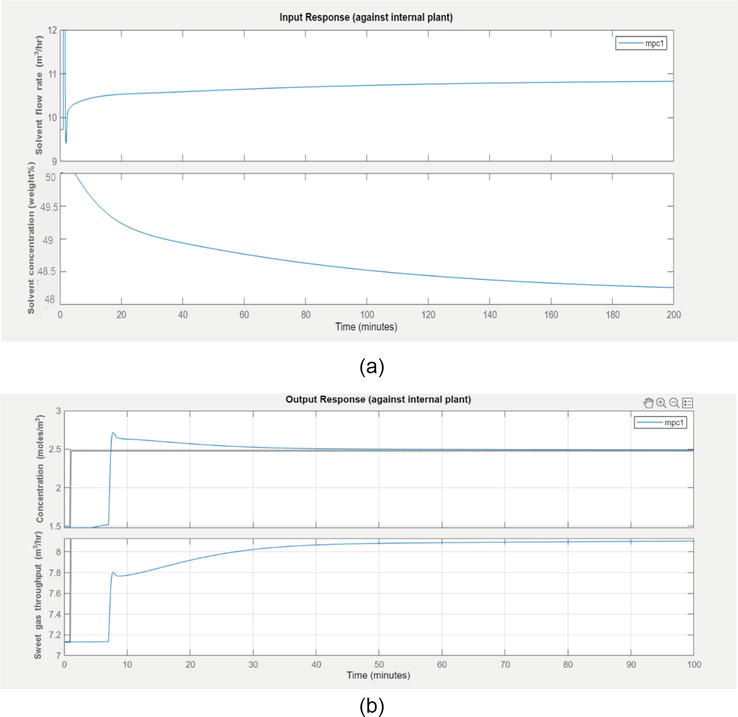
Figure 9.
(a) The movement of inputs under closed loop response (b) Set point tracking responses of sweet gas concentration (y1) and sweet gas throughput change (y2) under closed-loop response.
4.2.2 Set point tracking in closed loop response during step change in solvent flow rate
The following scenario has been done for manipulated step input change in solvent flow rate. The movements of manipulated variables are shown in Figure 10:
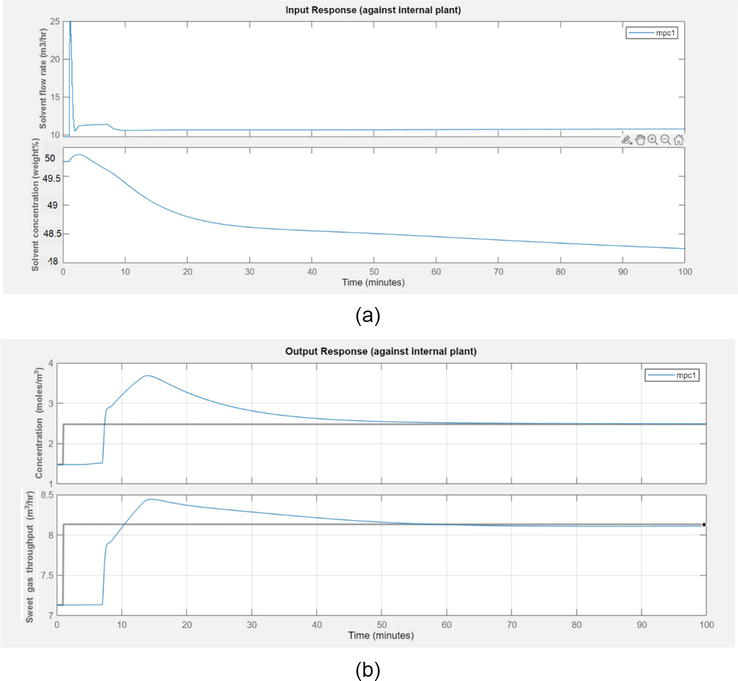
Figure 10.
(a) The input response of step change in solvent flow rate (b) Response of sweet gas concentration and sweet gas throughput due to step input change in solvent flow rate.
As we can observe from Figure 10b the controlled variable (sweet gas) response. This MPC simulation produced the concentration of H2S and CO2 to reach below its desired set point, during step change in solvent flow rate. After dead time, the concentration initially rises quickly and after 40 min, steady values have appeared. The throughput has been increased and reached a steady state value after 35 min. The change in throughput has appeared by the interaction effect with solvent flow rate.
4.2.3 Set point tracking of concentration in the aggressive mode of controller
The following section is presented with aggressive response of MPC for the concentration control of acid gases.
We can observe from Figure 11b the controlled variable sweet gases concentration has stayed below 4.633 moles/m3 for a step change in solvent flow rate. Initially, the concentration-response shot up within a shorter time duration and smoothly settled down after 40 min. The throughput also reached steady value after 35 min.
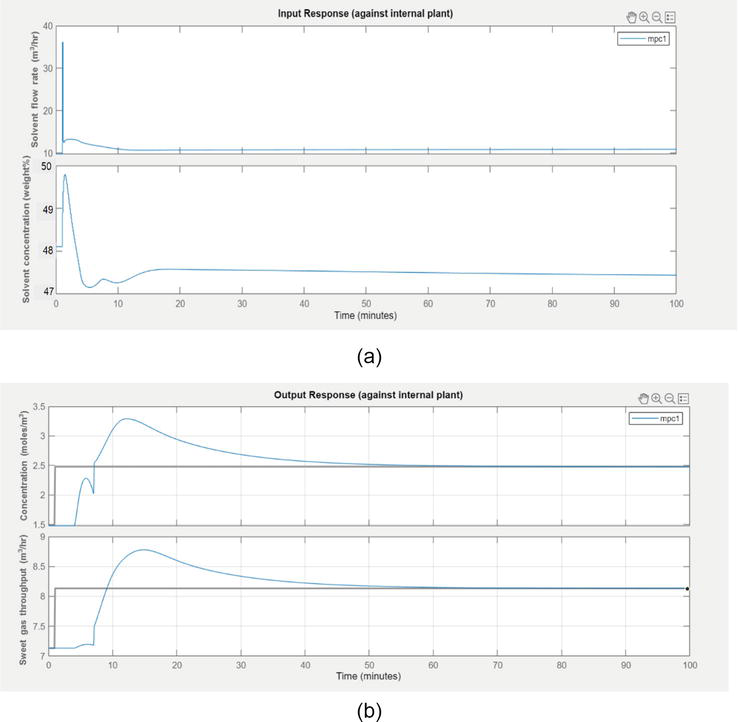
Figure 11.
(a) The movement of input variables during aggressive performance (b) The response in acid gases concentration and throughput during aggressive performance.
4.2.4 The response of output variables during load disturbance in closed-loop response
The scenario was analyzed for load disturbance (varying natural gas flow rate). The response of input variables is given in Figure 11. The solvent flow rate and concentration were adjusted according to variations in load.
When there is load disturbance, the handling of output concentration and throughput is given in the Figure 12. The concentration stayed at 2.5 moles/m3 and the throughput stayed at 7.15 m3/h. It can be observed from Figure that the MPC handles the load disturbance effectively and ensures the targeted set point in both output variables. Despite variation in load, the output response shows initial overshoots after longer duration.
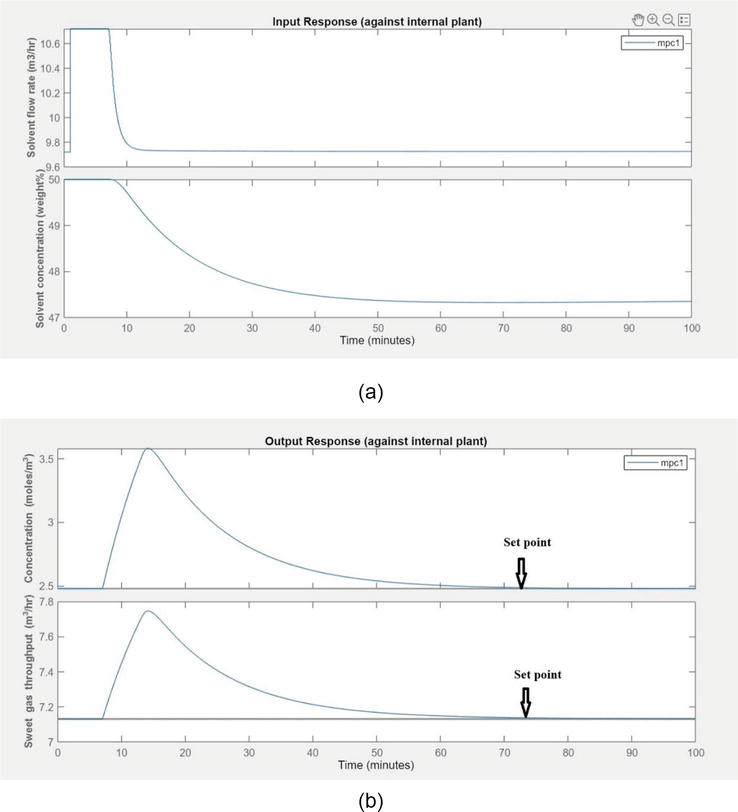
Figure 12.
(a) The input movements by the controller during load disturbances (b) The output response by controller during load disturbance.
4.2.5 The response of output variables in aggressive mode during load disturbance
The response of output during load disturbance is given in Figure 13. The acid gas concentration has reached the desired specification. The set point has reached by 25 min. The concentration steady values have taken place for 25 min. The throughput settling time is less than the concentration settling time as compared to settling of concentration. When load disturbance occurred, the sweet gas going out of the absorption tower increased marginally.
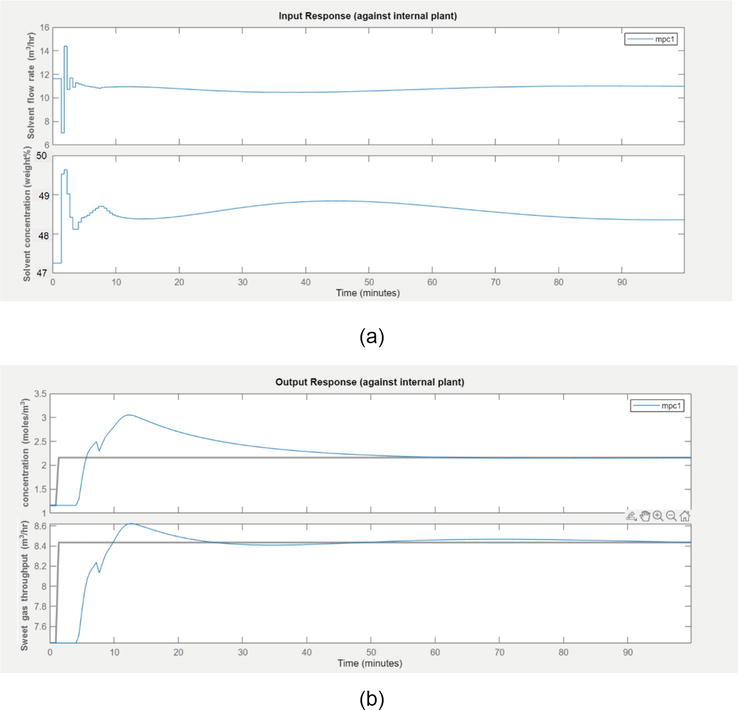
Figure 13.
(a) The input movements by the controller during aggressive mode (b) the response of output concentration and throughput during aggressive mode.
4.3 Performance assessment of controllers
The performance of controllers can be measured by set point tracking and disturbance rejection. The performance of MPC and PID have been assessed by Integral Absolute Error (IAE) criteria which measures the integral of absolute value of deviation from setpoints. The IAE values are tabulated in Table 2
| Type of controller | IAE values | |
|---|---|---|
| Concentration of sweet gas | Throughput of sweet gas | |
| PID closed loop | 3.49 | 3.23 |
| MPC closed loop | 1.69 | 1.47 |
| MPC aggressive | 0.91 | 0.80 |
Table 2.
IAE values of controllers.
The major objective of the chapter is to understand the controller’s handling ability in the efficient removal of sour gases during changes in manipulated variables and load disturbances. The closed-loop response were studied using decentralized PID controller and closed-loop response, robust response, and aggressive response were analyzed by MPC. The IAE values of MPC aggressive in Table 2 reveal that MPC aggressive mode efficiently handles the changes and has less error in maintaining the set point of concentration of sweet gas and throughput of sweet gas
4.3.1 PID controller response on sweet gas concentration
The PID controller responses revealed that the acid gas concentration showed an oscillatory behavior during the initial duration and the oscillation decayed quickly. The time to reach set point is very less in the case of PID but from the IAE value, it is observed that the error is higher.
4.3.2 PID controller response on throughput of sweet gas
The PID controller responses revealed that the acid gas throughput of acid gas evolved very slowly and took a long time to reach steady state. When the concentration of solvent is reduced, the throughput of acid gas increases. The IAE value of throughput is higher than MPC.
4.3.3 MPC controller closed loop response on sweet gas concentration and throughput
The MPC controller responses revealed that the acid gas concentration reached the set point without much error. Even though the attaining of steady values has longer duration, the IAE value in closed loop response is one among the less values as compared to other MPC response IAE values. In case of throughput, the closed loop response is effective and the IAE value is less.
4.3.4 MPC controller aggressive response on sweet gas concentration and throughput
The MPC controller responses revealed that the acid gas concentration reached the set point very quickly without much error. Even though the steady values took place very faster, the IAE value in closed loop response is less comparatively than PID and MPC response. In the case of throughput, the aggressive response is very effective. The aggressive response is very much faster and more efficient than all responses.
5. Conclusions
MPC can handle multi-input multi-output (MIMO) systems that have interactions. Because of these interactions, it is often challenging to design MIMO systems using traditional controller PID. Nevertheless, MPC concurrently controls all the outputs while taking into account input-output interactions. As far as reactive absorption is concerned, MPC has been proven for tracking setpoints even though the presence of disturbances along with handling constraints. MPC satisfies process intensification objects along with energy, mass, and environmental constraints. The MPC applications in post-carbon capture have proved that Integral square error in case of step change in flue gas flow rate of MPC is less than PI controller whereas the percentage carbon capture setpoint tracking is lower in case of MPC. A huge amount of heat duty has been saved by implementing an MPC controller that PI controller. The reboiler heat duty is significantly less than the PI controller. In case of settling time for reaching steady-state values, The MPC has taken less time to reach the setpoint quickly [20, 21].
The application of MPC in post-carbon capture (PCC) has been promising. In addition to that, MPC approach has exhibited the best performance compared to conventional PID control in the natural gas sweetening process. The MPC had performed well even under disturbance by avoiding large overshoots in case of step increment in solvent flow rate and acid gas composition. MPC allows the lean solvent flow rate to attain new operating points faster than the PID controller with no fluctuations. MPC has less computational speed when it has to calculate multiple trains. MPC has dynamic adaptability in maintaining different trains to achieve different individual acid gas rates to reach the overall acid gas rate. MPC has less integral square error for overall acid gases capture control on account of step increase/decrease in the manipulated variable as well as load changes. PID scheme has sluggish performance as compared to MPC controller. PID controller took a longer time and the responses are oscillatory to reach the desired set point. Whereas the MPC controller can handle the changes in manipulated variables along with satisfying constraints and can reach a set point within shorter time horizon. In all different cases of the MPC scheme, the trade-off observed is H2S concentration reaches nil value and concentration reduction of CO2 is compromised even though it is reduced to some extent.
Acknowledgments
To prepare a chapter, the authors gratefully acknowledge the support of A.C. Tech and CSIR-CLRI.
References
- 1.
Florez-Orregoa D, Shivom S, Seyed N. Editorial of the research topic:Integration and optimization in the chemical process industry. Frontiers in Chemical Engineering. 82 - 2.
Nikačević NM, Huesman AEM, Van den Hof PMJ, Stankiewicz AI. Opportunities and challenges for process control in process intensification. Chemical Engineering and Processing: Process Intensification. 2012; 52 :1-15 - 3.
Engell S, Fernholz G. Control of a reactive separation process. Chemical Engineering and Processing: Process Intensification. 2003; 42 (3):201-210 - 4.
Tian Y, Pappas I, Burnak B, Katz J, Pistikopoulos EN. Simultaneous design & control of a reactive distillation system–a parametric optimization & control approach. Chemical Engineering Science. 2021; 230 :116232 - 5.
Seborg DE, Edgar TF, Mellichamp DA, Doyle FJ III. Process Dynamics and Control. United States of America: John Wiley & Sons; 2016 - 6.
Eaton JW, Rawlings JB. Model-predictive control of chemical processes. Chemical Engineering Science. 1992; 47 (4):705-720 - 7.
Balasubramhanya LS, Doyle FJ III. Nonlinear model-based control of a batch reactive distillation column. Journal of Process Control. 2000; 10 (2–3):209-218 - 8.
Seban L, Kirubakaran V, Roy BK, Radhakrishnan TK. GOBF-ARMA based model predictive control for an ideal reactive distillation column. Ecotoxicology and Environmental Safety. 2015; 2015 :110-115 - 9.
Mahindrakar V, Hahn J. Model predictive control of reactive distillation for benzene hydrogenation. Control Engineering Practice. 2016; 52 :103-113 - 10.
Shin Y, Smith R, Hwang S. Development of model predictive control system using an artificial neural network: A case study with a distillation column. Journal of Cleaner Production. 2020; 277 :124124 - 11.
Najim K, Ruiz V. Long-range predictive control of an absorption packed column. Applied Mathematical Modelling. 1995; 19 (1):39-45 - 12.
Behroozsarand A, Shafiei S. Optimal control of amine plant using non-dominated sorting genetic algorithm-II. Journal of Natural Gas Science and Engineering. 2010; 2 (6):284-292 - 13.
Karthigaiselvan K, Panda RC. Dynamic modeling and solubility studies of sour gases during sweetening process of natural gas. Journal of Natural Gas Science and Engineering. 2021; 95 :104087 - 14.
Rake H. Step response and frequency response methods. IFAC Proceedings Volumes. 1979; 12 (8):519-526 - 15.
Arteaga FJ, Contreras JR. System Identification and model predictive control for the optimization of a gas sweetening process. In: IEEE International Symposium on Industrial Electronics. Proceedings. ISIE’98 (Cat. No. 98TH8357). IEEE; 1998 - 16.
Sahraei MH, Ricardez-Sandoval LA. Simultaneous design and control of the MEA absorption process of a CO2 capture plant. Energy Procedia. Elsevier. 2014; 63 :1601-1607 - 17.
Panda RC. Synthesis of PID controller for unstable and integrating processes. Chemical Engineering Science. 2009; 64 (12):2807-2816 - 18.
Rao A, Seshagiri VSR, Rao, and M. Chidambaram. Direct synthesis-based controller design for integrating processes with time delay. Journal of the Franklin Institute. 2009; 346 (1):38-56 - 19.
Deshpande PB, editor. Multivariable Process Control. USA: Instrument Society of America; 1989 - 20.
Patron GD, Ricardez-Sandoval L. A robust nonlinear model predictive controller for a post-combustion CO2 capture absorber unit. Fuel. Elsevier. 2020; 265 :116932 - 21.
Zhang Q, Turton R, Bhattacharyya D. Development of model and model-predictive control of an MEA-based postcombustion CO2 capture process. Industrial & Engineering Chemistry Research. 2016; 55 (5):1292-1308