Abstract
The lymphatic system is the immune system’s transport network (lymphatic vessels and lymph) that collects microbial antigens at the entrance and delivers them to the lymph nodes, where specific immune responses are stimulated. The lymphatic system maintains peripheral tolerance under normal conditions and rapidly develops protective immunity against foreign antigens after stimulation. Available evidence indicates that lymphatic function can be altered in various disease states such as cancer, infectious diseases, and autoimmunity. Many pathological conditions induce lymphangiogenesis, which is thought to provide an extensive lymphatic network that allows antigens and fluids to have greater access to the lymphatics. However, the role of lymphangiogenesis and lymphatic dysfunction in immune regulation is unclear. Understanding the causes of lymphatic dysfunction in pathological diseases will help develop new therapeutic approaches targeting the lymphatic system in various diseases. This chapter summarizes current knowledge about how lymphatic function is altered in autoimmune conditions, cancer, and infectious diseases, and how it modulates the immune response.
Keywords
- lymphatic function
- immune regulation
- cancer
- autoimmunity
- infection
1. Introduction
One component of the circulatory system is the lymphatic system, which plays an important role in both immunological function and the drainage of excess extracellular fluid. The lymphatic system is also considered part of the circulatory and immune systems. The role of the lymphatic system is to regulate the body’s fluid balance and filter pathogens from the blood, complementing the functions of the bloodstream. A complex network of lymphatic vessels connecting the local tissue site with secondary lymphatic organs such as lymph nodes, spleen, and mucosal lymphoid tissues constitutes the peripheral lymphatic system [1]. The peripheral lymphatic system is the main route for leukocyte transfer and antigen presentation. However, there is a broader view of how the lymphatic system influences immune responses beyond the physical connection between peripheral tissues and secondary lymphoid organs.
The lymphatic vessels, which provide structural and functional support for the distribution of antigens and antigen-presenting cells to drain lymph nodes, are known to be involved in an immune response. The lymphatic vasculature plays a critical role in maintaining peripheral tolerance or generating a protective immune response to infection or vaccination [2].
The immune system is regulated at several levels, both actively and passively. The active mechanism for regulating the immune response in the lymphatic system is to regulate the entry and migration of immune cells, expression of cytokines, chemokines, and adhesion molecules by lymphatic endothelial cells (LECs) [3]. LECs are specialized subsets of the endothelium of lymphatic vessels in tissues and lymph nodes that are essential for maintaining vascular integrity and proper lymphatic function. LECs in lymphatic vessels and lymph nodes provide a highly efficient pathway for initiating immune responses. LECs can recruit immune cells such as B cells, T cells, and dendritic cells (DCs) to the lymph nodes through the secretion of various chemokines and play a role in antigen presentation or exchange. Recruitment of immune cells is beneficial for the coordination of expansion and contraction of LECs and lymph nodes [3]. Recent studies have shown that cell surface molecules like PDL1 and interferon receptors are essential for the coordination of LEC division and death. During homeostasis, lymphatic endothelial cells play a role in immune regulation and tolerance induction through upregulation of PD-L1 expression and the absence of costimulatory molecules. In addition, after infection with or vaccination of viruses, LECs may be capable of obtaining, presenting, and exchanging foreign antigens [4]. However, in chronic disease, significant phenotypic changes in lymphatic endothelium due to transcription factor gene alterations have been reported [5]. There seems to be a need for more research into inflammation causing changes in lymphatic junctions, which are thought to influence immunological cell trafficking and the resolution of tissue inflammation.
Lymphoedema is the most obvious example of lymphatic and immune dysfunction interacting. During the progression of lymphoedema, the fluid build-up and congestion lead to several tissue changes, such as fibrosis and chronic inflammation. These changes, combined with the inability to deliver antigen and antigen-presenting cells to the lymph node, lead to a progressive decline in local immune function [6, 7, 8].
Under normal conditions, the lymphatic system is involved in controlling inflammatory responses and in maintaining tolerance. Not surprisingly, lymphatic dysfunction is associated with inflammation, cancer development and metastasis, infectious diseases, and sepsis, as the lymphatic system plays an important role in many physiological processes [9]. Many serious complications and abnormalities in the lymphatic system have recently been shown to be associated with changes in lymphatic transport function and lymphoid regulation of immune responses [6]. A wide range of diseases results in lymphangiogenesis, which can lead to an enlarged lymphatic network, allowing greater access for antigens and fluids into the lymphatic vessels. During inflammation and the progression of cancer, the growth of lymphatic vessels is often observed as lymphangiogenesis in the lymphatic capillaries and also in the lymph nodes. An enlarged lymphatic network appears to provide a greater surface area for fluid or cell entry into the lymphatic vessels [10, 11]. In any disease state, lymphatic function is also altered. However, the role of lymphangiogenesis and altered lymphatic function in regulating the immune system remains to be elucidated. The authors focus on cancer, autoimmunity, and infectious diseases in the review due to the following motivations:
Disease prevalence: Cancer, autoimmunity, and infectious diseases are significant health concerns globally, affecting a large number of individuals.
Impact on lymphatic system: These diseases have been observed to induce alterations in lymphatic function, including lymphangiogenesis and lymphatic dysfunction.
Immune response modulation: Understanding how the lymphatic system is affected in these diseases can provide insights into how it modulates the immune response, which is crucial for developing effective therapeutic approaches.
Clinical relevance: By studying the lymphatic system’s role in cancer, autoimmunity, and infectious diseases, researchers can potentially identify new targets for therapeutic interventions and improve patient outcomes.
Overall, the focus on these specific diseases allows for a comprehensive understanding of how lymphatic function is altered and its implications for immune regulation in various pathological conditions.
2. Immunological function of the lymphatic system in pathological conditions
The lymphatic system may be affected by a variety of conditions. Some occur during development or early childhood. Other diseases or injuries have caused the development of others [12]. Some of the most common problems in the lymphatic system include infections, blockage, and cancer [13]. The question of how lymphoid function or dysfunction contributes to the disruption of immunological homeostasis has been a long-standing topic in the field due to their diverse roles in regulating leukocyte trafficking and function. Here is a review of the immunological role of the lymphatic system in pathological conditions including cancer, autoimmunity, and infectious disease (Figure 1).
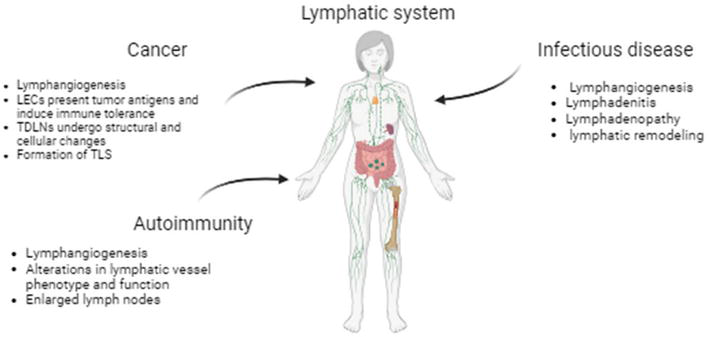
Figure 1.
A schematic diagram summarizing the effects of various pathological conditions on the lymphatic system. LECs: Lymphatic endothelial cells. TDLNs: Tumor-draining lymph nodes. TLS: Tertiary lymphoid structures.
2.1 Cancer
The tumor microenvironment is a complex and dynamic network composed of cellular and noncellular components. Cancer-associated fibroblasts and infiltrating immune cells constitute tumor stroma and are critical components of the tumor microenvironment. Through various cytokines, chemokines, growth factors, and the release of extracellular matrix (ECM) proteins, the cellular components of the tumor microenvironment form a complex crosstalk with the tumor [14].
In many types of tumors, the inflamed microenvironment, in combination with biochemical factors such as low oxygen concentration, contributes to tumorigenesis, angiogenesis, and lymphangiogenesis. These factors cause immune cell dysregulation through different mechanisms including apoptosis of cytotoxic T cells and activation of suppressor T cells [15, 16, 17]. Cancer-induced lymphangiogenesis contributes to an expanded lymphatic network that increases the delivery of molecular and cellular components to the draining lymph node. Before tumor seeding, lymphangiogenesis of the lymph nodes (LNs) is established and supports the initial regional metastatic progression. Indeed, lymphangiogenesis has been correlated with lymph node involvement and a poor prognosis in patients with cancer [11].
Immune tolerance to primary tumors may be the cause of poor prognosis in patients with lymph node metastases. However, the nature of these effects is not well defined. Peripheral tolerance may occur due to chronic inflammation mediators caused by cancer, tumor proliferation factors, or tumor antigens from apoptotic tumor cells. In addition, lymphatic endothelial cells can present tumor antigens and induce immune tolerance. Several studies have reported the immunomodulatory effect of the lymphatic endothelium in the tumor microenvironment [18]. The release of a number of suppressive cytokines and chemokines and the expression of inhibitory molecules by lymphatic endothelium create an immunosuppressive microenvironment that leads to the inhibition of dendritic cell maturation and the induction of T-cell tolerance, further suppressing anti-tumor immunity and facilitating tumor escape from the immune system, followed by its growth and metastasis [19, 20]. It is well established that lymphatic endothelial cells are competent to present antigens and have the ability to induce tolerance of tumor-specific T cells. In human and murine models of melanoma and breast cancer, LECs have been shown to present tumor-associated antigens (TAAs) to CD4+ and CD8+ T cells and induce their suppression. LECs are also able to induce reduced CD86 expression on dendritic cells [21, 22, 23]. A study using melanoma and colon cancer cell lines has shown that IFN-γ expression in tumor-specific CD8+ T cells induces PD-L1 expression in lymphatic endothelial cells [19]. This leads to functional impairment of T cells in tumor cell lysis. These findings, together with the observation that LECs increase the suppressive function of regulatory T cells [20], lead to the conclusion that LECs play an immunosuppressive role in the breast cancer microenvironment.
As lymphangiogenesis or immune modulation by lymphatic vessels appears to occur both locally and, in the tumor-draining lymph nodes, it is currently not possible to determine the relative importance of a process occurring at either site for tumor progression. The first sites where tumor-specific immune cells respond to tumor antigens are the draining lymph nodes. This occurs in the early stages of most cancers [24]. Sentinel LNs have attracted more attention because they are the first LNs to drain the tumor bed and are therefore expected to be the first site of tumor metastasis [25]. The presence of tumor cells in lymph nodes is considered evidence of the escape of the tumor from immune surveillance. Lymph nodes are specialized structures for the development of either cell-mediated or humoral immune responses [26].
The existence of tumor-specific effector T cells and immunosuppressive cells has been shown in tumor-draining lymph nodes [27]. The delicate balance between effector and regulatory T cells is suggested to be important in determining the outcome of immune responses to tumors [28]. On the other hand, in mouse models of cancer, B cells accounted for one-third of the lymphocyte population in tumor-draining lymph nodes [29], suggesting that B cells have crucial roles in the formation and/or modulation of anti-tumor immunity.
The three steps of immune surveillance – elimination, equilibrium, and escape – also take place in tumor draining lymph nodes (TDLNs) with LN involvement, which typically represents the escape route for the tumor [30]. Therefore, TDLNs are strategic for both anti-tumor responses and tumor metastasis. An important and still unanswered question is how an arsenal of immune cells can be so overwhelmed by tumor cells that they are eventually completely overpowered by them and become a major pathway for their spread. By analyzing the cellular composition and immune responses at different stages of cancer progression, researchers have sought to answer this question. Indeed, they found that when exposed to tumor products or tumor invasion, TDLNs undergo structural and cellular changes, usually favoring further tumor progression [31, 32, 33, 34]. According to this, investigation of the immune status of TDLN may help us understand the mechanism of immunosuppression associated with cancer patients. Several researchers studied the “immunomorphology” of draining lymph nodes of tumors by using hematoxylin and eosin staining and their association with disease parameters and outcomes [35, 36]. For example, head and neck cancer patients who have lymphocytic predominance in the draining lymph nodes have a lower risk of metastatic disease, whereas those who have germinal center predominance have a higher risk of metastatic disease [36, 37].
In addition, flow cytometry data showed a significant decrease in CD8+ T cell frequency in lymph nodes from head and neck cancer patients compared with control LNs [38]. The mechanisms responsible for this change may be complicated; however, the immunosuppressive effects mediated by tumor-associated factors, cytokines, and selective depletion of CD8+ T cells as a result of chronic antigenic stimulation might be important [38, 39]. In fact, suppression of the immune responses within TDLNs is a critical step for nodal invasion [27, 40]. It was shown that immunosuppressive events occur in regional LNs in melanoma even before nodal metastasis [41]. A comparison of the frequency of CD8+ T cells in TDLNs of melanoma patients with dormant and infectious inflamed LNs revealed that CD8+ T cells are decreased in both metastatic and nonmetastatic LNs. Furthermore, no difference was found in the expression of CD4, CD8, CD14, CD40, CD86, CD123, HLA-DR, and IL-10 in metastatic and nonmetastatic LNs of melanoma patients [41]. A similar observation was reported in breast cancer; CD4+ and CD8+ T cells were significantly decreased in sentinel (SLNs) and axillary (ALNs) lymph nodes of breast cancer patients compared to controls. A decrease in CD1a DCs in SLNs was also reported. The interesting finding was that the frequency of CD4+ T cells was reduced even in nonmetastatic ALNs, further supporting the idea that changes in the immune profile of TDLNs are dynamic and may precede nodal involvement. Another finding of the study was the association between the percentage of CD4+ T cells and DCs in ALNs and patients’ disease-free survival, independent of nodal involvement [42].
In situations of chronic inflammation, organized lymphoid structures containing DCs, T cells, and B cells are formed in nonlymphoid tissues, called tertiary lymphoid structures (TLS), similar to those seen in secondary lymphoid tissues [43]. The function of these TLS is probably to provide primary and local defense against microbes, and their constituent cells will disappear once the pathogen has been eliminated. TLS is seen in autoimmune and infectious diseases, graft rejection, and cancer at the site of inflammation. However, TLS has been shown to be a feature of autoimmune disease, where the large number of adaptive immune cells in these structures can exacerbate autoimmune disease [44]. On the other hand, TLS may play a role in cancer progression in the context of chronic inflammation, but it has been suggested that adaptive anti-tumor immune responses can be generated in TLS [45, 46]. In nonsmall cell lung cancer (NSCLC), TLSs are found in tumor tissues and are composed of mature DCs and T cells which are located adjacent to follicles containing GC B cells and FDCs. DC density in these TLS was reported to be a positive prognostic indicator in NSCLC patients [47]. TLSs are reported to be present in tumor tissues of breast cancer and the presence of Tfh cells in TLSs has been associated with better disease outcome. However, a recent study showed that breast cancer tissues usually contained TLS, but the presence of TLS was associated with high grade and aggressive form of the tumor [48].
Lymphangiogenesis process could be targeted by monoclonal antibodies (mAbs) including bevacizumab (anti-VEGF antibody), cediranib (anti-VEGFR antibody) alone, or in combination with kinase inhibitors. However, some clinical trials of anti-lymphangiogenic therapy for solid tumors failed to show an advantage in these patients [49]. In addition to lymphangiogenesis inhibitors, other approaches have been proposed in an attempt to target the lymphatic system. Given that TDLNs play a key role in generating an anti-tumor immune response and have also been shown to be important in suppressing tumor immunity, the most effective treatments are those that aim to tip the balance toward more effective T-cell responses. Further in vitro and in vivo studies are needed to determine whether immunotherapeutic approaches such as tumor-specific T-cell activation, elimination of immunosuppressive pathways, or a combination of these approaches have a blocking effect on tumor growth and metastasis.
Some strategies target tumor or tumor-draining LNs to circumvent tumor-induced immune suppression. Immunostimulatory strategies such as targeting CD40, Toll-like receptor (TLR) ligands, CTLA-4, PD-1 or using inflammatory or pro-inflammatory cytokines (IL-12, TNF-α, IFN-α or IL-2) have been used systemically in experimental models and clinical trials [50, 51]. Preclinical studies and clinical trials have shown that most of these therapies can enhance innate and/or adaptive immunity against tumors [52]. However, systemic approaches have several side effects, including severe toxicity due to systemic activation of the immune system [53].
2.2 Autoimmunity
The lymphatic system has not been the subject of much research about autoimmune diseases. The lymphatic system is a network of low-pressure vessels that provide a pathway for intercellular fluid to return to the blood vessel network. There is a network of lymphatic vessels throughout the body. In addition to returning intercellular fluid to the circulatory system, the lymphatic system also performs important immune functions in the body to keep tissues healthy and functioning properly [2]. The lymphatic vasculature is more conducive to immune induction and tolerance than the blood vasculature in peripheral tissues, which draws leukocytes from contaminated sites for effector functions. The lymphatic vessels are considered an important element of the immune system, not only in maintaining tissue fluid homeostasis but also in transporting antigens from the periphery to the lymph nodes, where lymphocytes are activated, expanded and ultimately transported to the site of inflammation or infection. In addition to the transport of lymphoid and immune cells, the lymphoid system is directly involved in the regulation of the immune system and the induction of tolerance to self-antigens [2, 54].
The ability of LECs to influence the activity of immune cells through a variety of mechanisms has been clearly demonstrated in recent studies. LECs secrete a wide range of cytokines to regulate the immune system. LEC-derived TGF-β has been reported to be a suppressor of dendritic cell maturation [55]. Increased IL-7 production by LECs can lead to the expansion of regulatory T cells, enhancing their immunoregulatory function [56]. LECs direct lymphocytes and DCs to infiltrate or exit the lymph nodes, while inflammation increases their ability to recruit cells. Through high levels of PD-L1 expression and their lack of costimulatory molecules, LEC also ensures that CD8 T cells are able to tolerate peripheral tissue antigens. Understanding how T-cell fate is influenced by other inhibitory molecules expressed in LEC will be extremely interesting. In addition, knowing whether LEC is able to induce CD4 T-cell tolerance or serves as a reservoir of peripheral tissue antigen in DC for presentation will provide more certainty about the general immunoregulatory role of LEC [57].
Alterations in the lymphatic system have also been observed in most autoimmune diseases, particularly in terms of lymphatic vessel phenotype and patterns of denovo lymphangiogenesis. For instance, in psoriasis, an autoimmune and inflammatory skin condition, many changes in the peripheral lymphatic system have been shown to play an important role in causing the disease to develop [58]. Interestingly, lymphatic vessels have shown the ability to modulate their expression of key immune mediators in response to most cytokines involved in the pathogenesis of psoriasis, including IL-27 or TNF-α [59, 60].
In the case of rheumatic autoimmune diseases, there is some evidence to suggest that lymphatic dysfunction may be a contributing factor in the development of these diseases [61]. This is due to the role of the lymphatic system in the immune system. One of the most studied autoimmune diseases regarding the role of the lymphatic system is rheumatoid arthritis (RA). RA is a chronic inflammatory disease. It causes pain, swelling, and stiffness (reduced flexibility) in the joints. It is a type of arthritis that occurs when the body’s immune system mistakenly attacks our joints, destroying and inflaming them [62]. The local lymphatic system is said to undergo two stages of change in association with the general inflammation of rheumatoid arthritis. In response to early rheumatoid arthritis or synovitis, lymphoid tissues undergo an “enlargement” phase, which increases their capacity to remove cellular debris and inflammatory cells from the site of infection, either through lymphangiogenesis or through increased vascular contraction frequency. During this expansion phase, in addition to changes in the lymphatic vessels, the draining lymph nodes themselves also expand characterized by a high infiltration of IgM+ CD23+ CD21hiCD1dhi B cells [63, 64]. This enlarged lymph node in the popliteal area may be a useful marker for the detection of arthritis in the early stages of disease activity. Based on RA model studies, an acute arthritis flare in the early stages of the disease has been observed, with increased lymphatic drainage from inflamed joints to enlarged draining lymph nodes. After a prolonged period of expansion, a stochastic event leads to the asymmetric collapse of LNs and lymphatics. In the collapsed phase, the local lymphatic system collapses, causing a loss of lymphatic flow and a reduction in lymphatic clearance. Coordinated with the collapse, B cells migrate from the follicle into the sinuses. B cell depletion therapy reduces arthritis flares by eliminating these B cells and improving passive lymphatic drainage from inflamed joints [65, 66].
Lupus is a type of autoimmune disease in which the body’s immune system attacks its tissues and organs. These attacks cause inflammation, swelling, and damage to different parts of the body, such as the joints, skin, kidneys, blood, heart, and lungs [67]. It is not well understood how the lymphatic system is related to lupus. However, the lymphatic vessels may expand and contract more easily than normal when an attempt is made to remove inflammatory cells in lupus, just as they do in other autoimmune diseases such as rheumatoid arthritis or scleroderma. The lymph nodes also enlarge due to the accumulation and filtration of lymph, resulting in the formation of inflammatory cells and fluid. This can lead to an increase in joint swelling and pain as the fluid volume builds up [68, 69]. Although the nodes do not always become enlarged, they can become swollen during periods of high disease activity or lupus flares. There have been cases of lymph leakage from the lymphatic system into the abdomen and upper spine in people with lupus. Lymphatic blockage or other abnormalities in the flow of lymph fluid may be the cause [68, 69, 70]. More needs to be known about what is going on here. The data show that lymphatic dysfunction is a factor contributing to photosensitivity in murine lupus and can even be alleviated by improving lymphatic flow with manual lymphatic drainage (MLD) [68]. The causes of lymphoedema in murine lupus and the mechanisms for reduced photosensitivity through improved lymphatic drainage will be investigated in future studies. Altering the lymphatic system may be a novel target for new drugs if similar immune circuit defects occur in patients with SLE.
Scleroderma is a chronic autoimmune disease that causes thickening and hardening of the skin, scarring, and damage to internal organs such as the heart and blood vessels, lungs, stomach, and kidneys. Scleroderma is caused by the overproduction and accumulation of collagen in the body’s tissues [71]. Collagen is a type of protein fiber that makes up the body’s connective tissues, including the skin. It is difficult to understand what causes the disease, but defects in vascular and cellular function are thought to be key driving factors. In the study of vascular dysfunction, blood endothelial cells have been highlighted, whereas lymphatic endothelial cells (LECs) have been much less studied. Skin samples from patients with scleroderma have been shown to be indicative of lymphatic dysfunction, based on studies conducted in 1999 by Leu et al. [72]. There has also been evidence of lymphatic changes in other diseases associated with fibrosis [73]. It also suggests that lymphatic dysfunction might be a therapeutic target for scleroderma.
With a better understanding of how the lymph system is affected, it is important to test approaches that can help treat lymph problems. There are several forms of manual therapy and manual lymphatic drainage (MLD) is one of them. Lymphatic massage is one of the most sensitive and important types of massage and is the drainage of lymph. Lymph drainage improves the function of the body’s lymphatic system and increases its efficiency. For example, people with scleroderma have edema, which is caused by fluid accumulation, and MLD has been shown to reduce swelling and have an effect on hand function [74]. Likewise, lymphatic dry brushing is recommended to reduce lymphatic congestion by reducing fluid accumulation and inflammation. This 5000-year-old technique is based on ancient Ayurvedic medicine in India [75].
2.3 Inflammation and infection
The lymphatic system removes infections and maintains a balance of fluids in the body. Abnormalities in lymphatic function are the cause of the disease, which is often characterized by reduced lymph flow and swelling in the affected limbs such as lymphedema, which can have immune-deficiency consequences. Infectious diseases are the main cause of acquired lymphoedema [76]. Despite the strong association between infection and lymphoedema, it is not well understood whether the lymphatic system is involved in the pathogenesis of bacterial infections or how it responds to microbial and viral infections.
Infections can also cause other problems with the lymphatic system, which are divided into two groups according to the site of damage: lymphadenitis or lymphadenopathy, which affects the lymph nodes, and lymphangitis, which affects the lymphatic vessels [77].
The lymph nodes play an important role in the immune system’s response to infection. Swollen lymph nodes are usually the result of a bacterial or viral infection. Several factors can cause lymph nodes to swell, including colds, flu, ear infections, tuberculosis, and strep throat [78]. In rare cases, this swelling can be caused by more serious conditions such as lymphoma. The amount of swelling depends on the severity of the infection or inflammation. The more serious the condition, the more swollen and painful the gland will be. Swollen lymph nodes can also be a sign of an autoimmune disease, such as rheumatoid arthritis and lupus, or an unusual infection, such as mononucleosis and AIDS. These diseases involve the immune system in all parts of the body and cause an accumulation of lymphocytes in the lymph nodes [54, 77].
Inflammation or infection has been associated with an increase in the number of dendritic cells that leave the site of disease and enter the afferent lymphatic vessels through the induction of chemokine receptors and adhesion molecules. The lymphatic system uses chemokines and counter receptors to regulate the circulation of immune cells within the lymph node [79]. In the inflamed lymph node, there is an increase in the mobilization of immune cells into the gland and a temporary decrease in lymphocytes, leaving the draining lymph nodes. These inflammatory changes, together with the highly specialized architecture of the lymph nodes, increase the likelihood of antigen presentation to the cognate lymphocyte [80]. During inflammation, the immune cells of the draining lymph node undergo changes in both phenotype and function [81]. In response to TNF-α stimuli, dendritic cells have been shown to upregulate MHC class II and the costimulatory molecules and downregulate the chemokine receptors, which contribute to the recruitment of leukocytes to the tissue [82]. In order to selectively increase the lymphatic trafficking of specific immune cells, the transcriptional profile of the lymphatic endothelial cells may also be altered [83]. In a study of human dermal LECs, LECs were shown to release exosomes that cluster around lymphatic vessels under inflammatory conditions and promote dendritic cell migration to lymph nodes [84]. The interaction of LECs with DCs has been studied, and the results show that cooperation between TNF-stimulated LECs and DCs through αMβ2 integrin: ICAM-1 interaction causes downregulation of the costimulatory molecule CD86 on DCs, which can lead to impairment of dendritic cells in activating T cells (Figure 2) [23].

Figure 2.
Schematic showing how lymphatic endothelial cells (LECs) interact with dendritic cells (DCs) and T cells in chronic inflammation (e.g., cancer/infection/autoimmunity). LECs acquire/exchange antigens. Transcription factor gene variations affect lymphatic endothelium phenotype. IFN-γ induces PD-L1 expression in LECs from antigen-specific CD8+ T cells. LECs induce reduced CD86 expression on DCs via TGF-β, impairing T-cell function and promoting immune tolerance.
Changes in lymphatic endothelial cell junction are also induced by inflammation. In endothelial cells of collecting lymphatic vessels, replacement of zipper junctions by button junctions was observed sometime after inflammation-induced mycoplasma infection and treatment with corticosteroids (anti-inflammatory drugs) could reverse these junctional changes [85]. These changes in the inflamed lymphatic vessels can affect the balance between fluid entry and drainage, and the removal of inflammation. However, the flexibility of these junctions makes them a suitable target for reducing chronic inflammation. In a mouse model of mycoplasma pulmonis, both blood and lymphatic vessels have been observed to undergo capillary to venular dilation and lymphangiogenesis. TNF-α has been shown to have a direct effect on both processes, but it appears that the effect on lymphangiogenesis requires further inflammatory mediators from leukocytes [86].
It is clear that lymphangiogenesis and lymphatic remodeling, which are associated with inflammation, are interesting therapeutic targets. An expanded lymphatic network would allow better lymphatic transport. This would presumably improve the removal of excess extracellular fluid caused by inflammation-induced vascular permeability. However, immune surveillance in the lymph nodes could be overwhelmed by trafficking and such proangiogenic signaling pathways need to be carefully controlled.
3. Conclusion
The critical role of the lymphatic system in maintaining tissue homeostasis as well as in disease has been well established over the last decades. Apart from their contribution to immune and inflammatory diseases, a large body of scientific literature documents the importance of lymph vessels for cancer biology and the prognostic value of cancer patients.
From a clinical point of view, targeting the lymphatic system has the potential to improve vaccine delivery and tolerance induction and increase the utility of therapies for a wide range of conditions, from infections like HIV to cancer, inflammation, and metabolism. Much progress could be made with tools that target lymphatic vessels in a specific tissue or lymph nodes, for example, using specific antibodies or mouse models that allow gene deletion at both sites. New insights into organ-specific differences in the lymphatic vasculature, such as those provided by newly developed transcriptomic analyses, may provide new opportunities in the future. More work is needed to better understand the complex interplay of LECs and the immune system, and ultimately, to translate this knowledge into therapeutic applications.
References
- 1.
Drayton DL et al. Lymphoid organ development: From ontogeny to neogenesis. Nature Immunology. 2006; 7 (4):344-353 - 2.
Breslin JW et al. Lymphatic vessel network structure and physiology. Comprehensive Physiology. 2018; 9 (1):207-299 - 3.
Jalkanen S, Salmi M. Lymphatic endothelial cells of the lymph node. Nature Reviews Immunology. 2020; 20 (9):566-578 - 4.
Zoltzer H. Initial lymphatics-morphology and function of the endothelial cells. Lymphology. 2003; 36 (1):7-25 - 5.
Tamburini BAJ et al. Chronic liver disease in humans causes expansion and differentiation of liver lymphatic endothelial cells. Frontiers in Immunology. 2019; 10 :1036 - 6.
Kataru RP et al. Regulation of immune function by the lymphatic system in lymphedema. Frontiers in Immunology. 2019; 10 :470 - 7.
Yuan Y et al. Modulation of immunity by lymphatic dysfunction in lymphedema. Frontiers in Immunology. 2019; 10 :76 - 8.
Lucas ED, Tamburini BAJ. Lymph node lymphatic endothelial cell expansion and contraction and the programming of the immune response. Frontiers in Immunology. 2019; 10 :36 - 9.
Padera TP, Meijer EF, Munn LL. The lymphatic system in disease processes and cancer progression. Annual Review of Biomedical Engineering. 2016; 18 :125-158 - 10.
Kim H, Kataru RP, Koh GY. Inflammation-associated lymphangiogenesis: A double-edged sword? The Journal of Clinical Investigation. 2014; 124 (3):936-942 - 11.
Christiansen A, Detmar M. Lymphangiogenesis and cancer. Genes & Cancer. 2011; 2 (12):1146-1158 - 12.
Radhakrishnan K, Rockson SG. The clinical spectrum of lymphatic disease. Annals of the New York Academy of Sciences. 2008; 1131 (1):155-184 - 13.
Margaris K, Black RA. Modelling the lymphatic system: Challenges and opportunities. Journal of the Royal Society Interface. 2012; 9 (69):601-612 - 14.
Anderson NM, Simon MC. The tumor microenvironment. Current Biology. 2020; 30 (16):R921-r925 - 15.
Mumprecht V et al. In vivo imaging of inflammation-and tumor-induced lymph node lymphangiogenesis by immuno–positron emission tomography. Cancer Research. 2010; 70 (21):8842-8851 - 16.
Qian C-N et al. Preparing the “soil”: The primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Research. 2006; 66 (21):10365-10376 - 17.
Murdoch C et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Reviews Cancer. 2008; 8 (8):618-631 - 18.
Clasper S et al. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Research. 2008; 68 (18):7293-7303 - 19.
Lane RS et al. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. Journal of Experimental Medicine. 2018; 215 (12):3057-3074 - 20.
Gkountidi AO et al. MHC class II antigen presentation by lymphatic endothelial cells in tumors promotes intratumoral regulatory T cell–suppressive functions. Cancer Immunology Research. 2021; 9 (7):748-764 - 21.
Lund AW et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Reports. 2012; 1 (3):191-199 - 22.
Lee E, Pandey NB, Popel AS. Lymphatic endothelial cells support tumor growth in breast cancer. Scientific Reports. 2014; 4 (1):5853 - 23.
Podgrabinska S et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via mac-1/ICAM-1-dependent mechanism. The Journal of Immunology. 2009; 183 (3):1767-1779 - 24.
Evans EM et al. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. Cancer Research. 1997; 57 (14):2943-2950 - 25.
Mabry H, Giuliano AE. Sentinel node mapping for breast cancer: Progress to date and prospects for the future. Surgical Oncology Clinics of North America. 2007; 16 (1):55-70 - 26.
Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunological Reviews. 2006; 213 (1):146-158 - 27.
Fransen MF, Arens R, Melief CJ. Local targets for immune therapy to cancer: Tumor draining lymph nodes and tumor microenvironment. International Journal of Cancer. 2013; 132 (9):1971-1976 - 28.
Mougiakakos D. Regulatory T cells in colorectal cancer: From biology to prognostic relevance. Cancers. 2011; 3 (2):1708-1731 - 29.
Li Q et al. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. The Journal of Immunology. 2005; 175 (3):1424-1432 - 30.
Swann JB, Smyth MJ. Immune surveillance of tumors. The Journal of Clinical Investigation. 2007; 117 (5):1137-1146 - 31.
Gai XD et al. Potential role of plasmacytoid dendritic cells for FOXP3+ regulatory T cell development in human colorectal cancer and tumor draining lymph node. Pathology-Research and Practice. 2013; 209 (12):774-778 - 32.
Shuang Z-Y et al. The tumor-draining lymph nodes are immunosuppressed in patients with hepatocellular carcinoma. Translational Cancer Research. 2017; 6 (6):1188-1196 - 33.
Munn DH et al. Expression of indoleamine 2, 3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of Clinical Investigation. 2004; 114 (2):280-290 - 34.
Norouzian M et al. Regulatory and effector T cell subsets in tumor-draining lymph nodes of patients with squamous cell carcinoma of head and neck. BMC Immunology. 2022; 23 (1):56 - 35.
Cernea C et al. Prognostic significance of lymph node reactivity in the control of pathologic negative node squamous cell carcinomas of the oral cavity. The American Journal of Surgery. 1997; 174 (5):548-551 - 36.
Chandavarkar V et al. Immunomorphological patterns of cervical lymph nodes in oral squamous cell carcinoma. Journal of Oral and Maxillofacial Pathology: JOMFP. 2014; 18 (3):349 - 37.
Yadav ST et al. Immunomorphological assessment of regional lymph nodes for predicting metastases in oral squamous cell carcinoma. Indian Journal of Dental Research. 2012; 23 (1):121 - 38.
Lapointe H, Lampe H, Banerjee D. Head and neck squamous cell carcinoma cell line-induced suppression of in vitro lymphocyte proliferative responses. Otolaryngology–Head and Neck Surgery. 1992; 106 (2):149-158 - 39.
Akbar AN et al. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. The Journal of Experimental Medicine. 1993; 178 (2):427-438 - 40.
Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Research. 2011; 71 (11):3792-3801 - 41.
Mansfield AS et al. Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Modern Pathology. 2011; 24 (4):487-494 - 42.
Kohrt HE et al. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Medicine. 2005; 2 (9):e284 - 43.
Chen J, Chen J, Wang L. Tertiary lymphoid structures as unique constructions associated with the organization, education, and function of tumor-infiltrating immunocytes. Journal of Zhejiang University. Science. B. 2022; 23 (10):812-822 - 44.
Shipman WD, Dasoveanu DC, Lu TT. Tertiary lymphoid organs in systemic autoimmune diseases: Pathogenic or protective? F1000Res. 2017; 6 :196 - 45.
Germain C, Gnjatic S, Dieu-Nosjean M-C. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Frontiers in Immunology. 2015; 6 :67 - 46.
Dieu-Nosjean M-C et al. Tertiary lymphoid structures in cancer and beyond. Trends in Immunology. 2014; 35 (11):571-580 - 47.
Dieu-Nosjean M-C et al. Long-term survival for patients with non–small-cell lung cancer with intratumoral lymphoid structures. Journal of Clinical Oncology. 2008; 26 (27):4410-4417 - 48.
Figenschau SL et al. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer. 2015; 15 :1-11 - 49.
Padera TP et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Molecular Cancer Therapeutics. 2008; 7 (8):2272-2279 - 50.
Melero I et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nature Reviews Cancer. 2007; 7 (2):95-106 - 51.
Vacchelli E et al. Trial watch: Immunostimulatory cytokines. Oncoimmunology. 2012; 1 (4):493-506 - 52.
Aranda F et al. Trial watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology. 2014; 3 (2):e27297 - 53.
Stucci S et al. Immune-related adverse events during anticancer immunotherapy: Pathogenesis and management. Oncology Letters. 2017; 14 (5):5671-5680 - 54.
Grant SM et al. The lymph node at a glance - how spatial organization optimizes the immune response. Journal of Cell Science. 2020; 133 (5):jcs241828 - 55.
Christiansen AJ et al. Lymphatic endothelial cells attenuate inflammation via suppression of dendritic cell maturation. Oncotarget. 2016; 7 (26):39421 - 56.
Schmaler M et al. IL-7R signaling in regulatory T cells maintains peripheral and allograft tolerance in mice. Proceedings of the National Academy of Sciences. 2015; 112 (43):13330-13335 - 57.
Dieterich LC et al. Tumor-associated lymphatic vessels upregulate PDL1 to inhibit T-cell activation. Frontiers in Immunology. 2017; 8 :66 - 58.
Henno A et al. Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque-type psoriasis. British Journal of Dermatology. 2009; 160 (3):581-590 - 59.
Shibata S et al. Possible roles of IL-27 in the pathogenesis of psoriasis. Journal of Investigative Dermatology. 2010; 130 (4):1034-1039 - 60.
Coimbra S et al. The roles of cells and cytokines in the pathogenesis of psoriasis. International Journal of Dermatology. 2012; 51 (4):389-398 - 61.
Schwartz N et al. Lymphatic function in autoimmune diseases. Frontiers in Immunology. 2019; 10 :519 - 62.
Radu A-F, Bungau SG. Management of rheumatoid arthritis: An overview. Cell. 2021; 10 (11):2857 - 63.
Bouta EM et al. The role of the lymphatic system in inflammatory-erosive arthritis. Seminars in Cell & Developmental Biology. 2015; 38 :90-97 - 64.
Rahimi H et al. Lymphatic imaging to assess rheumatoid flare: Mechanistic insights and biomarker potential. Arthritis Research & Therapy. 2016; 18 (1):194 - 65.
Li J et al. Expanded CD23+/CD21hi B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF-transgenic mice and are targets of anti-CD20 therapy. The Journal of Immunology. 2010; 184 (11):6142-6150 - 66.
Li J et al. Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow. Arthritis and Rheumatism. 2013; 65 (1):130-138 - 67.
Mok C, Lau C. Pathogenesis of systemic lupus erythematosus. Journal of Clinical Pathology. 2003; 56 (7):481-490 - 68.
Ambler WG et al. 205 lymphatic dysfunction in lupus photosensitivity. Archives of Disease in Childhood. 2021; 8 (Suppl 2):A1-A75 - 69.
Rajasekhar L et al. Lymphatic obstruction as a cause of extremity edema in systemic lupus erythematosus. Clinical Rheumatology. 2013; 32 :11-13 - 70.
Daniel A et al. Chylous ascites in a patient with an overlap syndrome: A surprising response to rituximab. Case Reports. 2017; 2017 :bcr-2017-222339 - 71.
Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. New England Journal of Medicine. 2009; 360 (19):1989-2003 - 72.
Leu A et al. Lymphatic microangiopathy of the skin in systemic sclerosis. Rheumatology (Oxford, England). 1999; 38 (3):221-227 - 73.
Garber S et al. Enlarged mediastinal lymph nodes in the fibrosing alveolitis of systemic sclerosis. The British Journal of Radiology. 1992; 65 (779):983-986 - 74.
Bongi SM et al. Manual lymph drainage improving upper extremity edema and hand function in patients with systemic sclerosis in edematous phase. Arthritis Care & Research. 2011; 63 (8):1134-1141 - 75.
Narahari S et al. Integrated management of filarial lymphedema for rural communities. Lymphology. 2007; 40 (1):3-13 - 76.
Sleigh BC, Manna B. Lymphedema. Treasure Island (FL): StatPearls Publishing; 2019 - 77.
Zeppa P, Cozzolino I. Lymphadenitis and lymphadenopathy. Lymph Node FNC. 2018; 23 :19-33 - 78.
Sahai S. Lymphadenopathy. Pediatrics in Review. 2013; 34 (5):216-227 - 79.
Neeland MR, Meeusen ENT, de Veer MJ. Afferent lymphatic cannulation as a model system to study innate immune responses to infection and vaccination. Veterinary Immunology and Immunopathology. 2014; 158 (1):86-97 - 80.
Tan KW et al. Expansion of cortical and medullary sinuses restrains lymph node hypertrophy during prolonged inflammation. The Journal of Immunology. 2012; 188 (8):4065-4080 - 81.
Johnson LA et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. The Journal of Experimental Medicine. 2006; 203 (12):2763-2777 - 82.
Sallusto F et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. European Journal of Immunology. 1998; 28 (9):2760-2769 - 83.
Vigl B et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood, The Journal of the American Society of Hematology. 2011; 118 (1):205-215 - 84.
Brown M et al. Lymphatic exosomes promote dendritic cell migration along guidance cues. The Journal of Cell Biology. 2018; 217 (6):2205-2221 - 85.
Baluk P et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. The Journal of Experimental Medicine. 2007; 204 (10):2349-2362 - 86.
Baluk P et al. TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. The Journal of Clinical Investigation. 2009; 119 (10):2954-2964