Proliferative potential in normal term placentas and placentas from pregnancies complicated by gestational (GDM) and insulin-dependent diabetes (DM 1) was expressed as mean number of Ki67-labeled nuclei per square millimeter of villous cross section.
Abstract
Placental capillary bed plays a key role in the bidirectional transport between mother and fetus. Its continuous growth and maturation accompany fetal growth and meet all fetal requirements to secure fetal well-being. Considerable growth of both capillary bed and area of villous syncytiotrophoblast comes on in third trimester of pregnancy, continues until the end of pregnancy, and is expressed by rapid development of terminal villi. The presented structural and quantitative data show enhanced villous capillary branching, higher proportion of capillaries displaying delayed maturation, and lower proliferative potential of cells forming capillary wall and cytotrophoblast in diabetic placenta at term. Too few studies have focused on the impact of other pathologies, i.e., preeclampsia and IUGR on development of placental capillary bed. The further research may contribute to better understanding of those disorders connected with pregnancy.
Keywords
- capillary
- nestin
- pericyte
- proliferation
- spatial arrangement
1. Introduction
The vascular system is inevitable organ component ensuring the supply and outflow of the blood and the blood distribution by arteries, arterioles, venules, and veins, as well as transport of gases, ions, nutrition, wastes, and signaling molecules between blood and cells at the level of capillaries. The development of vascular bed is one of processes of the organ growth, and after the end of its development the vascular component of the organ remains very stable under physiological conditions. Unlike other organs, there are locations in the human body displaying periodical development and demise of vascular bed, i.e., corpus luteum and endometrium.
The third organ, also bound to the fertile period of women life and showing vascular development, is placenta. The whole organ and obviously its vascular bed grow and develop during the whole pregnancy and follow the growth of fetus. Unlike endometrium and corpus luteum, the time of demise of vascular bed is not clear as the fetal well-being depends on the optimal placental function until the moment of birth. Therefore, studies on the placenta at the end of normal non-complicated gestation are performed on the functioning organ.
2. The spatial arrangement of placental capillaries
The capillary bed of the human
The spatial arrangement of villous capillaries is important for our knowledge of the normal blood flow in the capillary bed and thus for better understanding of its function in transport between mother and fetus. The routine light microscopy shows that voluminous placental capillaries of uneven diameter are found in tight relationship to the trophoblast covering the villus. Thinner parts of syncytiotrophoblast lacking nuclei together with adjacent capillary wall form frequent vasculosyncytial membranes taken as sites of preferential maternofetal transport (Figure 1).
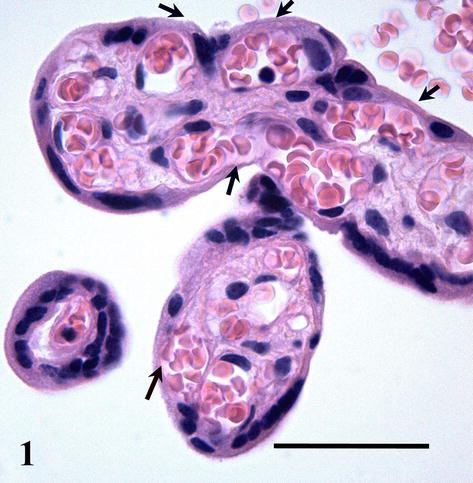
Figure 1.
Histological section showing terminal placental villi. Arrows indicate vasculosyncytial membranes. Scale bar = 50 μm.
Placental capillaries are of somatic type, i.e., the endothelial cells have tight (occluding) junctions on their contacts, the basal lamina is continuous, and perivascular pericytes and their projections surround the endothelial tube (Figure 2).
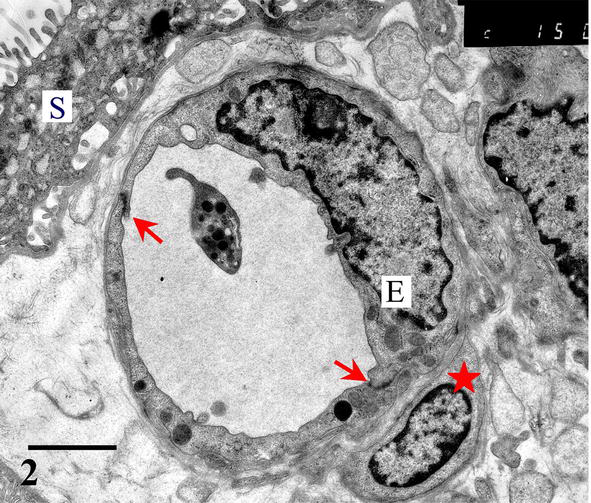
Figure 2.
Placental capillary in a tight relationship with syncytiotrophoblast (S). Endothelial cells (E) are interconnected with occluding junctions (arrows) and surrounded with pericytes (asterisk). Scale bar = 2 μm.
It is hardly possible to judge the spatial arrangement of villous capillaries from conventional two-dimensional pictures. Scarcity of papers dealing with spatial capillary arrangement has shown that it is difficult to achieve reliable data using injection of a dye into placental vessels, corrosion casts, or 3D reconstruction based on serially sectioned material [3, 4]. So, models based on those techniques do not meet the real spatial arrangement of placental capillaries. Only the application of advanced methods using combination of confocal laser scanning microscopy (CLSM) and software for 3D reconstruction yielded successful representation of villous capillary bed and allowed to obtain quite new findings on its real configuration [5].
Terminal villi of cylindrical shape arise from the mature intermediate villi. The simplest type of 3D arrangement is a U-like capillary loop parallel to the villous axis and continuous with capillaries of neighboring terminal villi or arising from a vessel (or also open into a vessel) of mature intermediate villus. It is evident that this type of capillary bed is a result of the longitudinal (nonbranching) capillary growth. The wavy course of some capillary segments represents their continuous elongation inside the villus. In contrast to the previously accepted model [6], the CLSM also revealed that the other type of angiogenesis, the branching angiogenesis is a common process taking part in placental capillary growth as well. It demonstrates itself by the appearance of blind capillary buds, longer blindly ending capillary projections, and of capillary beds formed by three or more capillary segments running parallel to the villous axis. Transversally running capillary segments interconnecting longitudinally oriented capillary segments originate also by the branching angiogenesis and make the capillary bed denser. All capillaries are located in tight relationship to (syncytio)trophoblast, and this way a larger area of vasculosyncytial membranes is formed. Further elongation of both longitudinally oriented capillary segments and transversally oriented capillary segments and blind capillary buds bring about the arching of adjacent trophoblast that is the first visible sign of a new villus formation. As demonstrated here, the continuous elongation and sprouting of capillaries lead to development of villous clusters with an intricately anastomosing capillary bed (Figures 3–5).
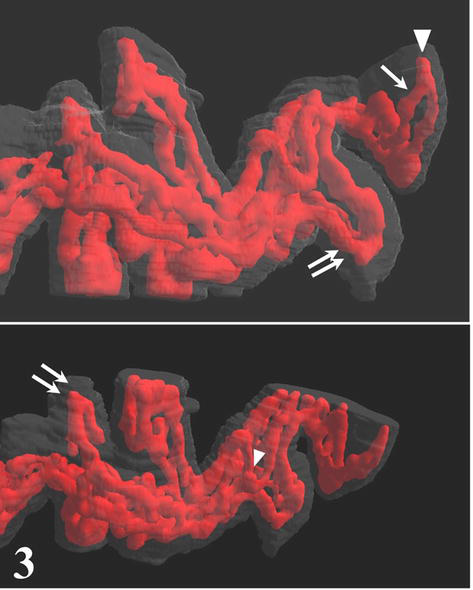
Figure 3.
Two projections of 3D presentation of the villous capillary bed. Blind capillary projections (arrowheads) and villous capillaries formed by three segments (double arrows) illustrate the branching angiogenesis. A simple arrow indicates U-like shape of the villous capillary.
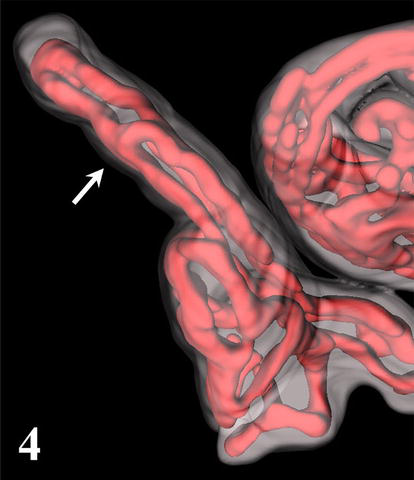
Figure 4.
3D reconstruction of terminal villus with three parallel capillary segments (arrow). Note the apical capillary dilation (arrowhead).
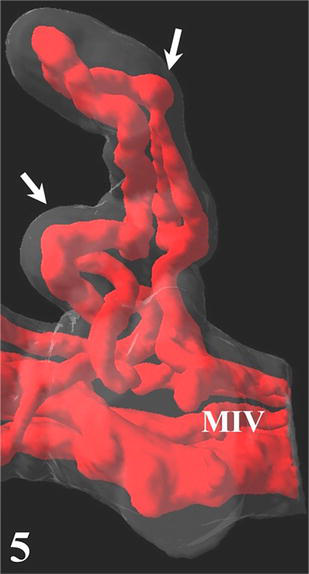
Figure 5.
Developing cluster of terminal villi (arrows) arising from a mature intermediate villus (MIV).
Concerning the control of villous development, little information is available. Nevertheless, placental vessels belong to the fetal circulatory system, and its capillary part together with trophoblast are fetal structure displaying the anatomically closest relationship to maternal organism and carrying out the transport between mother and fetus. The fetus that is completely dependent on the supply with oxygen and nutrition from the mother reacts on the maternal environment by influencing the growth of placental capillary bed via trophoblastic production of angiogenesis controlling factors [7, 8, 9].
As oxygen is essential to fetal survival, its decreased availability elicits hypoxia in fetoplacental unit. One of causes of fetal hypoxia is abnormal glucose metabolism in maternal organism, i.e., insulin-dependent and gestational diabetes. The introduction of insulin allowed the women suffering from diabetes to finish their pregnancy by the birth of viable newborn. But those pregnancies were accompanied with pathological, bulky edematous placentas. Nowadays, the management of maternal diabetes results in consistent metabolic control during pregnancy, well developed newborn, and placentas that do not deviate macroscopically from placentas in uncomplicated pregnancies. Nevertheless, despite the consistent metabolic control, the intermittent excess of glucose load taking place in diabetes has consequences in fetal capillary bed. Although nowadays most of placentas from pregnancies complicated by diabetes have histological structure like placentas of non-diabetic mothers, the villous vascularity may be variable, i.e., normally vascularized, edematous hypovascularized villi, and, on the other hand, hypervascularized terminal villi may be found (Figure 6).
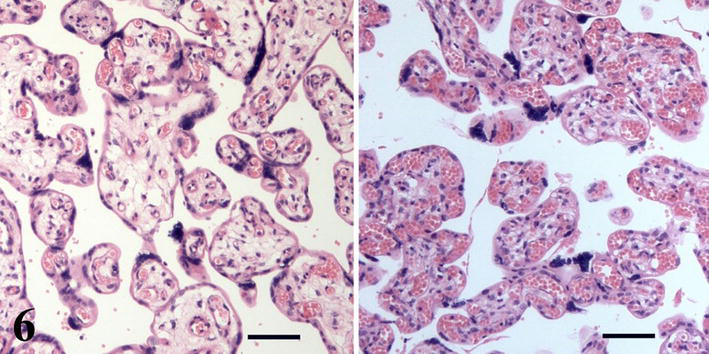
Figure 6.
Hypovascularized (left) and hypervascularized (right) villi of placentas from pregnancies complicated by insulin-dependent diabetes mellitus. Scale bars = 50 μm.
Quantitative studies based on the confocal microscopic analysis have shown enhanced capillary branching in terminal villi in both gestational and insulin-dependent diabetes [10, 11]. As demonstrated in Figure 5, the capillary growth stimulates villous growth and branching, and enhanced angiogenesis in diabetic placenta is probably the cause of higher villous branching described in [12]. Molecular factors conditioning those differences in diabetic placentas were studied, e.g., in [9]. Regarding the vascularization in abnormal villi, three-dimensional presentations show capillary beds formed by thin capillary segments of uniform diameter without dilated parts in hypovascularized villi and, on the other hand, very dilated capillary segments in the capillary bed of hypervascularized villi [3, 11]. The diameter of both forms of those villi are larger (Figure 6), and together with more branched villous tree they may disturb the blood flow in the intervillous space and dynamic processes necessary for normal maternofetal transport and capillary blood flow.
The 3D reconstructions of fetal capillaries also bear the potential of better understanding placental physiology and development of placental pathologies. The assessment of the cross-sectional areas of capillaries in capillary bifurcation has shown that villous capillaries branch asymmetrically and that their branching meets the condition for the plasma skimming, the rheological phenomenon important for blood flow in microcirculation [13]. The availability of three-dimensional representations of terminal villi and their capillaries also enabled the computational modeling for simulation and calculation of the influence of pressure drop in capillary dilations on vascular flow resistance and total oxygen transfer rate [14].
Topological characteristics and detailed structural information of villous capillary bed, i.e., length and diameter of capillary segments, their dilation, and branching angle, yielded by three-dimensional imaging, give a data for better insight to blood flow regulation, oxygen transport, blood pressure, and wall shear stress distribution. The further development of simulation methods used in the computational modeling may also contribute to better understanding of dynamic effects of, e.g., capillary diameter and its relationship to blood viscosity and erythrocyte concentration and thus to local differences of blood flow. And obviously, those methods used in the study of pathological placentas may help to elucidate the development and consequences of pathologic villous structure [15, 16].
3. Placental angiogenesis
The development of placental vasculature begins early in the embryonic period from cells of mesodermal origin. Those cells forming mesenchymal core of developing villi give rise to the new vessels in the process of vasculogenesis. Both types of angiogenesis, the nonbranching and branching one, represent the formation of new vessels by elongation or sprouting of preexisting vessels, respectively. Hand in glove with terminal villi, the placental capillaries undergo rapid development during third trimester of pregnancy. Three-dimensional reconstruction of serial confocal sections has shown signs of both nonbranching and sprouting capillary growth taking place inside villi and supporting the growth of new villi [4, 13]. This finding challenges the previously accepted opinion that the nonbranching angiogenesis predominates after 24th week of gestation [1].
The angiogenesis is a process covering the endothelial proliferation, differentiation, and migration allowed by disintegration of endothelial basement membrane. The recruitment of pericytes stabilizes newly formed part of capillary wall or capillary branch, and so, the new structurally and functionally mature part of capillary bed is available.
The cytoskeletal intermediate filament protein nestin occurs transiently among others also in villous capillary wall where it is confined to cytoplasm of endothelium and bodies and projections of pericytes during their differentiation [17, 18, 19]. Its immunocytochemical detection shows the distribution of angiogenic “hot spots” in villous capillaries (Figure 7). As shown in Figure 8, the angiogenic spot may take considerable proportion of capillary circumferrence resulting in enlargement of its diameter. Nestin-labeled cells with protruding nuclei showing typical picture of “ring stone “are differentiating pericytes.
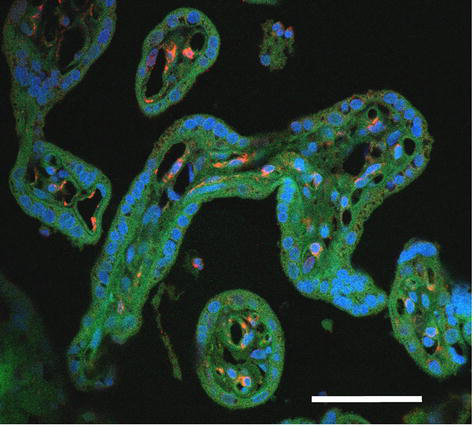
Figure 7.
Distribution of active angiogenic spots (red) in capillaries of terminal villi. Blue signal = cell nuclei (DAPI), green signal = autofluorescence of formalin fixed tissue. Scale bar = 50 μm.
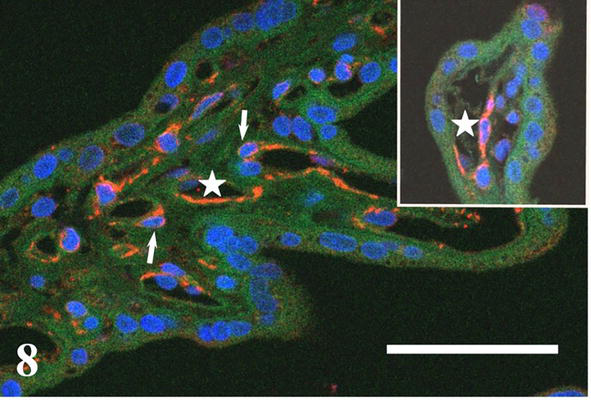
Figure 8.
Immunocytochemical detection of nestin (red) indicates active angiogenic spot of capillary endothelium (asterisks) and differentiating capillary pericytes (arrows). Blue signal = cell nuclei (DAPI), green signal = autofluorescence of formalin fixed tissue. Scale bar = 50 μm.
The nestin immunolocalization in the capillary wall reveals characteristic occurrence of nestin-positive endothelium beside quiescent pericytes and nestin-negative endothelium surrounded with nestin-positive pericyte bodies or projections. This picture demonstrates a time shift of differentiation between endothelium and pericytes during formation and maturation of capillaries. The higher proportion of the nestin-positive segments of the capillary circumference in diabetic placentas points stronger angiogenesis in placenta, but it may be also a sign of altered maturation of the cytoskeleton [20].
In semiquantitative study, the authors pointed higher nestin expression as a marker of higher capacity for angiogenesis in preeclamptic placentas [19]. Their finding seems to be in accordance with hyperramification of villous capillaries ascertained in pre-eclampsia using CLSM analysis [21].
In villous capillaries, pericytes are closely associated with endothelial cells and play important role in angiogenesis and vessel stabilization. At present, several new functions in placental development and homeostasis were discovered, i.e., in tissue regenerative and repair processes, lymphocyte activation or phagocytic properties [22].
The arrangement of pericyte coverage and its extent are two features potentially influencing the permeability of villous capillaries. The reduced thickness of tissues insulating maternal and fetal blood that is characteristic for term placenta is expressed most of all in vasculosyncytial membranes (Figure 1). As described in [23] and demonstrated in Figure 9, pericytes cover mainly endothelial junctions in regions of capillary wall turned away from the trophoblast. That distribution supports maternal-fetal transport and, at the same time, controls intercellular junctions of endothelium. Recently, an advanced method of electron microscopy, i.e., serial block-face scanning electron microscopy, revealed that there are up to 7 μm long interendothelial protrusions [24]. Their coincidence with pericytes maintains without questions the integrity of capillary wall.
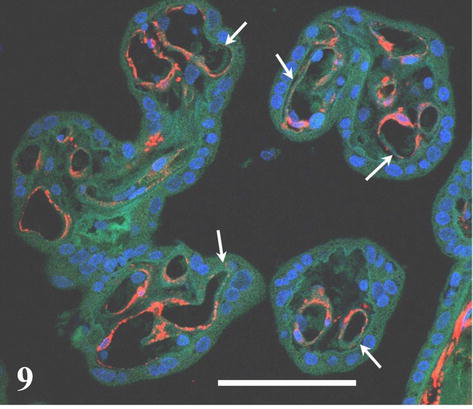
Figure 9.
Immunocytochemical detection of smooth muscle actin labels capillary pericytes (red signal). The pericyte coverage is markedly reduced in regions of capillary wall tightly adjacent to syncytiotrophoblast (arrows). Blue signal = cell nuclei (DAPI), green signal = autofluorescence of formalin fixed tissue. Scale bar = 50 μm.
Quantitative data on the extent of pericyte coverage of capillary endothelium are sparse. The proportion of pericyte coverage was found 15% in normal placenta [24]. The comparison of first trimester placentas and placentas at term has shown higher proportion of vessels surrounded with pericytes at term. Moreover, placental capillaries of pregnancies from high altitude were less covered with pericytes than those from downland [25]. In our study, non-significant differences were found in the proportion of pericyte-associated capillaries in placentas from pregnancies complicated by insulin-dependent diabetes (IDDM) (84% in controls vs. 79% in diabetic placentas) and in extent of pericyte coverage of capillary circumference (38% in controls vs. 33% in diabetic placentas) [26]. The data are hardly comparable among papers, as they were achieved on placental material from different diagnoses by different methodology. On the other hand, the continually lower oxygen pressure may cause lower pericyte coverage in placentas from pregnancies in high altitude. The reduced thickness of vascular wall produced in this case may express an adaptation to preplacental hypoxia caused by lower oxygen tension in high altitude [1]. In our study on diabetic placentas, the lower proportions of pericyte coverage were nonsignificant. Here, we have to take into consideration that the hypoxia in diabetic placenta is a consequence of non-enzymatic hemoglobin glycation leading to decreased release of oxygen from maternal erythrocytes. Diabetic mothers included in this study were well metabolically compensated, and thus, the intrauterine hypoxia, if came about, was low and unlike high altitude pregnancies rather intermittent because of obviously intermittent excess of the glucose load.
The angiogenesis and pruning of capillaries are two sides of formation of capillary bed. In the placenta at term, apoptotic cells were identified in cytotrophoblast and villous stroma including endothelium. The apoptosis was compared in normal and pathological placentas, but results are inconsistent due to studied material and used methodology [20, 27, 28, 29].
4. Proliferative potential in term placenta
The enlargement of the area of capillary wall and syncytiotrophoblast is essential for fetal growth that is most rapid in last weeks of pregnancy, and the requirement of optimal placental function overlaps the period of parturition. Thus, the potential of endothelial cells and pericytes to undergo mitotic division is retained in placenta at term. The Ki67 antigen is a proliferation marker expressed in the cell nucleus during the G1, S, G2, and M phase of the cell cycle, but not in quiescent cells. It detects cytotrophoblast, stromal cells, endothelium, and perivascular cells (Figure 10). The comparison of normal and diabetic placentas has shown the same occurrence of Ki67 positive cells. But the hypo- and hypervascularized villi of diabetic placentas were discovered free of Ki67-labeled cells [20].
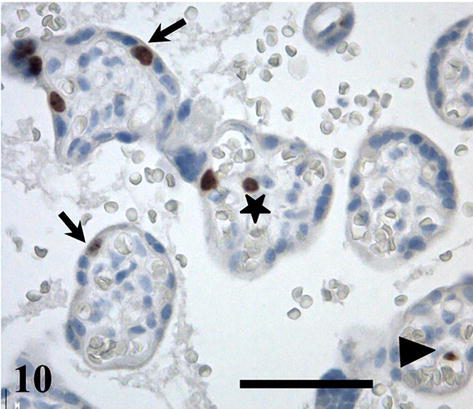
Figure 10.
Ki67-labeled cytotrophoblast (arrows), endothelial cell (arrowhead), and stromal cell (asterisk) in placental villi at term. Scale bar = 50 μm.
Many studies comparing normal and pathological placentas focus proliferative potential of cytotrophoblast only, e.g. [30]. The cytotrophoblast proliferation potential in peripheral villi was found not different between normal placentas and placentas in intrauterine growth restriction (IUGR), whereas lower proliferation potential was found in stromal compartment in IUGR [31]. The authors of two semiquantitative studies described significantly increased the PCNA and Ki67 signals in the villous compartment of preeclamptic and diabetic placentas [32, 33]. The positive influence of maternal physical activity on increased cell proliferation in both cytotrophoblast and endothelial and stromal cells was proved in [34].
Our analysis performed on terminal villi of normal and DM 1 placentas distinguished between cytotrophoblast, villous stroma, and capillaries. The proliferation index of cells of capillary wall was revealed significantly lower in diabetic placentas. Also, the counts of Ki67-labeled nuclei per villous area unit were significantly lower in cytotrophoblast and capillary wall in terminal villi of diabetic placentas, [20]. Later, the study was extended to the group of placentas from pregnancies complicated by gestational diabetes (GDM) and was also performed in stem and intermediate villi, see Table 1.
| Group (number of placentas) | IDDM (16) | GDM (13) | Control (9) |
|---|---|---|---|
| Stem villib | |||
| Cytotrophoblast | 6.21 ± 5.36 | 6.77 ± 5.27 | 7.60 ± 5.79 |
| Stroma | 5.11 ± 6.51 | 1.68 ± 1.35 | 2.91 ± 2.47 |
| Vascular wall | 1.76 ± 1.94* | 1.51 ± 1.53** | 3.71 ± 2.07 |
| Intermediate villib | |||
| Cytotrophoblast | 9.46 ± 6.12* | 10.93 ± 6.42* | 18.262 ± 7.741 |
| Stroma | 5.43 ± 6.16 | 1.65 ± 1.13 | 3.10 ± 4.30 |
| Vascular wall | 1.42 ± 1.54 | 2.07 ± 2.03 | 4.30 ± 5.51 |
| Terminal villia | |||
| Cytotrophoblast | 18.18 ± 11.90* | 17.67 ± 9.01* | 29.47 ± 15.87 |
| Stroma | 8.94 ± 9.78 | 1.57 ± 1.02 | 5.42 ± 7.17 |
| Vascular wall | 4.48 ± 3.28* | 7.39 ± 4.26 | 9.82 ± 4.46 |
In stem villi, no significant differences were found out in cytotrophoblast and stromal compartment. The vascular component consists of large arteries and vessels accompanied with capillaries that are taken as
Intermediate villi are branches of stem villi characterized by smaller diameter and occurrence of loose bundles of connective tissue fibers in stroma and arterioles, venules, and capillaries. The proliferative potential expressed as count of Ki67-labeled nuclei per villous area unit was discovered lower in cytotrophoblast of both diabetic groups.
The most peripheral branches of the villous tree, terminal villi, massively develop in the third semester to meet growing fetal requirements. Identically to other villous types they are covered with trophoblast, and their sparse stroma contain voluminous capillaries. Cytotrophoblast of those villi displayed significantly lower proliferative potential in both groups of diabetic placentas, whereas the proliferative potential in cells of capillary wall was significantly lower in DM 1 placentas only. Results of that study show that in the placenta at term, there are cell populations, i.e., cytotrophoblast and cells forming capillary wall, available and ready for mitotic division and thus for the enlargement of placental transport area, and that the proliferative potential is reduced in placentas from pregnancies complicated by maternal diabetes.
During their life, normal cells undergo limited number of replications and divisions of chromosomes. This fact limits their lifespan. Among other factors, the replication is regulated by the length of telomeres, and every chromosome replication and mitotic division shortens the telomere length. Telomere shortening induces cell senescence, apoptosis, or genome instability. Some papers showing that the telomere length in placenta decreases during pregnancy were published, but there are no unequivocal results of the assessment of telomere length in pathologic placentas [35]. As those studies were based mainly on the measurement of mean telomere length, the use of contemporary methods quantifying very short telomeres may better clarify processes related to placental senescence at the end of normal and pathologically complicated pregnancies [36, 37]. Nevertheless, it is possible to judge that the shortening of telomeres influences the proliferative potential including term placentas. The more branched villous capillary bed and foci of hypervascularized villi with extremely voluminous capillaries in diabetic placentas [11, 12] represent a compensation of hypoxia. However, such enlargement of capillary wall requires more frequent mitotic division. It is demonstrated by higher proportion of endothelial cells and pericytes labeled with nestin appearing in diabetic placentas. Due to this enhanced mitotic activity, the proliferative potential may be exhausted, and the area of syncytiotrophoblast and capillary wall cannot enlarge adequately to fetal requirements of nutrients and oxygen supply. Such situation might result in placental insufficiency, a serious complication of late pregnancy that is more frequent in maternal diabetes and brings a risk to the fetus.
5. Conclusions
Complications of pregnancy, i.e., maternal diabetes mellitus, preeclampsia, and intrauterine growth restriction threaten the normal intrauterine development of individual. Placenta, the organ ensuring optimal conditions for fetus during pregnancy, mirrors pregnancy pathologies in abnormal structural features. Here, we turned our attention to placental capillary bed. In pregnancies complicated by diabetes mellitus, maternal diabetes is treated nowadays very carefully to achieve consistent metabolic control. Thanks to it placentas look macroscopically as well as microscopically normal. Only the comparison of quantitative data, i.e., villous capillary branching, pericyte coverage, and proliferative potential uncovered differences that are important for our understanding of functional consequences. Unfortunately, similar data for placentas in preeclampsia or IUGR are not available, and those pregnancy complications remain a challenge for further research in this area.
Acknowledgments
This work was supported by a project Cooperation of the Charles University.
References
- 1.
Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. 6th ed. Berlin Heidelberg: Springer-Verlag; 2012. DOI: 10.1007/978-3-642-23941-0 - 2.
Benirschke K, Kaufmann P. Pathology of the Human Placenta. 6th ed. New York: Springer-Verlag; 1995 - 3.
Jirkovská M. The morphology of villous capillary bed in normal and diabetic placenta. In: Zheng J, editor. Recent Advances in Research on the Human Placenta. London, UK: InTech; 2012 - 4.
Jirkovská M. Spatial arrangement of the microvascular bed in the human placenta. Andrology and Gynecology: Current Research. 2013; 1 :4. DOI: 10.4172/2327-4360.1000110 - 5.
Jirkovská M, Kubínová L, Krekule I, Hach P. Spatial arrangement of fetal placental capillaries in terminal villi: A study using confocal microscopy. Anatomy and Embryology. 1998; 197 :263-272. DOI: 10.1007/s004290050136 - 6.
Kaufmann P, Bruns U, Leiser R, Luckhardt M, Winterhager E. The fetal vascularisation of term human placenta villi. II. Intermediate and terminal villi. Anatomy and Embryology. 1985; 173 :203-214 - 7.
Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014; 21 :15-25. DOI: 10.1111/micc.12093 - 8.
Loegl J, Nussbaumer E, Hiden U, Majali-Martinez A, Ghaffari-Tabrizi-Wizy N, Cvitic S, et al. Pigment epithelium-derived factor (PEDF): A novel trophoblast-derived factor limiting feto-placental angiogenesis in late pregnancy. Angiogenesis. 2016; 19 :373-388. DOI: 10.1007/s10456-016-9513-x - 9.
Loegl J, Nussbaumer E, Cvitic S, Huppertz B, Desoye G, Hidden U. GDM alters paracrine regulation of feto-placental angiogenesis via trophoblast. Laboratory Investigation. 2017; 97 :409-418. DOI: 10.1038/labinvest.2016.149 - 10.
Jirkovská M, Kubínová L, Janáček J, Moravcová M, Krejčí V, Karen P. Topological properties and spatial organization of villous capillaries in normal and diabetic placentas. Journal of Vascular Research. 2002; 39 :268-278. DOI: 10.1159/000063692 - 11.
Jirkovská M, Kučera T, Kaláb J, Jadrníček M, Niedobová V, Janáček J, et al. The branching pattern of villous capillaries and structural changes of placental terminal villi in type 1 diabetes mellitus. Placenta. 2012; 33 :343-335. DOI: 10.1016/j.placenta.2012.01.014 - 12.
Björk O, Persson B. Villous structure in different parts of the cotyledon in placentas of insulin dependent diabetic women. Acta Obstetrica Gynecologica Scandinavica. 1984; 6 :37-43 - 13.
Jirkovská M, Janáček J, Kaláb J, Kubínová L. Three-dimensional arrangement of the capillary bed and its relationship to microrheology in the terminal villi of normal term placenta. Placenta. 2008; 29 :892-893. DOI: 10.1016/j.placenta.2008.07.004 - 14.
Pearce P, Brownbill P, Janáček J, Jirkovská M, Kubínová L, Chernyavsky IL, et al. Image-based modeling of blood flow and oxygen transfer in feto-placental capillaries. PLoS One. 2016; 11 :e0165369. DOI: 10.1371/journal.pone.0165369 - 15.
Plitman Mayo R, Olsthoorn J, Charnock-Jones DS, Burton GJ, Oyen ML. Computational modeling of the structure-function relationship in human placental terminal villi. Journal of Biomechanics. 2016; 49 :3780-3787. DOI: 10.1016/j.jbiomech.2016.10.001 - 16.
Plitman MR. Advances in human placental biomechanics. Computational and Structural Biotechnology Journal. 2018; 24 :298-306. DOI: 10.1016/j.csbj.2018.08 - 17.
Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histology and Histopathology. 2005; 20 :665-671. DOI: 10.14670/HH-20.665 - 18.
Mokrý J, Němeček S. Angiogenesis of extra-and intraembryonic blood vessels is associated with expression of nestin in endothelial cells. Folia Biologica (Praha). 1998; 44 :155-161 - 19.
Hwang HS, Cho NH, Maeng YS, Kang MH, Park YW, Kim YH. Differential expression of nestin in normal and pre-eclamptic human placentas. Acta Obstetrica Gynecologica Scandinavica. 2007; 86 :909-914. DOI: 10.1080/00016340701417018 - 20.
Jirkovská M, Kučera T, Dvořáková V, Jadrníček M, Moravcová M, Žižka Z, et al. Impact of maternal diabetes type 1 on proliferative potential, differentiation, and apoptotic activity in villous capillaries of term placenta. Placenta. 2016; 40 :1-7. DOI: 10.1016/j. placenta.2016.02.003 - 21.
Resta L, Capobianco C, Marzullo A, Piscitelli D, Sanguedolce F, Schena FP, et al. Confocal laser scanning microscope study of terminal villi vessels in normal term and pre-eclamptic placentas. Placenta. 2006; 27 :735-739. DOI: 10.1016/j.placenta.2005.07.006 - 22.
Barreto RSN, Romagnolli P, Cereta AD, Coimbra-Campos LMC, Birbrair A, Miglino MA. Pericytes in the placenta: Role in placental development and homeostasis. Advances in Experimental Medicine and Biology. 2019; 1122 :125-151. DOI: 10.1007/978-3-030-11093-2_8 - 23.
Harris SE, Matthews KSH, Palaiologou E, Tashev SA, Lofthouse EM, Pearson Farr J, et al. Pericytes on placental capillaries in terminal villi preferentially cover endothelial junctions in regions furthest away from the trophoblast. Placenta. 2021; 104 :1-7. DOI: 10.1016/j.placenta.2020.10.032 - 24.
Palaiologou E, Goggin P, Chatelet DS, et al. Serial block-face scanning electron microscopy reveals novel intercellular connections in human term placental microvasculature. Journal of Anatomy. 2020; 237 :241-249. DOI: 10.1111/joa.13191 - 25.
Zhang EG, Burton GJ, Smith SK, Charnock-Jones DS. Placental vessel adaptation during gestation and to high altitude: Changes in diameter and perivascular cell coverage. Placenta. 2002; 23 :751-762. DOI: 10.1053/plac.2002.00856 - 26.
Kučera T, Vyletěl I, Moravcová M, Krejčí V, Žižka Z, Jirkovská M. Pericyte coverage of fetoplacental vessels in pregnancies complicated by type 1 diabetes mellitus. Placenta. 2010; 31 :1120-1122. DOI: 10.1016/j.placenta.2010.09.014 - 27.
Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. American Journal of Obstetrics and Gynecology. 1997; 177 :1395-1401. DOI: 10.1016/s0002-9378(97)70081-4 - 28.
Sgarbosa F, Barisan LF, Brasil MAM, Costa E, Calderon IMP, Gonçalves CR, et al. Changes in apoptosis and Bcl-2 expression in human hyperglycemic term placental trophoblast. Diabetes Research and Clinical Practice. 2006; 73 :143-140. DOI: 10.1016/j.diabres.2005.12.014 - 29.
Burleigh DW, Stewart K, Grindle KM, Kay HH, Golos TG. Influence of maternal diabetes on placental fibroblast growth factor-2 expression, proliferation, and apoptosis. Journal of the Society of Gynecological Investigation. 2004; 11 :36-41. DOI: 10.1016/j.jsgi.2003.06.001 - 30.
Can M, Guven B, Bektas S, Arikan I. Oxidative stress and apoptosis in preeclampsia. Tissue and Cell. 2014; 46 :477-481. DOI: 10.1016/j.tice.2014.08.004 - 31.
Chen C-P, Bajoria R, Aplin JD. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. American Journal of Obstetrics and Gynecology. 2002; 187 :764-769. DOI: 10.1067/mob.2002.125243 - 32.
Unek G, Ozmen A, Mendilcioglu I, Simsek M, Korgun ET. The expression of cell cycle related proteins PCNA, Ki67, p27 and p57 in normal and preeclamptic human placentas. Tissue and Cell. 2014; 46 :198-205. DOI: 10.1016/j.tice.2014.04.003 - 33.
Unek G, Ozmen A, Mendilcioglu I, Simsek M, Korgun ET. Immunohistochemical distribution of cell cycle proteins p27, p57, cyclin D3, PCNA and Ki67 in normal and diabetic human placentas. Journal of Molecular Histology. 2014; 45 :21-34. DOI: 10.1007/s10735-013-9534-3 - 34.
Bergmann A, Zygmunt M, Clapp JF. Running throughout pregnancy: Effect on placental villous vascular volume and cell proliferation. Placenta. 2004; 25 :694-698. DOI: 10.1016/j.placenta.2004.02.005 - 35.
Jirkovská M, Korabečná M, Laššáková S. Telomeres and telomerase activity in the human placenta. In: Morrish TA, editor. Telomerase and non-Telomerase Mechanisms of Telomere Maintenance. London, UK: IntechOpen; 2020. DOI: 10.5772/intechopen - 36.
Edelson PK, Sawyer MR, Gray KJ, Cantonwine DE, McElrath TF, Phillippe M. Increase in short telomeres during the third trimester in human placenta. PLoS One. 2022; 17 :e0271415. DOI: 10.1371/journal.pone.0271415 - 37.
Lai T-P, Simpson M, Patel K, Verhulst S, Noh J, Roche N, et al. Telomeres and replicative cellular aging of the human placenta and chorioamniotic membranes. Scientific Reports. 2021; 11 :5115. DOI: 10.1038/s41598-021-84728-2