Different species of Trichoderma genus against PPN and their effects.
Abstract
Plant parasitic nematodes are major pests of the agricultural industry in developing countries. This group is associated with different parts viz., flower, leaves, buds, roots, trunk etc., of approximately all crucial crops of agronomy due to their omnipresent nature. They are categorized as endo, ecto and semi-endoparasite based on the feeding habits. PPNs possess remarkable characteristics especially, parasitic adaptations which help in combating adverse conditions. Furthermore, they interact with other microorganisms (pathogens) forming complex diseases in crops. For effective management of the PPNs through biological control, it is essential to understand their parasitic mechanism, adaptation of J2 stages, feeding mechanism, host-nematode relationship and identification of associated microbiota. This review focuses on the basic biology of PPN, feeding habits, parasitic potential, molecular insights for understanding host-nematode relationship and their management by exploiting the inherent abilities of biocontrol agents.
Keywords
- plant parasitic nematodes
- parasitism
- biocontrol
- molecular
- pathogen
1. Introduction
Plant parasitic nematodes found in association with plants pose a major threat to the agriculture sector and are responsible for approximately $80–$118 billion dollars loss globally [1]. They are obligate parasites of plants which derive their nutrition from them by using their specialized structure viz., stylet. The stylet of PPNs required for feeding and also helps in the establishment of the nematode infection in the host as they used to pierce the plant cell wall for invasion. Furthermore, the stylet also showed connections to the glands present in the pharynx which helps in the production of the molecule’s requisite during the infection, invasion and other fundamental processes [2, 3]. These molecules released from the PPN affect the host immune system which ultimately enhances the parasitic association. These nematodes possess the potential to damage any part of the plant, however; roots found to be most susceptible for the PPN colonization especially endoparasites [4, 5]. The parasitic adaptations, feeding behavior and various lifestyle modes of PPN help in the survival in adverse conditions.
1.1 Morphology
Nematodes are the fascinating animals on earth after insects, with many free living and parasitic forms. It’s a noteworthy characteristic in case of nematodes that all the conceivable habitats viz., marine, soil and aquatic ecosystems are occupied by this amazing group. Diversity can also be seen in their shape and size as they occur in variable shapes such as melon shape (
Plant parasitic nematodes possess an accessory structure known as stylet which is utilized for association with the plants and deriving the nutrients. The shape, size and position of this stylet are of taxonomic importance for the identification of this nemic fauna. The stylet of PPN showed connections with the intestine because of attachment to the pharynx anteriorly (Figure 1). Order Tylenchida and Dorylaimida are found to be the pathogen of plants, invertebrates and fungi [8] and as parasites of agricultural crops and forest trees they have great economic importance. All possible habitats or ecological niches have been occupied by Tylenchids. The infection of PPN can be found in all the possible habitats; however, diversity occurred in the root parasites [5]. Over 4000 species of plant parasitic nematodes have been identified but it is very interesting to note that only few genera possess the potential of economic loss viz.,
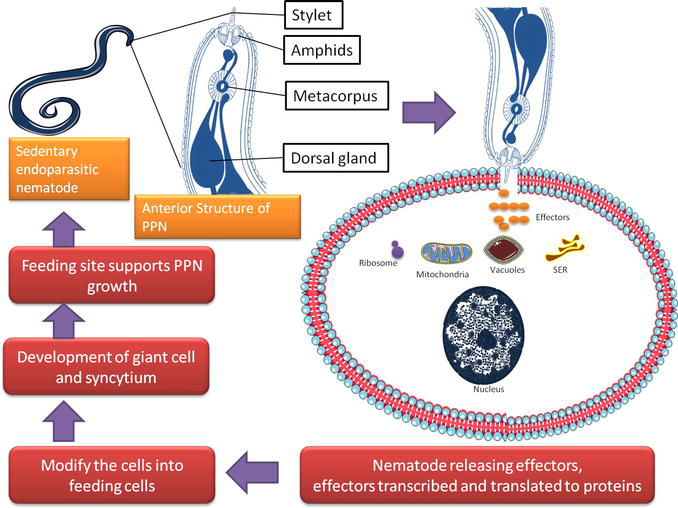
Figure 1.
The anterior end of plant parasitic nematode. The protrusible stylet helps in piercing the plant cell wall. The dorsal gland and other organs produced effectors which modify the surrounding cells for the development of feeding site.
1.2 Life cycle of plant parasitic nematodes
The life cycle of both root knot and cyst nematodes consists of 25–30 days on average. The second juvenile stage (J2) of the PPN finds the host roots for feeding. This stage showed attraction towards the roots and their penetration inside the host cells for satiating the nutrition needs developed by the synctial cells. The synctial comprises the host cells approximately 200 which lost their cell wall and contributed their protoplasts to the large feeding site [10]. After entering into the host cells, the J2 stage molts to J3, J3 molts to J4 and J4 molts to adults. The J2 stage once started feeding becomes larger in size and finally transforms to the males. Among all the larval stages, only the J2 stage showed mobility and others were immobile. Males possess a vermiform body and find their way out from the plants. The exterior environment consists of various chemical cues/pheromones released from the female’s body. These cues help males in locating the females for fertilization. Female body comprises eggs produced during fertilization. These eggs were protected in a cyst and all the cysts have the potential to release J2 stage. In case of cyst nematodes, J2 reached to the vascular cells and feeding site developed consists of syncytium cell, however, in root knot nematodes, J2 stage migrated to cortical cells where feeding site developed from the continuous mitosis without following cytokinesis [11]. The process leads to the development of a giant cell which becomes the feeding site for the J2 [12]. The J2 molts thrice and finally becomes an adult. Fertilization is not observed in the root knot, however, males formed during this entire process. Parthenogenesis observed in the root knot nematodes. Eggs are not present inside the female body, instead found outside the body in a protected matrix released from the female body itself. Feeding site either synctial cell or giant cells required repeat stimulus from J2 stage and both nematodes development and life cycle found dependent on these sites (Figure 2). This repeated thrust and maintenance of the feeding sites affects the roots which in turn affected the nutrient and water supply to the various parts of the plants, ultimately, affecting the yields.
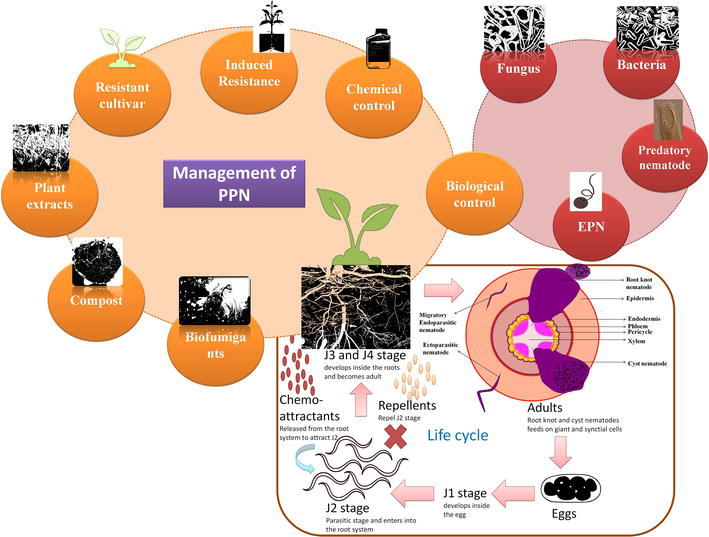
Figure 2.
Different feeding strategies and life cycle of plant parasitic nematode. The role of chemoattrants and repellents on the parasitic J2 stage during the host localization. Management of PPN using different strategies and biological control.
1.3 Feeding behavior strategies of PPN
PPNs are dependent on the plants for their survival and they are found in close proximity to the plant parts in order to complete their life cycle [6]. They are broadly categorized into above ground feeders and below ground feeders based on their feeding strategies adopted. Further, below ground feeders are classified into ectoparasites, semi-endoparasites and endoparasites.
1.3.1 Above ground feeders
1.3.1.1 Stem and bulb nematodes
Ditylenchus dipsac found responsible for causing disease in the plants as it largely affects the stem and bulb of the host hence referred to as stem and bulb nematodes. Hyacinth’s brown ring disease caused by this nematode in the plants where wilting, collapsing and yellowish color in the leaves was observed. In the garlic plants, the weight of the bulb decreases and they turn brownish and shriveled. These nematodes affect the storage of these plant bulbs ultimately decreasing the yield [7].
1.3.1.2 Seed gall nematodes
This nematode targets the seeds of the host plant affecting the yield. The disease which affects the seeds of wheat is caused by the Anguinatritici called ear-cockleseed or seed gall, hence commonly referred as seed gall nematode. They are ectoparasites but affect other areas of the plants as soon as it invades the seeds and inflorescence. The diseased plant showed symptoms such as wrinkles, swollen and bulged leaves, whitening, yellowish color of the stems with bending etc. [7].
1.3.1.3 Foliar nematodes
The nematodes belonging to genus
1.3.2 Below ground feeders
1.3.2.1 Ectoparasites
These nematodes found on the surface of the host and derive their nutrition from the root’s epidermis. They are further classified into sedentary ectoparasites and migratory ectoparasites. The sedentary nematodes showed specificity in the host and maintain a permanent attachment with the host, for example, Belonolaimus sp., Xiphenema sp., Trichodorus sp. etc. The J2 stage found to be the infective stage in these and only free-living stage. The migratory ectoparasites derive their nutrition from the roots itself but they do not maintain a permanent attachment with the host. Instead, if the roots get disturbed, they will show detachment from the roots and be found in the soil as free nematodes, for example, Criconemella sp., Paratylenchus sp., Hemicycliophora sp. etc. [7].
1.3.2.2 Semi endoparasites
These nematodes were attached with the host anteriorly, however, their posterior body was found free in the soil. They are further classified into sedentary and migratory semi-endoparasites. Insedentary semi-endoparasites, the anterior body showed permanent attachment with the host for example, Tylenchorchynchs, Hoplolaimus etc. TheJ3/J4–adult are the infective stages of sedentary nematodes. The migratory semi-endoparasites does not form any permanent attachment with the host andJ2/J4 are the infective stages, for example, Rotylenchulus sp., Tylenchulus sp. etc. [7].
1.3.2.3 Endoparasites
The endoparasites further categorized into migratory and sedentary. The migratory endoparasites enter into the host through roots and migrate to other areas as they feed on the cells, for exmple,
2. Adaptation to parasitism
2.1 Parasitic genes and effectors molecule of plant parasitic nematodes
2.1.1 Root-knot nematodes
PPN release effectors molecules to weaken the host immune system. One of the important and harmful root-knot nematodes viz., Meloidogyne releases a number of effectors which helps in their survival by combating the defense system. MiPFN3 gene in Meloidogyne incognita found to be coding for profilin (involved in disassembly of actin) which has the potential to bind the motor actin filament. The effectors molecule binds with the actin filament of the host cell disrupting its normal functioning facilitating the PPN survival [13]. The other effectors are secreted, encoded by gene MilSE5, which disrupts and interrupts important pathways of metabolism [14]. One of the gene viz., Misp12 secreted effectors which directly interfere with the defenses system of the host [15]. The Inflorescence Deficient in Abscission (IDA) like peptide is also encoded by the
2.1.2 Cyst nematodes
Cyst nematodes also released these effectors molecules which enhancing their survival inside the plant’s cells and tissues. The gene Hs30D08 in
2.1.3 Lesion nematodes
Lesion nematodes, one of migratory parasitic nematodes, releases effector molecules helping in either metabolism or suppression of the host defense system. The gene Ppen12895_c0_seq1 (FAR) encodes for the molecules that play a role in the metabolism of fatty acids in
2.1.4 Burrowing nematodes
The burrowing nematode especially,
2.1.5 Potato rot nematodes
In Ditylenchus destructor, two genes DD03093 (VAP-1) and DDC03397 (VAP-2) had been reported for coding the molecules which play an essential role in suppressing the host defense system [40]. The other gene viz., DD03835 (Sec-2) products facilitates the nematodes in overcoming the defense system of the host [40].
2.1.6 Pine wood/wilt nematodes
From the genome of
2.2 Adaptation of J2 stage during host invasion and colonization
2.2.1 Migratory endoparasites
The juvenile stages of PPNs such as Ditylenchus, Anguina and Pratylenchus require hosts for their survival purposes as they feed on them. Feeding is important for molting which ensures their survival. During extremities of temperature, pH, rain etc. the J2 stage enters into a dormant stage and possesses the ability of surviving without host for a certain period. The quiescent stage in
2.2.2 Sedentary endoparasites
The migratory stage in these PPNs is the second juvenile stage (J2) which is responsible for spreading the infection in the fields. The hatching of J2 stage from the egg found regulated from various chemical cues released from the specific host. The pre-J2 stage of sedentary nematodes can be quiescent in the environment for a longer period of time until it hatches out from the egg [48]. The signals from the host help in the hatching of the J2 from the egg and they showed similarity with the dauer larvae of free-living nematodes [49]. The J2 stage of cyst nematodes exhibit variations in the cuticle on the basis of lipophilicity as they perceive cues from the host [50]. These J2 stages are either pre- parasitic or parasitic, found to derive their energy from the deposited fat [51, 52]. The larvae of
2.3 Importance of chemotaxis in host recognition
Previous studies revealed that plant parasitic nematodes especially root knot nematodes (RKN) showed attraction to certain chemicals released from the roots exudates which helps them in finding their specific host [56, 57]. The chemotaxis behavior played an important role in the hatching, survival and development of the plant parasitic nematodes. Though chemicals/compounds released from the host helps in the attraction of J2 stage but some of them work as repellents, some act as stimulants for hatching of the J2 stage and some act as inhibitors [58, 59]. The chemoattractants of RKN found to be released from other parts of the plants also [60]. Likewise, cyst nematodes (CN) also showed chemotactic behavior in response to the compounds secreted from the different parts of the plants [59].
2.3.1 Factors influencing hatching
The hatching in
2.3.2 Chemo attractants
2.3.3 Repellents
There are some compounds which act as repellents in order to prevent the host from PPN infection. The bulb extracts of
3. Management of PPN
3.1 Biocontrol agent
3.1.1 Filamentous fungi
3.1.1.1 Trichoderma
This is considered as one of the important genera in the filamentous fungi for controlling the PPN population. They worked against both root knot and cyst nematodes. They are parasitic to the developmental stages of cyst nematodes. They penetrate the developmental stages by exploiting the enzymes chitinase and protease which break down the extracellular layer. Consequently, the egg number decreases due to fungus parasitism and low level of parasitic stage hatching occurred [87]. In South Africa, Romulus products are prepared from the T. harzianum wettable powder formulation by Dagutat Biolab for controlling the population. In India, there are two commercial products viz., ECOSOM® and commander fungicide prepared from the T. harzianum wettable powder formulation by Agri Life and H.T.C Impex Private Limited, respectively. In Columbia, two commercial products Trichobiol and Trifesol based on the T. harzianum wettable powder formulation were prepared by the Control Biologico Integrado; Mora Jaramillo Arturo Orlando—Biocontrol and Biocultivos Agricultura Sostenible, respectively, as fungal nematicide [88]. The potential of the Trichoderma genus against PPN was presented in Table 1.
| Fungus | PPN genera | Effects | References |
|---|---|---|---|
| inhibitory effect on the hatching of cysts | [89] | ||
| Effect on egg, J2 stage and females | [90] | ||
| Effect on egg, J2 stage and females | [91] | ||
| increased the mortality of the J2 | [92] | ||
| increased the mortality of the J2 | [93] | ||
| increased the mortality of the J2 | [94] | ||
| reduced infection and reproduction of the nematode; no effect on the eggs | [95] | ||
| decreased eggs hatching and increased the mortality of the J2 | [96] |
Table 1.
3.1.1.2 Mycorrhizal fungi
Previous findings on this genus did not report direct protection to the plants against PPN; however, it more efficiently worked on the plants, either by inducing morphological alterations in the roots, providing surplus nutrition or altering the environment for various interactions (Figure 3) [97, 98]. Recently, it’s been reported that they affect the PPN population and can be used in controlling the PPN infection. The
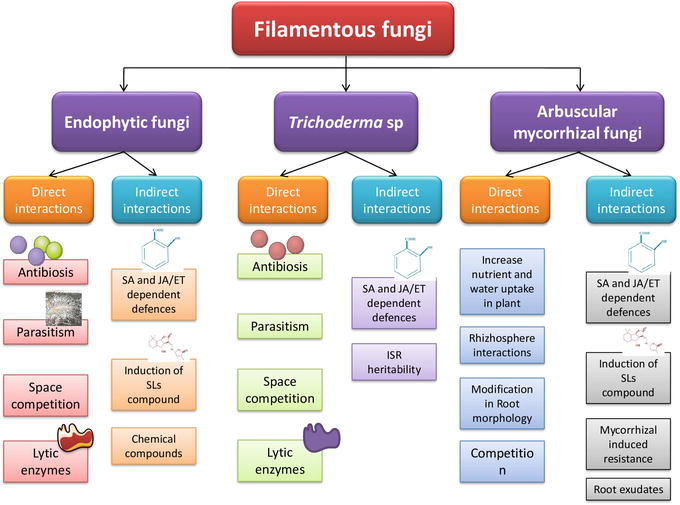
Figure 3.
Direct and indirect interactions of filamentous fungi with root system responsible for inducing induced resistance in plants against the PPN [
3.1.1.3 Endophytic fungi
The endophytic fungi are also considered for controlling the PPN infection in the field. The studies revealed that these fungi possess the potential of attacking, killing, immobilizing, repelling, interfering with the developmental cells and arouse confusion during host seeking in order to control the PPN population [105].
3.1.1.4 Aspergillus
This genus fungus showed parasitism to the PPN developmental stages and provided protection to the plants [110]. The
3.1.2 Bacteria
There are some bacterial species which are found efficacious against the phytoparasitica nematode. The
3.1.3 Entomopathogenic nematodes
Entomopathogenic nematodes (EPNs) gaining attention from the past few years due to their inherent ability of controlling the pest population. However, the characteristics of EPN as controlling agent of pest population also provide safer options from switching harmful insecticides and pesticides [114]. The omnipresent nature of EPN makes them suitable for including in the Integrated Pest Management (IPM) programs. The EPN belongs to two major families that is, Heterorhabditidae and Steinernematidae which includes 19 species from the
| S.no. | EPN | PPN managed | Crop | Conditions | Effects | References |
|---|---|---|---|---|---|---|
| 1. | turf grass | Reduced population | [118] | |||
| 2. | tomato | Greenhouse trials | Repelling Juveniles | [119] | ||
| 3. | Tomato, Soybeans | Greenhouse trials | reduction in root penetration | [120] | ||
| 4. | tomato | reduction in egg production and population of J2 | [111] | |||
| 5. | tomato | reduction in egg production and population of J2 | [121] | |||
| 6. | tomato | reduction in egg production and population of J2 | [121] | |||
| 7. | pecans | Greenhouse trials | reduction in egg masses | [116] | ||
| 8. | tomato | Inhibiting egg hatching and J2 infection | [122] | |||
| 9. | tomato | Inhibiting egg hatching and J2 infection | [122] | |||
| 10. | tomato | Greenhouse trials | reduction in reproduction factor | [123] | ||
| 11. | tomato | Greenhouse trials | reduction in reproduction factor | [123] | ||
| 12. | tomato | Greenhouse trials | reduced invasion of | [124] | ||
| 13. | pepper and summer squash | Greenhouse trials | reduction in egg masses | [125] | ||
| 14. | tomato | direct introduction of IJ/ | reduced RKN egg masses | [126] | ||
| 15. | tomato | Greenhouse trials | Decreased infection | [127] | ||
| 16. | tomato | reduced number of females | [128] | |||
| 17. | tomato | Greenhouse trials | reduction in egg masses and J2 population density | [117] | ||
| 18. | tomato | Greenhouse trials | reduction in egg masses and J2 population density | [117] | ||
| 19. | potato | reduction in reproduction and J2 population density | [129] |
Table 2.
The effects of different EPN species against PPN.
3.1.4 Predatory nematodes
The predatory nematodes feed on the plant parasitic nematode and can be exploited in controlling the PPN infection [130]. Most of the predators belong to Mononchida, Dorylaimida, Diplogasterida and Aphelenchida and each of the order possess specialized feeding apparatus [131].
3.2 Nematicides
3.2.1 Bionematicides
3.2.1.1 Antagonistic cultivated plants
There are some plants known for the production of compounds which restricts the growth of PPN [133]. These antagonistic/anthelmintic compounds are released in the soil where they regulate the PPN infection and protect the plants. There are so many species that release these anthelmintic compounds but some are found to play an important role in limiting the PPN infection [133]. The genus
3.2.1.2 Plant-related materials and compounds
There are some compounds isolated from the plants possessing anthelmintic properties that can be used for controlling the PPN infection. These compounds can be prepared from any part of the plant or sometimes whole plant for effective control. The prepared compound can be broadly categorized into acids, oils, alcohol etc. Though, most of these compounds have been prepared from the isolated metabolites of plants but this is not a necessary condition that these will be prepared only from plants. These can be prepared from other microorganisms as they also produce some similar compounds during metabolism [135]. The acetic acid production in the plants occurred during the metabolic pathways but this compound is also produced by the bacterium viz.,
3.3 Host resistance
ISR is an induced systemic resistance in the hosts against the parasites and pathogens causing disease. In this, the host defense system strengthens against the agents’ causing diseases through certain compounds [137]. The resistance in the host developed from a particular infection after suffering from the infection earlier and termed as induced resistance [138]. The induced resistance is further classified into two broad categories mainly SAR and ISR [139]. The SAR, that is, Systemic acquired resistance controls the disease-causing agent and decreases the harmful effects of the when compared with other plants. This SAR in the host plants found to be in coordination of the production of salicylic acid during the diseases [140]. The production of salicylic acid mediates the induction of PR-1 gene causing the degradation of pathogen walls. The ISR resistance is found to be regulated by the Jasmonic acid and ethylene pathways. The ISR does not depend upon the salicylic acid and does not show association with the PR genes expression [141]. There are some chemicals identified which provide resistance to the host plants against these parasites [142]. The successful management of lesion and burrowing nematodes found to be based on these chemicals which decrease the PPN infection and enhance the plant growth by providing resistance [143].
4. Conclusions
The PPN infection in the agricultural sectors is one of the major problems in the world. The exploitation of biological control agents especially, fungi, bacteria and EPN could be useful in management of PPN as suggested from research. Further, the use of these agents with other microorganisms or chemical/bionematicide can enhance their efficacy against the PPN. Till now, the use of chemical nematicide against the PPN has some drawbacks which draw the attention towards using the biopesticide. To use EPN agents in the fields it’s necessary to develop a successful formulation and extensive studies on their virulence activity against the PPN in
References
- 1.
Bernard GC, Egnin M, Bonsi C. The impact of plant-parasitic nematodes on agriculture and methods of control. Nematology-Concepts, Diagnosis and Control. 2017; 1 :121-151 - 2.
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology. 2013; 14 (9):946-961 - 3.
Mejias J, Truong NM, Abad P, Favery B, Quentin M. Plant proteins and processes targeted by parasitic nematode effectors. Frontiers in Plant Science. 2019; 10 :970 - 4.
Zinovieva SV. Co-adaptation mechanisms in plant-nematode systems. Parazitologiia. 2014; 48 (2):110-130 - 5.
Vieira P, Gleason C. Plant-parasitic nematode effectors—Insights into their diversity and new tools for their identification. Current Opinion in Plant Biology. 2019; 50 :37-43 - 6.
Hartman GL, Rupe JC, Sikora EJ, Domier LL, Davis JA, Steffey KL. Compendium of Soybean Diseases and Pests. St. Paul, MN: American Phytopathological Society; 2015 - 7.
El-Saadony MT, Abuljadayel DA, Shafi ME, Albaqami NM, Desoky ES, El-Tahan AM, et al. Control of foliar phytoparasitic nematodes through sustainable natural materials: Current progress and challenges. Saudi Journal of Biological Sciences. 2021; 28 (12):7314-7326 - 8.
Sasser JN, Freckman DW. World perspective on nematology: The role of society. In: Veech JA, Dickson DW, editors. Vistas on Nematology: A Commemoration of the 25th Anniversary of the Society of Nematologists. Hyattsville, Maryland: Society of Nematologists Inc.; 1987. pp. 7-14 - 9.
Ghaderi R, Karssen G. An updated checklist of Meloidogyne Göldi, 1887 species, with a diagnostic compendium for second-stage juveniles and males. Journal of Crop Protection. 2020; 9 (2):183-193 - 10.
Jones MG. Host cell responses to endoparasitic nematode attack: Structure and function of giant cells and syncytia. Annals of Applied Biology. 1981; 97 (3):353-372 - 11.
Von Mende N. Invasion and migration behaviour of sedentary nematodes. In: Fenol C, Grundler FMW, Ohl SA, editors. Cellular and Molecular Aspects of Plant-Nematode Interactions. The Netherlands: Kluwer Academic Publishers; 1997. pp. 51-64 - 12.
Jagdale S, Rao U, Giri AP. Effectors of root-knot nematodes: An arsenal for successful parasitism. Frontiers in Plant Science. 2021; 12 :800030 - 13.
Leelarasamee N, Zhang L, Gleason C. The root-knot nematode effector MiPFN3 disrupts plant actin filaments and promotes parasitism. PLoS Pathogens. 2018; 14 (3):e1006947 - 14.
Shi Q , Mao Z, Zhang X, Zhang X, Wang Y, Ling J, et al. A Meloidogyne incognita effector MiISE5 suppresses programmed cell death to promote parasitism in host plant. Scientific Reports. 2018; 8 (1):7256 - 15.
Xie J, Li S, Mo C, Wang G, Xiao X, Xiao Y. A novel Meloidogyne incognita effector Misp12 suppresses plant defense response at latter stages of nematode parasitism. Frontiers in Plant Science. 2016; 7 :964 - 16.
Wubben MJ, Gavilano L, Baum TJ, Davis EL. Sequence and spatiotemporal expression analysis of CLE-motif containing genes from the reniform nematode (Rotylenchulusreniformis Linford & Oliveira). Journal of Nematology. 2015; 47 (2):159 - 17.
Gleason C, Polzin F, Habash SS, Zhang L, Utermark J, Grundler FM, et al. Identification of two Meloidogyne hapla genes and an investigation of their roles in the plant-nematode interaction. Molecular Plant-Microbe Interactions. 2017;30 (2):101-112 - 18.
Lin B, Zhuo K, Chen S, Hu L, Sun L, Wang X, et al. A novel nematode effector suppresses plant immunity by activating host reactive oxygen species-scavenging system. New Phytologist. 2016; 209 (3):1159-1173 - 19.
Chen J, Lin B, Huang Q , Hu L, Zhuo K, Liao J. A novel Meloidogyne graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism. PLoS Pathogens. 2017; 13 (4):e1006301 - 20.
Naalden D, Haegeman A, de Almeida-Engler J, Birhane Eshetu F, Bauters L, Gheysen G. The Meloidogyne graminicola effector Mg16820 is secreted in the apoplast and cytoplasm to suppress plant host defense responses. Molecular Plant Pathology. 2018; 19 (11):2416-2430 - 21.
Zhuo K, Naalden D, Nowak S, Xuan Huy N, Bauters L, Gheysen G. A Meloidogyne graminicola C-type lectin, Mg01965, is secreted into the host apoplast to suppress plant defence and promote parasitism. Molecular Plant Pathology. 2019; 20 (3):346-355 - 22.
Zhuo K, Chen J, Lin B, Wang J, Sun F, Hu L, et al. A novel Meloidogyne enterolobii effector MeTCTP promotes parasitism by suppressing programmed cell death in host plants. Molecular Plant Pathology. 2017; 18 (1):45-54 - 23.
Verma A, Lee C, Morriss S, Odu F, Kenning C, Rizzo N, et al. The novel cyst nematode effector protein 30D08 targets host nuclear functions to alter gene expression in feeding sites. New Phytologist. 2018; 219 (2):697-713 - 24.
Habash SS, Sobczak M, Siddique S, Voigt B, Elashry A, Grundler FM. Identification and characterization of a putative protein disulfide isomerase (HsPDI) as an alleged effector of Heteroderaschachtii. Scientific Reports. 2017; 7 (1):13536 - 25.
Habash SS, Radakovic ZS, Vankova R, Siddique S, Dobrev P, Gleason C, et al. Heteroderaschachtii Tyrosinase-like protein-a novel nematode effector modulating plant hormone homeostasis. Scientific Reports. 2017; 7 (1):6874 - 26.
Vieira P, Eves-Van Den Akker S, Verma R, Wantoch S, Eisenback JD, Kamo K. The Pratylenchus penetrans transcriptome as a source for the development of alternative control strategies: Mining for putative genes involved in parasitism and evaluation of in planta RNAi. PLoS One. 2015; 10 (12):e0144674 - 27.
Barnes SN, Wram CL, Mitchum MG, Baum TJ. The plant-parasitic cyst nematode effector GLAND4 is a DNA-binding protein. Molecular Plant Pathology. 2018; 19 (10):2263-2276 - 28.
Vijayapalani P, Hewezi T, Pontvianne F, Baum TJ. An effector from the cyst nematode Heteroderaschachtii derepresses host rRNA genes by altering histone acetylation. The Plant Cell. 2018; 30 (11):2795-2812 - 29.
Liu J, Peng H, Cui J, Huang W, Kong L, Clarke JL, et al. Molecular characterization of a novel effector expansin-like protein from Heteroderaavenae that induces cell death in Nicotiana benthamiana. Scientific Reports. 2016; 6 (1):35677 - 30.
Noon JB, Qi M, Sill DN, Muppirala U, Eves-van den Akker S, Maier TR, et al. A plasmodium-like virulence effector of the soybean cyst nematode suppresses plant innate immunity. New Phytologist. 2016; 212 (2):444-460 - 31.
Mei Y, Wright KM, Haegeman A, Bauters L, Diaz-Granados A, Goverse A, et al. The Globodera pallida SPRYSEC effector Gp SPRY-414-2 that suppresses plant defenses targets a regulatory component of the dynamic microtubule network. Frontiers in Plant Science. 2018; 9 :1019 - 32.
Lilley CJ, Maqbool A, Wu D, Yusup HB, Jones LM, Birch PR, et al. Effector gene birth in plant parasitic nematodes: Neofunctionalization of a housekeeping glutathione synthetase gene. PLoS Genetics. 2018; 14 (4):e1007310 - 33.
Vieira P, Maier TR, Eves-van den Akker S, Howe DK, Zasada I, Baum TJ, et al. Identification of candidate effector genes of Pratylenchus penetrans. Molecular Plant Pathology. 2018; 19 (8):1887-1907 - 34.
Fosu-Nyarko J, Tan JA, Gill R, Agrez VG, Rao U, Jones MG. D e novo analysis of the transcriptome of P ratylenchuszeae to identify transcripts for proteins required for structural integrity, sensation, locomotion and parasitism. Molecular Plant Pathology. 2016; 17 (4):532-552 - 35.
Huang X, Xu CL, Chen WZ, Chen C, Xie H. Cloning and characterization of the first serine carboxypeptidase from a plant parasitic nematode, Radopholussimilis. Scientific Reports. 2017; 7 (1):4815 - 36.
Wang K, Li Y, Huang X, Wang DW, Xu CL, Xie H. The cathepsin S cysteine proteinase of the burrowing nematode Radopholussimilis is essential for the reproduction and invasion. Cell & Bioscience. 2016; 6 :1-5 - 37.
Li Y, Wang K, Xie H, Wang DW, Xu CL, Huang X, et al. Cathepsin B cysteine proteinase is essential for the development and pathogenesis of the plant parasitic nematode Radopholussimilis. International Journal of Biological Sciences. 2015; 11 (9):1073 - 38.
Li Y, Wang K, Xie H, Wang YT, Wang DW, Xu CL, et al. A nematode calreticulin, Rs-CRT, is a key effector in reproduction and pathogenicity of Radopholussimilis. PLoS One. 2015; 10 (6):e0129351 - 39.
Zhang C, Xie H, Cheng X, Wang DW, Li Y, Xu CL, et al. Molecular identification and functional characterization of the fatty acid-and retinoid-binding protein gene Rs-far-1 in the burrowing nematode Radopholussimilis (Tylenchida: Pratylenchidae). PLoS One. 2015; 10 (3):e0118414 - 40.
Peng H, Gao BL, Kong LA, Yu Q , Huang WK, He XF, et al. Exploring the host parasitism of the migratory plant-parasitic nematode Ditylenchus destuctor by expressed sequence tags analysis. PLoS One. 2013; 8 (7):e69579 - 41.
Hu LJ, Wu XQ , Li HY, Zhao Q , Wang YC, Ye JR. An effector, BxSapB1, induces cell death and contributes to virulence in the pine wood nematode Bursaphelenchusxylophilus. Molecular Plant-Microbe Interactions. 2019; 32 (4):452-463 - 42.
Shinya R, Morisaka H, Kikuchi T, Takeuchi Y, Ueda M, Futai K. Secretome analysis of the pine wood nematode Bursaphelenchusxylophilus reveals the tangled roots of parasitism and its potential for molecular mimicry. PLoS One. 2013; 8 (6):e67377 - 43.
Espada M, Silva AC, Eves van den Akker S, Cock PJ, Mota M, Jones JT. Identification and characterization of parasitism genes from the pinewood nematode Bursaphelenchusxylophilus reveals a multilayered detoxification strategy. Molecular Plant Pathology. 2016; 17 (2):286-295 - 44.
Tarjan AC. Longevity of Radopholussimilis (cobb) in host-free soil. Nematologica. 1961; 6 :170-175 - 45.
Bird AF, Buttrose MS. Ultrastructural changes in the nematode Anguinatritici associated with an hydrobiosis. Journal of Ultrastructure Research. 1974; 48 (2):177-189 - 46.
Perry RN, Moens M. Survival of parasitic nematodes outside the host. In: Molecular and Physiological Basis of Nematode Survival. Wallingford UK: CAB International; 2011. pp. 1-27 - 47.
Van Megen H, Van Den Elsen S, Holterman M, Karssen G, Mooyman P, Bongers T, et al. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology. 2009; 11 :927-950 - 48.
Hunt DJ, Luc M, Manzanilla-López RH. Plant parasitic nematodes in subtropical and tropical agriculture. In: Luc M, Sikora RA, Bridge J, editors. Identification, Morphology and Biology of Plant Parasitic Nematodes. 2nd ed. Wallingford: CABI publishing; 2005. pp. 11-52 - 49.
Yang D, Chen C, Liu Q , Jian H. Comparative analysis of pre- and postparasitic transcriptomes and mining pioneer effectors of heteroderaavenae. Cell & Bioscience. 2017; 7 :1-18 - 50.
Proudfoot L, Kusel JR, Smith HV, Harnett W, Worms MJ, Kennedy MW. Rapid changes in the surface of parasitic nematodes during transition from pre- to post-parasitic forms. Parasitology. 1993; 107 :107-117 - 51.
McCarter JP, Mitreva MD, Martin J, Dante M, Wylie T, Rao U, et al. Analysis and functional classification of transcripts from the nematode meloidogyne incognita. Genome Biology. 2003; 4 :1-19 - 52.
Popeijus H, Blok VC, Cardle L, Bakker E, Phillips MS, Helder J, et al. Analysis of genes expressed in second stage juveniles of the potato cyst nematodes globoderarostochiensis and G. pallida using the expressed sequence tag approach. Nematology. 2000; 2 :567-574 - 53.
Schroeder NE, Mac Guidwin AE. Behavioural quiescence reduces the penetration and toxicity of exogenous compounds in second-stage juveniles of heteroderaglycines. Nematology. 2010; 12 :277-287 - 54.
Sikder MM, Vestergård M. Impacts of root metabolites on soil nematodes. Frontiers in Plant Science. 2020; 10 :1-18 - 55.
Perry RN, Clarke AJ. Hatching mechanisms of nematodes. Parasitology. 1981; 83 :435-449 - 56.
Reynolds AM, Dutta TK, Curtis RH, Powers SJ, Gaur HS, Kerry BR. Chemotaxis can take plant-parasitic nematodes to the source of a chemo-attractant via the shortest possible routes. Journal of the Royal Society Interface. 2011; 8 (57):568-577 - 57.
Liu W, Jones AL, Gosse HN, Lawrence KS, Park SW. Validation of the chemotaxis of plant parasitic nematodes toward host root exudates. Journal of Nematology. 2019; 51 (1):1-10 - 58.
Oota M, Tsai AY, Aoki D, Matsushita Y, Toyoda S, Fukushima K, et al. Identification of naturally occurring polyamines as root-knot nematode attractants. Molecular Plant. 2020; 13 (4):658-665 - 59.
Ochola J, Coyne D, Cortada L, Haukeland S, Ng'ang'a M, Hassanali A, et al. Cyst nematode bio-communication with plants: Implications for novel management approaches. Pest Management Science. 2021; 77 (3):1150-1159 - 60.
Tsai AY, Iwamoto Y, Tsumuraya Y, Oota M, Konishi T, Ito S, et al. Root-knot nematode chemotaxis is positively regulated by l-galactose sidechains of mucilage carbohydrate rhamnogalacturonan-I. Science Advances. 2021; 7 (27):eabh 4182 - 61.
Masler E, Perry R. In: Perry RN, Moens M, Jones JT, editors. Hatch, Survival and Sensory Perception Cyst Nematodes. Wallingford, UK: C.A.B.I; 2018. pp. 44-73 - 62.
Bohlmann H. Introductory chapter on the basic biology of cyst nematodes. Advances in Botanical Research. 2015; 73 :33-59 - 63.
Nonaka S, Katsuyama T, Kondo T, Sasaki Y, Asami T, Yajima S, et al. 1, 10-Phenanthroline and its derivatives are novel hatching stimulants for soybean cyst nematodes. Bioorganic and Medicinal Chemistry Letters. 2016; 26 (21):5240-5243 - 64.
Renčo M, Sasanelli N, Papajová I, Maistrello L. Nematicidal effect of chestnut tannin solutions on the potato cyst nematode Globoderarostochiensis (Woll.) Barhens. Helminthologia. 2012; 49 :108-114 - 65.
Riga E, Topp E, Potter J, Welacky T, Anderson T, Tenuta A. The impact of plant residues on the soybean cyst nematode, Heterodera glycines . Canadian Journal of Plant Pathology. 2001;23 (2):168-173 - 66.
Charlson DV, Tylka GL. Heteroderaglycines cyst components and surface disinfestants affect H. Glycines hatching. Journal of Nematology. 2003; 35 (4):458 - 67.
Tanino K, Takahashi M, Tomata Y, Tokura H, Uehara T, Narabu T. Total synthesis of Solanoeclepin A. Nature Chemistry. 2011; 3 :484-488 - 68.
Jones P, Byrne J, Devine K. In vitro studies on the relative availability and mobility in soil of natural hatching factors for the potato cyst nematodes, Globodera rostochiensis andG. pallida . Nematology. 2001;3 (1):75-83 - 69.
Ganapati Reddy P, Chun B-K, Zhang H-R, Rachakonda S, Ross BS, Sofia MJ. Stereoselective synthesis of PSI-352938: A β-D-2′-deoxy-2′-α-fluoro-2′-β-C-methyl-3′,5′-cyclic phosphate nucleotide prodrug for the treatment of HCV. Journal of Organic Chemistry. 2011; 76 :3782-3790 - 70.
Zhou L, Wang J, Wang K, Xu J, Zhao J, Shan T, et al. Secondary metabolites with antinematodal activity from higher plants. Studies in Natural Products Chemistry. 2012; 37 :67-114 - 71.
Yu Q , Tsao R, Chiba M, Potter J. Selective nematicidal activity of allyl isothiocyanate. Journal of Food, Agriculture and Environment. 2005; 3 :218-221 - 72.
Ghisalberti EL. Secondary metabolites with antinematodal activity. Studies in Natural Products Chemistry. 2002; 26 :425-506 - 73.
Díaz-Tielas C, Graña E, Reigosa MJ, Sánchez-Moreiras AM. Biological activities and novel applications of chalcones. Planta Daninha. 2016; 34 :607-616 - 74.
Silva FJ, Campos VP, Oliveira DF, Gomes VA, Barros AF, Din ZU, et al. Chalcone analogues: Synthesis, activity against Meloidogyne incognita, and in silico interaction with cytochrome P 450. Journal of Phytopathology. 2019; 167 (4):197-208 - 75.
Perry R, Warrior P, Kerry B, Twomey U. Effects of the biological nematicide, DiTera, on hatching of Globoderarostochiensis and G. Pallida. Journal of Nematology. 2000; 2 :355-362 - 76.
Cox D, Dyer S, Weir R, Cheseto X, Sturrock M, Coyne D, et al. ABC transporters alter plant-microbe-parasite interactions in the rhizosphere. Science Reports. 2019; 9 :526582 - 77.
Wood C, Kenyon DM, Cooper JM. Allyl isothiocyanate shows promise as a naturally produced suppressant of the potato cyst nematode, Globodera pallida, in biofumigation systems. Nematology. 2017; 19 (4):389-402 - 78.
Zhang J, Li Y, Yuan H, Sun B, Li H. Biological control of the cereal cyst nematode (Heteroderafilipjevi) by Achromobacterxylosoxidans isolate 09X01 and Bacillus cereus isolate 09B18. Biological Control. 2016; 92 :1-6 - 79.
Fleming TR, Maule AG, Fleming CC. Chemosensory responses of plant parasitic nematodes to selected phytochemicals reveal long-term habituation traits. Journal of Nematology. 2017; 49 (4):462 - 80.
Wang C, Masler EP, Rogers ST. Responses of Heteroderaglycines and Meloidogyne incognita infective juveniles to root tissues, root exudates, and root extracts from three plant species. Plant Disease. 2018; 102 (9):1733-1740 - 81.
Kammerhofer N, Radakovic Z, Regis JM, Dobrev P, Vankova R, Grundler FM, et al. Role of stress-related hormones in plant defence during early infection of the cyst nematode Heteroderaschachtii in Arabidopsis. New Phytologist. 2015; 207 (3):778-789 - 82.
Būda V, Čepulytė-Rakauskienė R. The effects of α-solanine and zinc sulphate on the behaviour of potato cyst nematodes Globoderarostochiensis and G. Pallida. Nematology. 2015; 17 (9):1105-1111 - 83.
Būda V, Čepulyt Ė-Rakauskien Ė R. The effect of linalool on second-stage juveniles of the potato cyst nematodes Globoderarostochiensis and G. Pallida. Journal of Nematology. 2011; 43 (3-4):149 - 84.
Hu Y, You J, Li C, Williamson VM, Wang C. Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode heteroderaglycines. Scientific Reports. 2017; 7 (1):41282 - 85.
Hu Y, You J, Li C, Pan F, Wang C. Assessing the effects of water extract of Narcissus tazetta bulb on hatching, mortality, chemotaxis and reproduction of the soybean cyst nematode,Heterodera glycines . Nematology. 2019;22 (1):53-62 - 86.
Escudero Martinez CM, Guarneri N, Overmars H, van Schaik C, Bouwmeester H, Ruyter-Spira C, et al. Distinct roles for strigolactones in cyst nematode parasitism of Arabidopsis roots. European Journal of Plant Pathology. 2019; 154 :129-140 - 87.
Poveda J, Abril-Urias P, Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Frontiers in Microbiology. 2020; 11 :992 - 88.
Abd-Elgawad MM, Askary TH. Fungal and bacterial nematicides in integrated nematode management strategies. Egyptian Journal of Biological Pest Control. 2018; 28 (1):1-24 - 89.
Zhang S, Gan Y, Xu B, Xue Y. The parasitic and lethal effects of Trichoderma longibrachiatum against Heterodera avenae . Biological Control. 2014;72 :1-8 - 90.
Zhang S, Gan Y, Ji W, Xu B, Hou B, Liu J. Mechanisms and characterization of Trichoderma longibrachiatum T6 in suppressing nematodes ( Heterodera avenae ) in wheat. Frontiers in Plant Science. 2017;8 :1491 - 91.
Kumar P, Chand R. Bio-efficacy of Trichoderma harzianum against root knot nematodeMeloidogyne incognita on Brinjal. Annals of Plant Protection Sciences. 2015;23 (2):361-364 - 92.
Brahma U, Borah A. Management of Meloidogyne incognita on pea with bioagents and organic amendment. Indian Journal of Nematology. 2016;46 (1):58-61 - 93.
Devi TS, Mahanta B, Borah A. Comparative efficacy of Glomus fasciculatum ,Trichoderma harzianum ,carbofuran andcarbendazim in management ofMeloidogyne incognita andRhizoctonia solani disease complex on brinjal. Indian Journal of Nematology. 2016;46 (2):161-164 - 94.
Deori R, Borah A. Efficacy of Glomus fasciculatum ,Trichoderma harzianum for the management ofMeloidogyne incognita andRhizoctonia solani disease complex in green gram. Indian Journal of Nematology. 2016;46 (1):61-64 - 95.
Contina JB, Dandurand LM, Knudsen GR. Use of GFP-tagged Trichoderma harzianum as a tool to study the biological control of the potato cyst nematodeGlobodera pallida . Applied Soil Ecology. 2017;1 (115):31-37 - 96.
Khan MR, Ahmad I, Ahamad F. Effect of pure culture and culture filtrates of Trichoderma species on root-knot nematode,Meloidogyne incognita infesting tomato. Indian Phytopathology. 2018;71 :265-274 - 97.
Schouteden N, De Waele D, Panis B, Vos CM. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Frontiers in Microbiology. 2015;6 :1280 - 98.
Wani KA, Manzoor J, Shuab R, Lone R. Arbuscular mycorrhizal fungi as biocontrol agents for parasitic nematodes in plants. In: Mycorrhiza-nutrient uptake, biocontrol, ecorestoration. Cham: Springer; 2017. pp. 195-210 - 99.
Marro N, Caccia M, Doucet ME, Cabello M, Becerra A, Lax P. Mycorrhizas reduce tomato root penetration by false root-knot nematode Nacobbus aberrans . Applied Soil Ecology. 2018;124 :262-265 - 100.
Calvet C, Pinochet J, Hernández-Dorrego A, Estaún V, Camprubí A. Field microplot performance of the peach-almond hybrid GF-677 after inoculation with Arbuscular mycorrhizal fungi in a replant soil infested with root-knot nematodes. Mycorrhiza. 2001;10 :295-300 - 101.
Brito OD, Hernandes I, Ferreira JC, Cardoso MR, Alberton O, Dias-Arieira CR. Association between Arbuscular mycorrhizal fungi andPratylenchus brachyurus in maize crop. Chilean Journal of Agricultural Research. 2018;78 (4):521-527 - 102.
Ferreira BS, Santana MV, Macedo RS, Silva JO, Carneiro MA, Rocha MR. Co-occurrence patterns between plant-parasitic nematodes and arbuscular mycorrhizal fungi are driven by environmental factors. Agriculture, Ecosystems and Environment. 2018; 265 :54-61 - 103.
Emery SM, Reid ML, Bell-Dereske L, Gross KL. Soil mycorrhizal and nematode diversity vary in response to bioenergy crop identity and fertilization. GCB Bioenergy. 2017; 9 (11):1644-1656 - 104.
Alvarado-Herrejón M, Larsen J, Gavito ME, Jaramillo-López PF, Vestberg M, Martínez-Trujillo M, et al. Relation between arbuscular mycorrhizal fungi, root-lesion nematodes and soil characteristics in maize agroecosystems. Applied Soil Ecology. 2019; 135 :1-8 - 105.
Schouten A. Mechanisms involved in nematode control by endophytic fungi. Annual Review of Phytopathology. 2016; 54 :121-142 - 106.
Mwaura P, Dubois T, Losenge T, Coyne D, Kahangi E. Effect of endophytic Fusarium oxysporum on paralysis and mortality ofPratylenchus goodeyi . African Journal of Biotechnology. 2010;9 (8):1130-1134 - 107.
Khan B, Yan W, Wei S, Wang Z, Zhao S, Cao L, et al. Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiology Letters. 2019; 366 (14):fnz 169 - 108.
Liarzi O, Bucki P, Braun Miyara S, Ezra D. Bioactive volatiles from an endophytic Daldinia cf. concentrica isolate affect the viability of the plant parasitic nematode Meloidogyne javanica . PLoS One. 2016;11 (12):e0168437 - 109.
Lou J, Yu R, Wang X, Mao Z, Fu L, Liu Y, et al. Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif 01 and its bioactivities. Brazilian Journal of Microbiology. 2016; 47 :96-101 - 110.
Askary TH. Nematophagous fungi as biocontrol agents of phytonematodes. In: Biocontrol Agents of Phytonematodes. Wallingford, UK: CABI; 2015 - 111.
Bhat MY, Wani AH. Bio-activity of fungal culture filtrates against root-knot nematode egg hatch and juvenile motility and their effects on growth of mung bean (Vigna radiata L. Wilczek) infected with the root-knot nematode, Meloidogyne incognita . Archives of Phytopathology and Plant Protection. 2012;45 (9):1059-1069 - 112.
Zareen A, Zaki MJ, Khan NJ. Effect of fungal filtrates of aspergillus species on development of root-knot nematodes and growth of tomato (Lycopersicon esculentum mill). Pakistan Journal of Biological Sciences. 2001; 4 (8):995-999 - 113.
Mokbel AA, Obad IM, Ibrahim IK. The role of antagonistic metabolites in controlling root-knot nematode, Meloidogyne arenaria on tomato. Alexandria Journal of Agricultural Research. 2009;54 (1):199-205 - 114.
Khan RR, Ali RA, Ali A, Arshad M, Majeed S, Ahmed S, et al. Compatibility of entomopathogenic nematodes (Nematoda: Rhabditida) and the biocide, spinosad for mitigation of the armyworm, Spodoptera litura (F.)(Lepidoptera: Noctuidae). Egyptian Journal of Biological Pest Control. 2018; 28 :1-6 - 115.
Sharma H, Rana A, Bhat AH, Chaubey AK. Entomopathogenic nematodes: Their characterization, bio-control properties and new perspectives. In: Nematodes-Recent Advances, Management and New Perspectives. London: Books on Demand; 2021. p. 187 - 116.
Malan AP, Knoetze R, Kapp C, Tiedt L, Steinernemabakwenae n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology. 2023; 25 :275-293 - 117.
El Aimani A, Houari A, Laasli SE, Mentag R, Iraqi D, Diria G, et al. Antagonistic potential of Moroccan entomopathogenic nematodes against root-knot nematodes, Meloidogyne javanica on tomato under greenhouse conditions. Scientific Reports. 2022;12 (1):2915 - 118.
Grewal PS, Martin WR, Miller RW, Lewis EE. Suppression of plant-parasitic nematode populations in turfgrass by application of entomopathogenic nematodes. Biocontrol Science and Technology. 1997; 7 :393-399 - 119.
Grewal PS, Lewis EE, Venkatachari S. Allelopathy: A possible mechanism of suppression of plant-parasitic nematodes by entomopathogenic nematodes. Nematology. 1999; 1 (7):735-743 - 120.
Fallon DJ, Kaya HK, Gaugler R, Sipes BS. Effects of etomopathiogenic nematodes on Meloidogyne javanica on tomatoes and soybeans. Journal of Nematology. 2002;34 (3):239 - 121.
Pérez EE, Lewis EE. Use of entomopathogenic nematodes to suppress Meloidogyne incognita on greenhouse tomatoes. Journal of Nematology. 2002;34 (2):171 - 122.
Molina JP, Dolinski C, Souza RM, Lewis EE. Effect of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) on Meloidogyne mayaguensis Rammah and Hirschmann (Tylenchida: Meloidoginidae) infection in tomato plants. Journal of Nematology. 2007;39 (4):338 - 123.
Caccia M, Lax P, Doucet ME. Effect of entomopathogenic nematodes on the plant-parasitic nematode Nacobbus aberrans . Biology and Fertility of Soils. 2013;49 :105-109 - 124.
Javed N, Khan SA, Atiq M, Kamran M. Effect of Steinernema glaseri andHeterorhabditis indica on the plant vigour and root knot nematodes in tomato roots at different densities and time of applications. Pakistan Journal of Zoology. 2012;44 (4):1165-1170 - 125.
del Valle EE, Lax P, Rondán Dueñas J, Doucet ME. Effects of insect cadavers infected by Heterorhabditis bacteriophora andSteinernema diaprepesi onMeloidogyne incognita parasitism in pepper and summer squash plants. Agricultural Science and Research. 2013;40 :109-118 - 126.
Kepenekci I, Hazir S, Lewis EE. Evaluation of entomopathogenic nematodes and the supernatants of the in vitro culture medium of their mutualistic bacteria for the control of the root-knot nematodesMeloidogyne incognita andM. arenaria . Pest Management Science. 2016;72 (2):327-334 - 127.
Kepenekci I, Hazir S, Oksal E, Lewis EE. Application methods of Steinernema feltiae ,Xenorhabdusb ovienii andPurpureocillium lilacinum to control root-knot nematodes in greenhouse tomato systems. Crop Protection. 2018;108 :31-38 - 128.
Khan SA, Javed N, Kamran M, Abbas H, Safdar A, ul Haq I. Management of Meloidogyne incognita race 1 through the use of entomopathogenic nematodes in tomato. Pakistan Journal of Zoology. 2016;48 (3):763 - 129.
Osman HA, Ameen HH, Hammam MM, El-Sayed GM, Elkelany US, Abd-Elgawad MM. Antagonistic potential of an Egyptian entomopathogenic nematode, compost and two native endophytic bacteria isolates against the root-knot nematode ( Meloidogyne incognita ) infecting potato under field conditions. Egyptian Journal of Biological Pest Control. 2022;32 (1):137 - 130.
Esser E. Biological control of nematodes by nematodes I. Dorylaims (Nematoda: Dorylaimida). Nematology Circular. 1987; 144 :4 - 131.
Pervez R, Bilgrami AL, Yoshiga T, Kondo E. Feeding, attraction and aggregation behaviour of Mesodorylaimus bastiani andAquatides thornei at the feeding site usingHirschmanniella oryzae as prey. International Journal of Nematology. 2000;10 (2):207-214 - 132.
Kanwar RS, Patil JA, Yadav S. Prospects of using predatory nematodes in biological control for plant parasitic nematodes—A review. Biological Control. 2021; 160 :104668 - 133.
Grubišić D, Uroić G, Ivošević A, Grdiša M. Nematode control by the use of antagonistic plants. Agriculturae Conspectus Scientificus. 2018; 83 (4):269-275 - 134.
Abd-Elgawad MM. Optimizing safe approaches to manage plant-parasitic nematodes. Plants. 2021; 10 (9):1911 - 135.
Ntalli N, Ratajczak M, Oplos C, Menkissoglu-Spiroudi U, Adamski Z. Acetic acid, 2-undecanone, and (E)-2-decenal ultrastructural malformations on Meloidogyne incognita . Journal of Nematology. 2016;48 (4):248 - 136.
Seo HJ, Park AR, Kim S, Yeon J, Yu NH, Ha S, et al. Biological control of root-knot nematodes by organic acid-producing Lactobacillus brevis wikim 0069 isolated from kimchi. The Plant Pathology Journal. 2019;35 (6):662 - 137.
Conrath U. Systemic acquired resistance. Plant Signaling & Behavior. 2006; 1 (4):179-184 - 138.
Hammerschmidt R. Introduction: Definitions and some history. In: Induced Resistance for Plant Defense. United States: Wiley; 2014. pp. 1-10 - 139.
El-Deeb TS, Bakkar SM, Eltoony L, Zakhary MM, Kamel AA, Nafee AM, et al. The adipokine chemerin and fetuin-a serum levels in type 2 diabetes mellitus: Relation to obesity and inflammatory markers. The Egyptian Journal of Immunology. 2018; 25 (1):191-202 - 140.
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. The Plant Cell. 1996; 8 (10):1809 - 141.
Vallad GE, Goodman RM. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Science. 2004; 44 (6):1920-1934 - 142.
Malamy J, Sanchez-Casas P, Hennig J, Guo A, Klessig F. Dissection of the salicylic acid signaling pathway in tobacco. American Phytopathological Society. 1996; 9 :474-482 - 143.
Chałańska A, Łabanowski G, Maciorowski R. Control efficacy of selected natural products against chrysanthemum foliar nematode– Aphelenchoides ritzemabosi (Schwartz, 1911) Steiner & Buhrer, 1932 Ocena skutecznościdziałanianiektórychsubstancjiipreparatówpochodzenianaturalnego w zwalczaniuwęgorkachryzantemowca–Aphelenchoidesritzemabosi (Schwartz, 1911) Steiner i Buhrer, 1932. Progress in Plant Protection. 2013;53 (3):563-567