Milestones in immunization history.
Abstract
Within the realm of global health, the importance of immunization arises as a fundamental element of preventive medicine. The primary objective of this chapter is to offer an in-depth investigation of immunization. The present discussion on the topic commences by digging into the historical background, beginning with the ancient application of variolation techniques and culminating in Edward Jenner’s groundbreaking progress. Subsequently, the course proceeds to cover fundamental scientific principles within the field of immunology. This chapter offers a thorough review of various vaccine types, including DNA and mRNA vaccines, elucidating the mechanisms underlying each of them. Moreover, it clarifies the pivotal significance of adjuvants in enhancing immune responses and ensuring the effectiveness of vaccines. Moreover, it delves into the diverse phases encompassed in the process of vaccine development, ranging from preclinical investigations to post-marketing surveillance and regulatory approval. The next parts assess the challenges associated with immunizations, with a particular focus on vaccine hesitancy and ethical considerations. The chapter additionally evaluates the impacts of vaccines on various diseases, including polio, HPV, and COVID-19, by employing a range of case studies. Finally, it underscores the economic benefits and future advancements associated with immunization, emphasizing its significance in global health management.
Keywords
- vaccine
- immunization
- prevention
- smallpox
- variolation
- eradication
- types of vaccines
- adjuvants
- vaccine development
- regulatory approval
- vaccine safety
- vaccine effectiveness
- vaccine hesitancy
- emerging diseases
- future directions in vaccinology
1. Introduction
In a world that has witnessed the incredible power of scientific discovery, few advancements have transformed the course of human history as profoundly as the development of vaccines.
Immunization is not solely a medical procedure; it is a significant testament to human ingenuity, resilience, and an unwavering commitment to a healthier and safer future. This chapter explores the captivating tale of immunization, which encompasses the realms of history, advanced technology, ethical dilemmas, and life-preserving remedies. We initiate our investigation by examining the historical tracks, starting with the basic methods of variolation used in ancient civilizations and moving on to the significant advancements made by Edward Jenner. Exploring the past enables a deep comprehension of the current and future implications of immunization.
Immunization serves as a symbol of optimism in the field of global health. We commemorate the successful elimination of smallpox, the decline in polio cases, and the ongoing efforts to combat infectious diseases such as measles, mumps, rubella, hepatitis B, and influenza. Continuous scientific advancement is the driving force behind immunization. We explore the intricate mechanisms of vaccines, ranging from cell culture methodologies to recombinant DNA technologies, elucidating the scientific principles that form the foundation of their effectiveness. Immunization does not have a universal approach. This chapter examines different categories of vaccines and the complexities involved in their development, ranging from preclinical investigation to regulatory authorization.
Immunization goes beyond the realm of science and enters a complex ethical terrain. We aim to tackle issues related to fair allocation of resources, obtaining informed consent, vaccine hesitancy, and the spread of misinformation. We provide techniques to promote greater acceptance of vaccines. We also analyze empirical case studies, ranging from the successful elimination of polio in India to the worldwide response to the COVID-19 pandemic, to demonstrate the significant influence of vaccines on the management and prevention of diseases.
Immunization is a dynamic catalyst for global well-being. Here, we present a valuable source of insight for healthcare professionals, lawmakers, researchers, and the wider public to actively participate in a global society where the intertwined narratives of history, science, and ethics reveal the extraordinary impact of immunization on our global landscape.
2. A brief history of immunization
An investigation of the historical progression of immunization demonstrates its development from rudimentary techniques such as variolation to pioneering contributions made by Edward Jenner, thereby highlighting the remarkable capacity for human adaptability and inventiveness within this domain.
2.1 Variolation and the associated hazards
Prior to the inception of vaccines, an ancient method known as variolation was employed, which entailed purposefully infecting an individual with material derived from smallpox pustules. The practice, which emerged in China before 1000 AD, entailed the crushing of smallpox scabs and subsequent administration of the resulting powder, either by nasal insufflation or application onto an incision on the individual’s arm [1]. Although the effect of variolation was the development of immunity, it also came with inherent risks. The fatality rates varied between 0.5% and 3%, which, while lower than the mortality rates of naturally acquired smallpox ranging from 20 to 30%, on the other hand presented a remarkable risk [2].
2.2 Edward Jenner and the development of the smallpox vaccine
A popular idea in Edward Jenner’s era influenced his entry into the field of immunology. It was widely observed that dairy maids who had been infected with cowpox, a milder disease, appeared to possess immunity against smallpox [3]. In the year 1796, Dr. Edward Jenner carried out renowned scientific research. He administered pus from a cowpox pustule on the hand of a dairy maid (Figure 1) to James Phipps, an 8-year-old boy. Following this, it was observed that Phipps did not acquire the smallpox infection despite being exposed to it (Figure 2) [3]. Edward Jenner also vaccinated his own child, Edward, as part of his experimentation and research into the smallpox vaccine (Figure 3). Jenner coined the term “vaccine,” deriving it from the Latin word “vacca,” which means cow. The Jenner vaccination has had a profound influence on the domain of public health, resulting in notable advancements.
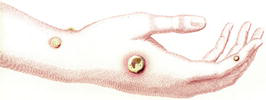
Figure 1.
The cowpox sores on the hands of Sarah Nelmes, from whom Jenner vaccinated James Phipps in 1796. Credit: Jenner, 1798, and Frontiers Media SA.
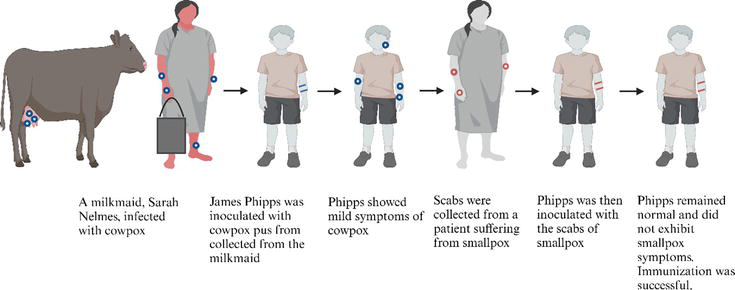
Figure 2.
The process above shows the steps taken by Edward Jenner to develop immunization. He did this by inoculating James Phipps with cowpox, a similar virus to smallpox.

Figure 3.
Edward Jenner vaccinating his young child in the 1800s. Credit: public domain, Wikimedia Commons.
During the latter part of the nineteenth century, several nations implemented organized smallpox vaccination campaigns, ultimately resulting in the worldwide eradication of the disease in 1980 [4]. The presentation of empirical proof of the concept by Jenner in the nineteenth and early twentieth centuries led to a substantial increase in the advancement of vaccinations. In the year 1885, Louis Pasteur made a significant advancement in the realm of immunization by successfully formulating a vaccine for the prevention of rabies. This achievement carries considerable importance in the progression of immunological science. Subsequently, the commencement of immunization programs targeting diseases such as diphtheria, tetanus, and pertussis was initiated [5].
2.3 The twentieth century: an era ruled by immunization
The twentieth century is widely recognized for its significant advancements in technology and science, namely in the field of vaccination, which has had a profound impact on human welfare. The practice of immunization has emerged as a pivotal component of modern medicine, serving as a critical factor in the decline of mortality rates and the advancement of the eradication or management of several infectious diseases. To provide a concise historical overview, please refer to Table 1.
| Year | Achievement |
|---|---|
| 1796 | Edward Jenner’s Smallpox Vaccine |
| 1800s | Rudimentary Variolation Techniques |
| 1940s | Development of Influenza Vaccine |
| 1955 | Introduction of Inactivated Poliovirus Vaccine (Salk) |
| 1961 | Development of Oral Polio Vaccine (Sabin) |
| 1963 | Licensing of Measles Vaccine |
| 1967 | Licensing of Mumps Vaccine |
| 1969 | Licensing of Rubella Vaccine |
| 1980 | WHO Declares Smallpox Eradicated |
| 1980s | Development of Hepatitis B Vaccine |
| twentieth century | Advancements in Vaccine Production and DNA Technology |
Table 1.
2.3.1 The eradication of smallpox
The process of eliminating smallpox on a global scale progressed over the span of the twentieth century, mostly due to the implementation of a comprehensive vaccination initiative. The World Health Organization (WHO) published an official announcement in 1980 regarding the eradication of smallpox, signifying an important achievement as it became the first disease to be eradicated through deliberate human intervention.
2.3.2 Polio vaccines
During the mid-twentieth century, the scientific community successfully developed two prominent vaccines for the prevention of polio. Jonas Salk first introduced the inactivated poliovirus vaccine in 1955, and Albert Sabin later created the oral polio vaccine in 1961. The effective distribution of these vaccines has resulted in the elimination of polio in a considerable number of geographic areas [6].
2.3.3 Immunizations against measles, mumps, and rubella
The measles vaccine was licensed in 1963, followed by the licensing of the mumps vaccine in 1967 and the rubella vaccine in 1969. Subsequently, the individual vaccines were combined to produce the MMR vaccines, resulting in a notable decrease in the prevalence of these diseases [7].
2.3.4 Hepatitis B
Hepatitis B is a liver disease resulting from a viral infection. The successful development of a hepatitis B vaccine during the 1980s was a notable milestone in ongoing efforts to address diseases affecting the liver. The vaccine now under discussion possesses the characteristic of being the inaugural immunization developed for a widespread manifestation of human malignancy [8].
2.3.5 The effectiveness of influenza vaccines
The development of the influenza vaccine during the 1940s and subsequent progress have greatly enhanced the ability to prevent and manage both seasonal influenza outbreaks and global pandemics.
2.3.6 Technological advancements
The impact of technological improvements on numerous sectors, particularly preventative medicine, has been perceived. The use of modern cell culture techniques has resulted in enhanced safety and effectiveness in vaccine production. Furthermore, the use of recombinant DNA technology has significantly propelled progress in the development of vaccines, such as the one for hepatitis B, while also establishing a foundation for forthcoming gene-based immunizations (Table 1).
3. Understanding immunology: the scientific study of vaccines
Immunology is a discipline of biology that scientifically studies the immune system, which serves as the innate protective mechanism of the human body against various pathogens, such as bacteria, viruses, and other external invaders. The subject of vaccines holds significance in the field of immunology, as they function as instrumental agents in instructing the immune system to identify and combat particular infections.
3.1 The workings of the immune system
The immune system is comprised of an intricate array of cellular elements, anatomical structures, and physiological mechanisms that operate in tandem to protect the organism against external hazards, such as harmful microbes like bacteria, viruses, and parasites. A vaccine delivers a non-pathogenic form or component of a pathogenic microbe, such as a virus or bacterium, into the human body. Consequently, the organism’s immune response is initiated, which enables the organism to retain its ability to protect itself against that particular pathogenic agent in subsequent encounters. The defense system can be categorized into two primary divisions: innate immunity and adaptive immunity (Table 2).
| Characteristic | Innate immunity | Adaptive immunity |
|---|---|---|
| Specificity | Non-specific, provides general defense against various pathogens. | Highly specific, it targets specific pathogens on the basis of their antigens. |
| Memory | Lacks memory; the response to an invader is typically the same each time. | Express immunological memory, leading to a quicker and more effective response if it encounters the same pathogen. |
| Response time | Exhibits a fast response to infections, acting instantly to prevent harm. | Slower response, taking days to weeks to exhibit a specific immune response |
| Mechanisms | Relies on physical barriers (intact skin, mucous membranes), inflammation, and phagocytosis. | Uses antibodies produced by B cells to neutralize pathogens, T cells play a key role in recognizing specific antigens on the surface of infected cells and responding to them. |
| Diversity | Exhibiting limited diversity with a fixed set of responses against different pathogens | Eexhibits high diversity and is able to adapt to a broader range of pathogens. |
Table 2.
Comparison of innate immunity and adaptive immunity.
3.1.1 The concept of innate immunity
The innate immune system functions as the principal protective mechanism against infections and demonstrates a non-discriminatory response, as it does not differentiate between various pathogenic agents.
3.1.1.1 The physical barriers
The first line of defense against pathogen invasion consists of physical barriers, including the integumentary system and mucosal surfaces. Mucous possesses the capacity to trap microorganisms, while the enzyme lysozyme, present in tears and saliva, exhibits the ability to degrade the cellular membranes of numerous bacterial species.
3.1.1.2 Cellular reactions
Cellular responses comprise a wide range of behaviors shown by cells in response to various stimuli or changes in their surrounding environment. Pathogens are recognized by innate immune cells, such as macrophages and neutrophils, after crossing physical barriers through the recognition of specific pattern-by-pattern recognition receptors (PRRs). Subsequently, the immune cells undertake the engulfment of the foreign intruders by a biological mechanism known as phagocytosis [9].
3.1.2 The concept of adaptive immunity
Adaptive immunity, the second line of defense in the immune system, demonstrates a significant degree of specificity. The process involves distinct cellular components, specifically T-cells and B-cells, that have the ability to identify specific antigens presented by pathogens. Upon identification, these cells undergo a process of activation, proliferation, and release of certain substances, such as antibodies, with the purpose of neutralizing the presence of the foreign invader.
3.1.2.1 The B lymphocytes
B lymphocytes are responsible for the production of antibodies that possess the capacity to specifically recognize antigens presented by invading pathogens. Following activation, specific B cells undergo a process of differentiation, resulting in the formation of memory B cells. This phenomenon facilitates a rapid and efficient immune response upon subsequent contact with the same antigen.
3.1.2.2 The T lymphocytes
T lymphocytes, namely CD8+ T lymphocytes, recognize and eliminate cells that have been infected. Helper T cells, also known as CD4+ T cells, play a crucial role in facilitating the activation of B cells and cytotoxic T cells. Additionally, memory T cells are generated to enhance and expedite immune responses in subsequent encounters with pathogens [10].
Regulatory T cells play a critical role in the maintenance of immunological homeostasis through the suppression of exaggerated immune responses, which have the potential to result in the development of autoimmune diseases [11].
3.1.3 Communication through cytokines and chemokines
Cytokines and chemokines help cells in the immune system communicate with each other and work together to fight off infections. Cytokines comprise a heterogeneous collection of low-molecular-weight signaling proteins that are secreted by several cell types, including immune cells like T cells, macrophages, and dendritic cells. In reaction to infection or injury, these cells secrete cytokines, therefore establishing a concentration gradient that guides immune cells toward the site of activity. These molecules exert their actions through three distinct pathways, specifically autocrine, paracrine, and endocrine [12]. Interleukins (ILs) are a class of cytokines that mediate intercellular communication among leukocytes. Tumor Necrosis Factor (TNF), however, is commonly linked to the initiation and progression of systemic inflammation. Interferons (IFNs) are frequently associated with antiviral reactions, serving as a pivotal component in the immune system’s defense.
Chemokines are small proteins that modulate immune responses and initiate inflammatory processes. CC Chemokine Ligand (CCL) and CXC Chemokine Ligand (CXCL) are two important groups of chemokines that affect how white blood cells move and join an immune system. Cytokines and chemokines possess regulatory functions that can either enhance or suppress immune responses. Some examples of regulatory cytokines are interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) [13]. They help slow down immune reactions and stop autoimmune responses.
The dysregulation of cytokine or chemokine expression has the potential to give rise to a range of diseases, such as autoimmune disorders, malignancies, and chronic inflammatory diseases [14].
3.2 Immunization vs. natural infection
Understanding how immunization and natural infection work is closely linked to comprehending the viral replication cycle.
3.2.1 Viral replication cycle
The viral replication cycle refers to the events that transpire when a virus invades a host organism. It generally entails multiple essential stages:
Now, let us establish a connection between the process of viral replication and the mechanisms of the host immune response, specifically in the context of immunization and natural infection.
3.2.2 Immunization and the viral replication cycle
Considering immunization, a vaccine is formulated to incorporate harmless components of the virus, such as attenuated viruses, inactivated viruses, or particular subunit components. The selection of these components is done cautiously to mimic specific characteristics of the viral replication cycle without inducing the complete onset of the disease.
3.2.3 Natural infection and the viral replication cycle
During a natural infection, the individual is exposed to the entire viral replication cycle. This entails the virus entering the host cells, undergoing replication, and generating a significant number of new virions. In the event of a natural infection, the immune system of the host identifies both the initial viral components (entry, uncoating) as well as the entire virus and all of its products. This generally leads to a stronger immunological response, characterized by the generation of antibodies, T cells, and other constituents of the immune system.
Natural infection frequently results in a vigorous immunological response, but it also carries the inherent risk of disease appearance, which can be moderate to severe and, in some cases, even fatal [15]. Vaccines, however, can be potentiated to elicit more robust or comprehensive immune responses compared to natural infection through the incorporation of adjuvants or the combination of numerous antigens in vaccines [16].
4. Types of vaccines and the role of adjuvants
Vaccines have played a crucial role in the prevention of a wide range of infectious diseases. They work by activating the immune system, prompting it to identify and fight specific pathogens. In this investigation, we will examine numerous types of vaccines (Table 3).
| Vaccine type | Mechanism of action | Advantages | Limitations | Examples |
|---|---|---|---|---|
| Live-attenuated vaccines | Weakened or modified viruses or bacteria | Robust and long-lasting immunity | Inappropriate for immunocompromised individuals | Measles, mumps, rubella, and yellow fever |
| Inactivated vaccines | Heat/chemically-inactivated pathogens | Safe for immunocompromised individuals | May require booster shots for sustained immunity. | Hepatitis A, influenza, polio, and rabies |
| Subunit vaccines | Vital antigens are derived from pathogens. | Low likelihood of adverse reactions | May necessitate booster shots for long-term immunity. | Hepatitis B, HPV, and whooping cough |
| Recombinant vaccines | Antigens are generated from pathogen DNA. | Rapid development | Challenges related to storage and stability | COVID-19, Hepatitis B |
| Polysaccharide vaccines | Sugar molecules from bacterial surfaces | Suitable for mimicking human cells | Limited effectiveness in young children | Pneumococcal, Meningococcal |
| Conjugate vaccines | Combination of polysaccharide and protein | Enhanced immune response in pediatric populations | Booster shots may be necessary for optimal immunity. | Hemophilus Influenzae, Meningococcal |
| Toxoid vaccines | Inactivated bacterial toxins | Highly effective in toxin neutralization | Restricted to diseases caused by toxin-producing bacteria | Diphtheria, Tetanus |
| mRNA-based vaccines | Synthetic mRNA encodes antigens. | High specificity and potency | Challenges in storage and stability | COVID-19, Diphtheria, and Tetanus |
| DNA vaccines | Plasmid DNA encodes pathogen antigens. | Stable at room temperature | Lower immunogenicity compared to other vaccines | Hepatitis B, Zika Virus |
| Viral-vector vaccines | Genetically modified non-pathogenic virus | Induces a robust immune response | Reduced efficacy in individuals with pre-existing immunity | Ebola, SARS-CoV-2, and tumor-specific antigens |
Table 3.
Comparison of different types of vaccines.
4.1 Types of vaccines
4.1.1 Live-attenuated vaccines
Live-attenuated vaccines are commonly known as one of the earliest and most efficacious approaches to immunization and often consist of a weak end or modified version of a virus or bacteria intentionally engineered to reproduce within the host organism. Live-attenuated vaccines have been employed for the prevention of several infectious diseases, such as measles, mumps, rubella, yellow fever, and tuberculosis (BCG).
4.1.1.1 The elucidation of the mechanism of action
Live-attenuated vaccines function by simulating the characteristics of a natural infection while refraining from causing disease. The weakened or modified microbes elicit an immunological response, resulting in the production of antibodies and the formation of memory cells. This technique enables the prompt detection and activation of the immune system in response to a second encounter with the same pathogen. These vaccines possess a unique attribute in their capacity to elicit both cellular and humoral protection. T-lymphocytes mediate the cellular immune response by selectively recognizing and eliminating infected cells. On the other hand, humoral immunity is characterized by the generation of antibodies through the activation of B cells. This dual response provides a wide spectrum of protection and demonstrates significant effectiveness against pathogens that necessitate intracellular invasion [17].
4.1.1.2 Advantages
Induce robust and lasting immunity
Typically, they require a lesser number of doses in comparison to other types of vaccines.
4.1.1.3 Limitations
In general, this is not suitable for those with compromised immune systems.
Live pathogens require careful storage and transportation procedures.
4.1.2 Inactivated or “Killed” vaccines
In contrast to live-attenuated vaccines, killed vaccines consist of microorganisms that have been inactivated through physical or chemical methods and exhibit particular utility in the case of pathogens that pose an excessive risk of being administered in a weakened live form or where live-attenuated variants are unsuccessful. Examples of such pathogens include hepatitis A, influenza, polio, and rabies.
4.1.2.1 Elucidation of the mechanism of action
Inactivated vaccines consist of viruses or bacteria that have undergone inactivation through the use of heat, chemicals, or radiation. While pathogens have lost their ability to induce illness, their fundamental structure remains intact. This mechanism enables the immune system to identify these agents and generate antibodies as a defensive reaction [18]. Upon administration, inactivated antigen interacts with antigen-presenting cells (APCs), thereby eliciting an immune response, leading to the production of antibodies and the generation of memory cells. Nevertheless, it is worth noting that inactivated vaccines typically do not elicit a robust cellular immunological response, primarily targeting humoral immunity [19].
4.1.2.2 Advantages
It is deemed safer for individuals with compromised immune systems.
These are more stable and easier to store in comparison to live vaccines.
4.1.2.3 Limitations
In general, the administration of booster shots is necessary to maintain ongoing immunity.
May induce a weaker immune response in comparison to live vaccines.
4.1.3 Subunit, recombinant, polysaccharide, and conjugate vaccines
Among the recent generations of vaccines are subunit, recombinant, polysaccharide, and conjugate vaccines. The vaccines have been specifically engineered to induce targeted protection by prioritizing the selection of pathogen components rather than the complete organism.
4.1.3.1 Subunit vaccines
Subunit vaccines exclusively comprise the vital antigens, typically a protein or a portion of it, derived from a pathogen. These antigens possess the capability to elicit an immune response, although their presence alone does not carry the necessary strength to induce disease. Subunit vaccines are commonly employed against hepatitis B, human papillomavirus (HPV), and whooping cough.
4.1.3.2 Advantages
There is a minimal likelihood of experiencing adverse reactions.
4.1.3.3 Limitations
It is possible that the administration of booster shots may be necessary in order to maintain long-term immunity.
4.1.3.4 Recombinant vaccines
Recombinant vaccines use a fragment of genetic material derived from the genome of the pathogen to generate antigens in a laboratory setting. Subsequently, these antigens are administered to elicit an immunological response. Recombinant vaccines are frequently used in the prevention and treatment of COVID-19 using mRNA vaccines, as well as in the management of hepatitis B.
4.1.3.4.1 Advantages
Rapid development
4.1.3.4.2 Limitations
Challenges related to storage and stability
4.1.3.5 Polysaccharide vaccines
Polysaccharide vaccines use long sequences of sugar molecules present on the external surface of specific bacteria in order to elicit an immunological response. These types of vaccines are primarily used for the prevention of pneumococcal and meningococcal diseases.
4.1.3.5.1 Advantages
This particular type of vaccine is suitable for bacteria that possess the ability to mimic human cells.
4.1.3.5.2 Limitations
The effectiveness of this intervention with young children is limited.
4.1.3.6 Conjugate vaccines
Conjugate vaccines consist of a combination of polysaccharide and protein vaccines to induce a more robust and durable immune response [20]. This approach is commonly employed for the administration of hemophilus influenzae type influenzae type b (Hib) and meningococcal vaccines.
4.1.3.6.1 Advantages
Enhance the immune response in the pediatric population
4.1.3.6.2 Limitations
Booster shots may be necessary to achieve optimum immunity
4.1.4 Toxoid vaccines
Toxoid vaccines use inactivated toxins to stimulate an immune response. These vaccines, in contrast to immunizations that specifically target the pathogen, aim to specifically neutralize the toxins produced by bacteria. Toxoid vaccines play a vital role in the prevention of diphtheria and tetanus.
4.1.4.1 Elucidation of the mechanism of action
The components of toxoid vaccines are bacterial toxins that have undergone chemical or thermal inactivation but are still capable of inducing an immune response. After a vaccine is given, antigen-presenting cells (APCs) show the toxoids to T cells, which then activate B cells to make antibodies. This gives the host the power to fight the real toxin if it is exposed [21].
4.1.4.2 Advantages
It is highly effective in neutralizing bacterial toxins.
Prolonged immunity is achieved through the use of booster shots.
4.1.4.3 Limitations
This type of vaccine is restricted to diseases that are attributable to bacteria that produce toxins.
To attain optimal immunity, it may be essential to administer multiple doses.
4.1.5 Vaccines based on nucleic acids
The domain of biotechnology has experienced notable advancements, resulting in the emergence of nucleic acid vaccines such as messenger RNA (mRNA) and deoxyribonucleic acid (DNA) vaccines.
4.1.5.1 Messenger RNA (mRNA)-based vaccines
In contrast to traditional vaccines, mRNA vaccines use a diverse approach. Following administration, the host cells take up the mRNA and use the cellular machinery to translate it, producing an original antigen. The immune system identifies antigens as exogenous substances, thus initiating the production of antibodies and the activation of T cells [22].
The mRNA vaccines for COVID-19 developed expeditiously have demonstrated significant efficacy in clinical trials [23]. Furthermore, investigations have been made to assess the therapeutic uses of these products for cancer treatment. Clinical trials are being conducted to evaluate the efficacy of tumor-specific antigens in cancer treatment. Likewise, the administration of these vaccines has a critical role in the prevention of diphtheria and tetanus.
4.1.5.1.1 Fundamental components
Synthetic messenger RNA (mRNA) used in vaccines is synthesized within a laboratory setting through
Lipid nanoparticles (LNPs) function as carriers for the transportation of mRNA into cellular structures and protect them from enzymatic destruction [25].
4.1.5.1.2 Advantages
Rapid development timelines
The characteristics of high specificity and potency are observed.
A lack of risk is associated with viral vectors.
4.1.5.1.3 Limitations
One of the primary concerns in this context pertains to the difficulties associated with storage and maintaining stability.
The potential consequences for the future remain uncertain
The possibility of incurring significant production costs
4.1.5.2 DNA vaccines
DNA vaccines use a small, circular DNA molecule known as a plasmid. The DNA has been genetically modified to facilitate the synthesis of a particular protein antigen upon its integration into the cellular machinery of the host organism. Following the administration, cells internalize the DNA and initiate the production of the antigen, thereby replicating the characteristics of a natural infection. This phenomenon induces both humoral and cellular immune responses, hence offering broad protection against infections [26]. DNA vaccines have been extensively investigated as a preventive measure against several viral diseases, including the Hepatitis B virus and the Zika virus, among others. Within the field of oncology, these vaccines are now being used in experimental settings with the objective of targeting tumor-associated antigens. The ultimate goal is to stimulate a strong immune response against cancer cells [27].
4.1.5.2.1 Fundamental components
Plasmid Deoxyribonucleic Acid (DNA): The genetic material contained within these vaccines is typically organized in the structure of a plasmid, which is a compact, circular DNA molecule. The genetic construct is designed to include the gene sequence responsible for the desired antigen, in addition to other regulatory components that facilitate its successful expression [28].
Modes of delivery: The issue of effective delivery continues to pose a significant difficulty. The present techniques involve electroporation, gene guns, and nanoparticle-mediated systems. Every approach possesses unique advantages and limitations.
4.1.5.2.2 Advantages
It is more convenient and cost-effective to produce.
The substance remains in a stable state when exposed to normal room temperature.
It has the ability to elicit a wide array of immunological responses [29].
4.1.5.2.3 Limitations
The immunogenicity of this vaccine type is comparatively lower when compared to other types of vaccines.
Challenges are associated with ensuring effective delivery.
Regulatory challenges are associated with genetic modification.
4.1.6 Viral-vector vaccines
Viral-vector vaccines are an innovative strategy within the evolving field of vaccine technology. The use of viruses’ inherent capacities to transport genetic material into host cells has been widely recognized as a groundbreaking approach to fighting a range of diseases, such as infectious diseases and malignancies. These vaccines have been used in the context of several diseases, including Ebola, Zika, and especially SARS-CoV-2 [30]. At present, there is a continuous assessment of these vaccines in relation to their potential effectiveness in the treatment of tumors by targeting tumor-specific antigens [31].
4.1.6.1 Elucidation of the mechanism of action
These vaccines use a genetically modified, non-pathogenic virus as a “vector” to get a specific genetic part from the target pathogen into the cells of the host organism. Once the genetic material gets into the cell environment, it commands the cells how to make a specific antigen, which eventually leads to an immune response. In essence, these vaccines harness the viral infection process to elicit a defensive immune response against another pathogen [32].
4.1.6.2 Types of viral vectors
Adenoviral vectors: These vectors have been widely used in both laboratory settings and public health campaigns. The COVID-19 vaccines produced by AstraZeneca and Johnson & Johnson use adenoviral vectors [33].
Lentiviral vectors: The vectors are obtained from retroviruses and provide favorable characteristics for the transportation of large genetic material. They have also been studied in relation to vaccines and gene therapy [34].
4.1.6.3 Advantages
Capacity to elicit a robust immunological response
This intervention is appropriate for both preventive and therapeutic applications.
Accessible to modifications
4.1.6.4 Limitations
The efficacy of the viral vector may be reduced due to pre-existing immunity.
Complexities and challenges associated with the manufacturing process
These vaccines face regulatory hurdles.
4.2 The role of adjuvants
Adjuvants are additional substances used in vaccines with the purpose of augmenting the immunological response of the host organism to the principal antigen. Adjuvants play a crucial role in enhancing the efficacy of vaccines by augmenting, expanding, and prolonging the immune response, hence conferring extended and heightened protection against the disease that is being addressed.
4.2.1 The historical background of adjuvants
Alum, which consists of aluminum salts, has been employed as the initial adjuvant for more than a century. The discovery of this phenomenon was accidental in nature, as researchers observed an augmented immune response upon the precipitation of allergens with alum [35].
4.2.2 Elucidation of the mechanism of action
Despite the diverse processes by which different adjuvants function, their collective objective is to enhance the immune response. They can:
4.2.3 The significance of adjuvants in modern vaccines
Many modern vaccines, particularly those designed to fight challenging infections, have adjuvants in order to enhance their effectiveness.
The immunological response to the influenza vaccine is generally diminished in elderly adults. Adjuvants have the capability to augment this immune response, hence providing improved protection.
The formulation of the human papillomavirus (HPV) vaccine includes an adjuvant that augments the immune system’s response. This is of particular significance due to the evasive nature exhibited by the virus.
The challenges encountered in the creation of a malaria vaccine have required the incorporation of adjuvants to enhance the immune response of potential vaccine candidates in experimental settings.
5. The life cycle of vaccine development
The production of vaccines is a multifaceted and intricate activity that requires thorough and prolonged research, extensive financial resources, and unwavering commitment. Each phase of the developmental process plays a vital role in ensuring that the vaccine is both safe and effective (Table 4).
| Development phase | Description | Primary focus | Scope | Key objectives |
|---|---|---|---|---|
| Preclinical phases | Initial research and testing before clinical trials | Evaluation in controlled settings and animal studies | In vitro, in silico, and animal testing | Identifying potential vaccine candidates and assessing safety and effectiveness |
| Clinical trials | Systematic scientific studies to evaluate vaccine safety and efficacy in humans | Human trials to assess safety and effectiveness. | Phase I, Phase II, Phase III, and Phase IV clinical trials | Establishing safety in humans (Phase I), optimizing dosage and assessing efficacy (Phase II), large-scale effectiveness evaluation (Phase III), and post-approval monitoring for rare side effects (Phase IV) |
| Phase I clinical trials | Initial testing is done on a small group of healthy volunteers or patients. | Safety assessment and suitable dosage schedules | Limited participants (usually <100) | Assessing vaccine safety in human participants, monitoring negative consequences, and examining immunological responses |
| Phase II clinical trials | Evaluation of a vaccine’s safety and efficacy in a substantial population | Dosage regimen optimization and efficacy assessment | Larger study populations | Optimizing dosage regimens and assessing vaccine safety and efficacy in a broader population |
| Phase III clinical trials | Large-scale evaluation of vaccine safety and efficacy with diverse participants | Significant data collection to evaluate effectiveness | Extensive study populations | Assessing vaccine effectiveness and identifying rare adverse responses for regulatory approval |
| Phase IV: post-marketing surveillance | Post-approval monitoring for infrequent or persistent side effects | A larger patient cohort and extended observation | Extended observation post-approval | Identifying rare side effects and conducting comparative effectiveness studies |
Table 4.
Comparison of vaccine development phases.
5.1 Preclinical phases
The preclinical phases refer to the primary stages of research and testing performed before clinical trials are conducted on human subjects.
5.1.1 Investigations conducted in vitro and in silico
Prior to conducting experiments on laboratory animals, a vaccine originates as a theoretical concept that undergoes first evaluation in controlled settings, such as petri dishes or computer simulations. The primary objective of these preliminary investigations is to gain an understanding of the basic attributes of the disease and identify viable candidates for the advancement of a vaccine.
5.1.2 Animal studies
After the identification of possible vaccine candidates, animal models, such as mice or monkeys, are employed to assess their safety and effectiveness in eliciting an immune response. Although it is acknowledged that the applicability of animal studies to human subjects may be limited, it is crucial to recognize the significance of these studies in evaluating the effectiveness and safety of potential vaccine candidates.
5.2 Clinical trials
Clinical trials are a fundamental component of the scientific research process in the field of medicine. These trials involve systematic investigation through rigorous scientific studies conducted to evaluate the safety of vaccines, particularly in humans.
5.2.1 Phase I clinical trials
These trials involve the initial testing of a new drug or vaccine on a small group of healthy volunteers or patients. The purpose of this preliminary phase is to assess the safety of the vaccine in human participants. Typically, these investigations involve a limited group of participants, often numbering less than 100, and primarily focus on evaluating the safety characteristics of the vaccine as well as establishing suitable dosage schedules. Researchers engage in simultaneous monitoring of potential negative consequences and assess the vaccine’s capacity to elicit an immunological response.
5.2.2 Phase II clinical trials
Phase II clinical trials represent a specific stage in the complete evaluation of the safety and efficacy of vaccines. During this phase, the vaccine is provided to a substantial portion of the population. Researchers are engaged in the optimization of dosage regimens and conducting a comprehensive assessment of safety parameters throughout the initiation of data collection pertaining to the efficacy of the intervention. At this stage, it is crucial to consider the inclusion of supplementary age cohorts and demographic variables in order to assess the wider scope of the vaccine’s efficacy.
5.2.3 Phase III clinical trials
Phase III clinical trials play a pivotal role in the progression of vaccine development. These clinical trials are done to find out if a new vaccine or therapeutic intervention is safe and effective for a large group of people with different types of health problems or comorbidities. The current phase is distinguished by its substantial magnitude and crucial significance due to the inclusion of a diverse array of people. In order to evaluate the effectiveness of the vaccine and identify any rare adverse responses, researchers collect a significant volume of data. The results obtained from this specific phase often form the basis for acquiring regulatory approval.
5.2.4 Phase IV: Post-marketing surveillance
After receiving approval from the relevant regulatory authorities, the vaccine proceeds to Phase IV, which is commonly referred to as post-marketing surveillance. The objective of this phase is to identify any infrequent or persistent side effects over a significantly broader patient cohort and an extended duration of observation. Additionally, this may encompass conducting comparative effectiveness studies in comparison to other available treatments [36].
5.3 Regulatory approval and ethical considerations
5.3.1 Regulatory approval
After the completion of Phase III studies, the collected data are subsequently submitted to regulatory authorities such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for the purpose of assessment.
In the context of the United States, it is customary for a biologics license application (BLA) to be formally presented to the FDA subsequent to the satisfactory conclusion of Phase III clinical studies. Similarly, the EMA and other international regulatory bodies adhere to their own distinct protocols for granting approval, which are informed by rigorous scientific evidence and overseen by ethical review committees. The purpose of this step is to ascertain that vaccines adhere to rigorous criteria in terms of safety, effectiveness, and quality. During times of crisis, governmental bodies possess the authority to provide Emergency Use Authorizations (EUAs) in order to streamline the approval process for vaccines, as exemplified by the COVID-19 vaccines.
5.3.2 Ethical considerations
In addition to the scrutiny of clinical trial data by regulatory agencies, ethical considerations play a crucial role in every stage of the development process. They involve a wide array of concerns, which include, but are not limited to, the principles of informed consent, the protection of vulnerable people, and the fair distribution of vaccines. It is imperative for all clinical trials to strictly adhere to the principles outlined in the Declaration of Helsinki. This international ethical framework emphasizes that the well-being and welfare of the trial participants should be given the utmost priority, superseding any other competing interests [37].
Once a vaccine is granted approval, there are ongoing ethical concerns, specifically about the fair and equal access to and distribution of the vaccines. Efforts such as COVAX have been established with the objective of equitably allocating vaccines among nations, particularly those that possess little resources [38].
6. Challenges in immunization
Immunization has emerged as a very efficacious intervention in the field of public health, yet it faces a number of obstacles that hinder its widespread global impact. The obstacles encompass a range of factors, including vaccine hesitancy, the propagation of misinformation, issues related to accessibility and distribution, and ethical considerations.
6.1 Vaccine hesitancy and myths
In the context of a global setting characterized by the successful reduction of mortality rates and the consequent saving of several lives by vaccination, the problem of vaccine hesitancy or complete rejection of vaccination poses a challenging problem. The phenomenon of vaccine hesitancy, which is defined by the World Health Organization (WHO) as the deliberate delay or refusal of vaccines, regardless of their availability, exhibits a complex and diverse character, influenced by various elements including cultural perspectives, the dissemination of inaccurate information, and a prevailing sense of skepticism toward healthcare systems. In the modern era of digitalization, social media platforms serve as both a beneficial tool for accessing credible information and a breeding ground for the dissemination of myths and misinformation.
The dissemination of inaccurate information reduces the acceptance and use of vaccines, hence playing a role in the occurrence of outbreaks of diseases that could have been prevented through vaccination. Public health initiatives and educational interventions play a crucial role in addressing this issue. Through a systematic awareness of the roots of the issue, the implementation of proficient communication strategies, and building trust, it is possible to establish a connection and agreement that individuals avail themselves of the protective effects of vaccines [39]. Vaccine hesitancy is a complex and multifaceted phenomenon. Exploring various dimensions is necessary to fully understand it, such as;
6.1.1 The phenomenon of complacency
Certain individuals, particularly those residing in areas with a low prevalence of diseases, may exhibit a lack of awareness regarding the necessity of vaccination, resulting in a state of complacency [40].
6.1.2 Mistrust in science or authorities
The combination of past instances of unethical medical experiments and the ongoing dissemination of misinformation may create mistrust among individuals toward both the healthcare system and government institutions.
6.1.3 Cultural or religious beliefs
The potential conflict between specific religious or cultural beliefs and medical interventions can result in hesitation or reluctance.
6.1.4 Concern regarding adverse effects
The dissemination of information on potential adverse effects, regardless of its truthfulness, heightens concerns related to the safety of vaccines.
6.2 Strategies for addressing hesitancy
The importance of tackling vaccine hesitancy cannot be overstated in the context of attaining and sustaining elevated vaccination rates and herd immunity. The following are ways to mitigate vaccination hesitancy:
6.2.1 Engaging with communities
Engaging religious, cultural, and community leaders in dialogs is vital in order to establish trust and promote cooperation within communities.
6.2.2 Addressing misinformation
Misinformation is a prevalent issue that needs to be effectively countered. The process of countering misinformation involves using proactive communication strategies and exploiting social media platforms to effectively distribute the correct information.
6.2.3 Personal stories
The act of narrating stories of those affected by preventable diseases or those who have experienced positive outcomes from immunization might serve to provide a sense of humanity, thereby enhancing its relatability.
6.2.4 Enhancing the convenience of vaccination
The reduction of logistical hurdles to immunization can be achieved by the implementation of measures such as easy access, flexible hours, and minimum prices.
6.2.5 Enhancing communication strategies
6.2.5.1 Active listening
Active listening is a crucial skill in effective communication. It involves fully engaging with the speaker and demonstrating attentiveness through verbal and non-verbal cues. By actively listening, individuals participate in dialogs wherein the concerns of persons who exhibit hesitancy are acknowledged in a non-judgmental manner. This cultivates a climate characterized by trust.
6.2.5.2 The empathy approach
The empathy method approaches the subject matter with a sense of empathy, recognizing that each individual who displays hesitancy possesses a unique narrative, concern, or fear.
6.2.5.3 Employ trusted authorities
Employ trusted authorities and use testimonials or endorsements from reputed community members or famous personalities to develop favorable attitudes toward vaccines.
6.3 Accessibility and distribution challenges
Despite the fact that vaccines are available, there may still be many barriers preventing access to them. In economically disadvantaged nations, the financial burden associated with vaccine procurement and the complex logistical hurdles involved in their distribution present significant challenges. Insufficient infrastructure, including deficient storage and transportation capabilities, may hinder the accessibility of vaccines. Efforts such as Gavi, the Vaccine Alliance, strive to address these obstacles by offering immunization to those with disadvantages [41, 42].
7. The impact of innovations on the future of immunizations
In modern vaccine technology, innovation plays a central role, encompassing several advancements such as mRNA technology and the development of personalized and therapeutic vaccines. As humanity prepares for forthcoming obstacles, the ongoing progress in vaccination technology is not only encouraging but also imperative for the well-being of the world population.
7.1 Advances in vaccine technology
The field of vaccine technology has experienced significant advancements, notably over the past 20 years. Traditional approaches using live-attenuated or inactivated pathogens are being enhanced and are being replaced by advanced technology such as mRNA vaccines. The COVID-19 pandemic, for instance, observed the rapid use and dissemination of mRNA vaccines throughout a remarkably limited timeframe. The mRNA vaccines possess numerous advantages, encompassing expedited development timelines, high potency, and cost-effective production.
Virus-like particles (VLPs) represent a growing area of interest in the field of vaccinology. The VLPs bear a structural resemblance to viruses, although they do not contain the viral DNA, consequently offering a more secure option for immunization. The human papillomavirus (HPV) vaccine has demonstrated effectiveness in reducing the incidence of HPV-related cancer cases [43].
7.2 Personalized vaccines and therapeutic vaccines
The concept of personalized vaccines and therapeutic vaccines has emerged as a promising approach in the field of immunotherapy. Personalized vaccines refer to immunizations that are custom designed according to an individual’s unique genetic or phenotypic traits [44]. The objective of these vaccines is to provide a more precise and effective immune response by considering the individual’s distinct biological composition. The use of this particular method is particularly advantageous for cancer, as it allows for the development of personalized vaccines that specifically target antigens unique to tumors. The initial findings from clinical trials evaluating the efficacy of neoantigen-based cancer vaccines have demonstrated encouraging outcomes [45].
Therapeutic vaccines represent a promising field of study that seeks to address not just preventing diseases but also the treatment of pre-existing conditions. These therapeutic interventions demonstrate significant use in the management of chronic diseases, malignancies, and established infections. One illustration of this is the objective of the therapeutic HIV vaccine, which is to enhance the management of viral replication to prolong the advancement of the disease [46].
7.3 Prospects for vaccines against emerging diseases
The presence of emerging and re-emerging infectious diseases is an ongoing and persistent challenge to the field of global health. Climate change, along with heightened human mobility and various other causes, all contribute to the dynamic and always-evolving environment. Zika, dengue, Ebola, MERS-CoV, and COVID-19 are illustrative instances of diseases that have emerged or re-emerged in recent times, underscoring the imperative for flexible vaccination technology. Given the rapid spread of such diseases, platforms enabling the swift development and production of vaccines are more critical than ever. Emerging advancements such as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) gene editing and artificial intelligence (AI)-based antigen prediction models offer potential for expedited and enhanced vaccine development procedures. In conjunction with enhanced surveillance systems and more international collaboration, these innovations have the potential to play a crucial role in mitigating future pandemics.
8. The effectiveness and safety of vaccines
The effectiveness and safety of vaccines is widely recognized as a very efficacious strategy for addressing the threat posed by infectious diseases. Although vaccines are helpful in saving numerous lives and reducing the burden of disease, it is imperative to conduct thorough evaluations of their effectiveness and safety. Acquiring a comprehensive understanding of the effectiveness and safety attributes of vaccines is important in order to make decisions pertaining to public health. While immunizations are widely acknowledged as safe and effective, it is imperative to underscore the significance of continuous monitoring and research to enhance and modify vaccination strategies.
8.1 Measures of vaccine effectiveness
The establishment of a precise differentiation between the concepts of vaccine efficacy and vaccine effectiveness holds significant importance. The evaluation of vaccine efficacy is commonly carried out in controlled environments, often through randomized controlled trials. Conversely, the assessment of vaccine effectiveness involves evaluating the performance of a vaccine in real-world scenarios, taking into account many elements such as demographic diversity, behavioral patterns, healthcare system capabilities, and disease prevalence [47].
8.1.1 Attack rate
The term “attack rate” pertains to the frequency at which new cases of a particular disease or illness arise within a clearly defined population during a specified duration. The evaluation of vaccine effectiveness often requires the use of a widely employed method that entails the computation of the attack rate within populations that have received vaccinations and those that have not.
Vaccine effectiveness (VE) can be calculated using the formula (Eq. (1)):
Where VE is the measure of how well a vaccine protects against a certain disease, ARv denotes the attack rate (incidence) of the disease in the vaccinated group, and ARu represents the attack rate in the unvaccinated group.
8.1.2 Case: control studies
Case–control studies are a prevalent form of observational study design frequently employed in the fields of epidemiology and medical research. Within the framework of case–control studies, the evaluation of vaccine effectiveness can be accomplished by analyzing the vaccination status of individuals who have contracted the disease in contrast to those who have not.
8.1.3 Longitudinal studies
The assessment of effectiveness might also encompass extended periods of time in order to examine the necessity of booster dosages and the gradual decline of immunity.
8.2 Prevalent adverse effects and their management strategies
8.2.1 Manifestation of mild symptoms
Typical moderate adverse effects encompass localized arm discomfort, low-grade pyrexia, and general fatigue. Typically, these conditions are inherently restricted in duration and can be effectively controlled through the use of non-prescription drugs such as acetaminophen [48].
8.2.2 Serious adverse effects
Although serious adverse effects are few, they might include severe allergic reactions or persisting neurological sequelae. Systems for reporting vaccine-related adverse events keep careful track of these occurrences [49].
9. Case studies
The case studies explore the impact of immunizations on the development of public health policies and their subsequent effects on health outcomes.
9.1 The eradication of polio in India
The global prevalence of polio, a debilitating disease that affected a significant number of individuals around the globe, has been diminished to a limited number of instances, primarily attributable to the implementation of extensive immunization programs. India stands as a notable triumph in this struggle. Despite being responsible for almost 50% of worldwide polio infections in 2009, the nation successfully declared itself free of polio in 2014, a remarkable accomplishment facilitated by an extensive and well-coordinated immunization campaign.
9.2 The relationship between HPV vaccination and public opinion
The reception of the Human Papillomavirus (HPV) vaccine, which was developed with the aim of preventing several types of malignancies, including cervical cancer, has been complicated. Despite extensive evidence of the vaccine’s effectiveness, opinions among the public are still conflicted. HPV vaccination has become a controversial topic in public discussion, partly due to false information spread on social media, which has affected vaccination acceptance rates [50].
9.3 The global response to COVID-19
The COVID-19 pandemic has highlighted how vital immunizations are to preventing global health problems. Several types of vaccines were rapidly developed, revealing the remarkable rate at which advances in vaccine science can occur when provided with adequate resources and prioritization. Despite these advancements, the pandemic has also brought to light major gaps in the distribution of vaccines among states, underscoring the imperative for global initiatives that guarantee fair access to vaccines.
10. Conclusion
In conclusion, it can be inferred that the aforementioned arguments support the notion that the topic at hand is deserving of more investigation and analysis.
10.1 Vaccines’ crucial role in Global Health
The significant impact of vaccines on shaping public health outcomes cannot be overlooked. The case studies on polio eradication in India, HPV vaccination, and the global response to COVID-19 demonstrate the significant potential of vaccines in effectively eradicating diseases, enhancing the quality of life, and prolonging human lifespans. The extensive use of vaccines has resulted in the saving of countless lives and has augmented global life expectancy. Vaccination campaigns have demonstrated a significant reduction in child death rates, particularly in underdeveloped nations.
10.2 The economic impacts of vaccines
The economic impacts of vaccines not only serve as a means of saving human lives but also possess a sound economic rationale. Based on a research study, it has been found that each dollar invested in childhood immunization yields a return of up to $44 in wider economic gains [52]. In spite of notable scientific progress, the ongoing fight against infectious diseases continues to present significant challenges, characterized by obstacles such as vaccine hesitancy, the dissemination of misinformation, and inequalities on a global level. However, the unwavering significance of immunizations remains a fundamental pillar of public health and an essential component of global health management.
10.3 Final thoughts and prospects for future directions
Vaccines currently find themselves at a critical juncture, characterized by the emergence of revolutionary advancements in vaccine technologies and the enduring obstacles of vaccine distribution and public acceptance. In order to enhance immunization rates and effectively fight emerging health risks, it is imperative to prioritize the resolution of vaccine hesitancy and the dissemination of accurate information. Further investigation is warranted to prioritize tailoring vaccines for different populations, taking into account factors like heredity and pre-existing medical conditions, in order to optimize both the effectiveness and safety of vaccines.
Acknowledgments
We acknowledge Wikimedia Commons and Frontiers Media SA for permitting us to use the images for completing the book chapter in both online and printed formats to ensure that the information reaches a wide and diverse audience.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.
Gross CP, Sepkowitz KA. The myth of the medical breakthrough: Smallpox, vaccination, and Jenner reconsidered. International Journal of Infectious Diseases. 1998; 3 (1):54-60. DOI: 10.1016/S1201-9712(98)90096-0 - 2.
Riedel S. Edward Jenner and the history of smallpox and vaccination. In: Baylor University Medical Center Proceedings. Vol. 18, No. 1. London, United Kingdom: Taylor & Francis; 1 Jan 2005. pp. 21-25.DOI: 10.1080/08998280.2005.11928028 - 3.
Willis NJ. Edward Jenner and the eradication of smallpox. Scottish Medical Journal. 1997; 42 (4):118-121. DOI: 10.1177/003693309704200407 - 4.
Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its Eradication. Geneva: World Health Organization; 1988. p. 540. DOI: 10.1163/182539189x01076 - 5.
Plotkin SL, Plotkin SA. Vaccines. 6th ed. Amsterdam: Elsevier; 2013. p. 458. DOI: 10.1016/B978-1-4557-0090-5.00017-3 - 6.
Pearce JM. Salk and Sabin: Poliomyelitis immunization. Journal of Neurology, Neurosurgery & Psychiatry. 2004; 75 (11):1552. DOI: 10.1136/jnnp.2003.028530 - 7.
Plotkin SL, Plotkin SA. Vaccines. 6th ed. Amsterdam: Elsevier; 2013. pp. 352-446. DOI: 10.1016/B978-1-4557-0090-5.00017-3 - 8.
Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. New England Journal of Medicine. 1997; 336 (26):1855-1859. DOI: 10.1056/NEJM199706263362602 - 9.
Akira S. Pattern recognition receptors & inflammation. Cytokine. 2009; 48 :4. DOI: 10.1016/j.cell.2010.01.022 - 10.
Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999; 401 (6754):708-712. DOI: 10.1038/44385 - 11.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133 (5):775-787. DOI: 10.1016/j.cell.2008.05.009 - 12.
Dinarello CA. Historical insights into cytokines. European Journal of Immunology. 2007; 37 (S1):S34-S45. DOI: 10.1002/eji.200737772 - 13.
O’Garra A, Vieira P. TH1 cells control themselves by producing interleukin-10. Nature Reviews Immunology. 2007; 7 (6):425-428. DOI: 10.1038/nri2097 - 14.
Feldmann M. Many cytokines are very useful therapeutic targets in disease. The Journal of Clinical Investigation. 2008; 118 (11):3533-3536. DOI: 10.1172/JCI37346 - 15.
Fine P, Eames K, Heymann DL. Herd immunity: A rough guide. Clinical Infectious Diseases. 2011; 52 (7):911-916. DOI: 10.1093/cid/cir007 - 16.
Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature Immunology. 2011; 12 (6):509-517. DOI: 10.1038/ni.2039 - 17.
Minor PD. Live attenuated vaccines: Historical successes and current challenges. Virology. 2015; 479 :379-392. DOI: 10.1016/j.virol.2015.03.032 - 18.
Vidor E, Plotkin SA. Immunogenicity of a two-component (PT&FHA) acellular pertussis vaccine in various combinations. Human Vaccines. 2008; 4 (5):328-340. DOI: 10.4161/hv.4.5.6008 - 19.
Orenstein WA, Ahmed R. Simply put: Vaccination saves lives. Proceedings of the National Academy of Sciences. 2017; 114 (16):4031-4033. DOI: 10.1073/pnas.1704507114 - 20.
Bryant KA, Marshall GS. Haemophilus influenzae type b–Neisseria meningitidis serogroups C and Y tetanus toxoid conjugate vaccine for infants and toddlers. Expert Review of Vaccines. 2011; 10 (7):941-950. DOI: 10.1586/erv.11.90 - 21.
Relyveld EH, Bizzini B, Gupta RK. Rational approaches to reduce adverse reactions in man to vaccines containing tetanus and diphtheria toxoids. Vaccine. 1998; 16 (9-10):1016-1023. DOI: 10.1016/S0264-410X(97)00288-0 - 22.
Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Current Opinion in Immunology. 2020; 65 :14-20. DOI: 10.1016/j.coi.2020.01.008 - 23.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine. 2020; 383 (27):2603-2615 - 24.
Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics—Developing a new class of drugs. Nature Reviews Drug Discovery. 2014; 13 (10):759-780. DOI: 10.1038/nrd4278 - 25.
Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005; 23 (2):165-175. DOI: 10.1016/j.immuni.2005.06.008 - 26.
Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990; 247 (4949):1465-1468. DOI: 10.1126/science.1690918 - 27.
Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Human Gene Therapy. 2009; 20 (11):1269-1278. DOI: 10.1089/hum.2009.067 - 28.
Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annual Review of Immunology. 1997; 15 (1):617-648. DOI: 10.1146/annurev.immunol.15.1.617 - 29.
Felber BK, Valentin A, Rosati M, Bergamaschi C, Pavlakis GN. HIV DNA vaccine: Stepwise improvements make a difference. Vaccine. 2014; 2 (2):354-379. DOI: 10.3390/vaccines2020354 - 30.
Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2020; 396 (10249):479-488. DOI: 10.1016/S0140-6736(20)31605-6 - 31.
Kaneko S, Mastaglio S, Bondanza A, Ponzoni M, Sanvito F, Aldrighetti L, et al. IL-7 and IL-15 allow the generation of suicide gene–modified alloreactive self-renewing central memory human T lymphocytes. Blood, The Journal of the American Society of Hematology. 2009; 113 (5):1006-1015. DOI: 10.1182/blood-2008-05-156059 - 32.
Graham BS. Rapid COVID-19 vaccine development. Science. 2020; 368 (6494):945-946. DOI: 10.1126/science.abb8923 - 33.
Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021; 397 (10269):99-111. DOI: 10.1016/S0140-6736(20)32661-1 - 34.
Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996; 272 (5259):263-267. DOI: 10.1126/science.272.5259.263 - 35.
HogenEsch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine. 2002; 20 :S34-S39. DOI: 10.1016/S0264-410X(02)00169-X - 36.
Kaitin KI, DiMasi JA. Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000-2009. Clinical Pharmacology & Therapeutics. 2011; 89 (2):183-188. DOI: 10.1038/clpt.2010.286 - 37.
World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association. 2013; 310 (20):2191-2194. DOI: 10.1001/jama.2013.281053 - 38.
Burki T. Equitable distribution of COVID-19 vaccines. The Lancet Infectious Diseases. 2021; 21 (1):33-34. DOI: 10.1016/S1473-3099(20)30949-X - 39.
Smith TC. Vaccine rejection and hesitancy: A review and call to action. In: Open Forum Infectious Diseases. Vol. 4, No. 3. Oxford, United Kingdom: Oxford University Press; 2017. p. ofx146. DOI: 10.1093/ofid/ofx146 - 40.
MacDonald NE. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015; 33 (34):4161-4164. DOI: 10.1016/j.vaccine.2015.04.036 - 41.
Ssebagereka A, de Broucker G, Ekirapa-Kiracho E, Kananura RM, Driwale A, Mak J, et al. Equity in vaccine coverage in Uganda from 2000 to 2016: Revealing the multifaceted nature of inequity. DOI: 10.21203/rs.3.rs-2002082/v1 - 42.
Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. Fair allocation of scarce medical resources in the time of Covid-19. New England Journal of Medicine. 2020; 382 (21):2049-2055. DOI: 10.1056/NEJMsb2005114 - 43.
Noad R, Roy P. Virus-like particles as immunogens. Trends in Microbiology. 2003; 11 (9):438-444. DOI: 10.1016/S0966-842X(03)00208-7 - 44.
Poland GA, Ovsyannikova IG, Kennedy RB. Personalized vaccinology: A review. Vaccine. 2018; 36 (36):5350-5357. DOI: 10.1016/j.vaccine.2017.07.062 - 45.
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017; 547 (7662):217-221. DOI: 10.1038/nature22991 - 46.
Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. The Lancet. 2013; 382 (9903):1525-1533. DOI: 10.1016/S0140-6736(13)61809-7 - 47.
Orenstein WA, Paulson JA, Brady MT, Cooper LZ, Seib K. Global vaccination recommendations and thimerosal. Pediatrics. 2013; 131 (1):149-151. DOI: 10.1542/peds.2012-1760 - 48.
McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. Journal of Allergy and Clinical Immunology. 2016; 137 (3):868-878. DOI: 10.1016/j.jaci.2015.07.048 - 49.
Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015; 33 (36):4398-4405. DOI: 10.1016/j.vaccine.2015.07.035 - 50.
Wilson SL, Wiysonge C. Social media and vaccine hesitancy. BMJ Global Health. 2020; 5 (10):e004206. DOI: 10.1136/bmjgh-2020-004206 - 51.
Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, et al. Assessment of the 2010 global measles mortality reduction goal: Results from a model of surveillance data. The Lancet. 2012; 379 (9832):2173-2178. DOI: 10.1016/S0140-6736(12)60522-4 - 52.
Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker DG. Return on investment from childhood immunization in low-and middle-income countries, 2011-20. Health Affairs. 2016; 35 (2):199-207. DOI: 10.1377/hlthaff.2015.1086