Classification of kyphosis based on the results of manual reduction.
Abstract
Thoracolumbar (TL) burst fractures occasionally result in severe instability, acute or delayed neurological dysfunction and require surgical intervention. Burst fractures can be reduced by manual reduction first and the following surgical approaches including anterior, posterior, or both have individual advantages and limitations. Even transpedicular decompression and augmentation with the body cages and short-segment fixation (TpBA) are regarded successful, yet they are limited in their ability to decompress the contralateral spinal cord and bilateral procedures are necessary. Thus, a posterior far-lateral subpedicle approach to open the lateral vertebral cortex window, creating a tunnel to remove retropulsed bony fragments and pass body cages for full-body augmentation (SpBA) to treat burst fracture was herein reported. The characteristics of SpBA include unilateral approach, direct decompression, short operation time, and no posterior instrumentation. While adjacent disc injury and degeneration may occur in burst fractures, Li’s short-term results indicate that SpBA is effective in preventing its adverse effects. This chapter describes the detailed advanced techniques and classification of the results obtained by a professional team manual reduction for post-traumatic kyphosis. The unilateral subpedicle approach with body cages and cementation without screw instrumentation rendering a minimally invasive solution for spinal burst fractures was demonstrated.
Keywords
- subpedicle approach
- Magerl incomplete burst fracture
- thoracolumbar burst fractures
- spinal trauma
- manual reduction
- posterior instrumentation
- body cage
- kyphosis
- kyphosis classification
1. Introduction
Thoracolumbar (TL) burst fractures occasionally lead to severe spinal instability and even cause acute or delayed neurological dysfunction, and surgical treatments are required [1, 2, 3]. The purpose of surgical intervention is to bring about nerve decompression, reconstruction of the vertebral body, and correction of angular deformity and stability [4]. Traditionally, decompression of the spinal cord and fixation of spinal construct can be performed either by anterior, posterior, or both approaches with individual advantages and limitations [5, 6, 7, 8]. A perfect minimally invasive approach with safe and fast procedure and leading to long-term good results and function recovery is still expected.
Transpedicular decompression and body augmentation (TpBA) with body cages combining short-segment fixation after indirect decompression by team manual reduction [9] has been reported to be a successful method to treat high-energy burst fractures or osteoporotic Kümmell’s disease [10, 11]. However, transpedicle decompression is limited in its decompression of the contralateral spinal cord, and bilateral procedures are needed. In 2014, Li et al. [12] reported a new approach, that is, unilateral subpedicle window operation: through a posterior far-lateral approach, so that the lateral vertebral cortex window can be opened by pushing the cortex shell laterally and ventrally. The subpedicle cortical window then works as a tunnel to remove all retropulsed bony fragments and passing body cage for full-body augmentation (SpBA). In addition, the screw instrumentation was not needed.
The problem is that adjacent disc injury usually occurs concurrently with burst fracture [13, 14, 15, 16], which was reported as the key mechanism for the progress in the kyphosis angle and the post-operative loss of the correction angle [17, 18, 19]. However, if the fracture of the endplate can be healed, resettlement of disc does not cause kyphosis progression [20]. The literature [21, 22] also confirmed that adjacent disc degeneration may occur in burst fractures; however, only 13% is severe and related to endplate fractures. According to the short-term results, SpBA was good in prevention of the adverse effects of adjacent disc injury [12]. However, up-to-date, the long-term results have not been reported yet.
This chapter will describe the detailed technique of team manual reduction and provide the classification of the outcome results. This chapter will also retrospectively evaluate the TpBA and SpBA outcomes based on the radiographic and clinical results of SpBA with a minimum 10-year follow-up. We hypothesized that unilateral mini-open SpBA to treat TL burst fractures is able to achieve cord decompression, vertebral reconstruction, and satisfactory long-term results as compatible or even better than TpBA, in particular with a smaller wound and a shorter operation duration.
2. Background of manual reduction
Manual reduction has been successfully applied in the correction of thoracolumbar fractures. From the biomechanical studies of cadaver burst fractures, traction force on the fractured cadaver vertebra can reduce retropulsed bony fragments in the spinal canal, restore anterior and posterior vertebral height, and the extension force is able to correct the kyphotic angles [23, 24]. Clinically, posture reduction in the thoracolumbar fractures has been considered to be safe and effective [25]. In 2003, Li et al. [26] reported a 5-member manual reduction (team-MR) for high-energy burst fracture while the patient was in prone position under general anesthesia with full muscle relaxation. The reduction rate was close to 100% in acute fracture without complications. The prone position was good for manual force control and next surgical approach. In 2004, Li et al. [27] reported team-MR for acute osteoporotic compression fracture and achieved similar results. Li’s clinical series have confirmed team-MR to be safe and effective in restoring thoracolumbar vertebral fractures [9, 10, 11, 12]. Tropiano [28] reported a reduction frame that was used to reduce the spine fracture without anesthesia. The patient was in supine position receiving cephalic axial traction and lordotic reduction with a strap under the fracture site. Then a thoracolumbar plaster cast was fabricated. The frame use is limited in non-operative acute fracture and cephalic traction force through cervical spine is poor in force quantity and direction adjustment. In 2018, Carlo et al. [29] reported a 3-member manual reduction for acute burst fracture as a technical note; in 2021, Li et al. [30] confirmed the effect of a 3-member manual reduction in acute spine fractures. However, a 3-member manual reduction is weak to correct chronic deformity following spine fracture because it cannot change the patient posture according to the deformity. In addition to acute spine fracture, even the chronic post-traumatic kyphosis was possibly reduced by team-MR by elevating the patient’s shoulders if at the thoracic spine or raising the patient’s lower limbs if at the low lumbar spine which may increase the extension forces to correct post-traumatic kyphosis (Figure 1). The success of manual reduction may prevent further operative decompression procedures or instrumentation reduction, which will decrease the necessity of open surgery in osteoporotic fractures or lessen the implant strain and bone-screw interface stress and prevent potential implant failure if open surgery is needed. In this chapter, we report our experiences of manual reduction for all spine fractures including fresh and old, high-energy and osteoporotic fractures, and ankylosing spondylosis in the past 25 years.
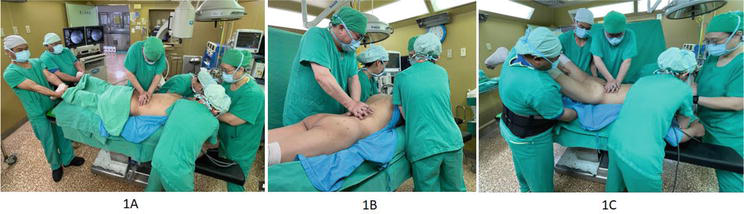
Figure 1.
Photographically shown operation steps; A: Team manual reduction to provide traction force and lordotic force to correct the kyphosis; B: If the thoracic kyphosis is not perfectly corrected, then the advanced step will elevate the shoulder and upper trunk and compression force over the kyphosis apex region, and C: If the lumbar lordosis is not well restored, then the next step will elevate the hip and lower trunk and compression force over the deformity apex point.
2.1 Techniques of team manual reduction
All the patients received manual reduction, using C-arm fluoroscopy to monitor the reduction if pre-operative MRI showed spinal cord compression and spinal angulation. Most of the patients with spinal fracture were anesthetized and put in a prone position with caution. (
Manual reduction includes traction and extension forces and should be operated step by step. Especially in kyphosis >60 degrees, slow traction without extension component should be applied first, which usually initiates a bony or disc opening. This procedure may last for more than 10 s and can be repeated 2–4 times. After checking in C-arm fluoroscopy, if the reduction is not good enough, then extension force can be followed with caution. The limitation of correction at one site is not more than 50 degrees. If the results of manual reduction were not satisfactory, then osteotomy should be considered as a substitute. There is no need to insist on manual reduction alone to achieve a perfect reduction.
2.2 Results of manual reduction
Twenty hundred and thirty cases of thoracolumbar kyphotic deformities were included in this study from January 1997 to December 2022. The male-to-female ratio was approximately 680:1350, and the mean age of the patients was 65 years (range 21 to 102 years). The inclusion criteria for this study dictated patients to have symptomatic thoracolumbar kyphosis (either primary or secondary, due to diseases, 197 cases) or spinal fracture due to trauma or osteoporosis (1833 cases) who were necessitated to undergo vertebroplasty or surgical intervention.
In the reducible cases, the spinal column height could be restored, and kyphosis corrected by manual reduction. According to the results from manual reduction in thoracolumbar kyphosis, the reduction patterns have been classified as follows and are shown in Table 1.
Table 1.
Type I. Opening of the unfused deformed vertebral body and disc was achieved, where the vertebral body fracture has not healed and interbody fusion was not established and could be restored by manual reduction (Figures 2–4). The most acute spinal fracture is easily reduced in this mechanism.
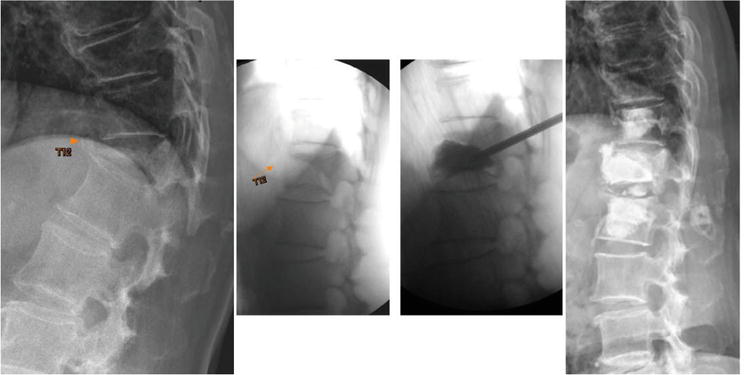
Figure 2.
Demonstrated radiographs of a 77-year-old female with T11-L1 kyphosis, T11-L1 lateral Cobb’s angle pre-op: 50′; post-manual reduction: 10′; post-vertebroplasty 10′. Cement vertebroplasties (T11,12,L1) and discoplasty (T12/L1) were done due to simultaneous reduction of vertebral body and disc.
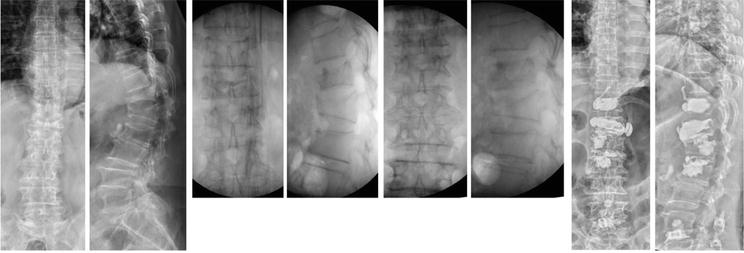
Figure 3.
Demonstrated radiographs of a 71-year-old female with T11-L2 kyphosis, T11-L2 lateral Cobb’s angle pre-op: 50′; postural reduction: 34′; post-manual reduction: 19′; post-vertebroplasty 12′. Cement vertebroplasties (T11,12,L1) and discoplasty (T12/L1) were done due to simultaneous reduction of vertebral body and disc.
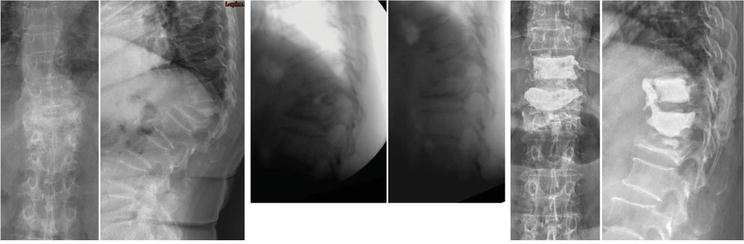
Figure 4.
Demonstrated radiographs of a 67-year-old female with T11–1 kyphosis, T11-L2 lateral Cobb’s angle pre-op: 64′; posture reduction: 53′; post-manual reduction: 28′; post-vertebroplasty 27′. Cement vertebroplasties (T11 & T12) and discoplasty (T12/L1) were done due to simultaneous reduction of vertebral body and disc.
Type II. Restoration of the disc space and successful realignment of the deformed vertebral body were achieved, corresponding to Type II kyphosis, where the vertebral body has healed with deformity, resulting in further tilting after the disc degeneration (Figure 5). The forces can open the narrowed disc space and correct the deformed vertebral column.
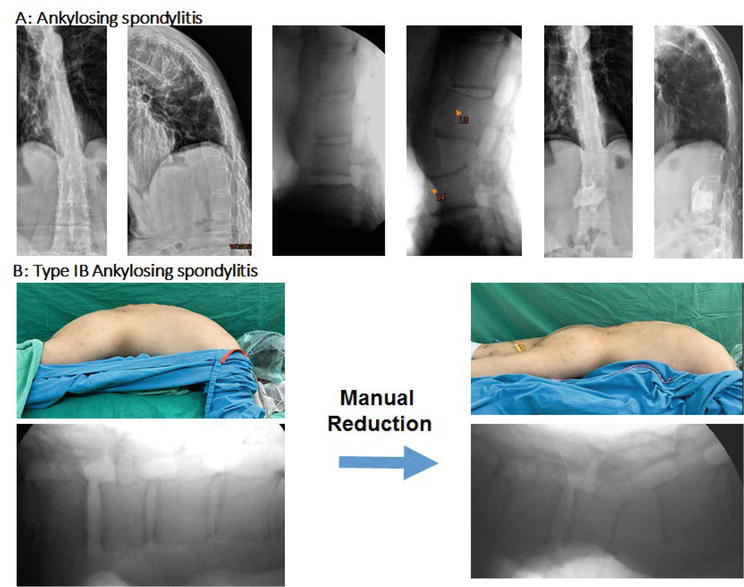
Figure 5.
Ankylosing spondylitis (A) and type 1B ankylosing spondylitis (B); 27YM, ankylosing spondylitis and kyphosis, manual reduction at the force concentrated at L3/4, inducing disc open wedge. L2/L5: Pre-op: 15′ kyphosis, posture reduction: 15′ kyphosis, post-manual reduction: 19′ lordosis; 33′ lordosis was corrected (measured at C-arm films). The interbody cementation was done.
Type III. Iatrogenic open-wedge fracture was induced by the team manual reduction (Figures 6–9). Because the healed deformed vertebrae and solid interbody fusion had been established, the force can open neither the original body breakage nor the disc space. When the vertebra was osteoporotic enough, the manual force will create a new open-wedge fracture, either in the originally healed vertebra or in the adjacent intact vertebral body, which can result in restoration of the anterior column height and thus correct the kyphosis.
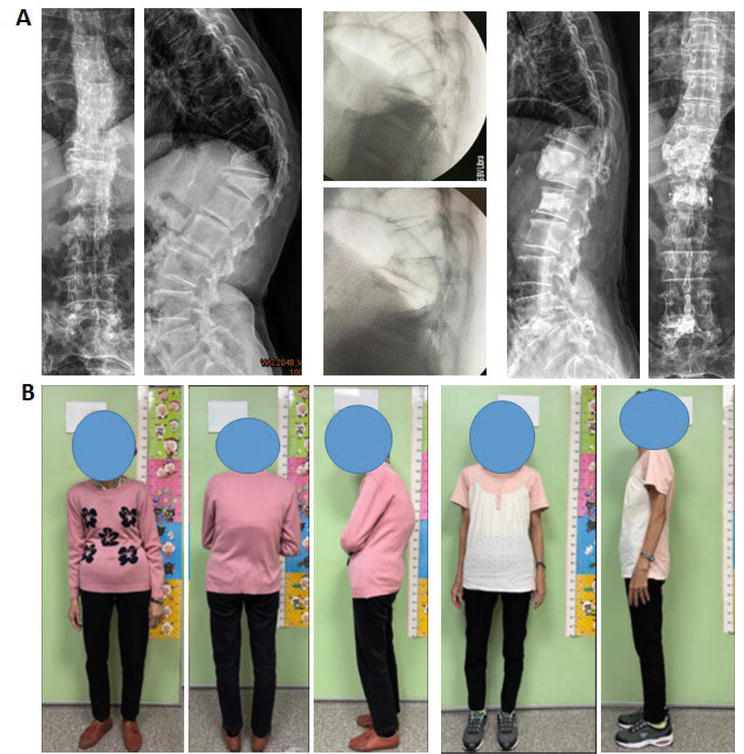
Figure 6.
Demonstrated radiographs of (A) and corresponding pictures (B) of a 78-year-old female with T10-L1 kyphosis, T10-L1 lateral Cobb’s angle pre-op: 78′; postural reduction: 77′; post-manual reduction: 34′; post-vertebroplasty 33′. T12 open-wedge reduction was noted. T12 and T11 vertebroplasties were done.
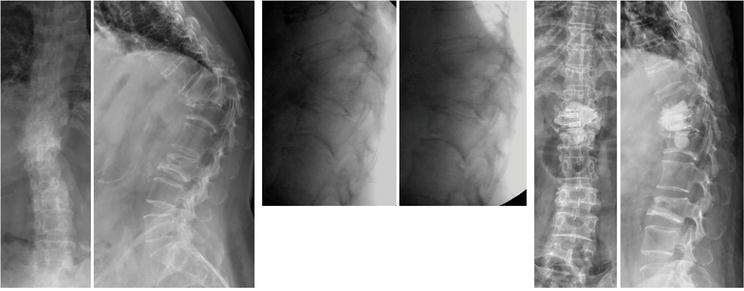
Figure 7.
Demonstrated radiographs of a 54-year-old female with T11-L1 kyphosis, T11-L1 lateral Cobb’s angle pre-op: 86′; posture reduction: 58′; post-manual reduction: 34′; post-vertebroplasty 34′. T12 open wedge was induced by manual reduction and restored by subpedicle decompression and body augmentation (SpBA) with cemented two polyetheretherketones (PEEKs).
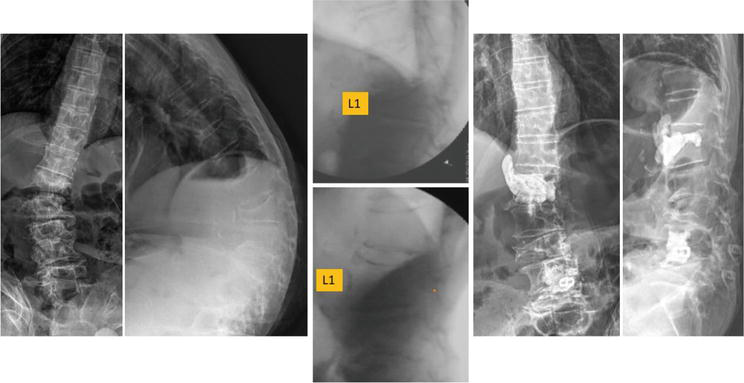
Figure 8.
Demonstrated radiographs of a 79-year-old male with T12-L2 Kypho-scoliosis, L1-L3 lateral Cobb’s angle pre-op: 50′; postural reduction: 38′; post-manual reduction: 3′; post-vertebroplasty 3′. L1 open-wedge correction was induced by manual reduction and cementation was done.
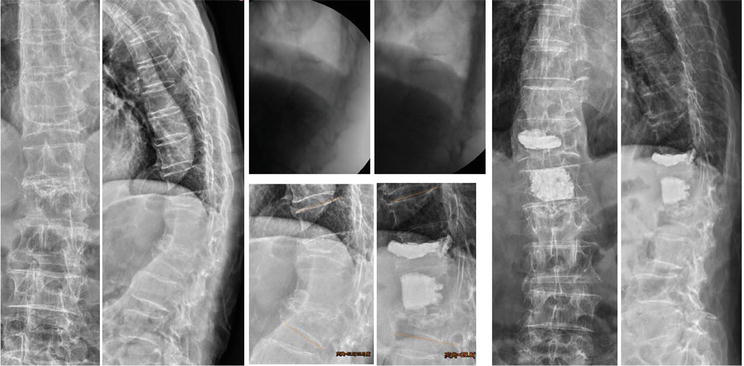
Figure 9.
Demonstrated radiographs of a 92-year-old male with T11-L2 kyphosis, T11-L2 lateral Cobb’s angle pre-op: 52′; posture reduction: 50′; post-manual reduction: 30′; post-vertebroplasty: 29′ T12 fracture site partial reduction and L1 iatrogenic open-wedge fracture.
Type IV. Failure of reduction, where solid interbody fusion was found in a non-osteoporotic spine. This type is usually predictable before operation by experienced surgeons.
The new classification of thoracolumbar kyphosis is based on the results of manual reduction and functionally guides the subsequent surgeries. In Type I kyphosis, except in infection case, the vertebroplasty, cement discoplasty, or posterior body reconstruction is the major operation after manual reduction and additional posterior instrumentation is needed if spinal instability is found. In Type II kyphosis, discectomy and interbody fixation are indicated if post manual reduction takes place. In Type III kyphosis, vertebroplasty or body augmentation with body cage with/without posterior instrumentation is indicated if manual reduction succeeds (Figure 4). In Type IV kyphosis, open or close osteotomy and posterior fixation are suggested.
Two major complications (0.1%) of manual reduction were found in the early learning curve in 1997. Hypotension and hemothorax were noted after manual reduction in a 78-year-old female with 900 kyphosis (Figure 10) who received blood transfusion, chest tube insertion, and intensive care unit (ICU) care and finally recovered. Another complication was reflected with iatrogenic L1 pars fracture without neurological injury; this was fixed with posterior instrumentation. After 2000, there were no major complications noted in the past 20 years. The common asymptomatic complication is cement leakage due to the opening of anterior vertebral cortex (Figure 11). Right now, if there is open wedge of anterior cortex, the cement vertebroplasty will be divided into two stages; that is, 1 cc liquid cement was first injected to form a thin cement membrane lining on anterior longitudinal ligament and then, 5 min later when the first cement was hard enough to prevent cement leakage, sufficient high viscous cement was injected to fulfill the open spaces, either in vertebral body defect or in disc space.
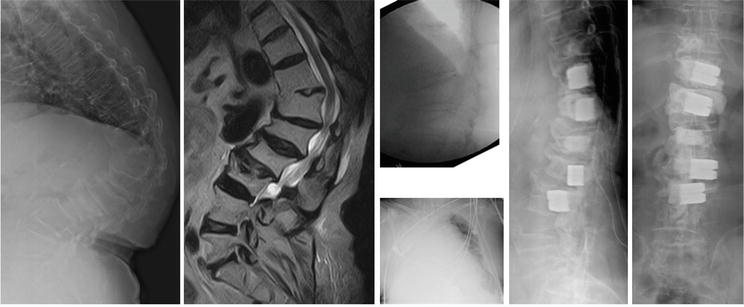
Figure 10.
Demonstrated radiographs of a 71-year-old female with T10-L3 95′ post-manual reduction: 24’ Hemothorax: T12 open-wedge fracture. Multiple SpBAs were done. SpBA indicates subpedicle decompression and body augmentation.
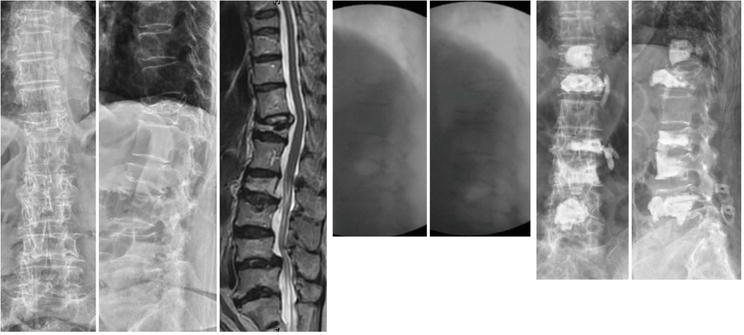
Figure 11.
Demonstrated radiographs of a 70-year-old female with T11-L1 kyphosis, T11-L1 lateral Cobb’s angle pre-op: 24′; posture reduction: 23′; post-manual reduction: 3′; post-vertebroplasty 2′. The cement leakage was noted at T12 and L2 vertebroplasties.
2.3 Discussion
Based on our 25-year experiences, the manual reduction of thoracolumbar kyphosis is effective and safe. Manual reduction is commonly used in orthopedic practice to manage long bone fractures and reduce limb joint dislocation [31]. Cervical spine injury is usually treated with traction [32]. In this chapter, manual reduction was designed based on basic biomechanical studies [23, 24, 33, 34] and hyperextension postural reduction [35]. Clinical application achieved a high success rate. The learning curve for manual reduction is short and no major complications were observed if the correction was not more than 50 degrees. Classification of thoracolumbar kyphosis was made according to retrospective study of the immediate results after manual reduction. This classification may be influenced by observer bias, since the post-reduction films were evaluated by the surgeons after performing the manual reduction. Since this is a qualitative analysis, the flaw had far less impact than if quantitative comparisons had to be made.
In Type I kyphosis with breakage of the vertebral body, manual reduction has led to a very good restoration of the vertebral body. Reduction of acute burst or compression fractures [36] was as easy by manual reduction as instrumentation reduction [37], followed by posterior fixation with transpedicle body augmenter [26, 38, 39]. Non-union osteoporotic compression fractures, also known as Kümmell’s disease [40, 41, 42], were also easily reduced by manual reduction accompanied by posterior body reconstruction with transpedicle body augmenter [27]. Vertebroplasty or kyphoplasty was shown to increase vertebral body height without instrumentation, but the restoration result was limited [36, 43, 44, 45]. The difference between manual reduction and postural hyperextension reduction lies in the amount of force used. Manual reduction can be repeated if the reduction is not complete and the forces can be increased to induce restoration close to the anticipated result. Therefore, manual reduction is a safe, effective, and economic method to reduce Type I kyphotic deformity.
Acute secondary body collapse in active spinal tuberculosis, multiple myeloma with compression fracture, or osteolytic metastasis can be easily reduced by the manual reduction. This finding may result in a new treatment policy in the future. Traditionally, body collapse with cord compression secondary to active tuberculosis [46] or metastasis [47, 48] has been explored through an anterior approach in order to remove the compression source. But with manual reduction, the kyphosis could be reduced and spinal cord can be decompressed, and confirmed intraoperatively by C-arm myelogram. For tuberculosis (TB) spine with body collapse, subpedicle debridement with bone graft and posterior instrumentation should be sufficient, but this should be followed by medical treatment for tuberculosis. For secondary body collapse due to spinal metastasis, the subpedicle corpectomy and discectomy in combination with posterior fixation should be able to prevent cord compression, and this should be followed with chemotherapy or radiotherapy. Manual reduction and posterior approach will be much easier than the combination of anterior decompression and posterior fixation.
In Type II kyphosis with healed or deformed body without anterior interbody fusion, manual reduction can open the disc and correct malalignment of the deformed vertebral body. Traditionally, such deformities may necessitate an anterior approach to decompress the cord, followed by anterior or posterior fusion [49]. Because tilting of the vertebra can be corrected by manual reduction, the posterior approach including transpedicle discectomy [50] and interbody fusion with transpedicle body augmenter would be sufficient. Manual reduction can change treatment modalities in Type II kyphosis.
In Type III kyphosis with anterior interbody fusion in osteoporotic spine, the iatrogenic open-wedge fracture can be induced by manual reduction and kyphosis should be corrected. That situation followed by percutaneous vertebroplasty with sufficient cement vertebral filling will lead to a good result. Usually, the degree of osteoporosis should be evaluated first. The success can be predicted when dual X-ray absorptiometry (DEXA) < −2.5. Theoretically, the force needed to create an iatrogenic open-wedge fracture must increase according to the strength of the vertebrae.
In Type IV kyphosis, if the fusion segment is strong, manual force may not create a new fracture, and thus fail to reduce the kyphosis. In such cases, transpedicular wedge osteotomy [51, 52] or the anterior approach must be needed. But in contrast, success using Type III will lead to simple posterior instrumentation with posterior body reconstruction, which prevents the technically demanding and highly risky transpedicle shortening osteotomy or the anterior approach. Manual reduction can change the selection of traditional operative procedures in Type III kyphosis.
2.4 Summary of team manual reduction
Manual reduction can reduce the thoracolumbar kyphosis through different mechanisms and change the followed treatments. New classifications of kyphosis and new concepts in treating thoracolumbar kyphosis have been developed after the development of team manual reduction.
3. TpBA and SpBA to fix burst fractures following manual reduction
This retrospectively observational, single-institute case series study was approved by the St. Martin De Porres Hospital, Chia-Yi, Taiwan ethics review board [IRB 20C-006].
This retrospective study evaluated patients with TL burst fractures from January 2004 to December 2012. The inclusion criteria were as follows: single-level high-energy non-osteoporotic burst fractures (Type A3.3 according to the classification of Magerl et al. [53] with more than five points graded by the load-sharing mechanism described by Gaines et al. [54], and involving T10–L2 with neurologic function limited to Frankel Grade C, D, or E [25]. Fifty-three non-operatively treated cases or DEXA < −2.5 and 16 patients with other major organ system or musculoskeletal injuries were excluded. Five patients who sustained multilevel involvement were also excluded. A total of 128 qualified patients completed the procedures. Because the cases with other organ trauma were excluded, the general conditions were stable in enrolled cases. The operation was done within 48 h after the patients were sent to the hospital within 10 days after the fracture happened. All cases were done by the same surgeons.
The clinical results were based on the latest follow-up before December 2022. Four patients died of unrelated medical illnesses and six patients were lost to follow-up. These 10 patients were excluded from this retrospective study. Finally, 118 cases with 41C, 58D, and 19E by the Frankel grading system [25] and average 7.1 ± 0.9 points graded by the load-sharing mechanism were included in this study. The follow-up rate was 92.2%. The male-to-female ratio was 71:47. The mean follow-up was 163.7 ± 27.1 (range, 124–227) months and age at the time of operation, 56.3 ± 6.7 (range, 32–67) years. The mechanisms of injury included fall (47%) and traffic accidents (53%). The demographic data are listed in Table 2.
| Items | TpBAa | SpBAa |
|---|---|---|
| Cases | 53 | 65 |
| Male:female | 33:20 | 38:27 |
| Age (yr) | 55.8 ± 6.6 | 56.7 ± 6.9 |
| T11:T12:L1:L2 | 7:20:18:8 | 11:22:23:9 |
| Load-sharing score | 7.2 ± 0.9 | 7.0 ± 1.2 |
| Fall: traffic accident | 25:28 | 31:34 |
| Follow-up (mo) | 173 ± 29 | 156 ± 23 |
| Frankel E:D:C | 21:23:9 | 20:35:10 |
| Final Frankel E:D:C | 49:4:0 | 60:5:0 |
Table 2.
Demographic data of the patients.
TpBA indicates transpedicle body augmentation; SpBA, subpedicle decompression and body augmentation.
The pre-operative evaluation protocol included anteroposterior (AP) and neutral lateral TL radiographs, and either computed tomography (CT) scans or MRI scans to evaluate fracture sites and cord compression status. The mean follow-up was 173 ± 29 (TpBA) and 156 ± 23 (SpBA) months (p = 0.001), and the age at the time of operation was 55.8 ± 6.6 and 56.7 ± 6.9 years (p = 0.47). All patients tolerated the TpBA or SpBA surgery smoothly.
In the radiographic analysis, the lateral Cobb’s angle was measured as described by Kuklo et al. [55] from the superior endplate of the vertebral body above the fracture to the inferior endplate of the vertebral body below the fracture level. The segment wedge angle was measured from the superior endplate to the inferior endplate of the fractured vertebral body wedge angle of the fractured vertebral body as described previously by Verlaan et al. [22]. The predicted anterior and posterior vertebral body heights were estimated by the mean of the heights of the upper and lower adjacent segments. The angles and body heights were measured on neutral thoracolumbar radiographs before the operation, immediately after surgery, and at the final follow-up. All digitization and measurements were done using EBM-viewer software (EBM Technologies Inc., Taipei, Taiwan) with an accuracy of ±0.1 mm by a graduate student. Clinical results were assessed by the performance scale (Grades A–E) described by Frankel et al. [25] and also evaluated pains using the Visual Analog Score (VAS).
Student’s paired t-tests were done between two groups to evaluate all radiographic and clinical parameters. All the data are presented as mean ± standard deviation. The level of statistical significance was set as p < 0.05.
3.1 Operative techniques using transpedicle decompression and transpedicle body augmentation with short-segment fixation (TpBA)
After manual reduction, short-segment fixation was followed. Pedicle screws were placed at the level above and below the fractured vertebrae (two levels, four screws) using the rod screw system (Reduction-Fixation Spinal Pedicle Screw System, Advanced Spine Technology Inc., Oakland, CA, USA; Diapason, Stryker Corporation, Allendale, NJ, USA; and UP spine system, TiTec Medical Co., Ltd., Taipei, Taiwan). Bilateral pedicle tunnels to the vertebral body were made by an awl, followed by serial custom-made trials (8 to 14 mm) to prepare transpedicle tunnel for decompression and body augmentation. The bony defect in the fractured vertebral body was filled through bilateral transpedicle tunnels [9], with autologous bone graft mixed with calcium sulfate (Osteoset, Wright Medical Technology, Arlington, TN, USA) if the autograft from the posterior iliac bone was insufficient. Then the augmenter was inserted into the vertebral body through the pedicle tunnel, and finally, bone graft was used to fill the pedicle tunnel space. Patients wore a thoracolumbar brace for 3 months. After discharge, patients were followed up regularly.
3.2 Operative techniques of subpedicle decompression (TpBA) and body augmentation without screw fixation (SpBA)
In the SpBA group, a paramedian incision of about 1 inch in length was made on the more painful side as reported by the patient. The transverse process was identified with muscle splitting and blunt dissection. The superior part of the transverse process was then punched out to expose the subpedicle region, that is, the pedicle-body junction area, where no nerve or vessel resides (Figure 12). A guiding pin was first inserted and confirmed by C-arm fluoroscopy, followed by serial custom-made dilators to prepare for the passage of body augmenters, the residual bone may be pushed into anterior and peripheral region, which served as autogenous bone graft and may help in vertebral fracture healing and anterior ankylosis. The outer vertebral cortex was pushed by the dilators to the lateral side, which then worked as a shield to protect the surrounding structures. After the preparation, a subpedicle working tunnel of about 13 × 22 × 40 mm was created for the removal of retropulsed bony fragments and passing two spacers simultaneously. The upper segmental nerve root was protected by a nerve retractor. Two pile-up body cages of 10 × 13 × 27 mm were inserted into the vertebral body through the subpedicle window. Body cages can be made of polyetheretherketone (PEEK) or titanium, solid or hollow, cylindrical or rectangular. Polymethyl methacrylate (radiopaque bone cement; Howmedica International S. De R.L., Limerick, Ireland) was used to stabilize the body cages and provide the initial partial internal support if stability was not perfectly established after the body cages were inserted or bone quality was not good enough. Usually, patients were able to walk on the same day of the operation. Both groups of patients were asked to wear a thoracolumbar brace for 3 months. After discharge, patients were followed up regularly.
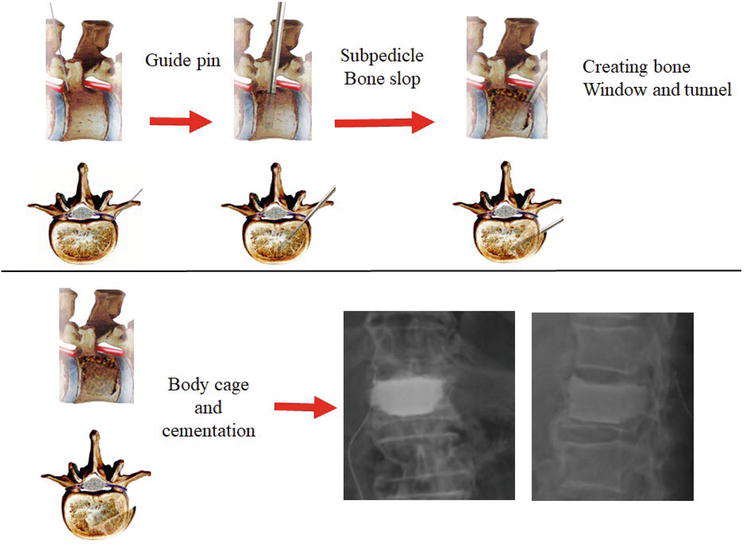
Figure 12.
Subpedicle decompression and body augmentation (SpBA) flowcharts of subpedicle decompression and body augmentation with body cage and cementation as shown in the lateral and transverse views. The first step is the insertion of the guiding pin as confirmed by C-arm fluoroscopy; second, the creation of a bone slop in the cephalocaudal direction; third, creation of the subpedicle bone window and tunnel; fourth, after the removal of retropulsed bone fragments and placement, the body cages with cementation were inserted. SpBA indicates subpedicle decompression and body augmentation.
3.3 Results of TpBA and SpBA
A summary of the results of the operative parameters is shown in Table 3. The average blood loss and hospitalization were not different between the two groups. The operation duration was significantly shorter in the SpBA group (40 ± 12 min), only 59% of that for the TpBA group (68 ± 17 min) (p < 0.001). All patients in the SpBA group could walk without or with assistance within 24 h after the operation; but ambulation for the TpBA group was achieved 1 to 3 days after the operation. Signs of fracture healing, that is, appearance of anterior vertebral cortical line could be documented radiographically within 3 to 5 months. Initial reduction and maintenance of reduction were mostly achieved in both groups. The final post-operative anterior vertebral restoration rates in the two groups were similar. There was no implant dislodgement in either group. The partial or complete spontaneous anterior or lateral ankylosing bridge was noted in every case of both groups (Figures 13–16).
| Operative parametersa | |||||
|---|---|---|---|---|---|
| Hospital stay (d) | Blood loss (mL) | Op. time (min) | |||
| TpBA | 4.7 ± 1.2 | 228 ± 77 | 68 ± 17 | ||
| SpBA | 4.4 ± 1.1 | 211 ± 70 | 40 ± 12 | ||
| P | 0.34 | 0.21 | <0.001 | ||
| Anterior body height (%) | |||||
| Pre-op | Post-op FU | Correction | Final FU | Correction loss | |
| TpBA | 43.0 ± 4.4% | 95.2 ± 1.9% | 52.2 ± 4.8% | 92.7 ± 2.0% | 2.4 ± 0.8% |
| SpBA | 42.6 ± 4.2% | 99.8 ± 1.5% | 57.1 ± 4.2% | 97.5 ± 1.2% | 2.3 ± 0.9% |
| P | 0.69 | <0.001 | <0.001 | <0.001 | 0.28 |
| Lateral Cobb’s Angle (°) | |||||
| Pre-op | Post-op FU | Correction | Final FU | Correction loss | |
| TpBA | 26.4° ± 13.9° | 2.5° ± 2.4° | 23.7° ± 3.9° | 5.5° ± 2.9° | 2.9° ± 2.2° |
| SpBA | 26.9° ± 4.2° | 2.6° ± 11.9° | 24.4° ± 4.3° | 5.4° ± 2.3° | 2.8° ± 2.4° |
| P | 0.41 | 0.94 | 0.41 | 0.77 | 0.79 |
Table 3.
Results of operative parameters, anterior body height, and kephotic angle (o).
TpBA indicates transpedicle body augmentation; SpBA, subpedicle decompression and body augmentation.
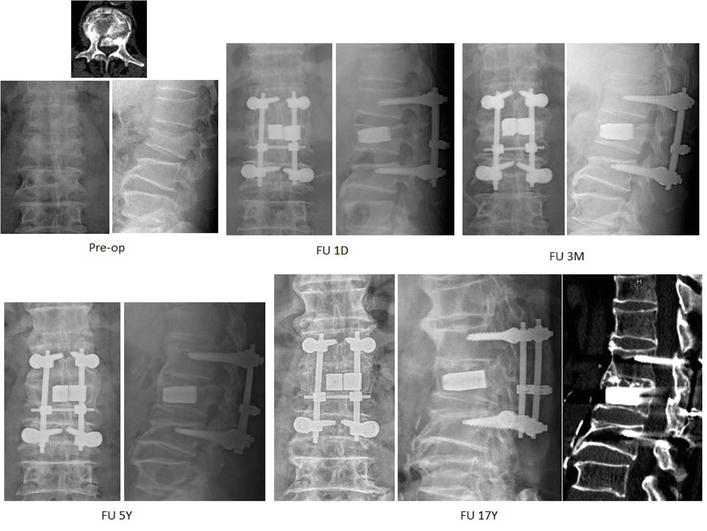
Figure 13.
Demonstrated radiographs of a 58-year-old male who fell from height with L2 burst fracture with Frankel grade D. He was treated with manual reduction and transpedicle body augmentation (TpBA). The fracture was healed with anterior ankylosing bridging 3 months post-operatively. He is still pain free and works at his farm.
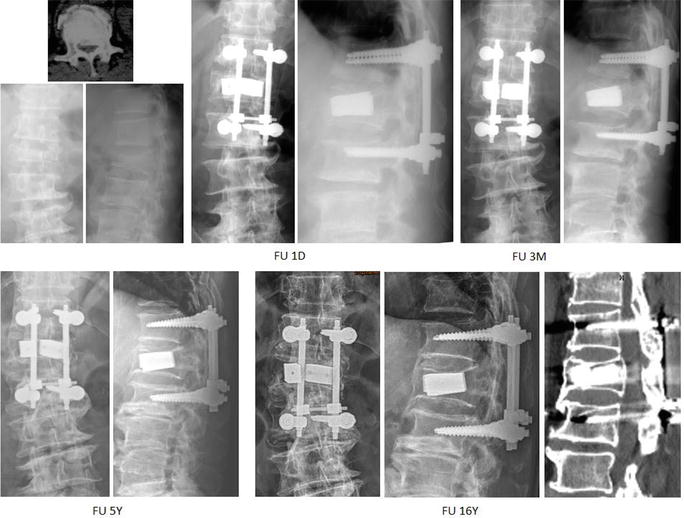
Figure 14.
Demonstrated radiographs of a 62-year-old male who fell from height with L1 burst fracture with Frankel grade D. He was treated with manual reduction and transpedicle body augmentation (TpBA). The fracture was healed smoothly and his previous lumbar degenerative scoliosis seems not to be affected too much by TpBA operation.
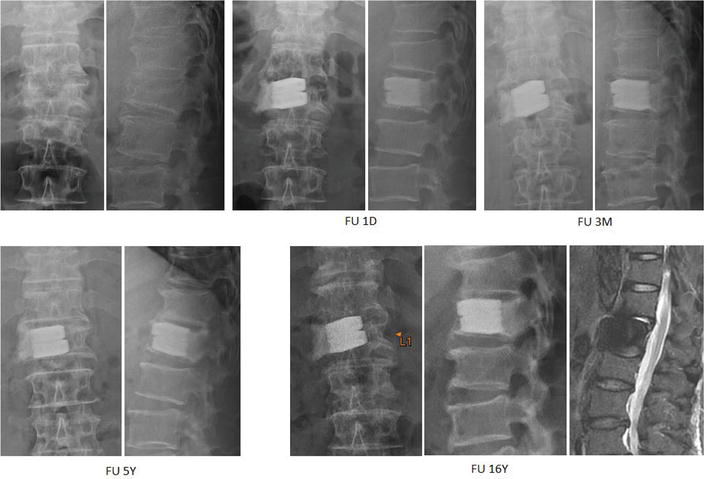
Figure 15.
Demonstrated radiographs of a 56-year-old male who got injured in a traffic accident with L1 burst fracture with Frankel grade E. He was treated with manual reduction, subpedicle decompression, and cement-augmented body cages. The spontaneous anterior ankylosis was noted from 1 month and continued to 16 years post-operatively and he is symptom free.
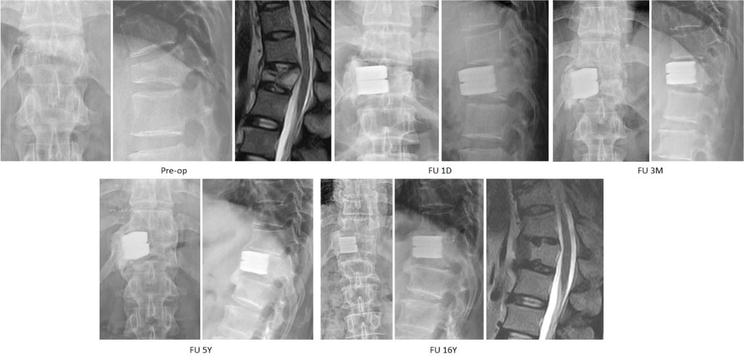
Figure 16.
Demonstrated radiographs of a 46-year-old male MD who had motorcycle accident and resulted in a L2 burst fracture with Frankel grade C with urinary incontinence. He was treated with manual reduction and transpedicle body augmentation (TpBA). He experienced the removal of implant and underwent decompression surgery due to residual spinal stenosis 6 years later. He is still an active physician.
The initial anterior vertebral height correction was 52.2 ± 4.8% (TpBA) versus 57.1 ± 4.2% (SpBA) (p < 0.001) and final loss reduction was 2.4 ± 0.8% versus 2.3 ± 0.9% (p = 0.28). Initial corrections of the lateral Cobb angle were 23.7° ± 3.9° versus 24.4° ± 4.3°) (p = 0.41) and of the final reduction loss were 2.9° ± 2.2° versus 2.8° ± 2.4° (p = 0.79).
Adjacent disc degeneration secondary to endplate fracture was noted in every case in the SpBA group; however, no late radiculopathy in the SpBA group was noted. In clinical evaluations, there were no significant intergroup statistical differences in VAS at pre-operative status (TpBA vs. SpBA = 7.5 ± 1.4 vs. 7.8 ± 1.3) as well as at the final visit (1.4 ± 0.4 vs. 1.2 ± 0.5). Totally, 109 patients were maintained or recovered to Frankel grade E. Four cases in the TpBA group and five in the SpBA group improved from grade C to grade D.
Complications included three superficial infections in the TpBA group, which were cured by antibiotics management, three post-operative seromas in the TpBA group and one in the SpBA group, which healed after debridement, and two deep vein thromboses in the TpBA group. Two patients in the SpBA group had an intraoperative first lumbar (L1) root overstretch injury, which caused numbness and neuralgia at the left inguinal region and leg weakness, but the symptoms gradually subsided in 4 and 6 weeks later. Three patients in the TpBA group had residual symptoms of incomplete decompression of the spinal stenosis, and laminectomy to decompress the spinal cord was done later (Figure 17). Four dura tears, which were caused by the penetration of bony spike, were observed during subpedicle decompression after the removal of retropulsed bony fragments; the tears were sutured or packed with gelatin sponge and healed without neurological impairment.
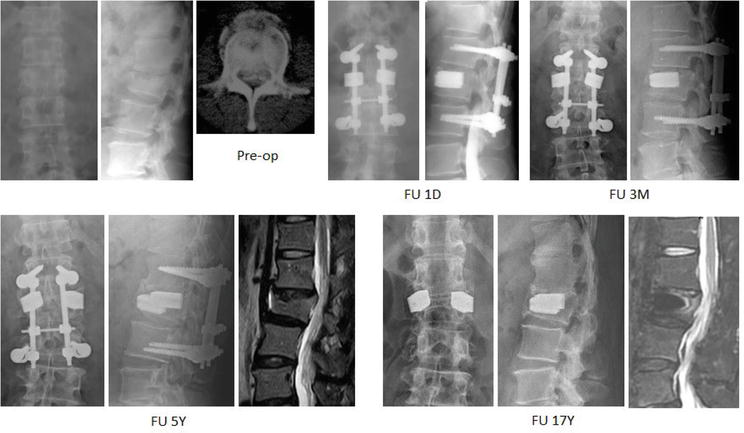
Figure 17.
Demonstrated radiographs of a 46-year-old male MD who had motorcycle accident and resulted in a L2 burst fracture with Frankel grade C with voiding dysfunction. He was treated with manual reduction and transpedicle body augmentation (TpBA). He experienced the removal of implant and underwent decompression surgery due to residual spinal stenosis 6 years later. He is still an active physician.
3.4 Discussion
Traditionally, there are three main surgical methods used for thoracolumbar Magerl incomplete burst fractures: anterior approach, posterior approach, and a combination of both [5, 6, 7, 8]. The anterior approach directly decompresses the spinal cord, but it comes with a risk of damaging the lungs, internal organs, and vascular structures. On the other hand, the posterior approach, including laminectomy, can only achieve indirect decompression. In this reported study, manual reduction [9] was used to provide indirect decompression, while the subpedicle approach [12] was used to directly remove the retropulsed bony fragments. This approach combines the advantages of both anterior and posterior decompression, but without the potential surgical complications. The study used a posterior far-lateral approach to create a unilateral subpedicle cortical window that served as a tunnel to remove all retropulsed bony fragments and passing body cage for full-body augmentation. The study demonstrated that the unilateral mini-open SpBA method for treating thoracolumbar burst fractures was effective in achieving cord decompression, vertebral reconstruction, and satisfactory long-term results, as good as those seen with TpBA, but with a smaller wound and shorter operation time, as evidenced by the results of a minimum 10-year follow-up.
In interpreting our data, it is important to consider some limitations. First, there may be nonhomogeneous inclusion protocols due to differences in pre-operative fracture classification methods. These protocols were studied and implemented by the trauma team in the emergency department, with some cases using computed tomography and others using MRI. This might result in different severities of neurological symptoms in each group at different times, leading to bias. Second, the study did not have a proper randomized control group. As this was a retrospective study, we performed fewer posterior short-segment fixations after learning that the results of SpBA were satisfactory. Consequently, the follow-up period of SpBA was significantly shorter than that of TpBA. Third, the clinical outcomes were determined by the treating surgeons, which may introduce bias in interpreting the findings. Fourth, all radiographs were obtained in the supine position, which could result in bias when compared to studies using standing radiographs. Finally, blinded evaluation of radiographic results was not possible, as the body cages or posterior screws were visible on the radiographs. However, independent reviewers were used to evaluate other criteria.
The long-term results of SpBA showed that adjacent disc degeneration did occur; however, there was no significant radiculopathy. Burst fractures are often accompanied by various degrees of intervertebral disc injury above the affected vertebrae [13, 14, 15], with reported rates as high as 63.4% [16]. The severity of intervertebral disc injury increased with the degree of fracture [13, 56]. The progression of kyphosis angle and loss of correction angle after surgery were mainly due to more severe intervertebral disc injuries and changes in the intervertebral disc shape [17, 18, 19]. However, if the fracture of the endplate can be healed, resettlement of disc does not cause kyphosis progression [20]. The literature [21, 22] also confirmed that adjacent disc degeneration does occur in burst fractures; however, only 13% are severe and related to endplate fractures. In the SpBA group, the vertebral body was fully restored by the body cage and fixed with cement. The endplate was restored and healed as completely as possible. Intervertebral disc injury is not only difficult to recover [57, 58, 59], but also increases apoptosis of intervertebral disc tissue [60], leading to intervertebral disc degeneration and spontaneous fusion [61]. Our findings support this, as every case in SpBA had disc degeneration and tended toward anterior ankylosis across the upper disc. Changes in the intervertebral disc angle and height reflect changes in the Cobb angle [19, 62]. Changes in the intervertebral disc morphology, based on the fractured and deformed endplate, may be the primary reason for this angle change [63]. However, body cages with cementation can restore the body and endplate well, and degenerative disc material can still link with the healed flat endplate without causing angle changes, even if the height is decreased. This may explain why Cobb’s angle has been maintained well after over a 10-year follow-up.
For high-energy burst fracture, PEEK cages and solid titanium cages lead to the same good clinical results. Originally, we used titanium cylindrical body cage to ensure the endplate reconstruction and prevent disc degeneration as slightly as possible. Later, we found that if cement could fulfill the body and support the endplate well, the PEEK cages, just as a spacer not a fusion device, would have led to the same results of titanium cages (Figure 18). The vertebral bone growth around the cage-cement complex was observed and finally stable fracture healing was achieved. Either titanium or PEEK cage would have the same mechanism. Due to interspinal process device being reported to decrease the disc stress [64, 65], the interspinal process device (IPD) (Rocker, Paonan Biotech Co., Ltd., Taiwan) was applied hopefully to decrease the disc stress and prevent the disc degeneration and kyphosis.
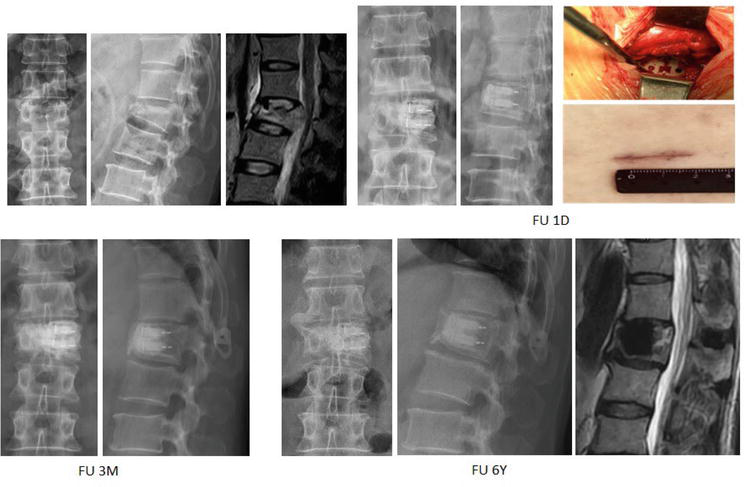
Figure 18.
Demonstrated radiographs of a 47-year-old male who fell from height with L1 burst fracture with Frankel grade D. He was treated with manual reduction, subpedicle decompression, and body cages with interspinal process device (IPD) (rocker, Paonan biotech Co., ltd., Taiwan). The photographs show the final results of cage insertion at the bone window and incision wound. The fracture was healed 3 months post-operatively and the patient returned back to be an active construction worker.
This study reveals that cemented body cages lead to similar clinical results as the body cage with short-segment fixation. The body cages are the internal supporter to the vertebral body to reconstruct the body height. The cement can fix the cage to the residual bony structure of the vertebra and prevent the cage dislodgement. The fractured vertebra will heal around the cage-cement complex and restore the spine stability. The long-term clinical results showed that the cemented body cages can work alone and the short-segment fixation is not needed. The cement lumbar interbody fixation without screw instrumentation was reported, leading to novel good clinical results with spontaneous ankylosis shown by a 10-year follow-up in lumbar degenerative spondylolisthesis and 6-year follow-up in degenerative lumbar scoliosis [66, 67]. All these findings suggest that cementation in the spine surgery can provide long-lasting stabilization, potentially inducing spontaneous ankylosis to further stabilize the spine construct over time.
4. Conclusion
This chapter describes the detailed techniques and results of treating post-traumatic kyphosis through team manual reduction, which has been developed and practiced 25 years in over 2000 patients. A practical classification system for thoracolumbar kyphosis was presented based on the results of team manual reduction.
The chapter further reports on surgical procedures used to treat thoracolumbar burst fractures, including TpBA with short-segment fixation and SpBA without instrumentation. The advanced SpBA procedure has advantages of being able to remove retropulsed bony fragments and reconstruct the fractured vertebra with body cages and cementation via a unilateral mini-open subpedicle window approach. The surgical outcomes of SpBA have been reported to be positive, with complete spinal cord decompression, maintenance of reduction, and further ensured fracture healing through anterior or lateral spontaneous interbody ankylosis.
Overall, the chapter concludes that combining team manual reduction and SpBA is a safe and efficient approach for treating thoracolumbar burst fractures, with good clinical outcomes observed in patients with a minimum 10-year follow-up.
Acknowledgments
The authors are grateful to the colleagues in the hospital for their professional contribution and assistance in data collection and analyses.
References
- 1.
Mulcahy MJ, Dower A, Tait M. Orthosis versus no orthosis for the treatment of thoracolumbar burst fractures: A systematic review. Journal of Clinical Neuroscience. 2021; 85 :49-56. DOI: 10.1016/j.jocn.2020.11.044 - 2.
Yan H, Ni M, Zhai W, Guo J, Huang Z, Zhang J, et al. Balloon kyphoplasty combined with posterior pedicle screw fixation for the treatment of osteoporotic thoracolumbar burst fractures. Annals of Palliative Medicine. 2021; 10 (7):7514-7524. DOI: 10.21037/apm-21-665 - 3.
Barakat AS, Elattar A, Fawaz K, Sultan AM, Koptan W, ElMiligui Y, et al. A comparative study between the universal spinal system®(USS) and the CD horizon® legacy™(CDH) in the management of thoracolumbar fractures. SICOT-J. 2019; 5 :42. DOI: 10.1051/sicotj/2019039 - 4.
Liao J-C, Chen W-P, Wang H. Treatment of thoracolumbar burst fractures by short-segment pedicle screw fixation using a combination of two additional pedicle screws and vertebroplasty at the level of the fracture: A finite element analysis. BMC Musculoskeletal Disorders. 2017; 18 :1-8. DOI: 10.1186/s12891-017-1623-0 - 5.
Piccone L, Cipolloni V, Nasto L, Pripp C, Tamburrelli F, Maccauro G, et al. Thoracolumbar burst fractures associated with incomplete neurological deficit in patients under the age of 40: Is the posterior approach enough? Surgical treatment and results in a case series of 10 patients with a minimum follow-up of 2 years. Injury. 2020; 51 (2):312-316. DOI: 10.1016/j.injury.2019.12.031 - 6.
De Iure F, Lofrese G, De Bonis P, Cultrera F, Cappuccio M, Battisti S. Vertebral body spread in thoracolumbar burst fractures can predict posterior construct failure. The Spine Journal. 2018; 18 (6):1005-1013. DOI: 10.1016/j.spinee.2017.10.064 - 7.
Tan T, Rutges J, Marion T, Gonzalvo A, Mathew J, Fitzgerald M, et al. Anterior versus posterior approach in traumatic thoracolumbar burst fractures deemed for surgical management: Systematic review and meta-analysis. Journal of Clinical Neuroscience. 2019; 70 :189-197. DOI: 10.1016/j.jocn.2019.07.083 - 8.
Wang T, Wang Z, Ji P, Zhang J, Zhang C, Zhang L. The efficacy and safety of anterior versus posterior approach for the treatment of thoracolumbar burst fractures: A systematic review and meta-analysis. Annals of Translational Medicine. 2022; 10 (6):309. DOI: 10.21037/atm-22-903 - 9.
Li K-C, Li AF-Y, Hsieh C-H, Chen H-H. Transpedicle body augmenter in painful osteoporotic compression fractures. European Spine Journal. 2007; 16 :589-598. DOI: 10.1007/s00586-006-0197-6 - 10.
Li K-C, Hsieh C-H, Lee C-Y, Chen T-H. Transpedicle body augmenter: A further step in treating burst fractures. Clinical Orthopaedics and Related Research (1976–2007). 2005; 436 :119-125. DOI: 10.1097/01.blo.0000158316.89886.63 - 11.
Li K-C, Li AF, Hsieh C-H, Liao T-H, Chen C-H. Another option to treat Kümmell’s disease with cord compression. European Spine Journal. 2007; 16 :1479-1487. DOI: 10.1007/s00586-006-0094-z - 12.
Li K-C, Yu S-W, Li A, Hsieh C-H, Liao T-H, Chen J-H, et al. Subpedicle decompression and vertebral reconstruction for thoracolumbar Magerl incomplete burst fractures via a minimally invasive method. Spine. 2014; 39 (5):433-442. DOI: 10.1097/BRS.0000000000000186 - 13.
Su Y, Ren D, Zou Y, Lu J, Wang P. A retrospective study evaluating the correlation between the severity of intervertebral disc injury and the anteroposterior type of thoracolumbar vertebral fractures. Clinics. 2016; 71 :297-301 - 14.
Pumberger M, Fuchs M, Engelhard N, Hermann KG, Putzier M, Makowski MR, et al. Disk injury in patients with vertebral fractures—A prospective diagnostic accuracy study using dual-energy computed tomography. European Radiology. 2019; 29 :4495-4502 - 15.
Mi J, Sun X-j, Zhang K, Zhao C-Q, Zhao J. Prediction of MRI findings including disc injury and posterior ligamentous complex injury in neurologically intact thoracolumbar burst fractures by the parameters of vertebral body damage on CT scan. Injury. 2018; 49 (2):272-278 - 16.
Kawakyu-O’Connor D, Bordia R, Nicola R. Magnetic resonance imaging of spinal emergencies. Magnetic Resonance Imaging Clinics. 2016; 24 (2):325-344. DOI: 10.1016/j.mric.2015.11.004 - 17.
Lee KY, Kim M-W, Seok SY, Kim DR, Im CS. The relationship between superior disc-endplate complex injury and correction loss in young adult patients with thoracolumbar stable burst fracture. Clinics in Orthopedic Surgery. 2017; 9 (4):465-471 - 18.
Aono H, Ishii K, Takenaka S, Tobimatsu H, Nagamoto Y, Horii C, et al. Risk factors for a kyphosis recurrence after short-segment temporary posterior fixation for thoracolumbar burst fractures. Journal of Clinical Neuroscience. 2019; 66 :138-143. DOI: 10.1016/j.jocn.2019.04.035 - 19.
Peng Z, Cui Z, Kuang X, Yu C, Ruan Y, Li C, et al. Intervertebral disc injury is the mainspring for the postoperative increase in cobb angle after thoracolumbar burst fracture. Journal of Orthopaedic Surgery. 2022; 30 (2):10225536221088753 - 20.
Oner FC, van der Rijt RR, Ramos LM, Dhert WJ, Verbout AJ. Changes in the disc space after fractures of the thoracolumbar spine. The Journal of Bone and Joint Surgery British. 1998; 80 (5):833-839. DOI: 10.1302/0301-620X.80B5.0800833 - 21.
Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Radiological study on disc degeneration of thoracolumbar burst fractures treated by percutaneous pedicle screw fixation. European Spine Journal. 2013; 22 :489-494 - 22.
Verlaan J-J, Dhert WJ, Oner FC. Intervertebral disc viability after burst fractures of the thoracic and lumbar spine treated with pedicle screw fixation and direct end-plate restoration. The Spine Journal. 2013; 13 (3):217-221. DOI: 10.1016/j.spinee.2012.02.032 - 23.
Fredrickson B, Edwards W, Rauschning W, Bayley J, Yuan H. 1992 Volvo award in experimental studies vertebral burst fractures: An experimental, morphologic, and radiographic study. Spine. 1992; 17 (9):1012-1021. DOI: 10.1097/00007632-199209000-00002 - 24.
Fredrickson BE, Mann KA, Yuan HA, Lubicky JP. Reduction of the intracanal fragment in experimental burst fractures. Spine. 1988; 13 (3):267-271. DOI: 10.1097/00007632-198803000-00008 - 25.
Frankel H, Hancock D, Hyslop G, Melzak J, Michaelis L, Ungar G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Spinal Cord. 1969; 7 (3):179-192 - 26.
Li K-C, Hsieh C-H, P68. Pedicle augmenter in treating burst fracture. The Spine Journal. 2003; 3 (5):148. DOI: 10.1016/S1529-9430(03)00351-6 - 27.
Li K-C, Wong T-U, Kung F-C, Li A, Hsieh C-H. Staging of Kümmell's disease. Journal of Musculoskeletal Research. 2004; 8 (01):43-55. DOI: 10.1142/S0218957704001181 - 28.
Tropiano P, Huang RC, Louis CA, Poitout DG, Louis RP. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine. 2003; 28 (21):2459-2465. DOI: 10.1097/01.BRS.0000090834.36061.DD - 29.
Carlo P, Francesco C. Preoperative manual on-table-traction for the reduction of thoracolumbar burst fractures: A technical note. Journal of Craniovertebral Junction & Spine. 2018; 9 (1):73. DOI: 10.4103/jcvjs.JCVJS_3_18 - 30.
Li Y, Du Y, Ji A, Wang Q, Li L, Wu X, et al. The clinical effect of manual reduction combined with internal fixation through Wiltse Paraspinal approach in the treatment of thoracolumbar fracture. Orthopaedic Surgery. 2021; 13 (8):2206-2215. DOI: 10.1111/os.13090 - 31.
Khan MR, Mirdad TM. Ipsilateral dislocation of the shoulder and elbow. Saudi Medical Journal. 2001; 22 (11):1019-1021 - 32.
Bohlman HH, Ducker TB. Spine and spine cord injuries. In: Herkowitz HN, Garfin SR, Balderston RA, et al., editors. Rothman-Simeone The Spine. 4th ed. Vol. 2. Philadelphia: WB Saunders Company; 1999. pp. 889-1002 - 33.
Harrington R, Budorick T, Hoyt J, Anderson P, Tencer A. Biomechanics of indirect reduction of bone retropulsed into the spinal canal in vertebral fracture. Spine. 1993; 18 (6):692-699. DOI: 10.1097/00007632-199305000-00003 - 34.
Lin RM, Panjabi MM, Oxland TR. Functional radiographs of acute thoracolumbar burst fractures. A biomechanical study. Spine. 1993; 18 (16):2431-2437. DOI: 10.1097/00007632-199312000-00011 - 35.
Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures: The clinical efficacy and outcome of nonoperative management. Spine. 1993; 18 (8):955-970 - 36.
Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate: Technique, indications, and results. Radiologic Clinics of North America. 1998; 36 (3):533-546. DOI: 10.1016/S0033-8389(05)70042-7 - 37.
Lindsey RW, Dick W. The fixateur interne in the reduction and stabilization of thoracolumbar spine fractures in patients with neurologic deficit. Spine. 1991; 16 (3 Suppl):S140-S145. DOI: 10.1097/00007632-199103001-00020 - 38.
Chen H-H, Wang W-K, Li K-C, Chen T-H. Biomechanical effects of the body augmenter for reconstruction of the vertebral body. Spine. 2004; 29 (18):E382-E3E7. DOI: 10.1097/01.brs.0000139308.65813.70 - 39.
Li K-C, Chen H-H, Li A, Hsien C-H. Safe zone for application of transpedicle body augmenter. Journal of Orthopaedic Surgery Taiwan. 2004; 21 (3):125-133 - 40.
Van Eenenaam DP, Georges Y. Delayed post-traumatic vertebral collapse (Kummell’s disease): Case report with serial radiographs, computed tomographic scans, and bone scans. Spine. 1993; 18 (9):1236-1241 - 41.
Golimbu C, Firooznia H, Rafii M. The intravertebral vacuum sign. Spine. 1986; 11 (10):1040-1043 - 42.
Osterhouse MD, Kettner NW. Delayed posttraumatic vertebral collapse with intravertebral vacuum cleft. Journal of Manipulative and Physiological Therapeutics. 2002; 25 (4):270-275. DOI: 10.1067/mmt.2002.123164 - 43.
Garfin SR, Yuan HA, Reiley MA. New technologies in spine: Kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001; 26 (14):1511-1515 - 44.
Hiwatashi A, Moritani T, Numaguchi Y, Westesson P-L. Increase in vertebral body height after vertebroplasty. American Journal of Neuroradiology. 2003; 24 (2):185-189 - 45.
McKiernan F, Jensen R, Faciszewski T. The dynamic mobility of vertebral compression fractures. Journal of Bone and Mineral Research. 2003; 18 (1):24-29. DOI: 10.1359/jbmr.2003.18.1.24 - 46.
Fukuta S, Miyamoto K, Masuda T, Hosoe H, Kodama H, Nishimoto H, et al. Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine. 2003; 28 (15):E302-E3E8. DOI: 10.1097/01.BRS.0000083318.40123.5E - 47.
Perrin RG, McBroom RJ. Spinal fixation after anterior decompression for symptomatic spinal metastasis. Neurosurgery. 1988; 22 (2):324-327 - 48.
Walker MP, Yaszemski MJ, Kim CW, Talac R, Currier BL. Metastatic disease of the spine: Evaluation and treatment. Clinical Orthopaedics and Related Research (1976-2007). 2003;415:S165-SS75. DOI: 10.1097/01.blo.0000092977.12414.f9 - 49.
Roberson JR, Whitesides TE Jr. Surgical reconstruction of late post-traumatic thoracolumbar kyphosis. Spine. 1985; 10 (4):307, 12. DOI: 10.1097/00007632-198505000-00003 - 50.
Daniaux HS, Genelin P, Lang A, T. Kathrein A. Application of posterior plating and modifications in thoracolumbar spine injuries: Indication, techniques, and results. Spine. 1991; 16 (3):S125-SS33 - 51.
Chen IH, Chien J-T, Yu T-C. Transpedicular wedge osteotomy for correction of thoracolumbar kyphosis in ankylosing spondylitis: Experience with 78 patients. Spine. 2001; 26 (16):E354-EE60 - 52.
Lehmer SM, Keppler L, Biscup RS, Enker P, Miller SD, Steffee AD. Posterior transvertebral osteotomy for adult thoracolumbar kyphosis. Spine. 1994; 19 (18):2060-2067. DOI: 10.1097/00007632-199409150-00009 - 53.
Magerl F, Aebi M, Gertzbein S, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. European Spine Journal. 1994; 3 :184-201. DOI: 10.1007/BF02221591 - 54.
Gaines RW, Satterlee CC, Groh GI. Experimental evaluation of seven different spinal fracture internal fixation devices using nonfailure stability testing: The load-sharing and unstable-mechanism concepts. Spine. 1991; 16 (8):902-909 - 55.
Kuklo TR, Polly DW Jr, Owens BD, Zeidman SM, Chang AS, Klemme WR. Measurement of thoracic and lumbar fracture kyphosis: Evaluation of intraobserver, interobserver, and technique variability. Spine. 2001; 26 (1):61-66 - 56.
Oner F, Van Gils A, Dhert W, Verbout A. MRI findings of thoracolumbar spine fractures: A categorisation based on MRI examinations of 100 fractures. Skeletal Radiology. 1999; 28 :433-443. DOI: 10.1007/s002560050542 - 57.
Che Y-J, Hou J-J, Guo J-B, Liang T, Zhang W, Lu Y, et al. Low energy extracorporeal shock wave therapy combined with low tension traction can better reshape the microenvironment in degenerated intervertebral disc regeneration and repair. The Spine Journal. 2021; 21 (1):160-177. DOI: 10.1016/j.spinee.2020.08.004 - 58.
Beekmans SV, Emanuel KS, Smit TH, Iannuzzi D. Stiffening of the nucleus pulposus upon axial loading of the intervertebral disc: An experimental in situ study. JOR Spine. 2018; 1 (1):e1005. DOI: 10.1002/jsp2.1005 - 59.
Duclos SE, Michalek AJ. Residual strains in the intervertebral disc annulus fibrosus suggest complex tissue remodeling in response to in-vivo loading. Journal of the Mechanical Behavior of Biomedical Materials. 2017; 68 :232-238. DOI: 10.1016/j.jmbbm.2017.02.010 - 60.
Tschoeke SK, Hellmuth M, Hostmann A, Robinson Y, Ertel W, Oberholzer A, et al. Apoptosis of human intervertebral discs after trauma compares to degenerated discs involving both receptor-mediated and mitochondrial-dependent pathways. Journal of Orthopaedic Research. 2008; 26 (7):999-1006. DOI: 10.1002/jor.20601 - 61.
Korres DS, Babis GC, Paraskevakou H, Stamos K, Tsarouchas J, Lykomitros V. Spontaneous interbody fusion after controlled injuries to the spine: An experimental study in rabbits. Clinical Spine Surgery. 2000; 13 (1):31-35 - 62.
Akeda K, Yamada T, Inoue N, Nishimura A, Sudo A. Risk factors for lumbar intervertebral disc height narrowing: A population-based longitudinal study in the elderly. BMC Musculoskeletal Disorders. 2015; 16 :1-9. DOI: 10.1186/s12891-015-0798-5 - 63.
Chen J-X, Xu D-L, Sheng S-R, Goswami A, Xuan J, Jin H-M, et al. Risk factors of kyphosis recurrence after implant removal in thoracolumbar burst fractures following posterior short-segment fixation. International Oorthopaedics. 2016; 40 :1253-1260. DOI: 10.1007/s00264-016-3180-9 - 64.
Huang W, Chang Z, Zhang J, Song R, Yu X. Interspinous process stabilization with rocker via unilateral approach versus X-stop via bilateral approach for lumbar spinal stenosis: A comparative study. BMC Musculoskeletal Disorders. 2015; 16 :1-8. DOI: 10.1186/s12891-015-0786-9 - 65.
Shen H, Fogel GR, Zhu J, Liao Z, Liu W. Biomechanical analysis of lumbar fusion with proximal interspinous process device implantation. International Journal for Numerical Methods in Biomedical Engineering. 2021; 37 (8):e3498. DOI: 10.1002/cnm.3498 - 66.
Li K-C, Hsieh C-H, Liao T-H. Cement lumbar interbody fusion (CLIF) for lowgrade degenerative spondylolisthesis with minimum 10-year follow-up. Journal of Surgery. 2022; 7 (16):1-9. DOI: 10.29011/2575-9760.001691 - 67.
Li K-C, Hsieh C-H, Liao T-H. Clinical outcome-supported advance of cement lumber interbody fusion for degenerative lumbar scoliosis. Journal of Surgery. 2023; 8 (1):1-8. DOI: 10.29011/2575-9760.001697