Change in PS after hexaraphane treatment.
Abstract
Hexaraphane (6-methylsulfinylhexyl isothiocyanate; 6-MSITC) is an isothiocyanate present in the rhizomes and roots of wasabi (Eutrema japonicum (Miq.) Kiudz.). It is known to induce detoxifying and antioxidant enzymes by activating the Keap1-Nrf2 system, ameliorating oxidative damage in the body. Hexaraphane was shown to inhibit brain damage and improve dementia symptoms in Alzheimer’s model mice. Moreover, in two randomized controlled trials conducted on middle-aged and elderly subjects, the extract powder “Wasabi sulfinyl™” containing 0.8% hexaraphane improved memory, attention, and judgment. In a clinical study of fifteen patients with chronic fatigue syndrome, treatment with Wasabi sulfinyl ™ for 3 months improved brain fog and other symptoms.
Keywords
- wasabi
- hexaraphane
- clinical study
- brain function
- dementia
- brain fog
1. Introduction
Wasabi (
Since ancient times, wasabi has grown in mountain streams throughout Japan and has been used as a medicinal herb for centuries because of its characteristic pungent flavor (Figures 1 and 2). The earliest written record is “Wasabi” written on a wooden strip unearthed from a ruin in the village of Asuka, Nara Prefecture, dating from the Aska period [2]. As the area was a medicinal herb garden, it is believed to have been used as a medicinal herb since ancient times.

Figure 1.
Wasabi cultivation. The wasabi fields in Izu City, Shizuoka Prefecture, Japan. The wasabi fields stretch from the upper reaches to the lower reaches, and the cold but abundant clear water nurtures the wasabi. Source: Kinjirushi Co., Ltd.

Figure 2.
Wasabi. Wasabi grows slowly for more than a year until the rhizome reaches about 15 centimeters. It has a distinctive flavor, sweetness, and consistency when ground. Source: Kinjirushi Co., Ltd.
“Wasabi” is also mentioned in “Honzo Wamyo,” Japan’s oldest encyclopedia of medicinal plants 1100 years ago. According to the dictionary of medicinal food published more than 300 years ago, “Gleanings from the Materia Medica,” it disperses depression, generates sweating, dispels wind (shallow evil poison as the cause of the disease), purges dampness (the first of the five tinctures as the cause of the disease), eliminates stagnation (pain-causing), and removes plumpness (plumpness, one of the five stagnations, refers to the spleen stagnation). It is the best of the seven flavors of colic herbs. It is also used to treat biofilms and is recognized as a medicinal plant [3]. In addition to its appetite-boosting properties, modern catalogs of medicinal plants state that the rhizomes of wasabi, when ground and thinly applied to a cloth and applied to the areas affected by rheumatism, neuralgia, and tonsillitis, can be effective in relieving pain and that the sap of the rhizomes is effective in preventing fish and horsemeat poisoning [4]. Finely chopped leaves can also be used as a moisturizing bath additive by placing them into a bathtub in bags [5].
Although wasabi has been used in Japan since ancient times, its medicinal properties have little scientific basis and have been regarded only folklore. Recent studies have elucidated the active ingredients in wasabi and explored their functions. These functional ingredients have been used to promote health and beauty. In particular, hexaraphane (6-methylsulfinylhexyl isothiocyanate, 6-MSITC) (Figure 3), which is relatively abundant in wasabi rhizomes and roots, has been reported to exhibit antioxidant, anticarcinogenic, and detoxifying enzyme-inducing effects. Moreover, it was reported to inhibit platelet aggregation and ameliorate dementia. Here, I would like to focus on the clinical data on the effect of hexaraphane in improving brain function. I would also like to discuss some of the other functional ingredients in wasabi.
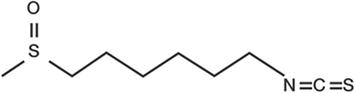
Figure 3.
Hexaraphane. It is found in trace amounts in the rhizomes of wasabi. It is a nonvolatile amphipathic substance with a slightly astringent flavor, but no pungent taste. 6-methylsulfinylhexyl isothiocyanate; molecular weight: 205.3, C8H15NOS2, CAS number: 4430-35-7. Source: Kinjirushi Co., Ltd.
2. Functional components of wasabi
The extracts of leaves, stems, and roots of wasabi were analyzed by MS chromatography, and 42 major peaks were detected, including glucosinolates, phenylpropanoid glycosides, flavone glycosides, and hydroxycinnamic acids [6]. Some of these constituents had the strongest anti-inflammatory, antimicrobial, and cytotoxic effects compared to others.
Wasabi is known to contain many isothiocyanates, responsible for its characteristic pungent flavor. In addition, its leaves contain many polyphenols, such as isosaponarin (4’-O-glucosyl-6-C-glucosyl apigenin) and isovitexin. In addition, several new components with various functional properties are being elucidated [7].
Wasabi contains at least 21 kinds of isothiocyanates [8, 9]. Among them, the most abundant isothiocyanate is allyl isothiocyanate (AITC), which is the main component responsible for the pungent flavor. Hexaraphane is another isothiocyanate that has recently attracted attention as a functional ingredient. It has been shown to induce detoxification of metabolic enzymes [10] and exhibit anticancer [11], anti-inflammatory [12], antidiabetic [13], and antiallergenic/anti-atopic [14] effects. Recent studies have demonstrated its ability to activate hair papilla cells and improve dementia [15].
In addition, 6-methylthiohexyl isothiocyanate (6-MTITC) is a volatile component with the characteristic green note of wasabi. It is mainly used as a flavoring agent in processed food, but it has also been reported to have functional properties including antimicrobial and anticarcinogenic effects [16], detoxification and antioxidant effects [17], anticarcinogenic effects [18], and antiallergic effects [12].
To date, wasabi leaves have been partially used as food, but mostly discarded. However, studies have gradually identified the components of wasabi leaves and their functional properties, such as anti-influenza virus [19], anti-obesity [20, 21], and anticancer [22] effects.
Experimental and clinical studies have demonstrated the effects of wasabi leaves on the skin. For example, wasabi leaf extract was shown to decrease tyrosinase activity, melanin content, and formation of advanced glycation end products in vitro and to improve skin condition in a clinical trial [23].
Further research on the functional ingredients may enable more efficient use of wasabi leaves.
2.1 Allyl isothiocyanate (AITC)
Studies have reported the antimicrobial properties of AITC. Its pungent flavor is believed to have antimicrobial properties. It is a relatively volatile ingredient with a low molecular weight of 99.15 and a boiling point of 152°C. Its antimicrobial activity is also very effective in gas-phase exposures. Its minimum growth inhibitory concentrations against bacteria, yeasts, and molds ranged between 16 and 110 ng/mL [24]. Industrially, AITC-based antimicrobial products for lunch boxes and rice storage container and antimicrobial deodorizers for car air conditioners are available.
Due to its highly irritant nature, an acute toxicity LD50 of 310 mg/kg has been reported in mice [25]. Moreover, it causes irritation of skin and mucous membranes on contact [26]. In addition, there are reports of suspected mutagenicity and genotoxicity [27]. Therefore, due caution should be exercised when using it as a functional material.
2.2 Hexaraphane
Hexaraphane is a nonvolatile isothiocyanate, mainly found in the rhizome and root, but at low levels of 200–500 μg/g [28]. The dietary intake ranges from 2.5 to 5.0 g per meal, with an estimated hexaraphane content of 0.5 to 2.5 mg per meal.
The LD50 values for hexaraphane in rats ranged from 338 to 451 mg/kg, but the LD50 values for the natural and synthetic extracts differed. Hexaraphane is detected in the blood 2 hours after ingestion, and approximately 50% of the ingested amount is excreted in the urine within 24 hours [29]. Animal studies have shown that sulforaphane (4-methylsulfinylbutyl isothiocyanate; 4-MSITC), an analog of hexaraphane, crosses the blood-brain barrier 15 minutes after ingestion [30].
Therefore, hexaraphane is considered to be an effective functional ingredient because it is also rapidly absorbed by the body. It is important to note that the most commonly used products are room temperature tubular ones. However, these processed products often contain no rhizomes or only a small amount of rhizomes and therefore very little hexaraphane [28]. Therefore, the consumption of ground rhizomes or the use of products or supplements primarily containing rhizomes is essential to utilize the benefits of hexaraphane.
Hexaraphane acts on the transcription factor Nrf2 (NF-E2-related factor 2)/Keap1 (Kelch-like ECH-associated protein 1) system and promotes the transcriptional expression of antioxidant response elements. It is known to increase the expression of phase 2 enzymes such as GST and NAD(P)H quinone reductase (NQO1), and its GST-inducing activity was reported to be the highest among 20 vegetables [31].
Hexaraphane has been reported to inhibit the production of reactive oxygen species (ROS) by neutrophils stimulated with phorbol myristate acetate (PMA) [32]. A study suggested that hexaraphane does not directly eliminate the ROS produced by stimulation, but inhibits the ROS production by neutrophils by acting on the site of ROS production. Studies have shown that benzyl isothiocyanate (BITC), a typical papaya isothiocyanate, modifies cytochrome b558 [33] of the NADPH oxidase complex on leukocyte membranes. Hexaraphane is also believed to have the same mechanism of action.
In a study, rat basophil-like cells RBL-2H3 were stimulated with specific IgE antibodies, and the released histamine and leukotrienes were measured by ELISA. The addition of hexaraphane [12] significantly inhibited the release of histamine and leukotrienes.
In addition, we investigated the effects of hexaraphane on dermal papilla cells (DPCs) and showed that hexaraphane activated hair papilla cells and upregulated vascular endothelial growth factor and adenosine receptors. This suggests that hexaraphane may promote the growth of hair [34].
It is important to note here that wasabi is primarily sold in tube form as processed, but horseradish is used as a substitute for it. In other words, many products are made by coloring dried horseradish powder, kneading it with water, and then putting it into tube form, which contains almost none of wasabi’s functional ingredients, including hexaraphane. Some wasabi products are made using the stems and leaves, but as the wasabi rhizomes and roots contain large amounts of hexaraphane, only processed products containing large amounts of these parts have these functional ingredients in adequate amounts.
2.3 Isosaponarin
Wasabi plants accumulate C-glucosyl flavones, including isosaponarin, in their leaves [7, 35]. Isosaponarin is one of the major flavonoids in wasabi and has been rarely reported from other species in the Plant Kingdom (Figure 4) [36].
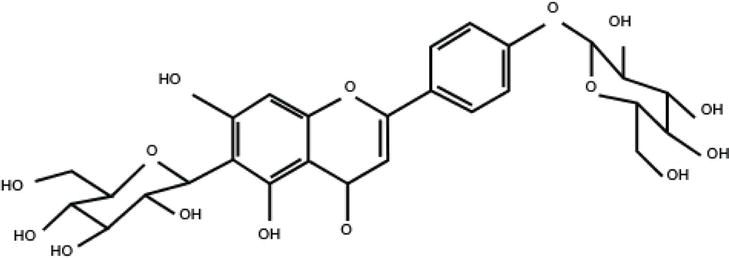
Figure 4.
Isosaponarin. It is a unique flavonoid with C-bond found in the leaves of wasabi and has the structure of apigenin with two sugars attached. An enzymatic reaction removes the sugar and converts it to isovitexin. Molecular weight: 594.5, C27H30O15, CAS number: 19416–87-6. Source: Kinjirushi Co., Ltd.
Isosaponarin and other flavonoids in wasabi leaves were shown to promote collagen production [37] and activate hair papilla cells.
Isosaponarin was found to increase collagen production in fibroblasts and enhance expression of transforming growth factor βII-type receptor (TβR-II) and prolyl 4-hydroxylase (P4H). These results suggested that isosaponarin promotes collagen synthesis by increasing TβR-II and P4H [37].
We also reported the effect of isosaponarin on DPCs. This was tested by adding wasabi leaf extract and isosaponarin to the culture of DPCs and assessing the cellular activity by the water-soluble tetrazolium method. Minoxidil and adenosine, which are used as ingredients in hair growth products, were used as positive controls. The results showed activation of DPCs at a low concentration of 0.1 μM, as well as an increase in vascular endothelial growth factor and other substances (Figure 5) [38]. Although further validation is needed, it is conceivable that isosaponarin could be used as a material to promote hair regrowth and inhibit hair loss. Indeed, formulations containing isosaponarin are now available as hair care materials.
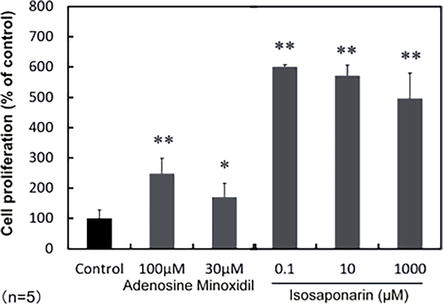
Figure 5.
Effect of isosaponarin on the proliferation of DPCs. The effect of isosaponarin on amounts and activity of mitochondria in DPCs. DPCs were cultured for 72 hours with the indicated concentrations of isosaponarin, solvent control (DMSO), and positive controls (adenosine and minoxidil). The amounts of mitochondria were measured by Mito Tracker staining and further divided by the number of nuclei obtained by Hoechist staining to evaluate the number per cell. The data represent mean ± SD of five replicates. * and ** indicates significant differences versus control (p < 0.05 and p < 0.01 respectively;
3. Cognitive function improvement by hexaraphane
3.1 Animal studies
Hexaraphane is known to express detoxification and antioxidant genes by activating the Keap1/Nrf2 system. Activation of Keap1/Nrf2 increases antioxidant enzymes such as HO-1, thereby inhibiting oxidative damage to neurons [39]. In the study by Morroni et al., the administration of hexaraphane to Parkinson’s disease model mice was found to improve symptoms [15].
Uruno et al. conducted animal studies on Alzheimer’s Disease model mice. The Keap1-Nrf2 system was shown to protect neurons in the hypothalamus against oxidative damage [40]. They also demonstrated that Nrf2 induction improved the impairment in AD model mice in the passive avoidance test (PAT) and suppressed the elevation of the oxidative stress marker 8-OHdG. Administration of hexaraphane improved the impaired cognition of AD model mice in the PAT [41].
These studies suggest that hexaraphane may inhibit oxidative damage in the brain, preventing the onset of dementia or alleviating symptoms.
3.2 Safety
The characteristic pungent component of wasabi is AITC, which is approximately five times more abundant than hexaraphane [28, 42]. Therefore, a wasabi extract prepared to obtain hexaraphane would contain large amounts of AITC and its original glycoside, sinigrin. Ingestion of this extract may cause abdominal pain due to their high stimulant properties. We developed a special manufacturing process to remove AITC and sinigrin and developed “Wasabi Sulfinyl™ (WS),” an extract containing 0.8% hexaraphane and almost no AITC or sinigrin. Four-week overdose studies of WS confirmed the safety, with intake ranging from 500–2000 mg (containing 4–16 mg of hexaraphane) [43, 44]. No adverse effects were observed in these overdose trials demonstrating the safety of WS.
3.3 Randomized controlled trial
Cognitive function is known to be influenced by diet [45, 46, 47] and lifestyle. Intake of vegetables, fruits, and spices was shown to improve cognitive function [48, 49, 50, 51]. Hexaraphane has antioxidant and anti-inflammatory properties and has been shown to improve memory and other functions in a mouse model of dementia. Thus, hexaraphane is expected to improve cognitive function. Below, we discuss several clinical trials on this subject.
3.3.1 Neurocognitive functions in cognitively intact middle-aged and older adults
Based on the antioxidant effects of hexaraphane and the results of animal studies, we conducted a randomized controlled trial for 8 weeks (UMIN000027407) [52]. Fifty cognitively intact elderly adults with memory complaints between the ages of 45 and 69 years were enrolled in this randomized double-blind, placebo-controlled trial. Subjects were randomized to receive 100 mg WS or placebo daily for 8 weeks. The effect on neurocognitive functions was assessed using a battery of four neuropsychological tests at baseline and after 4 and 8 weeks of treatment.
All subjects completed the study although four were excluded from the efficacy analysis due to adverse events or protocol violations. Analysis of the fully evaluable population indicated no significant differences between WS and placebo groups on any outcome measure. Because of the relatively small sample size, the unexpectedly high scores in the placebo group, the fact that all subjects were cognitively intact, and the fact that the eight-week treatment period was too short, it may not have been sensitive enough to discern the relatively subtle differences between the two groups. However, there were trends favoring WS in almost all parameters of the Group version of the Stroop Color Word Test (G-SCWT), of which the magnitude of improvement in correct responses in the Stroop interference task and the interference rate reached near-significant levels (
Exercise has been suggested to contribute to improved cognitive function [53, 54]. Therefore, we performed a subgroup analysis stratified according to their exercise habits or lack. A subset of nonexercisers exhibited significantly better performance in both the reverse-Stroop control task and the Stroop interference task of the G-SWCT, which assesses attention and judgment, at week 8. The Stroop test is divided into four levels of difficulty, and in this study, there was a significant difference between difficulty level 1 and difficulty level 4 (Figure 6). In difficulty level 1, an improvement in performance was observed after 8 weeks even with placebo, suggesting a learning effect; however, in difficulty level 4, there was no improvement in performance after a period of time with placebo, and only the WS intake group showed an improvement in performance. This suggests that the effect of WS is not just to maintain function by preventing impairment of brain function, but also to improve function. However, due to the small number of subjects and the stratified analysis of the results, more robust studies are required to obtain definitive evidence. Of note, no WS-induced side effects were observed in the study.
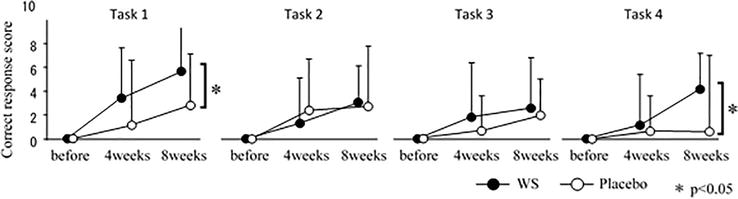
Figure 6.
The result of cognitive performance for the G-SCWT tasks 1 to 4 in the nonexercizer subgroup. Subjects who had a habit to perform exercise for 30 minutes or longer for more than twice a week were included in the exerciser subgroup, and the remaining subjects were included in the nonexercizer subgroup. Data presented as mean ± SD. Comparison of the mean change from baseline for independent samples. Source: Author.
3.3.2 Memory functioning in healthy adults aged 60 years and older
Nouchi et al. assessed cognitive function in 72 healthy subjects (19 males, 53 females; mean age: 65.4 years [range, 60–69 years]; mean MMSE score: 28.5) who received a capsule containing either 100 mg WS (0.8 mg hexaraphane/day) or a placebo for 12 weeks (UMIN000032694) [55].
A wide range of cognitive functions (e.g., executive function, episodic memory (immediate and delayed), processing speed, working memory, attention, reasoning, short-term memory, and visuospatial cognitive function) were assessed before and after the intervention.
There were no significant differences between the two groups baseline characteristics with respect to age (WS; 65.9 ± 3.9 years, placebo; 64.9 ± 3.6 years), education (WS; 12.5 ± 0.8, placebo; 12.9 ± 1.1), Mini-Mental State examination (WS; 28.5 ± 1.5, placebo; 28.8 ± 1.6), Frontal Assessment Battery at bedside (WS; 15.4 ± 1.9, placebo; 15.4 ± 1.9), Japanese Reading Ability Test (WS; 20.3 ± 3.9, placebo; 20.8 ± 3.6), and Geriatric Depression Scale (WS; 2.4 ± 1.4, placebo; 2.1 ± 1.1). And, the duration of supplement intake also did not differ between the two groups.
The WS group showed a significant improvement in working memory, measured by digit span backward (p = 0.000), and in episodic memory performance, measured by logical memory (LM) immediately (p = 0.00), LM delayed (p = 0.03), and the face and second name test (p = 0.00) (Figure 7). However, they did not find significant improvements in other cognitive functions, such as processing speed, Cd, short-term memory, attention, inhibition, rST, reasoning, and visuospatial performances.
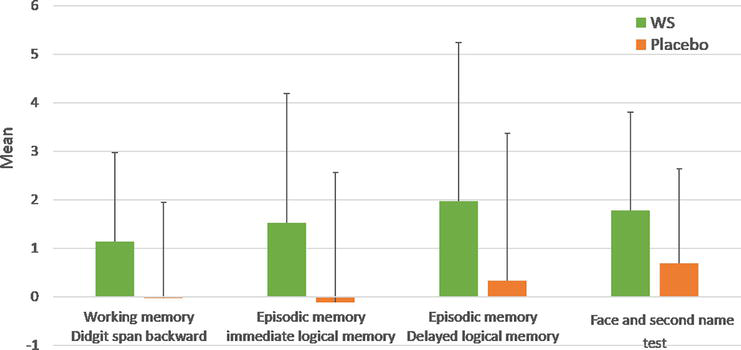
Figure 7.
Change in scores of cognitive functions in both groups. * indicates significant differences versus placebo for multiple comparisons using the Bonferroni method (p < 0.05). This figure was constructed from Ref. ([
There were no significant changes in the inhibition and processing speed performances after hexaraphane intake in older adults. This is inconsistent with a previous study (3.3.1) which showed a beneficial effect of hexaraphane on inhibition performance. However, they noted some differences in methods between this study and previous studies, such as the intervention period (12 weeks or 8 weeks), subjective cognitive complaints (healthy, subjective memory complaints, patients with chronic fatigue), psychological tests (trail making test, SS, or Cd), and participants’ age (older adults or middle-aged adults). Therefore, further studies are required to assess the effect of hexaraphane on inhibition and processing performances.
They further noted that antioxidant and anti-inflammatory effects are involved in the improvement of cognitive function [56, 57]. The hippocampus is involved in working and episodic memory [58], and the antioxidant and anti-inflammatory effects of hexaraphane on the hippocampus may improve cognitive functions. Antioxidant and anti-inflammatory effects are believed to be important in maintaining brain function, but as hexaraphane is thought to have a variety of effects, further studies are required to elucidate the underlying mechanisms.
3.4 ME/CFS and brain fog improvement
The antioxidant and anti-inflammatory effects of hexaraphane may be beneficial in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is a disease with an unknown etiology and no known cure. However, chronic inflammation of the brain has been implicated in its causation [59, 60]. An estimated 17 million people worldwide suffer from the syndrome [61]. In addition to general malaise, low-grade fever, and muscle aches, patients also experience a loss of thinking ability, referred to as brain fog. Symptoms of brain fog include an inability to understand what others are saying, an inability to hold a conversation, an inability to read books and understand their meaning, and an inability to comprehend music.
In a 12-week study by Oka et al., 15 patients with ME/CFS (3 males and 12 females, mean age 37.5 years [range 20–58], mean duration of illness 5.1 years) were administered 1.2 grams of WS (9.6 milligrams of hexaraphane per day). The results showed a significant improvement in the patients’ performance scores (PS), with six patients improving their scores (two patients improved their scores by 2 or more grades) (Table 1) [62].
| Performance status (PS) | Pre (n) | Post (n) | P-value |
|---|---|---|---|
| 0 | 0 | 0 | |
| 1 | 0 | 0 | |
| 2 | 0 | 2 | |
| 3 | 2 | 0 | |
| 4 | 0 | 1 | |
| 5 | 0 | 0 | |
| 6 | 1 | 1 | |
| 7 | 7 | 8 | |
| 8 | 4 | 2 | |
| 9 | 1 | 1 | |
| average | 6.8 ± 1.7 | 6.3 ± 2.1 | *0.001 |
Table 1.
indicates significant differences between pre- and post-treatment PS using Fisher’s exact test. A parametric paired Student’s
These findings demonstrate the potential of hexaraphane, considering that none of the patients in this study had responded to standard treatment for at least 3 months. Specifically, they showed improvement in headache frequency (4.1 to 3.0 times/week, p = 0.001), myalgia frequency (4.1 to 2.4 times/week, p = 0.019), numerical rating scale brain fog scores (5.7 to 4.5, p = 0.011), difficulty in discovering the right words (4.8 to 3.7, p = 0.015), photophobia (from 4.8 to 3.5, p = 0.008), and mood state vigor scores (from 46.9 to 50.0, p = 0.045), right occipital pressure pain threshold (from 17.3 to 21.3, p = 0,01), and trail making test-A (TMT-A) (from 53.0 to 38.1, p = 0.007) (Figure 8). In particular, 14 out of 15 participants perceived improvement in brain fog and cognitive function, as well as significant improvement in general health perception, vitality, and vigor. While the mechanisms of improvement remain to be elucidated and need to be validated in a larger sample, 10 of the 15 patients continued to take WS at their own request after the completion of the study.
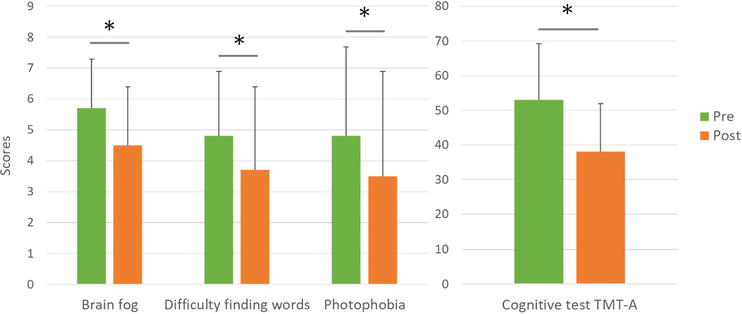
Figure 8.
Changes in neurocognitive symptoms and TMT-A test scores after Hexaraphane treatment. * indicates significant differences between pre- and post-treatment scores using a parametric paired Student’s
4. Conclusion
Wasabi is a pungent vegetable that contains several active ingredients, including hexaraphane. As wasabi is mainly grown and consumed in Japan, there is a paucity of research on its therapeutic effects in other parts of the world. Our basic research and clinical trials have demonstrated its efficacy in improving brain function, even in healthy individuals. The results of studies conducted in mice model of dementia also suggest its potential protective effect on the brain. It may not only help maintain brain function in healthy individuals but also improve symptoms in patients with MCI and dementia.
Acknowledgments
We are very grateful to Dr. Nouchi and Dr. Oka for their energetic research on the functionality of wasabi, and to NOMON Corporation for their tremendous efforts not only in research but also in the marketing of wasabi. The research fees paid by Kinjirushi Co., Ltd., over the years have been the cornerstone of our current research. We are very grateful to Kinjirushi Co., Ltd.
Conflict of interest
Isao Okunishi is an employee of Kinjirushi Co., Ltd., and received a research grant from the company.
References
- 1.
Yamane K et al. Genetic differentiation, molecular phylogenetic analysis, and ethnobotanical study of Eutrema japonicum andE. tenue in Japan andE. yunnanense in China. Horticultural Journal. 2016;85 :46. DOI: 10.2503/hortj.MI-065 - 2.
Chunichi Shinbun (Japanese newspaper). 2001; 26p. - 3.
Hitomi H. Honchoushokukagami. Chiyoda, Tokyo, Japan: Heibonsya; 1976. 180 p - 4.
Okada M. Newly Revised Illustrated Medicinal Plants of the World. Meguro, Tokyo, Japan: Hokuryukan; 2002. 154 p - 5.
Izawa B, Aida T. Yakuso zukan. Shinjuku-ku, Tokyo, Japan: Ie-no-Hikari, Association; 1999. 277 p - 6.
Dos Santos Szewczyk KDS et al. Chemical composition of extracts from leaves, stems and roots of wasabi ( Eutrema japonicum ) and their anti-cancer, anti-inflammatory and anti-microbial activities. Scientific Reports. 2023;13 :9142. DOI: 10.1038/s41598-023-36402-y - 7.
Hosoya T, Yun YS, Kunugi A. Five novel flavonoids from Wasabia japonica . Tetrahedron. 2005;61 :7037. DOI: 10.1016/j.tet.2005.04.061 - 8.
Ina K. Volatile components of wasabi, horse radish and mustard on allyl isothiocyanate. Koryo. 1982; 136 :45 - 9.
Etoh H et al. ω -methylsulfinylalkyl isothiocyanates in Wasabi, Wasabia japonica matsum. Agricultural and Biological Chemistry. 1990;54 :1587. DOI: 10.1080/00021369.1990.10870168 - 10.
Morimitsu Y et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. The Journal of Biological Chemistry. 2002; 277 :3456. DOI: 10.1074/jbc.M110244200 - 11.
Fuke Y et al. Wasabi-derived 6-(methylsulfinyl)hexyl isothiocyanate induces apoptosis in human breast cancer by possible involvement of the NF-κB pathways. Nutrition and Cancer. 2014; 66 :879. DOI: 10.1080/01635581.2014.916322 - 12.
Yamada-Kato T et al. Inhibitory effects of wasabi isothiocyanates on chemical mediator release in RBL-2H3 rat basophilic leukemia cells. Journal of Nutritional Science and Vitaminology. 2012; 58 :303. DOI: 10.3177/jnsv.58.303 - 13.
Yoshida J et al. Glycogen synthase kinase-3β inhibition of 6-(methylsulfinyl) hexyl isothiocyanate derived from wasabi (Wasabia japonica matsum). Bioscience, Biotechnology, and Biochemistry. 2011; 75 :136. DOI: 10.1271/bbb.100507 - 14.
Nagai M, Okunishi I. The effect of wasabi rhizome extract on atopic dermatitis-like symptoms in HR-1 hairless mice. Journal of Nutritional Science and Vitaminology (Tokyo). 2009; 55 :195. DOI: 10.3177/jnsv.55.195 - 15.
Morroni F et al. Neuroprotection by 6-(methylsulfinyl) hexyl isothiocyanate in a 6-hydroxydopamine mouse model of parkinson′s disease. Brain Research. 2014; 1589 :93. DOI: 10.1016/j.brainres.2014.09.033 - 16.
Yano T et al. The effect of 6-methylthiohexyl isothiocyanate isolated from Wasabia japonica (wasabi) on 4-(methylnitrosamino)-1-(3-pyridyl)-1-buatnone-induced lung tumorigenesis in mice. Syokuhin-Kogyo. 2000;115 (115):58. DOI: 10.1016/s0304-3835(00)00364-5 - 17.
Yano T et al. The effect of 6-methylthiohexyl isothiocyanate isolated from Wasabia japonica (wasabi) on 4-(methylnitrosamino)-1-(3-pyridyl)-1-buatnone-induced lung tumorigenesis in mice. Cancer Letters. 2000;155 :115. DOI: 10.1016/s0304-3835(00)00364-5. S0304-3835(00)00364-5 - 18.
Yoshinori K et al. Modulation of Nrf2/Keap1 system by wasabi 6-methylthiohexyl isothiocyanate in ARE-mediated NQO1 expression. Molecular Nutrition & Food Research. 2013; 57 :854. DOI: 10.1002/mnfr.201200689 - 19.
Mochida K, Ogawa T. Anti-influenza virus activity of extract of Japanese wasabi leaves discarded in summer. Journal of the Science of Food and Agriculture. 2008; 88 :1704. DOI: 10.1002/jsfa.3268 - 20.
Yamada-Kato T et al. Anti-obesity effects of wasabi leaf extract on rats fed a high-fat diet are related to upregulation of mRNA expression of β3-adrenergic receptors in interscapular brown adipose tissue. Food Science and Technology Research. 2016; 22 :665. DOI: 10.3136/fstr.22.665 - 21.
Oowatari Y et al. Wasabi leaf extracts attenuate adipocyte hypertrophy through PPARγ and AMPK. Bioscience, Biotechnology, and Biochemistry. 2016; 80 :1594. DOI: 10.1080/09168451.2016.1179093 - 22.
Okamoto M et al. Preventive effects of wasabi leaf extract on carcinogenic initiation. Journal of Clinical Biochemistry and Nutrition. 2008; 43 :251 - 23.
Chiang HM et al. Wasabi leaf supplementation had antioxidant, anti-glycation, and improved skin melanin, spot and moisture. Journal of Functional Foods. 2023; 100 :105398. DOI: 10.1016/j.jff.2022.105398 - 24.
Isshiki K et al. Allyl isothiocyanate and wholesomeness of food. Japanese Journal of Food Microbiology. 1993; 10 :1. DOI: 10.14840/jsfm1984.10.1 - 25.
European Food Safety Authority (EFSA). Scientific opinion on the safety of allyl isothiocyanate for the proposed uses as a food additive1 EFSA panel on food additives and nutrient sources added to food (ANS). EFSA Journal. 2010; 8 :1943 - 26.
IARC. IARC monographs on the evaluation of carcinogenic risks to humans. In: Some Chemicals That Cause Tumours of the Kidney or Urinary Bladder in Rodents and some Other Substances. Vol. 73. LYON CEDEX, France: IARC; 1998 - 27.
Human Health Assessment Branch, Department of Pesticide Regulation California Environmental Protection Agency. Allyl Isothiocyanate Risk Characterization Document Occupational and Bystander Exposures. Human Health Assessment Branch, Department of Pesticide Regulation. Sacramento, CA: California Environmental Protection Agency; 2022 - 28.
Murata M et al. Content of 6-methylsulfinylhexyl isothiocyanate in wasabi and processed wasabi products. Nippon Shokuhin Kagaku Kogaku Kaishi. 2004; 51 :477. DOI: 10.3136/nskkk.51.477 - 29.
Kinjirushi Co., Ltd. Exploration of New Functionality of Wasabi Derived Components and Development of Advanced Utilization Technology as Functional Food Ingredients, Development of Technologies to Improve the Functionality of Food Products. Chuo, Tokyo, Japan: New Food Creation Technology Research Association; 2004. pp. 69-100 - 30.
Tarozzi A et al. Neurodegeneration, neurogenesis, and oxidative stress. Oxidative Medicine and Cellular Longevity. 2013; 10 :415078. DOI: 10.1155/2013/415078 - 31.
Morimitsu Y et al. Antiplatelet and anticancer isothiocyanates in Japanese domestic horseradish, wasabi. BioFactors. 2000; 13 :271. DOI: 10.1002/biof.5520130141 - 32.
Okunishi I. Overview; functional studies on wasabi. In: The Annual Meeting of the Japanese Society for Food. Naka, Nagoya, Japan: Science and Technology Chubu branch; 2005 - 33.
Miyoshi N et al. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: Implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004; 25 :567. DOI: 10.1093/carcin/bgh051 - 34.
Yamada-Kato T et al. Stimulatory effects of 6-methylsulfinylhexyl isothiocyanate on cultured human follicle dermal papilla cells. Food Science and Technology Research. 2018; 24 :567. DOI: 10.3136/fstr.24.567 - 35.
Yoshida S et al. Component analysis of wasabi leaves and an evaluation of their anti-inflammatory activity. Food Science and Technology Research. 2015; 21 :247-253. DOI: 10.3136/fstr.21.247 - 36.
Mashima K et al. Identification and characterization of apigenin 6-C-glucosyltransferase involved in biosynthesis of isosaponarin in wasabi ( Eutrema japonicum ). Plant & Cell Physiology. 2019;60 :2733. DOI: 10.1093/pcp/pcz164 - 37.
Nagai M et al. The effect of isosaponarin isolated from wasabi leaf on collagen synthesis in human fibroblasts and its underlying mechanism. Journal of Natural Medicines. 2010; 64 :305. DOI: 10.1007/s11418-010-0412-y - 38.
Okunishi I et al. Wasabi components 6-methylsulfinylhexylisothiocyanate and isosaponarin for hair growth. In: 136th Annual Meeting of the Pharmaceutical Society of Japan. Shibuya, Tokyo, Japan: The Pharmaceutical Society of Japan; 2016 - 39.
Satoh T et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proceedings of the National Academy of Sciences of the United States of America. 2006; 103 (768):05057231. DOI: 10.1073/pnas.0505723102 - 40.
Yagishita Y et al. Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Reports. 2017; 18 :2030. DOI: 10.1016/j.celrep.2017.01.064 - 41.
Uruno A et al. Nrf2 suppresses oxidative stress and inflammation in app knock-in Alzheimer’s disease model mice. Molecular and Cellular Biology. 2020; 40 :e00467-e00419. DOI: 10.1128/MCB.00467-19 - 42.
Sultana T et al. Comparison of flavour compounds in wasabi and horseradish. Journal of Food, Agriculture and Environment. 2003; 1 :117 - 43.
Nakajima R et al. Oral administration of 6-methylsulfinylhexyl isothiocyanate extracted from is safe and improves the fatigue and sleep of healthy volunteers. BioPsychoSocial Medicine. 2023; 17 :30. DOI: 10.1186/s13030-023-00287-08 - 44.
Okunishi I et al. Safety evaluation of 6-(methylsulfinyl) hexyl isothiocyanate (hexaraphane) and wasabi sulfinyl, a hexaraphane-containing supplement. Food Science and Technology Research. 2020; 26 :813. DOI: 10.3136/fstr.26.813 - 45.
Solfrizzi V et al. Nutritional intervention as a preventive approach for cognitive-related outcomes in cognitively healthy older adults: A systematic review. Journal of Alzheimer's Disease. 2018; 64 :S229. DOI: 10.3233/JAD-179940 - 46.
Gardener SL, Rainey-Smith SR. The role of nutrition in cognitive function and brain ageing in the elderly. Current Nutrition Reports. 2018; 7 :139. DOI: 10.1007/s13668-018-0229-y - 47.
Nouchi R et al. Effects of lutein and astaxanthin intake on the improvement of cognitive functions among healthy adults: A systematic review of randomized controlled trials. Nutrients. 2020; 12 :617. DOI: 10.3390/nu12030617 - 48.
Khazdair MR et al. Neuroprotective potency of some spice herbs, a literature review. Journal of Traditional and Complementary Medicine. 2019; 9 :98. DOI: 10.1016/j.jtcme.2018.01.002 - 49.
Jiang TA. Health benefits of culinary herbs and spices. Journal of AOAC International. 2019; 102 :395. DOI: 10.5740/jaoacint.18-0418 - 50.
Panickar KS. Beneficial effects of herbs, spices and medicinal plants on the metabolic syndrome, brain and cognitive function. Central Nervous System Agents in Medicinal Chemistry. 2013; 13 :13 - 51.
Mirmosayyeb O et al. Possible role of common spices as a preventive and therapeutic agent for Alzheimer’s disease. International Journal of Preventive Medicine. 2017; 8 :5. DOI: 10.4103/2008-7802.199640 - 52.
Okunishi I et al. The effects of wasabi root-derived 6-(methylsulfinyl) hexyl isothiocyanate on neurocognitive functions in cognitively intact middle-aged and older adults—a randomized, double-blind, placebo-controlled trial. Japanese Pharmacology & Therapeutics. 2019; 47 :275 - 53.
Han H et al. Exercise improves cognitive dysfunction and neuroinflammation in mice through histone H3 lactylation in microglia. Immunity & Ageing. 2023; 20 :63. DOI: 10.1186/s12979-023-00390-4 - 54.
Valenzuela PL et al. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Research Reviews. 2020; 62 :101108. DOI: 10.1016/j.arr.2020.101108 - 55.
Nouchi R et al. Benefits of wasabi supplements with 6-MSITC (6-methylsulfinyl hexyl isothiocyanate) on memory functioning in healthy adults aged 60 years and older: Evidence from a double-blinded randomized controlled trial. Nutrients. 2023; 15 :4608. DOI: 10.3390/nu15214608 - 56.
Hajjar I et al. Oxidative stress predicts cognitive decline with aging in healthy adults: An observational study. Journal of Neuroinflammation. 2018; 15 :17. DOI: 10.1186/s12974-017-1026-z - 57.
Sartori AC et al. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. The Journal of Neuroscience Nursing. 2012; 44 :206 - 58.
Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research. 2013; 254 :34 - 59.
Fukuda S et al. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biological Psychology. 2016; 118 :88. DOI: 10.1016/j.biopsycho.2016.05.005 - 60.
Nakatomi Y et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An 11C-(R)-PK11195 PET study. Journal of Nuclear Medicine. 2014; 55 :945. DOI: 10.2967/jnumed.113.131045 - 61.
Lim EJ, Son CG. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Journal of Translational Medicine. 2020; 18 :289. DOI: 10.1186/s12967-020-02455-0 - 62.
Oka T et al. Clinical effects of wasabi extract containing 6-MSITC on myalgic encephalomyelitis/chronic fatigue syndrome: An open-label trial. BioPsychoSocial Medicine. 2022; 16 :26. DOI: 10.1186/s13030-022-00255-0