Comparative analysis of the kinetic data obtained in different reaction media.
Abstract
Solvent plays a significant role in the kinetics of any reaction in solution. The reactions that occur between ions of similar charges, ions with dissimilar charges, ion and neutral molecules, and-or, between neutral molecules/compounds, solvent or solvent-solvent mixture, and-or, reaction media show a distinct effect in each case. The solvent or solvent-solvent mixture, and-or, reaction media may either increase the rate of reaction or decrease it or may not even affect it when the rate constant is measured as a function of the dielectric constant of the reaction media. The transition state theory of reactions in solution correlates the rate constant with the dielectric constant, and provides a straight-line formulation. The charges on the reactants help to predict whether the rate of the reaction will be increased or decreased or will stay constant as the dielectric constant of the reaction media is varied. However, this is not always the case. There is no simple correlation of the rate constant and the dielectric constant of reaction media as described by the transition state theory. This chapter reveals the facts beyond this correlation. The kinetics of the redox reaction between Fe(III) based metal complex and iodide was studied in four different reaction media. The effect of the ionic strength was also revealed to identify the reactive species that are involved in the rate-determining step of the reaction to surface the role of the solvent or the mixture of solvents in the redox kinetics and mechanism. This study showed a prominent effect of the solvents on the kinetics and mechanism of the reaction.
Keywords
- dielectric constant
- rate constant
- dicyanobis(2
- 2′-dipyridyl)iron(III)
- iodide
- dimethyl sulfoxide
- dioxane
- tertiary butyl alcohol
1. Introduction
The reactions in solution phase are frequently studied because they play a significant role in the sustainability of life. There are numerous very crucial processes that involve such solution phase reactions. One example is the redox reactions. To understand the mechanism of the redox reactions, their kinetics is required to be studied to acquire sufficient knowledge of the reactions and to use them according to the need. The kinetics of reactions in solution is usually dealt with the formulation of the transition state theory. According to the formulation Eq. (1), the rate constant is dependent on the dielectric constant of the reaction media [1].
The symbols used in Eq. (1) correspond to rate constant (
Eq. (1) correlates the mathematical value of the rate constant (
A linear plot is acquired when the values of
For simplicity, the temperature is considered as 25°C (298 K). However, the temperature might be high or low according to the experimental or reaction conditions that may be inserted in Eq. (3) rather than 298 K. Eq. (2) may be reduced to Eq. (8) as follows.
Eq. (8) can be used to determine the value of
Eq. (1) correlates the rate constant and the dielectric constant of a reaction in solution phase. This might be a little confusing. For example, let us suppose the reaction is carried out at a range of dielectric constant such as 78, 68, 48, 40, 30 etc., and the rate constant is determined for a selected reaction and then a plot is drawn to deduce
The dielectric constant of a reaction medium may be varied by using a mixture of two or three solvents. For example, to decrease the dielectric constant of aqueous medium, alcohols such as methanol and ethanol are used because they are miscible with water and have low value of dielectric constant as compared to water. However, the alcohols such as methanol and ethanol have lower reduction potential value; they may get oxidized by the reactants and-or products during a redox reaction. Consequently, a redox reaction should be studied in such a mixture of solvents that does not produce parallel reactions by getting oxidized during the reaction. There are several inert solvents that do not get oxidized or reduced by the redox couple, and-or, redox pair during the reaction and do not show subsequent parallel reactions by the reaction media. For example, tertiary butyl alcohol (TBA) and 1,4-dioxane (Diox) are inert solvents with low dielectric constant as compared to water and are miscible to water in all proportions. Similarly, dimethyl sulfoxide (DMSO) can also be used as a solvent to change the dielectric constant of water but it may be oxidized, and-or, reduced during the redox reaction. Therefore, its redox reaction with the reactants of target redox reaction could be studied to confirm its rate prior to using it as a solvent for the target redox reaction. If the rate of oxidation, and-or, reduction of DMSO is much slower than the target redox reaction, DMSO can be used as a solvent to reduce the dielectric constant of reaction media to deduce the rate constant of the target redox reaction as a function of the dielectric constant of the solution/reaction media.
The chemical structure of either the reactants and-or the solvent(s) leads the kinetics of any chemical reaction including electron transfer or redox reaction [2, 3]. The structure of solvent, its dielectric constant, its viscosity, hydrogen bonding, polarity, and protic or aprotic characteristic play a crucial role in the kinetics and therefore mechanism of the redox reaction [4, 5, 6, 7]. A redox reaction is one that involves the donation and acceptance of electron or electrons. The electron donating species is termed as reductant or reducing agent and the electron accepting species is known as the oxidant or oxidizing agent. The donation of electron is oxidation and its acceptance is reduction. Therefore, the reaction medium has a significant effect on the electron transfer process, its rate, and mechanism.
Kinetics unfolds several facts regarding a specific reaction. It correlates the rate of reaction with the concentration of reactants by introducing a constant value known as the rate constant. The rate of a reaction is defined as the consumption of reactant or formation of product with respect to time. It is measured in molar concentration per second. The rate is correlated to the power of the concentration terms involved in a reaction. This power is known as the order of reaction. The order of reaction is classified as zero, fractional, first, second, and third order reaction. The zero order reaction indicates the concentration independent nature of the rate of reaction. First order reaction identifies the first power dependence of the rate on the concentration of the reactant. Meanwhile fractional (0.5 or 1.5), second, and third order reactions state the fractional, second, and third power dependence of the rate on the concentration of the reactant(s), respectively. The zero order reaction is described by the zero order rate constant with dimension M s−1. Likewise, fractional (0.5 or 1.5), first, second, and third order reactions are illustrated by fractional (M0.5 s−1 or M–0.5 s−1), first (s−1), second (M−1 s−1), and third order (M−2 s−1) rate constants, respectively.
The electron carries a negative charge. Similarly, the ions also carry charges either positive or negative. If the electron transfer reaction occurs between the ions in the solvent or the mixture of solvents that are polar and protic, then, the rate of reaction and the reaction mechanism may certainly be influenced by the reaction media. Similarly, if the solvent is polar but aprotic, this may lead the kinetics and reaction mechanism differently. Additionally, when the solvent is non-polar and aprotic, the reaction’s kinetics and mechanism may vary differently. As a result, if the mixture of such solvents is used as the reaction media and the kinetics and the mechanism of the redox reactions are studied carefully, then, the reactions may either be controlled or catalyzed to be used in a fruitful and environmentally benign way to get the most out of the reaction. Such as the sensitizer-mediator redox reaction in the dye-sensitized solar cells (DSSC) may be controlled by such mixes of solvents that may increase not only the efficiency of the solar cell but also be a green approach to keep environment safe with a durable and stable energy producing system.
As a result, to reveal whether the rate constant is a function of the dielectric constant in terms of decrease, increase or no effect in its value, or, there is something beyond the correlation of the rate constant and the dielectric constant of the reaction media, the redox reaction between dicyanobis(2,2′-dipyridyl)iron(III) and iodide was studied in different solvent mixtures. Dicyanobis(2,2′-dipyridyl)iron(III) is a potential sensitizer for DSSC because of its photosensitive nature [8, 9]. Meanwhile, its hydrophobic and hydrophilic nature in its reduced and oxidized forms, respectively, makes it attractive and suitable for a DSSC. Another advantage is its inert and outer sphere structural property with a high value of the reduction potential [10]. The effect of the structural variation of the solvent-solvent mixtures and their corresponding properties were studied on the kinetics of the reduction of dicyanobis(2,2′-dipyridyl)iron(III) by iodide. Iodide is a well known mediator for DSSCs, and therefore, it has been explored frequently [11, 12, 13]. The potential sensitizer that is dicyanobis(2,2′-dipyridyl)iron(III) can oxidize iodide in all the selected reaction media without need of any external triggering [14, 15]. The redox reaction was studied in aqueous medium (100% water) that is a polar protic solvent. The same reaction was also studied in the reaction media consisted of 10% (volume/volume) DMSO in 90% volume/volume water. DMSO is a polar aprotic solvent. The third solvent mixture was used with 10% volume/volume 1,4-dioxane (Diox) in 10% volume/volume DMSO and 80% volume/volume water. Diox is a non-polar aprotic solvent. However, the fourth reaction media was maintained by mixing 10% volume/volume TBA and 90% volume/volume water. TBA is polar and very weak protic solvent. The three electron donating methyl groups in TBA build an aprotic character to release proton from OH group in it.
2. Methodology
Analar grade (Sigma-Aldrich/Merck) materials and solvents were used for this study. The reagents include potassium iodide, potassium nitrate, DMSO, 1,4-dioxane, and TBA. Dicyanobis(2,2′-dipyridyl)iron(III) nitrate was prepared as cited earlier [10]. Deionized water was used for preparation of the aqueous solution or maintaining the aqueous composition of the reaction mixture. The kinetic analyses were carried out by using fluorescence/UV-visible spectrophotometer (Vernier, USA).
The kinetic studies were performed spectrophotometrically in the visible region of electromagnetic spectrum considering the intense red color and high molar absorptivity of dicyanobis(2,2′-dipyridyl)iron(II) as compared to dicyanobis(2,2′-dipyridyl)iron(III) nitrate and other reactants such as potassium iodide or potassium nitrate and iodine. The wavelength maximum of dicyanobis(2,2′-dipyridyl)iron(II) in the visible region was appropriate to probe the reaction rate without interference of other reactants and product. It is important to note that the wavelength shift associated with dicyanobis(2,2′-dipyridyl)iron(II) manifests itself in various solvent media. Thus, in the selected reaction media, the kinetic traces, or time course graphs, were acquired in accordance with the wavelength maximum of dicyanobis(2,2′-dipyridyl)iron(II) (Figure 1). It is also crucial to note that because dicyanobis(2,2′-dipyridyl)iron(II) has a neutral structure—that is, no charge on it—its solubility decreases with increase in its concentration. In the meantime, as the solvent’s organic content rises, so does its molar absorptivity in the organic-aqueous medium. It therefore produces the following molar absorptivity values: 3266 M−1 cm−1 at 522.8 nm (100% water), 4938 M−1 cm−1 at 528.2 nm (10% DMSO–90% water), 5026 M−1 cm−1 at 533.3 nm (10% Diox–10% DMSO–80% water), and 6093 M−1 cm−1 at 522.3 nm (10% TBA–90% water).
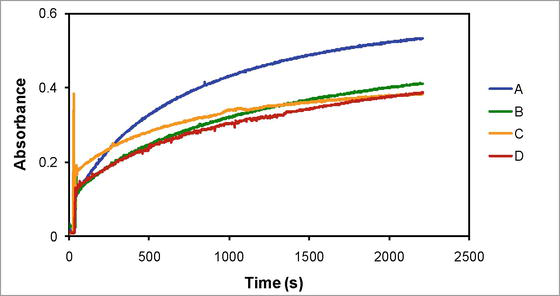
Figure 1.
The kinetic traces corresponding to (A) 100% water (aqueous medium) at 522.8 nm, (B) 10% DMSO-90% water at 528.2 nm, (C) 10% Diox-10% DMSO-80% water at 533.3 nm, and (D) 10% TBA-90% water at 522.3 nm.
The pseudo-first order condition was applied by keeping the concentration of iodide in excess over dicyanobis(2,2′-dipyridyl)iron(III) in all the reaction media. The integration method was implemented to identify the order of reaction and that was observed to be zero corresponding to dicyanobis(2,2′-dipyridyl)iron(III) in all the reaction media (Figure 2). Each experiment was repeated multiple (3–6) times and the average value of the observed zero order rate constant (
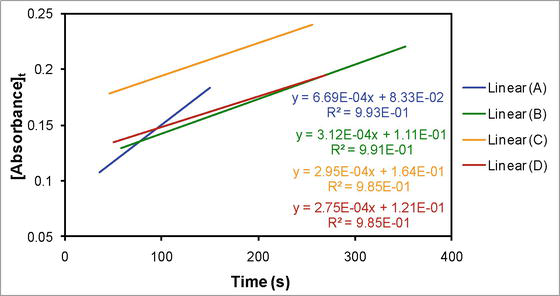
Figure 2.
Zero order straight-line plots corresponding to (A) 100% water (aqueous medium), (B) 10% DMSO-90% water, (C) 10% Diox-10% DMSO-80% water, and (D) 10% TBA-90% water.
The limited solubility of dicyanobis(2,2′-dipyridyl)iron(II) causes the absorbance corresponding to it to decrease somewhat as the reaction proceeds, even at large concentrations. Therefore, in order to deduce the reaction’s order, the absorbance data were used before it bent. The best linear fit (R2 value) served as the criterion for figuring out the reaction order when employing the straight-line equations of integration method for different orders. The data between the two absorbance bends were processed using the integration approach in order to verify the reaction’s order. After that, this process was carried out again till the absorbance did not change that is reaction’s completion stage. In all cases, the zero order plot yielded the best linear fit when compared to the first, fractional, second, and third orders. However, the slope decreased in value after each absorbance bend in comparison to the one before it. Consequently, the absorbance data prior to the absorbance’s first bend were used to calculate the observed zero order rate constant.
It is worth mentioning that the reaction was studied at room temperature that is 33°C when the reaction medium was 100% water, and 37°C when the reaction media were 10% DMSO-90% water, 10% Diox-10% DMSO-80% water, and 10% TBA-90% water. The ionic strength was maintained constant at 0.06 M by using potassium nitrate in all reaction media.
3. Results of kinetic analyses and discussion
The results of the redox reaction between dicyanobis(2,2′-dipyridyl)iron(III) and iodide in different solvent mixtures revealed that the overall rate constant and therefore the reaction mechanism are significantly influenced by the nature of the solvent. This is not just the dielectric constant of the solvent mixtures that either increases or decreases the rate of reaction or shows no effect on it. The role of solvent is beyond the acceleration or deceleration of the rate of reaction by variation in the dielectric constant of the reaction media. This effect is catalyzing or inhibitory, where the solvent takes part in the reaction mechanism and then forms back at the end of the reaction without getting consumed in the process. Different mixture of solvents with same dielectric constant may lead the reaction mechanism and may change the reaction pathways completely for the same reaction in different reaction media.
For elucidation of such promising role of the solvents that is beyond the dielectric constant of the reaction media, the kinetics of the redox reaction of dicyanobis(2,2′-dipyridyl)iron(III) and iodide was studied in 100% water (aqueous medium), 10% DMSO-90% water, 10% Diox-10% DMSO-80% water, and 10% TBA-90% water. The integration method helped to identify the zero order kinetics with respect to dicyanobis(2,2′-dipyridyl)iron(III) in all the reaction media. However, when the concentrations of the reducing agent or reductant (electron donor; iodide) and the oxidizing agent or oxidant (electron acceptor; dicyanobis(2,2′-dipyridyl)iron(III)) were varied and their effect was studied on the observed zero order rate constant (
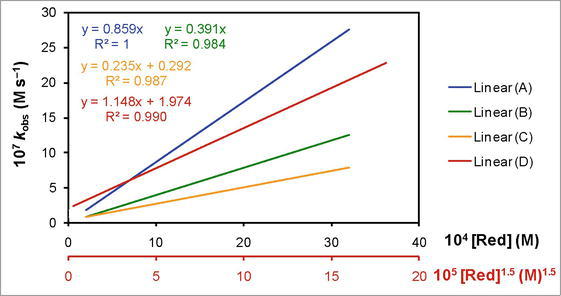
Figure 3.
The correlation of the observed zero order rate constant (
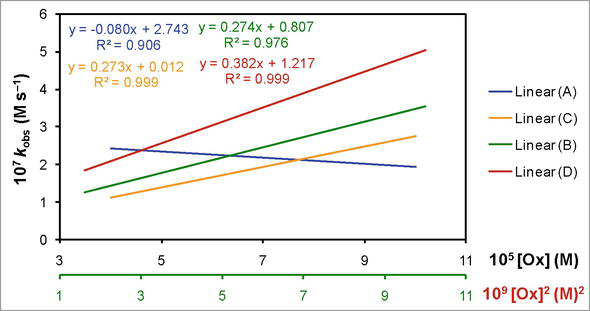
Figure 4.
The correlation of the observed zero order rate constant (
In 100% water (aqueous medium), the reaction followed a first order with increasing concentration of the reducing agent (Red). The linear correlation was produced between the observed zero order rate constant (
A similar kinetics was observed in 10% DMSO-90% water (Figure 3, line (B)). The increase in the concentration of iodide (Red) increased the value of
However, in 10% Diox-10% DMSO-80% water (Figure 3, line (C)), the behavior was totally different. An increase in the concentration of iodide (Red) enhanced the value of
Similarly, in case of the reaction kinetics in 10% TBA-90% water, the results are completely different as compared to the other results in other reaction media, as can be seen in Figure 3, line (D). The correlation between the observed zero order rate constant (
The effect of variation in the concentration of oxidizing agent (Ox or dicyanobis(2,2′-dipyridyl)iron(III)) on the observed zero order rate constant (
The integration method revealed the zero order reaction in dicyanobis(2,2′-dipyridyl)iron(III) as mentioned earlier in methodology. The slope of the straight-line equation according to integration method for zero order yielded the value of the observed zero order rate constant with respect to dicyanobis(2,2′-dipyridyl)iron(III). The results illustrated that the rate of reaction was independent of the concentration of dicyanobis(2,2′-dipyridyl)iron(III) because zero order means no effect of variation in the concentration of dicyanobis(2,2′-dipyridyl)iron(III) on the rate of reaction as mentioned in Eq. (12).
Eq. (10) demonstrates that the observed zero order rate constant (
In 100% water (aqueous medium), the results are somehow consistent with Eq. (12). However, there is an inverse effect of variation in the concentration of oxidant on the value of
In the polar aprotic and polar protic solvents mixture such as 10% DMSO-90% water (Figure 4, line (B)), the value of
The kinetics of the redox reaction corresponding to non-polar aprotic, polar aprotic, and polar protic solvents mixture that is 10% Diox-10% DMSO-80% water (Figure 4, line (C)), has also showed the electron transfer between the reactants by the parallel reactions. The intercept of the line (C) shows zero order in oxidant. The first order correlation between the concentration of oxidant and the value of
Similarly, in polar weak aprotic and polar protic solvents mixture that is 10% TBA-90% water, the results revealed parallel or concurrent reactions (Figure 4, line (D)). One among them is second order in oxidant and the other is zero order in it. Consequently, the reaction that is zero order in oxidant has fractional (1.5) order in reductant and the one that is zero order in reductant has second order in oxidant.
For further confirmation of the kinetics and reaction mechanism, the effect of ionic strength was studied on the observed zero order rate constant (
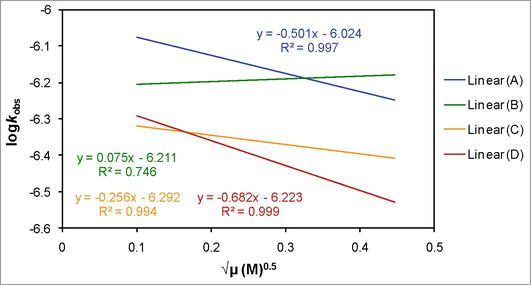
Figure 5.
The correlation of the observed zero order rate constant (
Eq. (13) represents that the rate constant (
From the slope of the plot, it is identified that whether similar or opposite charge carriers are involved in the rate-determining step, when it is positive and-or negative, respectively. And if, there is any neutral reactant or both of the reactants are neutral that is/are formed during the reaction and then lead the rate-determining step or exist(s) since the beginning of the reaction, the reaction will bear no effect of variation in the ionic strength on the rate constant.
The effect of ionic strength on the observed zero order rate constant of the redox reaction between dicyanobis(2,2′-dipyridyl)iron(III) and iodide in 100% water shows that opposite charge carriers are involved in the reaction mechanism (Figure 5, line (A)). The value of the slope of the plot of log
There is no effect of variation in the ionic strength on the observed rate constant in case of 10% DMSO-90% water (Figure 5, line (B)). The value of the slope of the plot of log
The comparative analysis of the results shows that in each solvent media the multiplication product of charges on the reactants are different. These findings help to identify the involvement of ion or polar or neutral species in the rate-determining step and revealing different reaction mechanism in each reaction media.
4. Conclusion
The results of this study revealed that the effect of solvent or solvent-solvent mixture is not limited to the correlation of dielectric constant and the rate constant as illustrated by Eq. (1). The real effect of solvent or solvents mixture is beyond this concept where Eq. (1) stands insufficient to be implemented to deduce the inter-nuclear distance between the reacting species that are involved in the rate-determining step and though lead the reaction mechanism. The solvent or solvent-solvent mixture may lead the whole mechanism of one reaction in a very different way in one reaction media than in other and so on. Therefore, it is very crucial to study the complete kinetics of any reaction in any reaction media or mixture of solvents because the same reaction follows different mechanism in different solvents or their mixtures. Until and unless, the detailed kinetic model is not revealed in any specific reaction media, the reaction could not be used in a process for expected outputs. The comparative analysis of this study regarding kinetic data in all solvent media is mentioned below (Table 1). The dielectric constants of different solvents are listed in Table 2.
| 100% water (polar protic) | 10% DMSO-90% water (polar aprotic-polar protic) | 10% Diox-10% DMSO-80% water (non-polar aprotic-polar aprotic-polar protic) | 10% TBA-90% water (polar weak protic-polar protic) |
|---|---|---|---|
Table 1.
| Solvent | Dielectric constant at 20°C |
|---|---|
| water | 80.1 |
| Dimethyl sulfoxide | 46.7 |
| 1,4-Dioaxne | 2.25 |
| Tertiary butyl alcohol | 10.9 at 30°C, (below 30°C it is solid) |
Table 2.
Dielectric constant of different solvents used in this study.
According to Eq. (1), as the reactants of this study are opposite charge carriers, therefore, with decreasing dielectric constant, the rate of reaction should be accelerated and hence, the value of rate constant should be increased. The reaction must follow the same kinetics in either of the solo solvent and-or the mixture of solvents. However, in real, there is no such restriction on the reactants to follow same kinetics and same mechanism in different solvents and their mixture. Consequently, Eq. (1) fails to correlate the rate constant and the dielectric constant of reaction media. Table 1 illustrates that the decrease in dielectric constant did not increase the value of rate constant if we compare the first order rate constant in reductant, and, zero order rate constant corresponding to oxidant, as an example. In every media, the kinetics is different. Consequently, the mixture of solvents may yield a stable and an efficient reaction for DSSC, only if the complete kinetic knowledge is available and the reaction is controlled accordingly. This is how the reaction may worth according to the prediction.
References
- 1.
Wright MR. An Introduction to Chemical Kinetics. England: John Wiley & Sons Ltd; 2004 - 2.
Zhao Y, Freeman GR. Solvent effects on the reactivity of solvated electrons with ions in tert-butanol/water mixtures. Canadian Journal of Chemistry. 1995; 73 (3):392-400 - 3.
Walker TW et al. Universal kinetic solvent effects in acid-catalyzed reactions of biomass-derived oxygenates. Energy & Environmental Science. 2018; 11 (3):617-628 - 4.
Slakman BL, West RH. Kinetic solvent effects in organic reactions. Journal of Physical Organic Chemistry. 2019; 32 (3):e3904 - 5.
Besbes R, Ouerfelli N, Latrous H. Density, dynamic viscosity, and derived properties of binary mixtures of 1,4 dioxane with water at T=298.15 K. Journal of Molecular Liquids. 2009; 145 (1):1-4 - 6.
Ben-Naim A, Yaacobi M. Hydrophobic interaction in water-p dioxane mixtures. The Journal of Physical Chemistry. 1975; 79 (13):1263-1267 - 7.
Ying Guang W, Masaaki T, Toshiyuki T. A local solvent structure study on 1,4-dioxane-water binary mixtures by total isotropic Rayleigh light scattering method. Journal of Molecular Liquids. 2001; 94 (3):273-282 - 8.
Ferrere S. New photosensitizers based upon [Fe(L)2(CN)2] and [Fe(L)3] (L) substituted 2,2′-bipyridine: Yields for the photosensitization of TiO2 and effects on the band selectivity. Chemistry of Materials. 2000; 12 :1083-1089 - 9.
Ferrere S. New photosensitizers based upon [FeII(L)2(CN)2] and [FeIIL3], where L is substituted 2,2′-bipyridine. Inorganica Chimica Acta. 2002; 329 (1):79-92 - 10.
Khattak R. Comparative kinetic study for the electron transfer reactions of some iron complexes. In: Department of Chemistry. Karachi: University of Karachi; 2011 - 11.
Boschloo G, Hagfeldt A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Accounts of Chemical Research. 2009; 42 (11):1819-1826 - 12.
Wang X, Stanbury DM. Copper catalysis of the oxidation of iodide by [FeIII(bpy)2(CN)2]+ in acetonitrile. The Journal of Physical Chemistry. A. 2004; 108 (38):7637-7638 - 13.
Wang X, Stanbury DM. Oxidation of iodide by a series of Fe(III) complexes in acetonitrile. Inorganic Chemistry. 2006; 45 (8):3415-3423 - 14.
Khattak R et al. Catalytic effect of 1,4-dioxane on the kinetics of the oxidation of iodide by dicyanobis(bipyridine)iron(III) in water. Catalysts. 2021; 11 (7):840 - 15.
Khattak R et al. Effect of the ionic strength on the redox reaction of dicyanobis(bipyridine)iron(III)-iodide in binary and ternary solvent systems. International Journal of Chemical Kinetics. 2021; 53 (1):16-26 - 16.
Wilkinson F. Chemical Kinetics and Reaction Mechanisms. New York: Van Nostrand Reinhold Company; 1980. pp. 170-173 - 17.
Sánchez F et al. Micellar, microemulsion, and salt kinetic effects upon the reaction Fe(CN)2(bpy)2 + S2O82−. Langmuir. 1997; 13 (12):3084-3089 - 18.
Múñoz E et al. Salt effects upon reactions of different charge type reactants: Peroxodisulphate oxidations of Fe(CN)4(bpy)2−, cis-Fe(CN)2(bpy)2 and Fe(bpy)32+ and iron(II) oxidation by Co(NH3)5Cl2+. International Journal of Chemical Kinetics. 1994; 26 (2):299-307