Sequence types of
Abstract
Blastocystis is a very common gastrointestinal protozoan globally distributed; it colonizes humans and non-humans, and in some communities, it reaches prevalences of up to 100%. Blastocystis is transmitted through the fecal-oral route, contaminated food and water, and close contact with animals. There are 34 known subtypes of Blastocystis, and subtypes 1 to 4 (ST1–ST4) are the most common in humans. It should be remembered that its pathogenicity is controversial since some studies have shown that Blastocystis is more prevalent in healthy individuals; who have greater diversity and richness of the intestinal microbiota; other studies suggest that Blastocystis infections occur in individuals with intestinal dysbiosis. In America and Africa, a high incidence of ST1 and ST2 is observed in rural areas. Recent data indicate that Blastocystis is linked with specific gut microbiota profiles and health indicators. Convincing information and tools that distinguish asymptomatic colonization from infection in children have yet to be demonstrated. Although this protozoan can cause disease under certain circumstances, but the attention of Blastocystis may change, as the frequency of Blastocystis subtypes in children may vary depending on the geographic area and local health conditions.
Keywords
- Blastocystis
- controversial pathogenicity
- frequency
- health
- gut microbiota
1. Introduction
A complex and diverse population of microorganisms lives in the human intestine: bacteria, fungi, viruses, archaea, and protozoans [1].
It has been identified 34 subtypes of
In America, a high incidence is observed for ST1 and ST2 [23]. In Mexico, higher prevalence was reported for ST1 (51%) [24] in 2023. A global prevalence of 44.0% was reported in school-age children in a rural area, with a prevalence of 56.5% for ST1, followed by ST2 (26.3%) and ST3 (19.7%). In Colombian children, a study carried out in 2021 reported a global prevalence of 58.2% of
Some African countries have reported a high prevalence of
The classification of
More sensitive tools that differentiate asymptomatic colonization from infection in children have yet to be demonstrated. Although the parasite can cause disease under certain circumstances, the focus on
2. Blastocystis spp.
It was described for the first time in 1912 [43] by Alexeieff, who named it considered it a yeast and named it
2.1 Taxonomy
2.2 Morphology
There are many variations between sizes and the form of presentation according to subtypes, but the primary peculiarity is the appearance of a central vacuole (occupies 90% of the cytoplasm) with metabolic and storage functions, easily observable after staining [48]. In humans, different forms of
It presents four well-differentiated morphological stages:
2.2.1 Vacuolar form
The form most easily identified in feces, they have a size of 5–15 μm and binary fission reproduction. The diagnosis is made based on vacuolar form. They have a central corpuscle or vacuole composed of lipids and carbohydrates with reserve functions, which compress the cell nucleus and cytoplasm [49].
2.2.2 Ameboid form
This form measures 10 μm, does not have a central body, but has 1 or 2 slow-moving pseudopodia. Cellular debris has been found inside, which suggests that it has a significant role in the nutrition of the microorganism [49].
2.2.3 Granular form
It has 1 to 4 nuclei and measures between 6 and 8 μm. This form is scarce, and three types of granules are distinguished: metabolic, reproductive, and lipidic [50].
2.2.4 Cyst form
They measure 3–10 μm and are ovoid or spherical; the cells include lipid and glycogen deposits and vacuoles. Generally, the isolated nuclei are binucleate. They survive approximately 1 month at room temperature; however, they are sensitive to disinfectants and extreme temperatures [49].
2.3 Life cycle
The life cycle is similar to most protists. Two types of cycles have been described: binary fission and autogamy for the formation of cysts that can be thick or thin-walled, which help in the transmission of the parasite. The avacuolar cell is present in the intestine, passing through the intestinal tract. After the disintegration of vesicles in the cytoplasm, the multi-vacuolar form is generated, which is covered by a thick cell wall. The cystic wall forms under the cell cover disintegrates, resulting in a cyst, which is the infectious cell of
2.4 Transmission
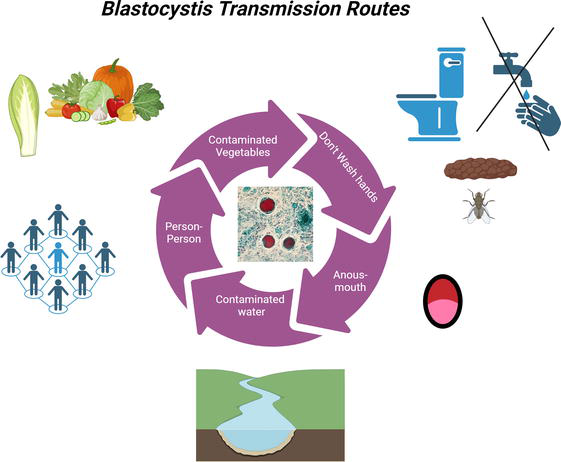
Figure 1.
2.5 Pathogenicity
The pathophysiology of
2.5.1 Blastocystis and immune system
In vivo, studies with rats have shown that the microorganism’s proteases stimulate the production of Interleukin 8 (IL-8) by colon epithelial cells through mechanisms dependent on nuclear factors, which are responsible for fluid loss and intestinal inflammation in affected individuals. Therefore, it is believed that these enzymes produce hyperplasia of the goblet cells and cecal mucosa in infected rats [49]. It was also experimentally demonstrated that
However, it should be noted that recent studies have identified
2.6 Subtypes
As previously mentioned, 34 subtypes of
2.6.1 Distribution of subtypes by hosts
ST1 and ST2 are also found in other animal species, including monkeys, cows, chickens, pigs, rats, dogs, and nonhuman primates. ST3 has been found in nonhuman primates, pigs, and cattle. ST4 has been described in rodents and monkeys. The rarer subtypes in humans (ST5–8) are found most frequently in other hosts: ST5 is common in cattle, apes, pigs, rats, dogs, and Old World monkeys, while ST6 and ST7 are mainly found in birds and cattle (ST6). ST8 has been recognized in marsupials, different species of captive primates and their caretakers. These rarer subtypes in humans have been suggested to be of zoonotic derivation, and there is some confirmation that ST8 has been found in zookeepers of nonhuman primates [15, 59].
Based on next-generation sequencing [60],
2.6.2 Prevalence of Blastocystis subtypes according to geographic region
2.6.3 Differences between subtypes
Currently, the
Subtype 3 (ST3) is one of the most common in humans, as well as the most frequently found in fecal samples from patients with both gastrointestinal and dermatological clinical manifestations (urticaria) [62]. About the pathogenicity of ST4, in a meta-analysis that included studies from Europe, Asia, Africa, and South America this subtype has a much higher global sequence conservation than the others. Furthermore, heat shock proteins (such as 0PHA3 and KOG3047, a ubiquitously expressed prefoldin-like chaperone) and cytosolic Ca2+ ion-dependent cysteine proteases (such as KOG0045, which is a cysteine-like peptidase similar to calpain) were found in the ST4 genomes that were not present in other ST genomes, which may represent virulence factors unique to ST4 [63]. The activities of cysteine proteases in ST4 and ST7 isolates have shown significant variations, which may be one of the reasons for the differences in their virulence [10]. Several studies worldwide have demonstrated that ST5 is derived from animals since it has been found in people who live in rural areas and have close contact with animals. In addition, poor hygiene plays a vital role in contagion [62, 64].
About subtype 7 (ST7), in vitro assays revealed that this subtype caused alterations in the intestinal epithelial barrier by altering binding proteins such as occludin and zonula occludens-1 (ZO-1) [38], which was associated with greater adhesiveness to intestinal epithelial cells and more significant cysteine protease activity than ST4. In the same study, histopathological results showed that mice infected with ST7 had more colon damage and ulceration than control mice [32]. Also, it has shown that ST7 in isolate H (with greater adhesiveness than other isolates) binds preferentially to colonic tissue concerning the cecum and terminal ileum. ST7 has also been associated with more significant colonization of other parasites [60]. Furthermore, comparing sensitivity and resistance to certain antibiotics showed that ST7 has greater resistance to metronidazole than ST4 whereas it has greater sensitivity to emetine than ST4 [61]. In ST7, the 0IZK7 Cystatin B has a potential role in parasitic cysteine protease function and inhibition of host proteases; this protein is also present in ST2 but not in ST1, ST3 and ST4 [62].
2.6.4 Distribution of subtypes in children from different countries
With the advent of new-generation sequencing (Figure 2), since 2020, more
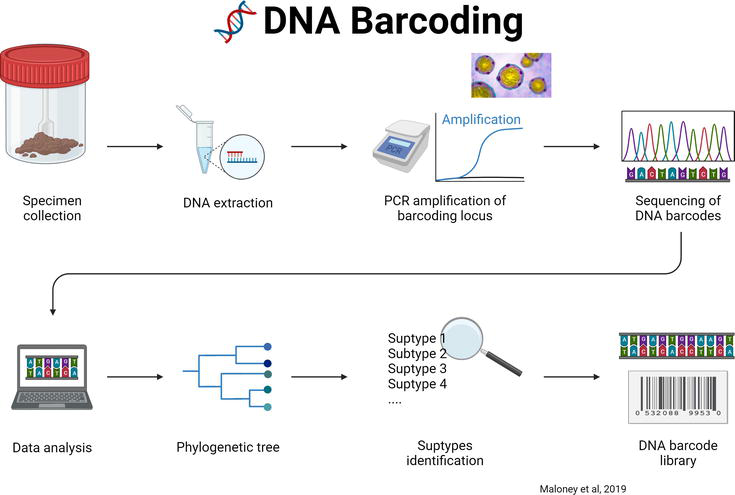
Figure 2.
Next generation sequencing method for the subtyping of
| Country | Year | Age (years) | N of samples sequenced n | ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | ST7 | ST16 | ST26 | Methodology | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jakarta | 2018 | 6–12 | 16 | 12 (67.95%) | 5 (26.4%) | 2 (3.8%) | 1 (1.9%) | — | — | — | — | — | Sanger | [65] |
| Panama | 2020 | 1–12 | 49 | 28 (57.1%) | 21 (42.8%) | — | — | — | — | — | — | Sanger | [27] | |
| Senegal | 2020 | 6–19 | 588 | 113 (19.2%) | 226 (38.4%) | 107 (18.19%) | — | — | — | — | — | — | Genoscreen | [28] |
| Colombia | 2021 | 0–5 | 59 | 12 (20.3%) | 14 (23.7%) | 18 (30.5%) | 3 (5.0%) | — | 1 (1.7%) | — | 9 (15.3%) | — | Barcoding | [13] |
| Azerbaijan | 2021 | 6.4–13.4 | 22 | 3 (14%) | 5 (23%) | 14 (64%) | 0 | — | 0 | — | — | — | Barcoding | [66] |
| Czechia | 2021 | 8.1–12.7 | 8 | 1 (13%) | 3 (38%) | 3 (38%) | 1 (25%) | — | 0 | — | — | — | Barcoding | [66] |
| Jordan | 2021 | 7.9–13.4 | 5 | 1 (20%) | 1 (20%) | 4 (80%) | 0 | — | 0 | — | — | — | Barcoding | [66] |
| Nigeria | 2021 | 13.9–16.9 | 14 | 8 (57%) | 4 (29%) | 5 (36%) | 0 | — | 1 (7%) | — | — | — | Barcoding | [66] |
| Sudan | 2021 | 7.4–12.9 | 24 | 14 (58%) | 5 (21%) | 10 (42%) | 0 | — | 0 | — | — | — | Barcoding | [66] |
| Tanzania | 2021 | 11.1–14.6 | 8 | 2 (25%) | 2 (25%) | 4 (50%) | 0 | — | 0 | — | — | — | Barcoding | [66] |
| Tukey | 2022 | 1–18 | 14 | 3 (21.4%) | 6 (42.8%) | 5 (35.7%) | — | — | — | — | — | — | Sanger | [67] |
| Thailand | 2023 | 4–12 | 17 | 5 (29.4%) | 1 (5.8%) | 11 (64.7%) | — | — | — | — | — | — | Barcoding | [68] |
| Rural Thailand | 2023 | 6–14 | 93 | 7 (7.5%) | 19 (20.4%) | 42 (45.1%) | — | 1 (1.07%) | — | 14 (15.05%) | — | 1 (1.07%) | Barcoding | [69] |
| Ecuador | 2023 | 3–11 | 84 | 22 (26.2%) | 22 (26.2%) | 24 (28.6%) | 12 (14.3%) | — | — | — | — | — | Bigdye Terminator | [70] |
| Mexico | 2023 | 3–15 | 78 | 43 (56.5%) | 18 (23.6%) | 15 (19.7%) | — | — | — | — | — | — | Barcoding | [24] |
Table 1.
n number of samples; % of sequenced samples.
In Latin America, in a study carried out in Colombia in a child population that attended daycare centers in Medellín, where the population was urban, the global prevalence of
2.6.5 Association of Blastocystis and the gut microbiota
The association of
A previous study in 2016 with subjects aged 9 to 70 years found a higher relative abundance of Bacteroides in
3. Conclusions
The studies described above aim to understand the dynamics of the transmission of
References
- 1.
Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers. 2018; 6 :1-21 - 2.
Farré EM. The brown clock: Circadian rhythms in stramenopiles. Physiologia Plantarum. 2020; 169 :430-441 - 3.
Tan TC, Suresh KG. Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients.Parasitology Research . 2006;98 (3):189-193. Epub ahead of print. DOI: 10.1080/01421590500312847 - 4.
Scanlan PD, Stensvold CR. Blastocystis : Getting to grips with our guileful guest. Trends in Parasitology. 2013;29 :523-529 - 5.
El Safadi D, Gaayeb L, Meloni D, et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infectious Diseases. 2014;14 :1-11 - 6.
Tan KSW. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clinical Microbiology Reviews. 2008;21 :639-665 - 7.
Zou Y, Bin YW, Zou FC, et al. Molecular detection and subtype distribution of Blastocystis in farmed pigs in southern China. Microbial Pathogenesis. 2021;151 :104751 - 8.
Domínguez-Márquez MV, Guna R, Muñoz C, et al. High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain). Parasitology Research. 2009;105 :949-955 - 9.
Ramírez JD, Sánchez LV, Bautista DC, et al. Blastocystis subtypes detected in humans and animals from Colombia. México: Infection, Genetics and Evolution. 2014;22 :223-228 - 10.
Kumarasamy V. Blastocystis sp., parasite associated with gastrointestinal disorders: An overview of its pathogenesis, immune modulation and therapeutic strategies. Current Pharmaceutical Design. 2018;24 :3172-3175 - 11.
Jinatham V, Maxamhud S, Popluechai S, et al. Blastocystis one health approach in a rural community of Northern Thailand: Prevalence, subtypes and novel transmission routes. Frontiers in Microbiology. 2021;12 :746340. Epub ahead of print. DOI: 10.3389/fmicb.2021.746340 - 12.
Maloney JG, Molokin A, Seguí R, et al. Identification and molecular characterization of four new Blastocystis subtypes designated ST35-ST38. Microorganisms. 2023;11 :46. Epub ahead of print. DOI: 10.3390/microorganisms11010046 - 13.
Osorio-Pulgarin MI, Higuera A, Beltran-álzate JC, et al. Epidemiological and molecular characterization of Blastocystis infection in children attending daycare centers in medellín, Colombia. Biology (Basel). 2021;10 :669. Epub ahead of print. DOI: 10.3390/biology10070669 - 14.
Alfellani MA. Genetic diversity of Blastocystis in livestock and zoo animals. Protist. 2013;164 :497-509 - 15.
Skotarczak B. Genetic diversity and pathogenicity of Blastocystis . Annals of Agricultural and Environmental Medicine. 2018;25 :411-416 - 16.
Scanlan PD, Stensvold CR, Rajilić-Stojanović M, et al. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiology Ecology. 2014;90 :326-330 - 17.
Lukeš J, Stensvold CR, Jirků-Pomajbíková K, et al. Are human intestinal eukaryotes beneficial or commensals? PLoS Pathogens. 2015; 11 :e1005039. Epub ahead of print. DOI: 10.1371/journal.ppat.1005039 - 18.
Andersen LO, Stensvold CR. Blastocystis in health and disease: Are we moving from a clinical to a public health perspective? Journal of Clinical Microbiology. 2016;54 :524-528 - 19.
Nourrisson C, Scanzi J, Pereira B, et al. Blastocystis is associated with decrease of fecal microbiota protective bacteria: Comparative analysis between patients with irritable bowel syndrome and control subjects. PLoS ONE. 2014;9 :1-9 - 20.
Lepczyńska M, Dzika E, Kubiak K, et al. The role of Blastocystis sp. as an etiology of irritable bowel syndrome. Polish Annals of Medicine. 2016;23 :57-60 - 21.
Yakoob J, Jafri W, Beg MA, et al. Blastocystis hominis andDientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitology Research. 2010;107 :679-684 - 22.
Audebert C, Even G, Cian A, et al. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Scientific Reports. 2016;6 :1-11 - 23.
Seyer A, Karasartova D, Ruh E, et al. Epidemiology and prevalence of Blastocystis spp. in North Cyprus. The American Journal of Tropical Medicine and Hygiene. 2017;96 :1164-1170 - 24.
Hidalgo-Gonzalez LA, Salgado-Lopez J, Pineda-Rodriguez SA, et al. Identification of Blastocystis sp. in school children from a rural Mexican village: Subtypes and risk factors analysis. Parasitology Research. 2023;122 :1701-1707 - 25.
Casero RD, Mongi F, Sánchez A, et al. Blastocystis and urticaria: Examination of subtypes and morphotypes in an unusual clinical manifestation. Acta Tropica. 2015;148 :156-161 - 26.
Ramírez JD, Flórez C, Olivera M, et al. Blastocystis subtyping and its association with intestinal parasites in children from different geographical regions of Colombia. PLoS ONE. 2017;12 :1-13 - 27.
Perea M, Vásquez V, Pineda V, et al. Prevalence and subtype distribution of Blastocystis sp. infecting children from a rural community in Panama. Parasite Epidemiology and Control. 2020;9 :1-8 - 28.
Khaled S, Gantois N, Ly AT, et al. Prevalence and subtype distribution of Blastocystis sp. in Senegalese school children. Microorganisms. 2020;8 :1-17 - 29.
Lepczyńska M, Dzika E. The influence of probiotic bacteria and human gut microorganisms causing opportunistic infections on Blastocystis ST3. Gut Pathogens. 2019;11 :1-11 - 30.
Becerril-Flores MA. Blastocystosis. In: Parasitología Médica . México: McGraw Hill; 2019. pp. 165-167 - 31.
Stensvold CR, van der Giezen M. Associations between gut microbiota and common luminal intestinal parasites. Trends in Parasitology. 2018; 34 :369-377 - 32.
Gentekaki E, Curtis BA, Stairs CW, et al. Extreme genome diversity in the hyper-prevalent parasitic eukaryote Blastocystis . PLoS Biology. 2017;15 :1-42 - 33.
Forsell J, Bengtsson-Palme J, Angelin M, et al. The relation between Blastocystis and the intestinal microbiota in Swedish travellers. BMC Microbiology. 2017;17 :1-9 - 34.
Kodio A, Coulibaly D, Koné AK, et al. Blastocystis colonization is associated with increased diversity and altered gut bacterial communities in healthy Malian children. Microorganisms. 2019;7 :1-11 - 35.
Tito RY, Chaffron S, Caenepeel C, et al. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut Microbiota. 2019;68 :1180-1189 - 36.
Castañeda S, Muñoz M, Villamizar X, et al. Microbiota characterization in Blastocystis -colonized andBlastocystis- free school-age children from Colombia. Parasit Vectors. 2020;13 :521. Epub ahead of print. DOI: 10.1186/s13071-020-04392-9 - 37.
Nieves-Ramírez ME, Partida- Rodríguez O, Laforest-Lapointe I, et al. Asymptomatic intestinal colonization with protist Blastocystis is strongly associated with distinct microbiome ecological patterns. mSystems. 2018;3 :1-18 - 38.
Yason JA, Liang YR, Png CW, et al. Interactions between a pathogenic Blastocystis subtype and gut microbiota: In vitro and in vivo studies. Microbiome. 2019;7 :1-13 - 39.
Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress. 2017; 7 :124-136 - 40.
García Peña C, Álvarez Cisneros T, Quiroz Baez R, et al. Microbiota and aging. A review and commentary. Archives of Medical Research. 2017; 48 :681-689 - 41.
Stensvold CR, Sørland BA, Berg RPKD, et al. Stool microbiota diversity analysis of Blastocystis -positive andBlastocystis -negative individuals. Microorganisms. 2022;10 :1-6 - 42.
Deng L, Wojciech L, Gascoigne NRJ, et al. New insights into the interactions between Blastocystis , the gut microbiota, and host immunity. PLoS Pathogens. 2021;17 :1-15 - 43.
Yoshikawa H, Nagano I, Wu Z, et al. Genomic polymorphism among Blastocystis hominis strains and development of subtype-specific diagnostic primers. Molecular and Cellular Probes. 1998;12 :153-159 - 44.
Poirier P, Meloni D, Nourrisson C, et al. Molecular subtyping of Blastocystis spp. using a new rDNA marker from the mitochondria-like organelle genome. Parasitology. 2014;141 :670-681 - 45.
Zhan T, He S, Liu T, et al. A novel genotype screening and phylogenetic analysis of Blastocystis hominis based on EF-1α. International Journal of Clinical and Experimental Pathology. 2017;10 :8314-8323 - 46.
Sierra RL, Muñoz SGD, Lora SFM, et al. Identificación de subtipos de Blastocystis sp. asociados a fuentes de transmisión en una zona rural del departamento del Quindío. Revista de la Asociación Colombiana de Ciencias Biológicas. 2023;35 :113-127 - 47.
Stensvold CR, Traub RJ, von Samson-Himmelstjerna G, et al. Blastocystis : Subtyping isolates using pyrosequencing™ technology. Experimental Parasitology. 2007;116 :111-119 - 48.
Ajjampur SSR, Tan KSW. Pathogenic mechanisms in Blastocystis spp.—Interpreting results from in vitro and in vivo studies. Parasitology International. 2016;65 :772-779 - 49.
del Coco VF, Molina NB, Basualdo JA, et al. Blastocystis spp.: Advances, controversies and future challenges. Revista Argentina de Microbiología. 2017;49 :110-118 - 50.
Jeremiah S, Parija S. Blastocystis : Taxonomy, biology and virulence. Tropenmedizin und Parasitologie. 2013;3 :17 - 51.
Popruk S, Adao DE V, Rivera WL. Epidemiology and subtype distribution of Blastocystis in humans: A review. Infection, Genetics and Evolution. 2021;95 :1-14 - 52.
Mohamed RT, El-bali MA, Mohamed AA, et al. Subtyping of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Makkah, Saudi Arabia. Parasites & Vectors. 2017;10 :1-7 - 53.
Basak S, Rajurkar MN, Mallick SK. Detection of Blastocystis hominis : A controversial human pathogen. Parasitology Research. 2014;113 :261-265 - 54.
Andersen LOB, Bonde I, Nielsen HBHB, et al. A retrospective metagenomics approach to studying Blastocystis . FEMS Microbiology Ecology. 2015;91 :1-9 - 55.
Kurt Ö, Doğruman Al F, Tanyüksel M. Eradication of Blastocystis in humans: Really necessary for all? Parasitology International. 2016;65 :797-801 - 56.
Stensvold CR, Clark CG. Current status of Blastocystis : A personal view. Parasitology International. 2016;65 :763-771 - 57.
Eme L, Gentekaki E, Curtis B, et al. Lateral gene transfer in the adaptation of the anaerobic parasite Blastocystis to the gut. Current Biology. 2017;27 :807-820 - 58.
Chandramathi S, Suresh K, Sivanandam S, et al. Stress exacerbates infectivity and pathogenicity of Blastocystis hominis : In vitro and in vivo evidences. PLoS ONE. 2014;9 :1-11 - 59.
Jiménez PA, Jaimes JE, Ramírez JD. A summary of Blastocystis subtypes in North and South America. Parasites & Vectors. 2019;12 :1-9 - 60.
Maloney JG, Molokin A, Santin M. Next generation amplicon sequencing improves detection of Blastocystis mixed subtype infections. Infection, Genetics and Evolution. 2019;73 :119-125 - 61.
Mohamed AM, Ahmed MA, Ahmed SA, et al. Predominance and association risk of Blastocystis hominis subtype I in colorectal cancer: A case control study. Infectious Agents and Cancer. 2017;12 :1-8 - 62.
Cakir F, Cicek M, Yildirim IH. Determination the subtypes of Blastocystis sp. and evaluate the effect of these subtypes on pathogenicity. Acta Parasitologica. 2019;64 :7-12 - 63.
Beghini F, Pasolli E, Truong TD, et al. Large-scale comparative metagenomics of Blastocystis , a common member of the human gut microbiome. ISME Journal. 2017;11 :2848-2863 - 64.
Yañez CM, Hernández AM, Sandoval AM, et al. Prevalence of Blastocystis and its association withFirmicutes /Bacteroidetes ratio in clinically healthy and metabolically ill subjects. BMC Microbiology. 2021;21 :1-11 - 65.
Sari IP, Benung MR, Wahdini S, et al. Diagnosis and identification of Blastocystis subtypes in primary school children in Jakarta. Journal of Tropical Pediatrics. 2018;64 :208-214 - 66.
Cinek O, Polackova K, Odeh R, et al. Blastocystis in the faeces of children from six distant countries: Prevalence, quantity, subtypes and the relation to the gut bacteriome. Parasites & Vectors. 2021;14 :1-16 - 67.
Semra Güreser A, Comba A, Karasartova D, et al. Detection of Blastocystis subtypes in children with functional abdominal pain and celiac disease in Çorum, Turkey. Iranian Journal of Parasitology. 2021;17 :296-305 - 68.
Abu A, Sutthikornchai C, Mahittikorn A, et al. Prevalence and subtype distribution of Blastocystis isolated from school-aged children in the Thai-Myanmar Border, Ratchaburi Province, Thailand. International Journal of Environmental Research and Public Health. 2022;20 :1-9 - 69.
McCain A, Gruneck L, Popluechai S, et al. Circulation and colonisation of Blastocystis subtypes in schoolchildren of various ethnicities in rural northern Thailand. Epidemiology and Infection. 2023;151 :e85. Epub ahead of print. DOI: 10.1017/S0950268823000596 - 70.
Tapia-Veloz E, Gozalbo M, Guillén M, et al. Prevalence and associated risk factors of intestinal parasites among schoolchildren in Ecuador, with emphasis on the molecular diversity of Giardia duodenalis ,Blastocystis sp. andEnterocytozoon bieneusi . PLoS Neglected Tropical Diseases. 2023;17 :1-21 - 71.
O’Brien Andersen L, Karim AB, Roager HM, et al. Associations between common intestinal parasites and bacteria in humans as revealed by qPCR. European Journal of Clinical Microbiology and Infectious Diseases. 2016; 35 :1427-1431