Abstract
The temporomandibular joint (TMJ) is an important structure of the facial skeleton and is an important source of pain when inflammatory processes are occurring in it. It is located inferior the skull base, above the neck and anterior to the ear. The principal components include: bone structures, ligaments, intrarticular content, and muscles. Because of the anatomic relations, the temporomandibular joint affections can produce a limitation of the masticatory function and express headache of dental, sinusal, nervous, or muscular type. For this reason, the patients require multiple medical and dental specialties in the search of a solution for the current illness. The myofascial pain refers to a chronic, inflammatory condition of the TMJ and the muscular system of the head and neck. It has an important demand in the health sector, can incapacitate significantly the quality of life of the patients, and requires an appropriate diagnosis and treatment. The aim of this chapter is to guide the clinical practice in the etiology, diagnosis, prevention, and treatment of the myofascial pain as a clinical presentation of the temporomandibular dysfunction.
Keywords
- myofascial pain
- trigger point
- TMJ dysfunction
- referred pain
- botulinum toxin
1. Introduction
The temporomandibular joint “TMJ” is a ginglymoid, arthrodial, and compounded joint. It presents an articular biconcave disk that is interposed between the temporal and mandibular bone. It is located inferior the skull base, above the neck, anterior to the ear, and behind the ear. Its anatomical structures of relevance are divided in 4 big components: bone structures, ligaments, intrarticular components, and muscles [1].
Because of the masticatory function, TMJ has an important muscular component. The main muscles related to the masticatory function and the TMJ are the temporal, masseter, medial pterygoid, and lateral pterygoid. Nevertheless, there is a wide muscular network that extends from the head, neck, and upper back that could be affected in articular dysfunction presentations [2].
The temporomandibular dysfunction refers to a group of disorders that affect the TMJ; this affections can have an origin such as anatomic, growth and development, tumoral, traumatic, systemic disease, and acute or chronic inflammatory conditions, among others [3, 4].
It is estimated that more than 5% of the population presents some type of temporomandibular dysfunction, and a 6–12% of the general population could have a sign or symptom suggestive of TMD [2]. Generally, the temporomandibular dysfunction is presented in 2/3 of the feminine population, between 20 and 40 years of age, and exists an antecedent that triggers the symptomatology in the patients such as recent dental treatments, anxiety, depression, and tension type conditions, among others [2, 5]. Although the temporomandibular dysfunction could present signs and symptoms at early ages, the myofascial pain is often diagnosed at an older age. This is because the psychosocial triggers appear at an elderly age and the patients usually consult when the symptomatology has years of evolution.
Myofascial pain síndrome (MPS) is a chronic condition of dysfunction and inflammatory joint with multifactorial etiology that located the pain in the head and neck region [6].
Because of the anatomic relation, a lot of patients with TMJ dysfunction require health services, which can involve specialists such as neurologists, otolaryngologists, pain medicine, rheumatologists, physiotherapists, and dentists [7]. This pain can stimulate other conditions in the head and neck, so the diagnosis is very important to bring an adequate attention to patients with TMJ dysfunction [8].
2. Diagnostic of the myofascial pain syndrome
The main symptom of the patients with joint dysfunction is the pain and the most difficult to evaluate. The pain in the temporomandibular region could appear in different ways. An electric, burning, or searing type of pain refers to disorders of nervous of neuralgic type. The auriculotemporal nerve is the principal nerve of the TMJ. The compression of the auriculotemporal nerve may be due a disk displacement or an anatomical variation of the joint [8].
A deep, dull, irradiated, and not located pain frequently relates with a muscular disorder. The myalgia refers as a muscle band that suffered an inflammatory process (trigger point) that radiates the pain [9].
The main challenge for the clinician is to establish the origin of the pain. Detailing if the pain has a dental, articular, nervous, or muscular origin could be difficult. The anesthetic blockade is a useful tool to differentiate from a dental, neuralgic, or muscular pain [10].
The anesthetic blockade can be done for a tooth or a group of teeth on a regular basis so that you can exclude a possible dental cause.
The anesthetic blockade of the auriculotemporal nerve can be done by placing the needle behind the neck of the condylar process to the posterior aspect of the mandibular ramus. The patient can open and close the mouth, so you can feel the position of the needle on the bone and place 0.5 ml of 1% simple lidocaine [11].
The aim of the anesthetic blockade is to differentiate the articular pain such as capsulitis, synovitis, or any neuropathic pain of the myofascial pain (Figure 1).
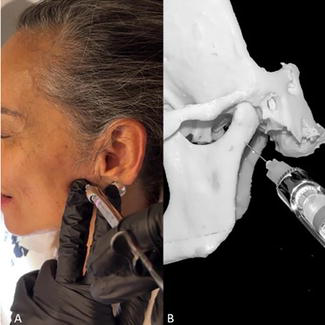
Figure 1.
A. Anesthetic block of the auriculotemporal nerve. B. Anatomical reference.
2.1 Medical history and e interrogatory
The information about the beginning and type of pain is fundamental to achieve an adequate diagnosis. The health questionnaire has to include questions addressed to systemic diseases that could be related to the myofascial pain or joint dysfunction. Because the TMJ is a synovial joint, the disorders that affect other joints could affect the TMJ. Interrogating the patient about another symptom of other joints of the body could relate an autoimmune affection or a rheumatoid systemic condition. The main illnesses that affect the joint system are fibromyalgia, rheumatoid arthritis, and others [2].
72% patients with joint dysfunction and myofascial pain can have ear symptomatology. It is important to investigate about symptoms such as vertigo, dizziness, sensation of plugged ear, tinnitus, or ear pain [12].
The pain is the main symptom that patients refer and has lots of presentations. A pain that appears in the morning or when they wake up could be related with bruxism. A pain that appears ending the day could be related to tensional conditions or stress. On many occasions, the psychological and emotional condition of the patients could affect the muscular system and the sleep cycle producing tensional states. Generally, mourning, unemployment, or anxious or depressive personal situations could trigger a muscular painful condition [13, 14].
2.2 Articular exam
A healthy temporomandibular joint has a mouth opening between 35 to 60 mm, but this measure can vary from age to age; movements of opening that are increased (hypermobility) could be related to an hyperlaxity of the ligaments, a flat and joint and a condilar luxation or subluxation. In contrast, a reduced opening (hypomobility) could be related to a degradation and entrapment of the articular disk or active inflammatory processes [15].
Deviation, clicking, and joint sounds can show a bone asymmetry with chronic deterioration and degenerative of the articular disk, but this is part of another chapter. However, the integral examination has to include palpation, auscultation, and the registration of deviations at the opening or closure of the mandible [16].
2.3 Muscular exam
The muscular exam of the head and neck must be exhaustive and as important as the others for the diagnosis of the myofascial pain.
The first step before the initial exam is to educate the patient in order to determine the level of pain. Under a visual analogue scale (VAS) of the pain, the patient can refer when the pain is mild (1–3 points), moderate (4–6 points), or severe (7–10 points) (Figure 2).
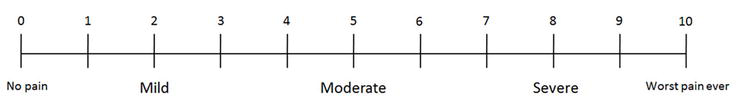
Figure 2.
Visual analog scale used for all of our patients.
The second step is to guide the patient to identify the most painful palpation zone, if the pain radiates behind another anatomical structure of the head or neck (e.g., eyes, periorbital area, frown, head, neck, back of the neck, shoulders, teeth, etc.).
The third step is to begin the exploration of the musculature of the head and neck in an orderly way. The professional stands behind the head of the patient and mak-ing a bilateral exploration. Attention must be paid to the gestures that the patient does when the palpation is applied. Many patients present a high threshold, even if they qualified the pain as mild; this could be moderate or severe (Figure 3). Some patients, even if they present a high threshold, express a palpebral reaction or a departure with severe pain [2].
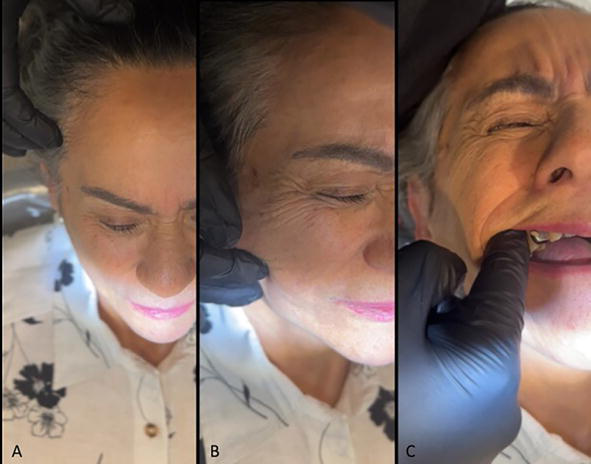
Figure 3.
Head and neck muscle examination: A. Temporal muscle. B. Masseter muscle. C. Lateral pterygoid muscle.
The palpation strength has to be steady, oriented, and local. Some muscles show multiple fascicles, and these must be palpated. It is needed an adequate anatomical study of the zone, to identify correctly the origin, insertion, and the layout of the fibers of every muscle. In this manner, we can identify the trigger points and the hypertrophic and symptomatic muscle bands.
Care should be taken to the blood vessels of the neck region, for not generating a vasopressor effect. In the intraoral region, the muscular band and the referred pain must be identified properly in order to differentiate neuropathic pain with radiate pain [9].
2.4 Intraoral exam
The intraoral exam consists of several points; it must include the dental analysis, demonstrating the health or illness of any dental organ, through a meticulous examination of the dental arches, dental position, and evaluation of Spee and Wilson curves. Percussion tests must be done with the suggesting dental organs to present any associated pathology.
Evaluating the dental position is important for the diagnosis, because it can guide the clinician to the existence of an imbalance between dental arches; this can be done with a previous interrogatory about the stability perceived from the patient when eating, swallowing, and so forth, with the colocation of dental paper, to verify the presence of any premature contact points and if there is any midline discrepancy or differences between the molar relationship [17].
As a complement to the muscular examination, in the intraoral examination, we must palpate the lateral pterygoid muscles. To achieve this, we must direct palpation towards the infratemporal fossa [2].
2.5 Imaging studies
The use of imaging studies is very useful to complete the diagnosis. The periapical radiographs are helpful to determine the origin of dental pain or any other pathologies. The cone-beam computed tomography scans and the panoramic radiographs can provide better details about the bone and joint structures of the TMJ, but not the articular disk (Figure 4). However, MRI could be considered as the best option to evaluate the articular disk; it allows to see the position, morphology, and degenerative changes of the bones [2].
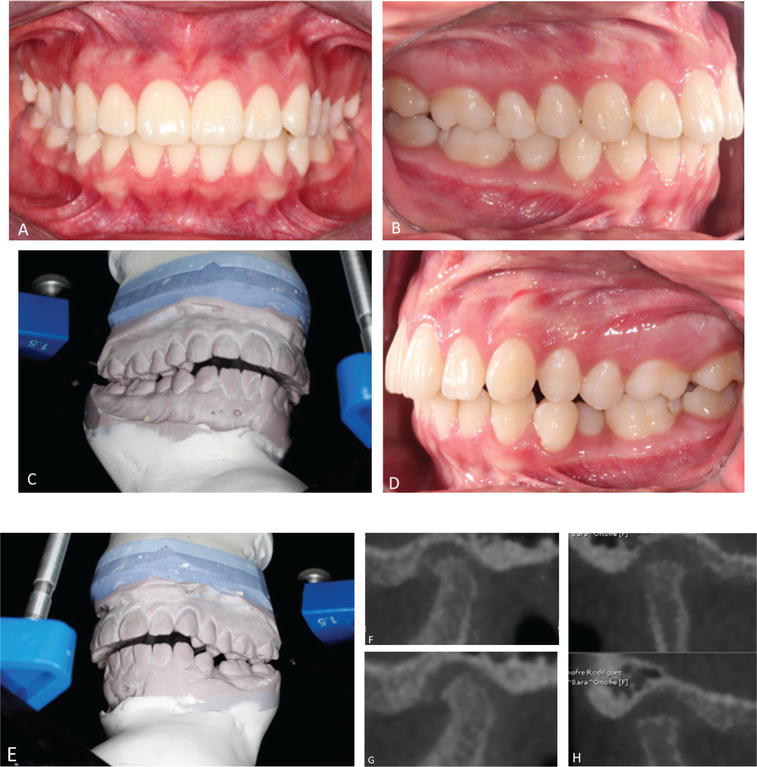
Figure 4.
A–E. Occlusion with diagnostic mounting. F–H. TC scan (note the irregularity of the cortical bone of the mandibular condyle).
3. Myofascial pain syndrome treatment
The MPS treatment is complex; there are different initiators or trigger factors. As already mentioned, MPS is composed of peripheral inflammatory processes with a huge psychosocial compound.
The treatment must have an integral and multidisciplinary focus. Particularly at a muscular level, the treatment must be addressed in the muscular trigger points, seeking to correct the structural and muscular imbalance. This can be done through different actions, such as pharmacologic strategies, physical therapy, and intraoral devices, among others [6, 18].
3.1 Pharmacological management
Because of the multifactorial nature of the myofascial pain syndrome (MPS), it can appear with an insidious, trauma, or injury onset [6]. There will be a release of inflammatory mediators that sensitize the peripheral nociceptors [19]. Actually, it can develop a “central sensitization” it consists of an amplified response of the CNS, characterized for a hyperexcitability of the neurons of the dorsal spinal horns, causing hypersensitivity and/or allodynia. This response is present in patients with MPS; that is why you should seek pharmacological treatment to reduce it [20].
3.1.1 Nonsteroidal anti-inflammatory drugs (NSAIDs)
These drugs inhibit the cyclooxygenase (COX) 1 and 2 blocking the prostaglandins synthesis; this reduces the sensitization and excitation of the peripheral nociceptors [21].
It is important to say COX-1 is present in most of the tissues; it participates in the formation of relevant prostaglandins (PG) to regulate the hemostasis, kidney integrity, platelet function, protection of gastric mucosa (PG12), and so on. Meanwhile, COX-2 is inducible especially in inflammatory processes [21, 22].
3.1.2 NSAIDs classification
The NSAIDs are divided in 3 groups depending on their selectivity to the inhibition of COX-2, such as Nonselective (Aspirin, Diflunisal, Ibuprofen, Ketoprofen, Naproxen, Meclofenamate, and Piroxicam), Semiselective or preferential to COX-2 (Diclofenac, Etodolac, and Meloxicam), and Highly Selective (Celecoxib, Etoricoxib, Lumiracoxib, Rofecoxib, and Valdecoxib) [23].
Also, these drugs can be classified depending on their chemical structure, such as derived from salicylic acid (acetylsalicylic, nonselective but very aggressive with gastric mucosa), aryl-propionic (Ibuprofen and naproxen, nonselective), acetic acid (diclofenac and ketorolac) and anthranilic acid (both are hematologically toxic), enolic acid or oxicams (piroxicam, tenoxicam, and meloxicam, the last one is preferential to COX-2), sulfonanilide (nimesulide, acts in the thermoregulatory center in the CNS), coxib (etoricoxib and celecoxib, highly selective to COX-2), and NSAIDs without inflammatory activity (acetaminophen and dipyrone), among others [22].
3.1.3 Adverse effects of NSAIDs and recommendations
It is important to be careful with the drug interactions, because they are responsible of multiple effects. It is not recommended to mix NSAIDs with any of these drugs: lithium (increases its toxicity), anticoagulants and/or alcohol (increases risk of GI bleeding), other NSAIDs (increase toxicity and produce kidney failure in the long-term), antihypertensive (Loop) and/or hypoglycemic drugs (decreases hypoglycemic effect), antihypertensive (Loop) and anticonvulsants drugs (increases toxicity of anticonvulsant), antihypertensive drugs such as diuretics or ACE inhibitors (decreases the effect of these drugs), and acetaminophen and alcohol (risk of hepatic damage in patients with previous hepatic damage) [24].
At the level of the gastrointestinal (GI) tract, NSAIDs can cause abdominal pain, nausea, vomit, hemorrhage, gastroduodenal ulcer; also, they can cause peripheral edema and hypertension (particularly if they are nonselective). In the long term, they are associated with congestive heart failure with risk of infarction, hepatic insufficiency, and kidney failure, so they are not recommended for prolonged periods [22, 24, 25].
Furthermore, NSAIDs have demonstrated hypersensitivity in a lot of patients, showing multiple clinical expressions such as pruritus, urticaria, bronchospasm, and anaphylaxis in patients with analgesic intolerance to this group of drugs [26].
Because of all the evidence previously mentioned, the use of selective to COX-2 NSAIDs is associated with less GI adverse effects, therefore these drugs result useful to prevent GI bleeding. However, it is suggested to use a proton bomb inhibitor (PBI) regardless of the NSAID, because to see anti-inflammatory effect, the treatment with NSAIDs must be of 2 weeks [23, 27].
In patients with GI bleeding risk, the use of Celecoxib 200 mg twice a day is recommended, for its low adverse effects, only when there are no cardiovascular risk factors, because of the cardiovascular toxicity of the highly selective to COX-2 NSAIDs [28].
In the group of nonselective NSAIDs, both naproxen and ibuprofen appear to be the safest drugs concerning cardiovascular effects. Moreover, the use of preferential NSAIDs such as meloxicam at a dose of 7.5–15 mg once daily for 2 to 4 weeks achieves pain relief, but it is temporary if the treatment is suspended [28].
Furthermore, Hsieh and cols. in a prospective, randomized, double-blind study of 153 patients with MPS of the trapezius muscle compared the use of topic diclofenac sodium (60 mg) with placebo, 3 times a day for 7 days, concluding it is useful for MPS decreasing the pain without adverse effects [29].
The authors of this chapter suggest individualizing the case, knowing the risks of each patient, and choosing the pharmacologic treatment consciously.
The use of naproxen or ibuprofen with gastric protectors can be fair, and the use of preferential or highly selective to COX-2 NSAIDs without cardiac risk is valid. A pharmacological therapy for 2 weeks could be enough to resolve or improve the clinical condition.
3.1.4 Analgesics
The acetaminophen (Paracetamol) is the most used analgesic; this drug has affinity for the COX-3 at CNS level and almost no affinity for COX-1 and COX-2 [30]. For this reason, an analgesic and antipyretic effect can be achieved, but minimal anti-inflammatory effect. The main advantage is that it acts in a different site from the NSAIDs; this allows enhancing the analgesic effects in addition when it is prescribed with NSAIDs.
Kurita and cols. in a triple-blind study, in 18 patients, compared the effectivity of the diclofenac sodium alone; together with acetaminophen, caffeine, and carisoprodol; and placebo for the treatment of temporomandibular disorders (TMD). They concluded that both diclofenac sodium alone and the group of drugs together reduced the pain until the third day of treatment [31], so acetaminophen in addition with other drugs could be beneficial.
3.1.5 Opioids
These drugs are weak agonists that bind their muscarinic, kappa, and delta receptors at a central level and other tissues getting a similar effect as well as endorphins, altering the pain perception and modulating the painful response of the CNS [32].
These drugs can be combined with other analgesics and NSAIDs staggering the pharmacological therapy in cases of severe pain where it cannot be treated with the previous options. Cigerim and cols. in a double-blind, randomized study of 169 patients with myofascial pain compared naproxen (550 mg) alone, with codeine (30 mg), with dexamethasone (8 mg, single dose), and acetaminophen (550 mg) alone. They concluded that naproxen sodium with codeine has the greatest analgesic effect at the first and fourth week of treatment, suggesting a synergic effect on the part of the codeine unto the naproxen [33]. Moore et al. in a systematic review in 2008 analyzed acetaminophen alone or with codeine (60 mg) for the management of the postoperative pain, showing it can produce pain relief even with a single dose [34].
Although the opioids have shown analgesic effectivity in the chronic musculoskeletal pain, they should be used with care because these drugs can cause abuse, dependence, and adverse effects such as depression of the CNS [35].
For the authors, the use of opioids should be reserved for the last instance in severe pain where multimodal therapy of peripheral action has not solved them.
3.1.6 Musculoskeletal relaxants
The musculoskeletal relaxants act on the CNS interrupting the nociceptive signal but are considered to act in the muscle trigger points [6, 36].
These drugs can be classified into neuromuscular blockers, antispasticity, antispasms, and nerve blockers. The most used are cyclobenzaprine, baclofen, tizanidine, carisoprodol, methocarbamol, and metaxalone, among others [27, 28]. Other type of drugs such as diazepam, Dantrolene and botulinum toxin, can also be mentioned [19].
The most studied musculoskeletal relaxant is cyclobenzaprine, showing analgesic efficacy in MPS with antispasmodic effect in the cervical region; that is why it is recommended as the first treatment option for myogenous TMD. It also has shown better effect with 10 mg than 0.5 mg of clonazepam [6, 23].
Some authors recommend pharmacological treatment with muscle relaxants until 30 days [23, 28]. However, for the authors of this chapter, 2 weeks of musculoskeletal relaxant and multimodal pharmacological therapy are enough to evaluate an improvement of the clinical condition.
The use of ibuprofen with codeine or with cyclobenzaprine has been studied, but has not shown better analgesia and does not even decrease muscle spasms, so it is not recommended as a therapeutic [37, 38].
Also, tizanidine (36 mg daily) has shown pain relief, improvement of sleep, and antispasmodic effect after 5 weeks of treatment for MPS, with doses of 36 mg daily (but lower doses can be used with success) [6].
In addition, Clonazepam (benzodiazepine) as a therapeutic propose acts in the chloride channels increasing the GABA-A receptors; this achieves an inhibition of the pre- and postsynaptic sites on the spinal cord. Even the use of muscle relaxants has therapeutic utility; the depressant effect of CNS can appear [35]. That is why it is important to individualize each therapy and start the treatment with minimal doses and escalate them as required.
3.1.7 Central action pain modulators
In this group are commonly antidepressants, anticonvulsants, and benzodiazepines, among others.
The most common antidepressants are tricyclic antidepressants (TCAs) and serotonin and norepinephrine reuptake inhibitors such as amitriptyline, nortriptyline, duloxetine, and venlafaxine [27].
The gabapentin has shown to be effective for pain relief in myogenous TMD at doses of 300 mg daily, increasing the dose every third day, without exceeding the maximum dose of 4200 mg a day, showing decrease in pain spontaneously at 8 weeks of treatment [27].
Kimos and cols. Evaluated 50 women with chronic masticatory myalgia, comparing the effectivity of gabapentin (300 mg initially) with placebo, concluding that gabapentin was effective for musculoskeletal disorders, suggesting a superiority to the TCAs due to a lower drug interaction [39].
The benzodiazepines have demonstrated good effectiveness for pain. In combined therapy with NSAID, the effect increases. Therefore, benzodiazepines are recommended as a second line and not as initial therapy [27].
Based on this evidence, for the authors, it is convenient to carry out a multimodal treatment for pain management and muscular affection. With the use of drugs with direct effect in the: inflammation, pain, muscle spasm as well as the management of chronic pain at a central level. However, everything depends of the severity of the pain, the individual state of each patient, and the possible pharmacokinetics and pharmacodynamics interactions that may occur. Keep in mind that the MPS affects in its majority the elderly population. For this reason, patients tend to have systemic affections and pharmacologic therapies for those conditions, to consider when to choose a multimodal pharmacological therapy.
4. Physiotherapy
Physiotherapy can be done by the patient at home or by a professional physiotherapist. Myofascial pain can be caused by an excessive functional muscular load, tensional issues (e.g. family problems), bruxism, long dental visit, history of trauma, among others muscles triggers. As a result, we always have to decrease the masticatory function when treating myofascial pain. Physiotherapy carried out in a rehabilitation center or by a physiotherapist for the MPS management is shown in Table 1 [20].
| Physiotherapy at home | Physiotherapy at a rehabilitation center |
|---|---|
|
|
Table 1.
The physical therapy (or manual) refers to the mobilization of the TMJ, soft tissues of the muscles involved, active and passive movements, gentle isometric tension with resistance, and guided mandibular movements [40].
It is also recommended that a specialist (physiotherapist) performs it, and so do the patient (at home) getting better results [41].
Furthermore, when compared with manual therapy, pain relief is achieved for MPS, followed by dry needling [42].
Extracorporeal shockwave therapy has shown to reduce P substance on the application area and a relief in patients with pain in lower back who undergo this type of therapy [43].
In the case of MPS of the trapezius muscle, it can be treated with extracorporeal shockwave therapy with 6 weekly sessions (1000 pulsations each one). Also, there are reports of similar effects with dry needling, laser, infiltration of trigger points, and so on [43].
Ahi and Sirzai realized a randomized, blind, controlled study in 108 patients with MPS of the neck and upper back; they compared high-intensity laser therapy with dry needling, both together with physical exercise, concluding that both therapies where effective for the MPS management [44].
Regarding to electrode therapy (TENS), Johnson and cols. Conducted a systematic review about the use in patients with any pain condition. TENS showed pain relief during or at the end of each session [45]. Kato and cols. as well in a randomized, controlled study in 18 patients with myogenous TMD compared the use of TENS (with low level pulse of 1.5 sec each one) and laser therapy (of 830–904 nm with power of 100 mW), concluding both therapies can reduce the symptoms of TMD significantly [46].
Celakil and cols. observed favorable results with ozone therapy for TMD management in a prospective, double-blind, randomized study in 40 patients with chronic masticatory muscle pain. They compared the therapy with bio-oxidative ozone (intensity of 60%) placed on the masticatory muscles and placebo, both 3 times a week for 2 weeks. They concluded that ozone therapy can decrease the pain and improve the mandibular function in myogenous TMD [47].
5. Botulinum toxin
The botulinum toxin (BTX) is produced by the clostridium botulinum bacteria; the action mechanism consists in the inhibition of the acetylcholine on the neuromuscular union, causing muscular paresia. It has also been seen that BTX can act at a CNS level with the central endogenous opioid system, reducing the inflammatory response, being type A the most used for TMD (Botox, Xeomin, and Dysport) [27]. Under this mechanism, the striated muscle is forced to rest of the muscle and masticatory function. Although BTX is widely used for cervical dystonia, overactivity of the bladder, and chronic migraine, there is no consensus for its use for TMD [48].
Systematic reviews about the use of BTX in TMD have been conducted; however, the irregular evidence does not allow to reach a conclusion, even though it has been seen to improve the outcome with conservative treatment [48, 49].
The use of BTX in muscular hypertrophy cases has shown a good response, decreasing the painful symptomatology, getting from high levels of pain (8.2 VAS) to minimal levels (1.8 VAS) after treatment [50].
Some authors recommend the use of 50 IU of BTX initially with 12 week intervals between each infiltration and after a few cycles; BTX can be administered every 6 months reducing the possibility of presenting adverse effects such as facial asymmetry or muscular weakness [51].
For other authors, the application of BTX is indicated only when the patient’s condition has not been resolved at all with pharmacologic and physiotherapeutic therapy after 3 weeks of treatment [52].
5.1 BTX application protocol
The first step is to realize an anatomical marking of the zone to identify the affected muscle band and its anatomical relations. These marks are realized exploring the muscle contraction at opening, closing, and maximum intercuspation [53].
The second step is the dilution of the BTX as the supplier recommends [54]. Some suppliers feature diluted BTX, and others need to be diluted in injectable water. The marking of the syringe at the units per ml of solution allows us to quantify the amount of BTX that is going to be injected to each muscle group.
The third step is to perform the asepsis and antisepsis of the area and inject the amount of the desired drug for each muscular condition and for every muscle. For the skin areas, simply perform asepsis with alcohol solution or topical antiseptic. For the intraoral injections, it must be rinsed with chlorhexidine or any other antiseptic solution for oral use (Figure 5).
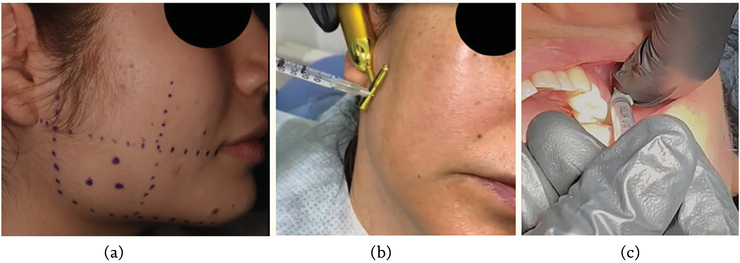
Figure 5.
A, B. Marks on masseterine region and infiltration of the masseter muscle. C. Intraoral infiltration of lateral pterygoid muscle.
6. Intraoral devices
An occlusal splint is a deprogrammer or a mandibular repositioner to establish the ideal relation between maxilla and mandible, relieving pain and giving correct function. According to the American Academy of Orofacial Pain, the purpose of this device is to give occlusal stability, protect the teeth, redistribute the occlusal forces, relax the muscles, and reduce bruxism [55].
A poorly designed occlusal device or with the wrong material could increase the muscle activity and thus increase the myofascial pain in the patient.
The intraoral devices must meet certain conditions so that the treatment and the orofacial pain control can be effective.
6.1 Basic conditions of efficacy
Condylar Stability
Guard Design
Permanent Action
Correct and Timely Adjustments of the Guard
6.2 Condylar stability
The intraoral device must be designed considering the dental contact and the stability at a condylar level. Patients with myofascial pain frequently have an important level of temporomandibular dysfunction and dental alterations. All these situations can cause the patient to present a “double occlusion.” This condition refers to a forced occlusion where the patient feels more comfortable but can be prejudicial for the joint and other anatomical structures [56].
A correct design of the intraoral device will provide the patient the necessary occlusal stability to not having to look for a new mandibular position, so we will eliminate that “double” occlusion [57].
6.3 Guard ydesign
The elaboration of the intraoral device requires an articulator mounting. Through the facial arch, we will determine the position of the maxillary occlusal plane and thus find the appropriate maxilla–mandible relation for the patient. A registration must be made with interoclusal wax without teeth contact, to ensure the elimination of the proprioception with the selected technique by the operator seeking to establish the condyles in the most stable possible position [58].
The diagnostic mounting allows realizing a detailed analysis of the occlusion, determining the existence of any fulcrum that could cause any sort of pry over the TMJ, as well as interferences on the mandibular excursion. This same diagnostic mounting could be used for the design of the intraoral device; we could determine the thickness of it by separating the first point of contact by 2–3 mm; after that, we will try to simulate with the splint a perfect functional occlusion giving what is known as “mutually protected occlusion.” This can be achieved by having uniform contacts and with the same intensity in every work cusp of the opposite arch and a slight disocclusion of a thousandth of an inch in the anterior teeth. The anterior ramp of the intraoral device should have an inclination of 45° approximately, which gives immediate posterior disocclusion in any mandibular movement (laterality and protrusion) [58].
The treatment with splint should be considered as a permanent action in order to get the desired results; that is why patient cooperation is indispensable. The splint should stay in the mouth 24 hours a day, 7 days of the week, so patients should perform all their activities with the splint. The splint can only be retired after each meal in order to perform the dental and guard hygiene [59].
6.4 Correct and timely adjustments of the guard
The treatment with the splint must have follow-up for several months, at least 3 to 4 months, in which the splint will be checked, verifying the permanence of the ideal simultaneous dental contacts and with the same intensity. At the beginning, those revisions should be done every week, posteriorly fortnightly; however, when we will get stability, they could be monthly [58]. If the adjustments are not performed, which consists in the wear of possible interferences or premature points in the acrylic, the patient could experience periods of incommodity or even an increase of the pain. That is why adequate adjustments are important.
6.5 Clinical case
We present a case of a female patient of 15 years old, who presents bilateral clicking and pain on the masseter muscle, in the left ear, neck, upper back, and shoulders. We carry out: CT scan of both mandibular condyles (with signs of active degenerative process), diagnostic mounting of casts (it was observed the change of occlusion from Class I to Class II with a considerably increased projection) (Figure 4).
The gnathologic guard is done with multiple contacts and with anterior guide (Figure 6). Achieving favorable outcome as all the symptoms disappear after 2 months of treatment without needing of other alternative therapies (e.g., pharmacologic). The tomography showed that the degenerative process stopped and achieved recorticalization of the condyles and articular eminence (Figure 7).
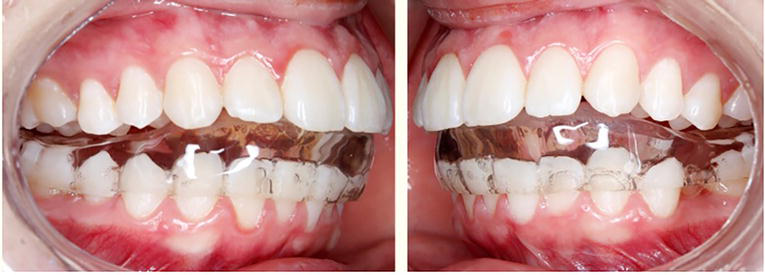
Figure 6.
A, B. Lower gnathologic guard.
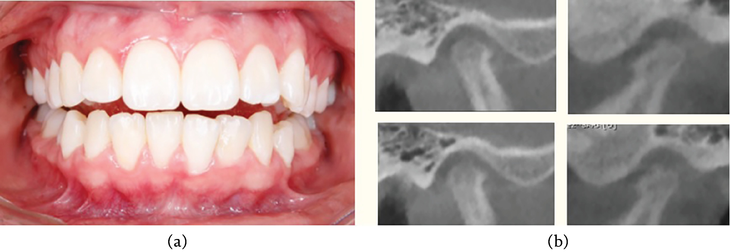
Figure 7.
A. Posttreatment occlusion. B–E. TC scan after 8 months of treatment. Note the corticalization of the condylar processes and the real malocclusion ready to be treated with surgical-orthodontic treatment.
References
- 1.
Alomar X, Medrano J, Cabratosa J, Clavero JA, Lorente M, Serra I, et al. Anatomy of the temporomandibular joint. Seminars in Ultrasound, CT, and MR. 2007; 28 (3):170-183 - 2.
Liu F, Steinkeler A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dental Clinics of North America. 2013; 57 (3):465-479 - 3.
Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. Journal of Dental Research. 2008; 87 (4):296-307 - 4.
Hisatomi M, Munhoz L, Asaumi J, Arita ES. Parotid mandibular bone defect: A case report emphasizing imaging features in plain radiographs and magnetic resonance imaging. Imaging Science in Dentistry. 2017; 47 (4):269-273 - 5.
Ohrbach R, Slade GD, Bair E, Rathnayaka N, Diatchenko L, Greenspan JD, et al. Premorbid and concurrent predictors of TMD onset and persistence. European Journal of Pain. 2020; 24 (1):145-158 - 6.
Borg-Stein J, Iaccarino MA. Myofascial pain syndrome treatments. Physical Medicine and Rehabilitation Clinics of North America. 2014; 25 (2):357-374 - 7.
Tran C, Ghahreman K, Huppa C, Gallagher JE. Management of temporomandibular disorders: A rapid review of systematic reviews and guidelines. International Journal of Oral and Maxillofacial Surgery. 2022; 51 (9):1211-1225 - 8.
Lomas J, Gurgenci T, Jackson C, Campbell D. Temporomandibular dysfunction. Australian Journal of General Practice. 2018; 47 (4):212-215 - 9.
Chan NHY, Ip CK, Li DTS, Leung YY. Diagnosis and treatment of Myogenous temporomandibular disorders: A clinical update. Diagnostics (Basel). 2022; 12 (12):2914 - 10.
Nascimento MM, Vasconcelos BC, Porto GG, Ferdinanda G, Nogueira CM, Raimundo RD. Physical therapy and anesthetic blockage for treating temporomandibular disorders: A clinical trial. Medicina Oral, Patología Oral y Cirugía Bucal. 2013; 18 (1):e81-e85 - 11.
Donlon WC, Truta MP, Eversole LR. A modified auriculotemporal nerve block for regional anesthesia of the temporomandibular joint. Journal of Oral and Maxillofacial Surgery. 1984; 42 (8):544-545 - 12.
Mejersjö C, Pauli N. Ear symptoms in patients with orofacial pain and dysfunction—An explorative study on different TMD symptoms, occlusion and habits. Cllinical and Experimental Dental Research. 2021; 7 (6):1167-1174 - 13.
Vlăduțu D, Popescu SM, Mercuț R, Ionescu M, Scrieciu M, Glodeanu AD, et al. Associations between bruxism, stress, and manifestations of temporomandibular disorder in young students. International Journal of Environmental Research and Public Health. 2022; 19 (9):5415 - 14.
Fernandes G, Franco AL, Siqueira JT, Gonçalves DA, Camparis CM. Sleep bruxism increases the risk for painful temporomandibular disorder, depression and non-specific physical symptoms. Journal of Oral Rehabilitation. 2012; 39 (7):538-544 - 15.
Dhanrajani PJ, Jonaidel O. Trismus: Aetiology, differential diagnosis and treatment. Dental Update. 2002; 29 (2):88-94 - 16.
Taşkaya-Yilmaz N, Oğütcen-Toller M. Magnetic resonance imaging evaluation of temporomandibular joint disc deformities in relation to type of disc displacement. Journal of Oral and Maxillofacial Surgery. 2001; 59 (8):860-865 discussion 865-866 - 17.
Fushima K, Inui M, Sato S. Dental asymmetry in temporomandibular disorders. Journal of Oral Rehabilitation. 1999; 26 (9):752-756 - 18.
Ivan Urits I, Charipova K, Gress K, Schaaf AL, Gupta S, Kiernan HC, et al. Treatment and management of myofascial pain syndrome. Best Practice & Research Clinical Anaesthesiology. 2020; 34 (3):427-448 - 19.
Heir GM. The efficacy of pharmacologic treatment of temporomandibular disorders. Oral and Maxillofacial Surgery Clinics of North America. 2018; 30 (3):279-285 - 20.
Ferrillo M, Giudice A, Marotta N, Fortunato F, Di Venere D, Ammendolia A, et al. Pain management and rehabilitation for central sensitization in temporomandibular disorders: A comprehensive review. International Journal of Molecular Sciences. 2022; 23 (20):12164 - 21.
García-Rayado G, Navarro M, Lanas A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert Review of Clinical Pharmacology. 2018; 11 (10):1031-1043 - 22.
Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2012; 11 (1):52-64 - 23.
Hersh EV, Balasubramaniam R, Pinto A. Pharmacologic management of temporomandibular disorders. Oral and Maxillofacial Surgery Clinics of North America. 2008; 20 (2):197-210 - 24.
Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clinical Therapeutics. 2007; 29 (Suppl):2477-2497 - 25.
Grosser T, Smith EM, FitzGerald GA. Pharmacotherapy of inflammation, fever, pain, and gout. In: Brunton LL, Hilal R, Knollmann BC, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. USA: McGraw Hill Education; 2019. pp. 673-684 - 26.
Celik G, Erkekol FO, Bavbek S, Dursun B, Misirligil Z. Long-term use and tolerability of cyclooxygenase-2 inhibitors in patients with analgesic intolerance. Annals of Allergy, Asthma & Immunology. 2005; 95 (1):33-37 - 27.
Andre A, Kang J, Dym H. Pharmacologic treatment for temporomandibular and temporomandibular joint disorders. Oral and Maxillofacial Surgery Clinics of North America. 2022; 34 (1):49-59 - 28.
Ouanounou A, Goldberg M, Haas DA. Pharmacotherapy in temporomandibular disorders: A review. Journal of the Canadian Dental Association. 2017; 83 :h7 - 29.
Hsieh LF, Hong CZ, Chern SH, Chen CC. Efficacy and side effects of diclofenac patch in treatment of patients with myofascial pain syndrome of the upper trapezius. Journal of Pain and Symptom Management. 2010; 39 (1):116-125 - 30.
Botting R, Ayoub SS. COX-3 and the mechanism of action of paracetamol/acetaminophen. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2005; 72 (2):85-87 - 31.
Kurita Varoli F, Sucena Pita M, Sato S, Issa JP, do Nascimento C, Pedrazzi V. Analgesia evaluation of 2 NSAID drugs as adjuvant in management of chronic temporomandibular disorders. Scientific World Journal. 2015; 2015 :359152 - 32.
Inturrisi CE. Clinical pharmacology of opioids for pain. The Clinical Journal of Pain. 2002; 18 (4 Suppl):S3-S13 - 33.
Cigerim L, Kaplan V. Analgesic efficacy of naproxen-codeine, naproxen+dexamethasone, and naproxen on myofascial pain: A randomized double-blind controlled trial. Cranio. 2023; 41 (2):119-125 - 34.
Moore A, Collins S, Carroll D, McQuay H, Edwards J. Single dose paracetamol (acetaminophen), with and without codeine, for postoperative pain. Cochrane Database of Systematic Review. 2000;(4). Article ID: CD001547. DOI: 10.1002/14651858.CD001547 - 35.
Galasso A, Urits I, An D, Nguyen D, Borchart M, Yazdi C, et al. A comprehensive review of the treatment and management of myofascial pain syndrome. Current Pain and Headache Reports. 2020; 24 (8):43 - 36.
Desai MJ, Bean MC, Heckman TW, Jayaseelan D, Moats N, Nava A. Treatment of myofascial pain. Pain Management. 2013; 3 (1):67-79 - 37.
Turturro MA, Frater CR, D'Amico FJ. Cyclobenzaprine with ibuprofen versus ibuprofen alone in acute myofascial strain: A randomized, double-blind clinical trial. Annals of Emergency Medicine. 2003; 41 (6):818-826 - 38.
Childers MK, Borenstein D, Brown RL, Gershon S, Hale ME, Petri M, et al. Low-dose cyclobenzaprine versus combination therapy with ibuprofen for acute neck or back pain with muscle spasm: A randomized trial. Current Medical Research and Opinion. 2005; 21 (9):1485-1493 - 39.
Kimos P, Biggs C, Mah J, Heo G, Rashiq S, Thie NM, et al. Analgesic action of gabapentin on chronic pain in the masticatory muscles: A randomized controlled trial. Pain. 2007; 127 (1-2):151-160 - 40.
de Melo LA, Bezerra de Medeiros AK, Campos MFTP, Machado B, de Resende CM, Barbosa GAS, et al. Manual therapy in the treatment of myofascial pain related to temporomandibular disorders: A systematic review. Journal of Oral & Facial Pain and Headache. 2020; 34 (2):141-148 - 41.
Tuncer AB, Ergun N, Tuncer AH, Karahan S. Effectiveness of manual therapy and home physical therapy in patients with temporomandibular disorders: A randomized controlled trial. Journal of Bodywork and Movement Therapies. 2013; 17 (3):302-308 - 42.
Menéndez-Torre Á, Pintado- Zugasti AM, Zaldivar JNC, García-Bermejo P, Gómez-Costa D, Molina-Álvarez M, et al. Effectiveness of deep dry needling versus manual therapy in the treatment of myofascial temporomandibular disorders: A systematic review and network meta-analysis. Chiropractic & Manual Therapies. 2023; 31 (1):46 - 43.
De la Corte-Rodríguez H, Román-Belmonte JM, Rodríguez- Damiani BA, Vázquez-Sasot A, Rodríguez-Merchán EC. Extracorporeal shock wave therapy for the treatment of musculoskeletal pain: A narrative review. Healthcare (Basel). 2023; 11 (21):2830 - 44.
Ahi ED, Sirzai H. Comparison of the effectiveness of dry needling and high-intensity laser therapy in the treatment of myofascial pain syndrome: A randomized single-blind controlled study. Lasers in Medical Science. 2022; 38 (1):3 - 45.
Johnson MI, Paley CA, Jones G, Mulvey MR, Wittkopf PG. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: A systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open. 2022; 12 (2):e051073 - 46.
Kato MT, Kogawa EM, Santos CN, Conti PC. TENS and low-level laser therapy in the management of temporomandibular disorders. Journal of Applied Oral Science. 2006; 14 (2):130-135 - 47.
Celakil T, Muric A, Gokcen Roehlig B, Evlioglu G, Keskin H. Effect of high-frequency bio-oxidative ozone therapy for masticatory muscle pain: A double-blind randomised clinical trial. Journal of Oral Rehabilitation. 2017; 44 (6):442-451 - 48.
Thambar S, Kulkarni S, Armstrong S, Nikolarakos D. Botulinum toxin in the management of temporomandibular disorders: A systematic review. The British Journal of Oral & Maxillofacial Surgery. 2020; 58 (5):508-519 - 49.
Gil-Martínez A, Paris-Alemany A, López-de-Uralde-Villanueva I, La Touche R. Management of pain in patients with temporomandibular disorder (TMD): Challenges and solutions. Journal of Pain Research. 2018; 11 :571-587 - 50.
Farrier JN, Farrier S, Haworth S, Beech AN. Can we justify the continued use of botulinum toxin A in the management of myofascial pain? The British Journal of Oral & Maxillofacial Surgery. 2020; 58 (9):1133-1138 - 51.
González MF, Miranda LM, Malagón HH, González AV. Use of botulinum toxin for treatment of hypertrophy of the masseter muscle. Cirugía Plástica Ibero-Latinoamericana. 2012; 38 (3):297-302 - 52.
Yoshida K. Effects of botulinum toxin type a on pain among trigeminal neuralgia, myofascial temporomandibular disorders, and oromandibular dystonia. Toxins. 2021; 13 :605 - 53.
Serrera-Figallo MA, Ruiz-de-León-Hernández G, Torres-Lagares D, Castro-Araya A, Torres-Ferrerosa O, Hernández-Pacheco E, et al. Use of botulinum toxin in orofacial clinical practice. Toxins (Basel). 2020; 12 (2):112 - 54.
Kandhari R, Kaur I, Gupta J, Al-Niaimi F. Microdroplet botulinum toxin: A review. Journal of Cutaneous and Aesthetic Surgery. 2022; 15 (2):101-107 - 55.
Albagieh H, Alomran I, Binakresh A, Alhatarisha N, Almeteb M, Khalaf Y, et al. Occlusal splints-types and effectiveness in temporomandibular disorder management. The Saudi Dental Journal. 2023; 35 (1):70-79 - 56.
Ahmed MMS, Shi D, Al-Somairi MAA, Alhashimi N, Almashraqi AA, Musa M, et al. Three dimensional evaluation of the skeletal and temporomandibular joint changes following stabilization splint therapy in patients with temporomandibular joint disorders and mandibular deviation: A retrospective study. BMC Oral Health. 2023; 23 (1):18 - 57.
Al-Moraissi EA, Farea R, Qasem KA, Al-Wadeai MS, Al-Sabahi ME, Al-Iryani GM. Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: Network meta-analysis of randomized controlled trials. International Journal of Oral and Maxillofacial Surgery. 2020; 49 (8):1042-1056 - 58.
Lundeen TF. Occlusal splint fabrication. The Journal of Prosthetic Dentistry. 1979; 42 (5):588-591 - 59.
Matsumoto H, Tsukiyama Y, Kuwatsuru R, Koyano K. The effect of intermittent use of occlusal splint devices on sleep bruxism: A 4-week observation with a portable electromyographic recording device. Journal of Oral Rehabilitation. 2015; 42 (4):251-258