Binding energy of compounds docked into tubulin beta protein.
Abstract
Oxidative stress is an imbalance between oxidation and antioxidant responses caused by the accumulation of free radicals in the body. Cells produce free radicals that cause oxidative damage such as aging, atherosclerosis, arthritis, cancer, and neurological diseases. Antioxidants are substances that can neutralize free radicals, thereby reducing the damage to the body. Antioxidants are classified as synthetic and natural antioxidants. Natural products, mainly extracted from some medicinal plants, have strong antioxidant activity and low toxicity and side effects. Moreover, antioxidant activities are also detected in probiotics. Therefore, this chapter summarized the roles of antioxidants and then suggested antioxidants from natural sources have good prospects in the prevention and treatment of various diseases related to oxidative stress. In this study, antioxidant activities were mentioned in plants such as Basella alba, Cistanche sp., and representative probiotics such as Bifidobacterium breve ATCC 15700, Lactobacillus rhamnosus PN04, and Lactococcus lactis PN05 which could serve in DNA damage protection and cytotoxicity oriented for cancer treatment. With the results obtained in these medicinal herbs and probiotics, the antioxidant activities of more and more medicinal herbs and probiotics should be exploited in health care and pharmaceutical product development.
Keywords
- antioxidant activities
- medicinal herbs
- probiotics
- DNA damage protection
- cytotoxicity
- cancer
1. Introduction
Cells maintain reactive oxygen species (ROS) homeostasis through various systems, such as the superoxide dismutase (SOD) system, catalase (CAT) system, and glutathione peroxidase (GSH-Px) system [1]. Antioxidants are a group of substances that help trap and neutralize free radicals, thereby reducing the damage to the body caused by free radicals. Additionally, antioxidants play a protective role against several diseases including inflammation and cancer caused by oxidative stress [2]. Antioxidants are classified as synthetic antioxidants and general natural antioxidants. However, synthetic antioxidants sometimes have toxicity and side effects, especially with the long-term use [3]. The exploration of natural antioxidants that are safe and free of side effects has become a hot spot in the past few years. Some natural products, mainly extracted from some medicinal plants and microorganisms, have strong antioxidant activity and low toxicity and side effects [3]. For this reason, antioxidants from natural sources have good application prospects in the prevention and treatment of various diseases related to oxidative stress. Due to reducing the chemical drugs’ negative effects, herbal medicines have gained their attractiveness in recent years. The natural sources contain vast components having antioxidant activities which are essential in cancer prevention, damaged DNA protection, and preventing lipid peroxidation [4]. For example, phenylethanoid glycosides (PhGs) in medicinal herbs act as a hydrogen donor to reductive radicals, allowing them to scavenge free radicals and regulate antioxidant enzymes involved in in vivo free radical metabolism [5].
Oxidation affects humans causing many diseases. Importantly, DNA is easy to spoil, leading to health problems in humans. DNA, the genetic material of all living organisms on the planet, is often influenced by a variety of variables, including exogenous and endogenous agents, which may alter its activity inside cells and ultimately lead to dramatic changes in living animals. The main exogenous factors can include UV light, ionizing radiation, toxic chemicals, and so on, whereas the primary endogenous factor is commonly caused by ROS [6]. The endogenous agents include superoxide anion, hydroxyl (OH) radicals, and hydrogen peroxide radicals. Furthermore, endogenous agent-caused damage occurs more often than exogenous agent-caused harm. If the damage is not addressed or corrected, it may lead to mutations and other health issues including aging, cancer, diabetes, reperfusion, cataracts, and lung failure.
Because of these, the therapeutic approach to alternative conventional medicine has expanded, such that increased research focused on the use of natural metabolites produced by medicinal herbs and lactic acid bacteria.
2. Roles of antioxidants
Life needs oxygen element for many biological activities. When oxygen damages the cells, oxidation occurs. Cells use oxygen to produce energy and free radicals. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are the by-products produced by cellular redox processes. These reactive species play dual roles in humans toxically and beneficially. At low or moderate levels, reactive species have beneficial effects on cellular and immune redox signaling function; however, high concentrations cause oxidative stress which can produce harmful substances, leading to spoiling cell function and structure. The toxic properties may be free radicals [6]. Free radicals play a role in the aging process. Free radicals cause oxidative stress, involving cardiovascular, neurological, and other human disorders. Antioxidants are our important defense against free radicals. ROS species such as superoxide, hydroxyl, and hydrogen peroxide are equivalent to the reduced oxygen levels. Oxidation stress is due to unhealthy lifestyles, exposure to chemicals, pollution, smoking, drugs, illness and sol. Antioxidants from plants, animals, and mineral sources have been shown to be beneficial to human health and effectively reduce the incidence of free radicals. Antioxidants are also involved in reducing free radicals, improving the normal function, and treating those diseases. Low levels of antioxidants or low levels of antioxidants in the blood increase the risk of diseases such as cancer. So, antioxidants in a healthy diet and natural antioxidants are now being recognized for protecting health from oxidative stress. Oxidative stress is through four important steps including membrane lipid peroxidation, protein oxidation, DNA damage, and disorders in reduction cellular equivalent, leading to cell destruction and changes in signaling pathways. Consequently, these mechanisms are implicated in the pathogenesis of diseases such as mitochondrial diseases in which cells cannot produce energy, leading to death [7]. Every biological molecule presenting in our body is at risk of free radicals. Such damaged cellular molecules may impair cellular function or may result in cell death. Lipids in the membranes of subcellular organelles are very susceptible to free radical damage. Lipid peroxidation leads to the generation of large amounts of toxic substances. Lipid peroxidation in cell membranes can damage the cell membranes by disrupting fluidity and permeability. Lipid peroxidation begins by removing hydrogen atoms from the methylene group, leading to the formation of a single electron on the carbon atom or carbon radical. Proteins are also susceptible to free radicals. Free radicals cause the destruction of many proteins and loss of enzyme activities. Protein hydroperoxide can be generated by ROS/RNS-induced protein oxidation, forming radicals. Although most oxidized proteins are essentially nonfunctional and eliminated quickly, some may accumulate gradually over time and thus contribute to aging-related damage as well as various diseases [5].
3. Antioxidant activities in DNA damage protection and cytotoxicity
3.1 Medicinal herbs
3.1.1 Phytochemicals served as antioxidants
Antioxidants are found in many vegetables, fruits, grains, tea, legumes, nuts, herbs and so on. Antioxidants may provide protection against diseases caused by free radicals. Phytochemicals or plant components are the main sources of antioxidants. The majority of these phytochemicals are redox-active molecules; therefore, they work to maintain a redox balance that is defined as an antioxidant. Phytochemicals are often classified as primary or secondary metabolites. The primary metabolites are sugars, amino acids, proteins, purines, and pyrimidines of nucleic acids, chlorophyll, etc., while secondary components include alkaloids (derived from amino acids), terpenes (a group of lipids), and phenolics (derived from carbohydrates). Plants produce an extremely impressive range of antioxidant compounds such as carotenoids, flavonoids, cinnamic acid, benzoic acid, folic acid, ascorbic acid, tocopherols, and tocotrienols to prevent oxidation of sensitive substrates. The plant-based antioxidants are believed to have better biological effects than synthetic ones because the plant components are part of the physiological processes of living flora, and thus, they are believed to have better compatibility with the human body. In this chapter, the plant extracts were studied for antioxidant activities. These plants have been used as daily vegetables in Vietnam.
3.1.2 Representative medicinal herbs
3.1.2.1 Basella alba (B. alba )
To clarify the roles of antioxidants, the study focused on the daily vegetable that can be used as a medicinal herb named

Figure 1.
3.1.2.2 Cistanches sp.
Cistanches belong to the family Orobanchaceae, and based on different chemical positions and genetic variations, the accepted genus
Figure 2.
They play a pivotal role in strong antioxidant effects in animal models and cell cultures. For instance, ginsenoside Rb1 exerts its antioxidant activity through two major mechanisms: chemical scavenging of certain types of ROS and a receptor-mediated genomic effect on antioxidant protein expression, such as SOD [13]. Secondarily, ginseng saponins have been shown to stimulate the formation of blood vessels and improve blood circulation in the brain, thereby enhancing memory and cognitive ability [14]. Additionally, ginsenoside Rg1 inhibits platelet activation via the inhibition of the ERK pathway and attenuates arterial thrombus formation
Of all the flavonoids, apigenin (4′,5,7-trihydroxyflavone) is one of the most widely distributed flavonoids in the plant kingdom, and one of the most studied phenolics [16]. Apigenin can be found in significant amounts in vegetables, herbs, plants, and plant-based beverages. Like other flavonoids, apigenin reported numerous functional activities such as antioxidant properties [17], anti-hyperglycemic [18], anti-inflammatory, and (in myocardial ischemia) anti-apoptotic effects [19]. Therefore, the various biological activities of apigenin and several methods of extraction from natural sources, or also with the use of modern extractive approaches, are further analyzed.
ROS/RNS interferes with DNA, leading to oxidative damage. DNA is vulnerable to free radicals such as the hydroxyl group (∙OH). The C4-C5 double bond of pyrimidine is very sensitive to hydroxyl group attack, which generates a series of oxidative pyrimidine destruction products, such as thymine glycol, uracil glycol, urea residue, 5-hydroxydeoxyuridine, 5-hydroxydeoxycytidine, hydantoin, and others. Likewise, purines are also susceptible to the hydroxyl group, leading to the formation of 8-hydroxydeoxyguanosine (8-OHdG), 8-hydroxydeoxyadenosine, formamidopyrimidines, and purine products of oxidation. Free radical attack also caused the activation of the enzyme poly(ADP-ribose) synthetase; thus, DNA fragmentation and programmed cell death occur. This process is due to exhausting NAD+ levels in the cells, thereby disrupting the electron transport chain function. Free radicals such as the hydroxyl group react with carbohydrates. Plant extracts gave antioxidant activities. Based on this activity, the study evaluated the ability of the extract for the protection of DNA from UV/H2O2 effects.
3.1.3 DNA damage prevention and cytotoxicity
Different amounts of dry extracts showed similar protection levels, while there was no band of DNA exposed in UV/H2O2 (Figure 3). Figure 3 shows the loss of DNA under UV effect, but still remained in plant treatments.
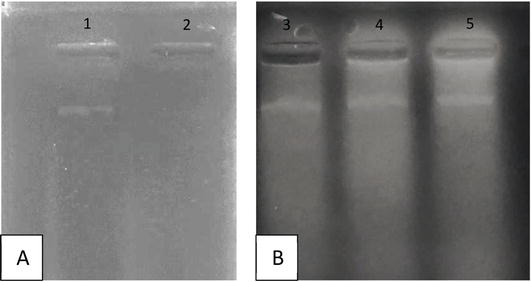
Figure 3.
DNA damaged protection of
DNA damaged by UV was recovered because ginsenoside accelerated nucleotide excision repair (NER). NER is the important pathway that mammals can use to eliminate DNA lesions [20]. Rg2 contributed to p53 upregulation, but the mode of activation had not been reported [21]. Upregulation of p53 caused posttranslational modification that includes targeting murine double minute 2 (MDM2). MDM2 is an E3 ubiquitin ligase. MDM2 has oncogenic activities that might be dependent and in-dependent on p53. MDM2 was increased in many kinds of human cancer. Another protein also involves p53 that is wild-type p53-induced phosphatase 1 (WIP1) [22]. Moreover, the antioxidant property of ginseng was also reported to be involved in cell cycle arrest. Ginsenosides arrest the cell cycle via the c-Myc/p53 pathway [23]. The Myc gene is one of the oncogenes relating a nuclear protein involved in p53 expression and cell cycle. It is also reported that ginsenoside Rg3 reduces DNA damage by alkylating agents in normal cells [24]. In this study, tubulin was selected as the target protein to investigate different mechanisms in cell cycle arrest. Beside these tumor proteins, O6-methylguanine-DNA methyl transferase (MGMT) was also used to study on the interaction with ligands. Ligand-protein complexes were investigated on potential inhibition on MDM2, a primarily negative regulator of p53 and MGMT.
Additionally, tubulin beta class I (TUBB) protein belonging to globular protein is the major component of the cytoskeleton. It takes part in many important processes including cell mobility, cellular transport, and DNA segregation (i.e., mitosis). Residues involved in the inhibitor-binding site of TUBB validated by taxol molecule are Val23, Asp26, Leu217, Leu219, His229, Leu230, Ala233, Ser236, Phe272, Pro274, Leu275, Thr276, Ser277, Arg278, Pro260, Arg369, Gly370, and Leu371. Among them, the residues Pro274, Leu275, Thr276, Ser277, and Arg278 are claimed to be important for the taxol molecule. The selected constituents showed interaction to maximum two out of five aforementioned residues. Docking results showed that all the ligands mainly displayed hydrophobic interaction with the active residues. Only four ginsenosides could display hydrogen bonds with Asp26, mimicking the interaction between Taxotere and TUBB (Table 1, Figure 4). Ginsenoside Rh4 had the lowest binding energy as it showed three H-bonds with both Asp26 and Pro274. Despite occupying four H-bonds including Asp26 and Thr276, ginsenoside Rg3 was ranked 6 as it lacked hydrophobic interaction to stabilize. Due to the limit of hydrogen bonds, PN constituents showed weak interaction with TUBB featuring protein-ligand complexes that may be reversible. This confirmed PN compounds only temporarily halt the cell cycle allowing DNA to repair.
| Rank | Ligands | Protein target | Binding energy value (kcal/mol) | Residues involved in interaction |
|---|---|---|---|---|
| 1 | Ginsenoside Rh4 | TUBB | −8.12 | Val23, Asp26, His229, Ala233, Phe272, Pro274,Arg284, Leu286, and Leu371 |
| 2 | Ginsenoside Rh2 | TUBB | −6.34 | Asp26, Leu217, Lys218, Leu219, Phe272, Arg278,Gly370, and Leu371 |
| 3 | Ginsenoside Rg5 | TUBB | −6.17 | Glu22, Asp26, His229, Pro274, Arg284, Leu286, and Leu371 |
| 4 | Panaxydol | TUBB | −6.0 | Phe272, Leu275, Thr276, Pro360, Arg369, Gly370, and Leu371 |
| 5 | Falcarindiol | TUBB | −5.99 | Leu217, His229, Leu230, Pro274, Leu275, Thr276,Arg284, Leu286, and Leu371 |
| 6 | Ginsenoside Rg3 | TUBB | −5.67 | Glu22, Asp26, His229, Thr276, Leu286, Leu371, andLys372 |
| 7 | Quercetin | TUBB | −4.61 | Phe272, Pro274, Thr276, Pro360, Leu371, and Gly370 |
| 8 | Panaxynol | TUBB | −4.36 | Leu217, His229, Leu230, Ala233, Phe272, Leu275,Ser277, and Arg278 |
Table 1.
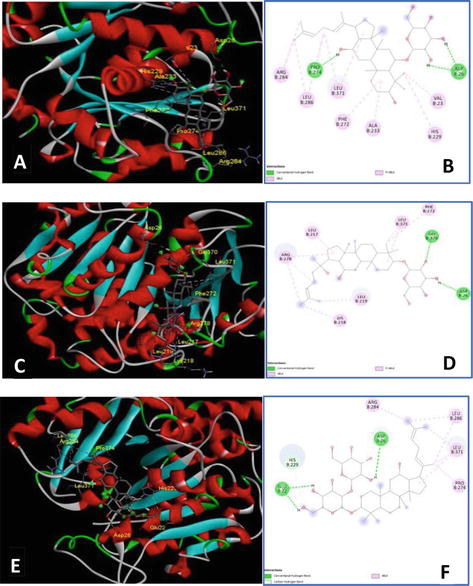
Figure 4.
Best docking conformations and interactions between TUBB (shown in colored cartoon mode) and ginsenoside Rh4, Rh2, and Rg5 (gray stick), respectively. The 3D illustration demonstrated the interaction of TUBB with ginsenoside Rh4 (A), Rh2 (C), and Rg5 (E). 2D diagrams depicted interaction types of ginsenoside Rh4 (B), Rh2 (D), and Rg5 (F) and the active sites of TUBB.
MGMT, commonly known as the suicide repair enzyme, plays a pivotal role in maintaining genome stability. MGMT repairs DNA-alkylated lesions at the O6 position of guanine. Such lesions may lead to DNA double-strand breaks or DNA mismatch resulting in mutations. The binding pocket of this enzyme is located at Tyr114, Pro138, Pro140, Cys145, Val148, Asn157, Tyr158, Ser159, and Lys165. The residue Cys145 is considered an important site for the occurrence of alkyl transfer. Only quercetin and the ligand group of polyacetylene could form hydrogen bonds with Cys145, whereas the ginsenoside showed H-interaction mainly with Ser159. Plus, the ginsenosides Rh2 and Rh4 were ranked second and third, respectively, but they only occupied one or two orientations with key residues, leaving most of the pocket empty. Although quercetin possessed the best binding energy, it created an unfavorable connection with Val128.
MDM2 is a regulator suppressing the expression of p53 protein [25]. At homeostasis, p53 is maintained at a low level via the MDM2-p53 complex (Table 2, Figure 5). This complex is mediated by hydrophobic interaction via three key residues of p53, namely, Phe19, Trp23, and Leu26. Inhibition of this negative regulator leads to the stability of p53 by mimicking p53 hydrophobic interaction with MDM2. Residues of MDM2 involved in the p53-MDM2 complex are Leu54, Leu57, Ile61, Met62, Gln72, His73, Val75, Val93, His96, Ile99, and Tyr100. MDM2 binding pocket can be divided into one large and main pocket Phe19/Trp23 and the subpocket Leu26. One ring of Rh2 formed hydrophobic bonds with Leu54, Leu57, Ile61, Met62, Val93, and Ile99 occupying pocket Phe19/Trp23. It also showed an H-interaction with Gln24 and a hydrophobic bond with Ile99 which was similar to Leu26 subpocket. Ginsenoside Rh4 ranked the second displayed linkage and interactions to the main Phe19/Trp23 pocket but left the subpocket Leu26 mostly empty (no interactions were found with Ile99). All the ginsenosides, flavonoids, and polyene ligands displayed strong affinity to MDM2; however, as this is an in silico test, further in vitro and in vivo experiments need to be performed to confirm this mode of interaction.
| Rank | Ligands | Protein target | Binding energy value (kcal/mol) | Residues involved in interaction |
|---|---|---|---|---|
| 1 | Ginsenoside Rh2 | MDM2 | −8.16 | Gln24, Lys51, Leu54, Leu57, Ile61, Met62,Tyr67, Val93, and Ile99 |
| 2 | Ginsenoside Rh4 | MDM2 | −7.64 | Lys51, Leu54, Leu57, Ile61, Met62, Tyr67, Ala69,and Val93 |
| 3 | Panaxydol | MDM2 | −6.3 | Lys51, Leu54, Leu57, His96, and Ile99 |
| 4 | Ginsenoside Rg5 | MDM2 | −6.23 | Gln24, Lys51, Met62, Tyr67, Val93, and Tyr100 |
| 5 | Falcarindiol | MDM2 | −5.57 | Ile19, Lys51, Leu54, Leu57, His96, Ile99, and Tyr100 |
| 6 | Panaxynol | MDM2 | −5.3 | Ile19, Lys51, Leu54, Leu57, Phe91, Ile99, His96and Tyr100 |
Table 2.
Binding energy of compounds docked into murine double minute 2 protein.
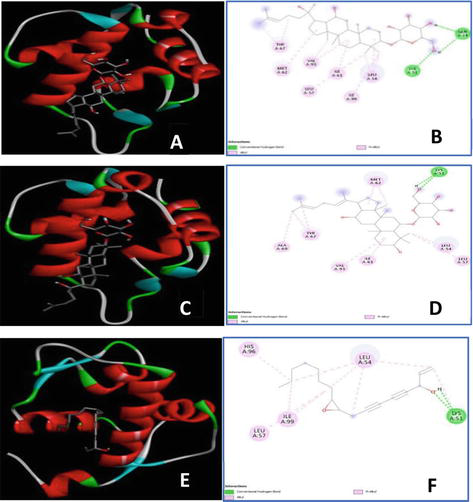
Figure 5.
Best docking conformations and interactions between MDM2 (shown in colored cartoon mode) and ginsenoside Rh2, Rh4, and panaxydol (gray stick), respectively. The 3D illustration demonstrated the interaction of MGMT with ginsenoside Rh2 (A), Rh4 (C), and panaxydol (E). 2D diagrams depicted interaction types of ginsenosideRh2 (B), Rh4 (D), panaxynol (F) and the active sites of MDM2.
From the results in Tables 1 and 2 and Figures 4 and 5, ginsenosides were predicted to interact with the oncogenes (MDM2 and TUBB). Then, via antioxidant activities of ginsenosides, these genes will suppress p53 and cell cycle [23, 24]. The prediction was also performed with MGMT to help with the effect of ginsenosides in DNA damage prevention and cancer treatment. Together with the roles of medicinal herbs in antioxidation relating to cancer treatment toward, probiotics are also discussed.
3.2 Probiotics
3.2.1 Probiotic concepts
Lactic acid bacteria are important organisms honored for their fermentative capability as well as their health and nutritive benefits. They produce colorful composites similar to organic acids, diacetyl, hydrogen peroxide, and bacteriocin or bactericidal proteins during lactic restlessness. The lactic acid bacteria used commonly are
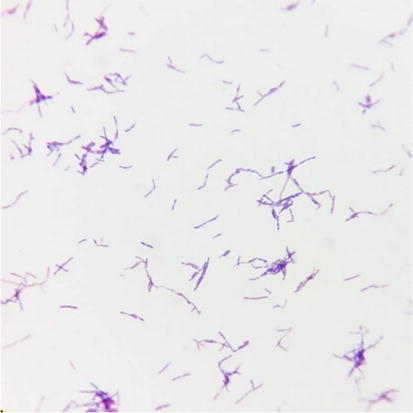
Figure 6.
The morphology of
To date, bifidobacteria are regulated as dietary supplements and specific products. These species are also called as “generally recognized as safe” (GRAS) microorganisms [30]. A number of clinical studies claim that
Besides, the genera
To find out the point of view, the study showed the antioxidant activity, DNA damage, and cancer prevention of
3.2.2 Representative probiotics
This chapter gave the research as an illustration of lactic acid bacteria benefits, especially the antioxidant activities involving DNA damage protection and cytotoxicity toward anticancer activity. Table 3 shows the survival rate of
| Components | % Survival | ||
|---|---|---|---|
| CE (20 mg/mL) | |||
| H2O2 (1 mM) + HU (40 mM) | H2O2 (1 mM) + HU (100 mM) | ||
| Case 1 | (hH)1 | 78.1003 ± 1.4394 | 54.2366 ± 1.4394 |
| (hH)2 | 61.5917 ± 1.9819 | 47.5754 ± 0.79 | |
| (hH)3 | 67.8872 ± 2.8716 | 41.8788 ± 2.9496 | |
| Case 2 | (hH)1E | 67.214 ± 0.6169 | 53.8313 ± 0.0353 |
| (hH)2E | 69.2042 ± 0.2447 | 45.266 ± 0.3657 | |
| (hH)3E | 68.4472 ± 2.5178 | 38.3967 ± 1.35927 | |
| Case 3 | (hHE)1 | 68.1475 ± 1.2307 | 65.5729 ± 1.4192 |
| (hHE)2 | 67.9151 ± 0.273 | 59.2573 ± 0.6829 | |
| (hHE)3 | 69.9547 ± 2.7488 | 69.5909 ± 1.1288 | |
| Case 4 | E(hH)1 | 79.5539 ± 2.7549 | 68.9468 ± 0.9499 |
| E(hH)2 | 74.7305 ± 0.7937 | 68.8953 ± 0.7768 | |
| E(hH)3 | 67.5318 ± 1.5285 | 40.75504 ± 0.217604735 | |
Table 3.
Survival percentage of
Case 1:
When comparing with case 1, the survival rate was significant higher. Furthermore, gradually increasing incubation time resulted in increased survival (53.8314 ± 0.0353% to 57.9562 ± 1.5168% when extracts were added and incubated at 1–2 hours after adding H2O2 – HU (100 mM) (case 2) and 65.5729 ± 1.4192% to 69.5909 ± 1.1288% when adding extracts and H2O2 – HU (100 mM) at the same time (case 3). For case 4, extract was added in yeast culture to protect the yeast survival capacity, and then the mixture was contacted with H2O2 – HU (40 mM and 100 mM). It seemed that the yeast survival rate was improved than case 1, case2, and case 3 during the 2-hour incubation. Especially, H2O2 – HU (100 mM) caused the lower survival rates, but case 4 could improve survival rate when comparing to cases 1 and 2. However, the survival rate was low in case 4 during the 3-hour incubation. Probably, any active component of bacteria was degraded by yeast during longer incubation. Actually, the incubation time of the extract in case 4 was longer than 3 h because the extract was incubated with yeast before and then prolonged 3 h incubation in HU and H2O2. Anyway, the bacterial extract could prevent the toxicity of HU and H2O2 by means of the antioxidant activity.
The protection of incentive cells by the expert under the effect of H2O2 – HU is harmonious with the compliances above, suggesting the possible part of the excerpt in the junking of hydrogen peroxide (H2O2) revolutionaries. This supports the excerpt’s antioxidant exertion by demonstrating its part in guarding cells from DNA dangerous chemicals that the release of OH- revolutionaries from H2O2 – HU complex is the main reason for converting DNA damage in cells [33, 34]. The protection of Bifidobacteria for prokaryotic and eukaryotic cells involved in numerous DNA mismatch form (DMR) protein. DMR’s top function is to ameliorate replication dedication by repairing replication-associated base-base mismatches. Modulating DNA recombination and easing DNA damage signaling are significant primary functions [35]. DMR blights are connected with cancer vulnerability, including heritablenon-polyposis colorectal cancer, resistance to certain chemotherapeutic agents, and anomalies in meiosis and sterility in mammalian systems [36]. Inactivation of DMR in cells causes sporadic malice in humans. In response to some forms of DNA damage, the DMR system is necessary for cell cycle arrest and/or programmed cell death. This capability was inactivated when exposed to high attention of H2O2 (1 mM). As a result, when adding HU− a medicine applied to treat neoplastic conditions but has a side effect of inhibits DNA conflation,
Lactic acid bacteria including
| Growth phase | % DPPH scavenging activity of culture supernatant |
|---|---|
| Early exponential phase | 93.17 ± 0.075 |
| Late exponential phase | 94.33 ± 0.117 |
| Stationary phase | 94.69 ± 0.075 |
| Death phase | 94.31 ± 0.075 |
Table 4.
Antioxidant activities of
Consequently, antioxidant activities of probiotics/lactic acid bacteria could involve in DNA damage protection. Many metabolites were produced in the bacteria; however, exopolysaccharides were studied most in cytotoxicity. This activity of lactic acid bacteria was like other species such as seaweeds [44]. It is undoubtable. Therefore, the lactic acid bacteria could be exploited for cancer therapy development by finding their antioxidants in the future.
4. Conclusion
Antioxidant conditioning can be detected in both medicinal plants and lactic acid bacteria. Antioxidant agents could prevent cytotoxicity and DNA damage. This chapter showed the antioxidant activities of daily vegetables such as
References
- 1.
He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiology and Biochemistry. 2017; 44 (2):532-553 - 2.
Cao L, Xu X, Cao LL, Wang RH, Coumoul X, Kim SS, et al. Absence of full-length Brca1 sensitizes mice to oxidative stress and carcinogen-induced tumorigenesis in the esophagus and forestomach. Carcinogenesis. 2007; 28 :1401-1407 - 3.
Mahmoud AM, Wilkinson FL, Sandhu MA, Dos Santos JM, Alexander MY. Modulating oxidative stress in drug-induced injury and metabolic disorders: The role of natural and synthetic antioxidants. Oxidative Medicine and Cellular Longevity. 2019; 2019 :3206401 - 4.
Conrad M, Kagan VE, Bayir H, et al. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes and Development. 2018; 32 (9-10):602-619 - 5.
Knecht KT, Bradford BU, Mason RP, Thurman RG. In vivo formation of a free radical metabolite of ethanol. Molecular Pharmacology. 1990; 38 (1):26-30 - 6.
Collins AR. Oxidative DNA damage, antioxidants, and cancer. BioEssays. 1999; 21 (3):238-246 - 7.
Rahaman MM, Hossain R, Herrera-Bravo J, et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Science & Nutrition. 2023; 11 (4):1657-1670 - 8.
Deshmukh SA, Gaikwad DK. A review of the taxonomy, ethnobotany, phytochemistry and pharmacology of Basella alba (Basellaceae). Journal of Applied Pharmaceutical Sciences. 2014;4 (01):153-165 - 9.
Güder A, Korkmaz H. Evaluation of in-vitro antioxidant properties of hydroalcoholic solution extracts Urtica dioica L., Malva neglecta Wallr. and their mixture. Iranian Journal of Pharmaceutical Research. 2012;11 (3):913-923 - 10.
Song Y, Zeng K, Jiang Y, Tu P. Cistanches Herba, from an endangered species to a big brand of Chinese medicine. Medicinal Research Reviews. 2021; 41 (3):1539-1577 - 11.
Huang J, Zhao D, Cui C, Hao J, Zhang Z, Guo L. Research progress and trends of phenylethanoid glycoside delivery systems. Food. 2022; 11 (5):769 - 12.
Sui ZF, TingMin G, Liu B, Peng SW, Zhao ZL, Li L, et al. Water-soluble carbohydrate compound from the bodies of Herba Cistanches: Isolation and its scavenging effect on free radical in skin. Carbohydrate Polymers. 2011; 85 (1):75-79 - 13.
Salekdeh GH, Siopongco J, Wade LJ, Ghareyazie B, Bennett J. Proteomic analysis of rice leaves during drought stress and recovery. PROTEOMICS: International Edition. 2022; 2 (9):1131-1145 - 14.
Lü JM, Jiang J, Jamaluddin M, Liang Z, Yao Q , Chen C. Ginsenoside Rb1 blocks ritonavir-induced oxidative stress and eNOS downregulation through activation of estrogen receptor-beta and upregulation of SOD in human endothelial cells. International Journal of Molecular Sciences. 2019; 20 (2):294 - 15.
Oliynyk S, Oh S. Actoprotective effect of ginseng: Improving mental and physical performance. Journal of Ginseng Research. 2023; 37 (2):144-166 - 16.
Zhou Q , Jiang L, Xu C, et al. Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thrombosis Research. 2014; 133 (1):57-65 - 17.
Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of apigenin. International Journal of Molecular Sciences. 2019; 20 (6):1305 - 18.
Villa-Rodriguez JA, Kerimi A, Abranko L, et al. Acute metabolic actions of the major polyphenols in chamomile: An in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Scientific Reports. 2018; 8 (1):5471 - 19.
Lim R, Barker G, Wall CA, Lappas M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Molecular Human Reproduction. 2013; 19 (7):451-462 - 20.
Zhou Z, Zhang Y, Lin L, Zhou J. Apigenin suppresses the apoptosis of H9C2 rat cardiomyocytes subjected to myocardial ischemia-reperfusion injury via upregulation of the PI3K/Akt pathway. Molecular Medicine Reports. 2018; 18 (2):1560-1570 - 21.
Cai BX, Jin SL, Luo D, Lin XF, Gao J. Ginsenoside Rb1 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Biological and Pharmaceutical Bulletin. 2009; 32 (5):837-841 - 22.
Chung YH, Jeong SA, Choi HS, Ro S, Lee JS, Park JK. Protective effects of ginsenoside Rg2 and astaxanthin mixture against UVB-induced DNA damage. Animal Cells and Systems. 2018; 22 (6):400-406 - 23.
Ladds M, Laín S. Small molecule activators of the p53 response. Journal of Molecular Cell Biology. 2019; 11 (3):245-254 - 24.
He NW, Zhao Y, Guo L, Shang J, Yang XB. Antioxidant, antiproliferative, and pro-apoptotic activities of a saponin extract derived from the roots of panax notoginseng (Burk.) F.H. Journal of Medicinal Food. 2012;15 (4):350-359 - 25.
Li T, Sun W, Dong X, et al. Total ginsenosides of Chinese ginseng induces cell cycle arrest and apoptosis in colorectal carcinoma HT-29 cells. Oncology Letter. 2018; 16 (4):4640-4648 - 26.
Zhang YH, Li HD, Li B, Jiang SD, Jiang LS. Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells. Oncology Reports. 2014; 31 (2):919-925 - 27.
Hidalgo-Cantabrana C, Delgado S, Ruiz L, Ruas-Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their health-promoting effects. Microbiology Spectrum. 2017; 5 (3):10 - 28.
Alessandri G, van Sinderen D, Ventura M. The genus bifidobacterium: From genomics to functionality of an important component of the mammalian gut microbiota running title: Bifidobacterial adaptation to and interaction with the host. Computational and Structural Biotechnology Journal. 2021; 19 :1472-1487 - 29.
Whitman WB, Rainey F, Kämpfer P, Trujillo M, Chun J, DeVos P, et al., editors. Bergey’s Manual of Systematics of Archaea and Bacteria. 1st ed. Wiley; 2015 - 30.
Turroni F, Duranti S, Milani C, Lugli GA, van Sinderen D, Ventura M. Bifidobacterium bifidum : A key member of the early human gut microbiota. Microorganisms. 2019;7 (11):544 - 31.
Petersen AB, Gniadecki R, Vicanova J, Thorn T, Wulf HC. Hydrogen peroxide is responsible for UVA-induced DNA damage measured by alkaline comet assay in HaCaT keratinocytes. Journal of Photochemistry and Photobiology. B, Biology. 2000; 59 (1-3):123-131 - 32.
Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, et al. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Frontiers in Bioengineering and Biotechnology. 2021; 9 :612285 - 33.
Koziol S, Zagulski M, Bilinski T, Bartosz G. Antioxidants protect the yeast Saccharomyces cerevisiae against hypertonic stress. Free Radical Research. 2005;39 (4):365-371 - 34.
Fakruddin M, Hossain MN, Ahmed MM. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complementary and Alternative Medicine. 2017;17 (1):64 - 35.
Golla U, Bhimathati SS. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Scientific World Journal. 2014;2014 :215084 - 36.
Garbacz K. Anticancer activity of lactic acid bacteria. Seminars in Cancer Biology. 2022; 86 (Pt 3):356-366 - 37.
Davies BW, Kohanski MA, Simmons LA, Winkler JA, Collins JJ, Walker GC. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli . Molecular Cell. 2009;36 (5):845-860 - 38.
Li GM. Mechanisms and functions of DNA mismatch repair. Cell Research. 2008; 18 (1):85-98 - 39.
Stojic L, Brun R, Jiricny J. Mismatch repair and DNA damage signalling. DNA Repair (Amst). 2004; 3 (8-9):1091-1101 - 40.
Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World Journal of Gastroenterology. 2014; 20 (24):7878-7886 - 41.
Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M. Probiotic Lactobacillus acidophilus andLactobacillus casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutrition and Cancer. 2010;62 (3):371-378 - 42.
Kim SH, Moon JY, Lim YJ. Dietary intervention for preventing colorectal cancer: A practical guide for physicians. Journal of Cancer Prevention. 2022; 27 (3):139-146 - 43.
Nguyen DT, Nguyen TH. Detection on antioxidant and cytotoxicity activities of exopolysaccharides isolated in plant-originated Lactococcus lactis . Biomedical and Pharmacology Journal. 2014;7 (1):33-38 - 44.
Pradhan B, Bhuyan PP, Ki JS. Immunomodulatory, antioxidant, anticancer, and pharmacokinetic activity of Ulvan, a seaweed-derived sulfated polysaccharide: An updated comprehensive review. Marine Drugs. 2023; 21 (5):300