Subcellular localization of enzymatic and non-enzymatic antioxidant systems.
Abstract
Stress caused by challenging environmental conditions is often associated with the rapid production of reactive oxygen species (ROS) that dramatically alter cellular redox homeostasis. ROS generation is tightly connected with its utilization, as impaired equilibrium of these processes results in oxidative stress having profound consequences for cell physiology. The balanced action of both antioxidant enzymes and non-enzymatic antioxidants counterbalances the harmful effects of ROS. Despite the functional antioxidant system of the cell, excessive ROS leads to disruption of vital cellular processes which is associated with the development of various lifestyle diseases, mainly cardiovascular diseases, and cancer. Enhanced consumption of foodstuffs with high and balanced antioxidant bioactive compounds is linked with the positive effects of beneficial antioxidants impacting cellular protection resulting in health promotion. In this chapter, we present an outline of how bioactive compounds determine their health-promoting properties.
Keywords
- oxidative stress
- cellular protection
- ROS
- antioxidant defense
- bioactive substances
1. Introduction
1.1 ROS formation and utilization
Under physiological conditions, over 90% of oxygen (O2) the living organism receives is utilized in the electron transport chain as a terminal acceptor of electrons. Human beings require oxygen for energy production through the oxidative metabolism of the mitochondria. They generate adenosine triphosphate (ATP), from which the energy necessary for almost all vital processes is released. Remaining oxygen molecules undergo partial one-electron reduction through stepwise addition of electrons resulting in the formation of oxygen products called reactive oxygen species (ROS). The superoxide anion is formed by the univalent reduction of triplet-state molecular oxygen (3O2). The stepwise molecular oxygen reduction has the following progress [1]:
In general, ROS are formed as by-products of aerobic metabolism, however, several other sources are also involved. Cellular ROS levels can increase as a consequence of UV irradiation, ionizing radiation, exposure to toxins such as heavy metals, due to chemotherapy treatment, or neighboring inflammatory cells. In a healthy organism, ROS, which include superoxide radical (O2•−), singlet oxygen (1O2), H2O2, perhydroxyl radical O2H•, or hydroxyl radical (OH•), regulate various physiological processes such as differentiation, stress signaling, long-distance signaling, redox levels, immune response, cell death, etc. [2].
1.2 Oxidative stress
Formation of ROS as a deteriorative by-product of aerobic metabolism, can occur in any organelle of the cell consisting of high redox-potential molecules capable to excite the atmospheric oxygen. Transition metal ions such as iron (Fe), that reacts with H2O2 in the Fenton reaction, are involved in the conversion of oxygen intermediates into highly reactive forms (ROS or free radicals).
Consequently, due to the high reactivity of ROS, their levels are maintained under strict control to prevent unintentional oxidation of biological macromolecules. Formed ROS, that are not utilized for physiological processes, are removed, or detoxified by pathways of enzymatic defense systems or compounds with antioxidant properties to control ROS signaling reactions. The imbalance between ROS generation and their sequestration/utilization, with certain consequences for cell physiology, is called oxidative stress [3]. Since stress-free conditions are nearly unreachable, living organisms have to develop mechanisms to resist stress in order to survive. Even though the majority of chronic diseases of the modern age are multifactorial in origin, they share one common factor, oxidative stress. Oxidative stress, resulting in the oxidation of lipids, nucleic acids, or proteins, has been suggested to contribute to several pathologies like cancer, cardiovascular diseases, diabetes, neurological disorders (Alzheimer’s, Parkinson’s, and Huntington’s diseases and amyotrophic lateral sclerosis), genetic disorders (such as Down or Klinefelter syndrome), psychiatric diseases (including depression, schizophrenia, and bipolar disorder), renal or lung diseases, and aging.
1.3 Antioxidants
The protective activity of molecules with antioxidant properties to reduce oxidative stress and thereby prevent negative consequences of its action, is ensured either by antioxidant enzymes or small molecular weight antioxidants, or by the combination of both [4, 5]. The most prominent and well characterized molecules with antioxidant properties are depicted in Table 1.
| Antioxidant | Abbreviation | Subcellular localization |
|---|---|---|
| Superoxide dismutase | Cu/Zn-SOD | Cytosol, plastid, peroxisome |
| Mn-SOD | Mitochondrion | |
| Fe-SOD | Plastid | |
| Catalase | CAT | Peroxisome |
| Ascorbate peroxidase | APX | Cytosol, mitochondrion, plastid, peroxisome |
| Dehydroascorbate reductase | DHAR | Cytosol |
| Glutathione reductase | GR | Cytosol, mitochondrion, plastid |
| Glutathione peroxidase | GPX | Cytosol, mitochondrion, plastid |
| Monodehydroascorbate reductase | MDAR | Cytosol, mitochondrion, plastid |
| Peroxiredoxin | Prx | Chloroplast, mitochondrion |
| Ascorbate | AsA | Apoplast, cytosol, mitochondrion, plastid peroxisome, nucleus, vacuole |
| β-Carotene | CAR | Plastid |
| Glutathione reduced | GSH | Cytosol, mitochondrion, plastid, nucleus |
| Polyamines | Cytosol, mitochondrion, plastid, nucleus | |
| α-Tocopherol | Membranes | |
| Zeaxanthin | Plastid | |
Table 1.
Antioxidants inhibit or delay the oxidation of a substrate. The protective mechanism of antioxidants is classified in accordance with the general criteria as enzymatic or non-enzymatic, preventative or system-repairing, endogenous or exogenous, primary and secondary, hydrosoluble and liposoluble, and natural and synthetic. Antioxidant molecules capable of sequestering free radicals by donation of hydrogen (chain breakers) are called primary antioxidants. Secondary antioxidants belong to compounds capable of singlet oxygen quenching, peroxide decomposition, chelating of metal, inhibiting oxidative enzymes, or absorbing UV radiation.
Trace elements Cu, Se, Mn, and Zn represent the essential ions in the structure of antioxidant enzymes in the cytosol, mitochondria, or in plasma: Cu/Zn superoxide dismutase (SOD), Mn-SOD, catalase (Cu, Fe), and glutathione peroxidases (GPX:Se). The antioxidant processes are initiated by SODs that transform the superoxide anion into hydrogen peroxide, followed by catalase and GPX-mediated neutralization of the various peroxides at both intra- and extra-cellular levels [6].
In addition, the harmful effects of oxidative stress can be reduced via the ingestion of dietary antioxidants present in various foods. Nutritional antioxidants vary in their composition and act through various mechanisms and in different compartments, however, the predominant type of action is free radicals scavenging. This is realized either by the direct neutralization of ROS, reduction of the peroxide levels, and reconstruction of oxidized membranes, or by iron quenching to limit ROS formation, or via lipid metabolism, as short-chain free fatty acids and cholesteryl esters neutralize ROS [5].
2. Natural antioxidants
Generally, the function of antioxidants is to delay or prevent oxidation of chemical molecules in the cell. Initially, the studies on the antioxidant molecules have been aimed at investigating their ability to prevent the degradation of unsaturated fatty acids. The main source of natural substances with health-promoting properties is plants. Although the use of plants and their metabolites to promote health dates back to ancient times, nowadays they still belong to the constitutive and inseparable part of medicine and pharmacology. Most of the compounds with bioactive properties do not play an essential role in plant growth or development. Rather, they are important for organism protection against pathogens or toxic elements. Alternatively, they serve as signaling substances for various physiological processes. The synthesis of substances with bioactive properties is not common for all plant species. Mostly it is limited to a genus or, in some cases, even to individuals. In general, these natural compounds are not considered as stand-alone drugs. They are usually used as supportive substances that contribute to the reduction of side effects of the drug treatment or enhance the curative effect of the pharmaceutical. Natural compounds constitute a base for the design and development of new pharmaceuticals (Figure 1) [7, 8].
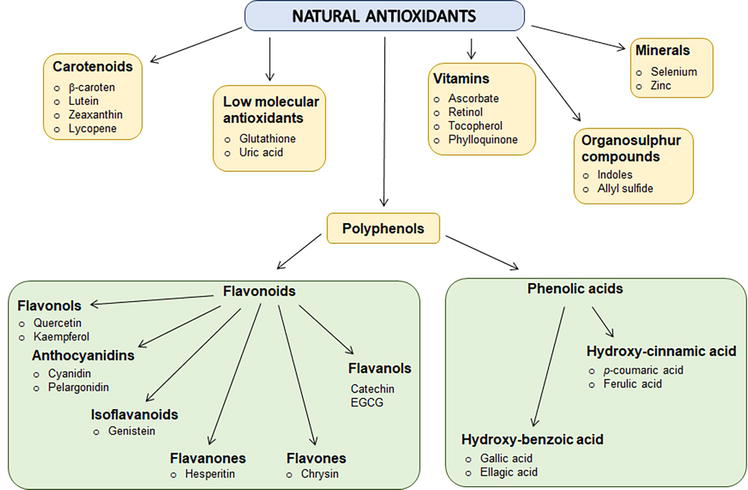
Figure 1.
Schematic overview of the major natural antioxidants.
2.1 Natural antioxidants of plant origin
Plants as an invaluable source of natural substances with versatile medicinal properties have been used to treat various diseases and discussed for centuries. Multidirectional activities exhibited by natural substances and their ability to influence key signaling pathways in the cell, mainly related to cancer and other civilization diseases, drive these substances in an important research direction. Over the last decades, research has advanced the discovery and identification of plant components with beneficial properties and revealed their underlying mechanisms [7, 9].
2.1.1 Phenolic compounds
The chemical composition of a specific group of secondary metabolites, phenolic compounds, comprises one or more aromatic rings triggering one or more hydroxyl groups capable of stabilizing and relocating unpaired electrons. In general, phenolic compounds are a heterogeneous group of phytocompounds that can be categorized as phenolic acids, flavonoids, coumarins, and tannins. The function of phenolic acids in plants has been associated with various physiological processes that include nutrient uptake, protein synthesis, enzyme activity, photosynthesis, or allelopathy. They exist in virtually all plants and are dispersed throughout the plant in seeds, leaves, roots, flowers, and stems [10, 11]. Except for their significant role in plant physiology, phenolic compounds included in the human diet have been connected to a variety of benefits associated with reduced risk of chronic diseases acting as an anti-allergenic, anti-atherogenic, anti-inflammatory, or antimicrobial medium. Most of the beneficial effects of phenolic compounds result from their antioxidant impact as they are capable to neutralize free radicals (i.e., superoxide anion, hydroxyl- radicals, and peroxy-radicals) thereby protecting a person from degenerative diseases such as cardiovascular disease, diabetes mellitus, neurodegenerative diseases, and cancer. They represent the largest known group of natural antioxidants [12, 13, 14]. The antioxidant activity of phenolic compounds is often determined by their ability to protect LDL lipids from oxidation. Several studies on various individuals of all ages under different health conditions such as overweight, obesity, metabolic syndrome, heavy smokers, patients with peripheral vascular disease, or subjects at high cardiovascular risk have been involved in clinical trials that involve the consumption of extracts obtained from various plant substances (e.g., different types of berries, grapes, olives, citrus fruits, various nuts, coffee, tea, or chocolate) the same way as particular phenolic compounds (e.g., gallic acid (GA), resveratrol, luteolinidin, coumaric acid, apigenidin, rosmarinic acid, caffeic acid, catechin, quercetin, ellagic acid, and rutin). These studies have been performed to confirm the strong antioxidant effect of dietary phenolics consumption at retarding LDL oxidation
2.1.2 Phenolic acids
Phenolic acids, a subclass of plant phenolics, possess a structure capable of the H-atom donation resulting in antioxidant activity through radical scavenging mechanism, radical quenching
Gallic acid (GA) is a naturally occurring organic compound that belongs to the family of phenolic acids. Its derivatives are widely found in fruits and plants. The esters of gallic acid possess a wide range of industrial applications, including their use as antioxidants in food, cosmetics, and pharmaceuticals. It has been demonstrated that gallic acid can mediate various health-promoting activities involved in anti-inflammation, anti-obesity, and anti-cancer effects. More recently, the anti-cancer activities of GA have been described to act via several biological pathways that include induction of programmed cell death, reticence of vasculature, tumor migration, metastasis, inflammation, cell cycle arrest, and oncogene expression. The activation of the NF-κB and Akt signaling pathways, as well as the activities of COX, ribonucleotide reductase, and glutathione reduced (GSH), are all inhibited by gallic acid, which has an impact on biochemical signaling pathways. Additionally, gallic acid inhibits the processes of carcinogenesis through activating ATM kinase signaling pathways. Furthermore, gallic acid is found to show synergism with other existing chemotherapeutic drugs [18, 19, 20, 21, 22].
Syringic acid is a phenolic molecule produced by plants through the shikimic acid pathway. It is commonly present in plant-based materials such as fruits, vegetables, and medicinal plants. It has been demonstrated by many studies that it can be used in a wide range of therapeutic applications in the prevention of diabetes, cardiovascular diseases, cancer, and cerebral ischemia due to its potent antioxidant, antimicrobial, anti-inflammatory, antiendotoxic, chemoprotective, anti-angiogenic, anti-glycating, anti-proliferative, anti-hyperglycaemic, neuro, and hepatoprotective activities. The therapeutic potential of syringing acid is largely attributed to the presence of methoxy groups onto the aromatic ring operating as an effective free radical scavenger leading to the oxidative stress alleviation [23]. In addition, syringic acid alters protein dynamics, the activity of enzymes, and a variety of transcription factors associated with angiogenesis, diabetes, inflammation, and cancer. In recent studies, it has been shown that treatment with syringic acid may help neurological dysfunction or behavioral impairments management [24]. Rats fed with syringic acid-enriched food displayed protective effects on diabetic cardiomyopathy by reducing lipid peroxidation and protein carbonylation related to the antioxidant activity of this phenolic acid. Furthermore, syringic acid administration has been linked to the amelioration of cardiac ischemia-reperfusion injury in the rat model, by blocking mitochondria-induced apoptosis that is controlled by the PI3K/Akt/GSK-3β signaling pathway [25, 26]. It is noteworthy that syringic acid effectively suppressed the frequency of UVB-induced skin tumors in mice by inhibiting the phosphorylation of the epidermal growth factor receptor (EGFR), Akt signaling pathways, and mitogen-activated protein kinases. Moreover, administration of syringic acid inhibited nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity resulting in intracellular ROS reduction and inhibited protein-tyrosine phosphatase-κ activity, a regulator of EGFR activation. Based on these findings, syringic acid may be a useful chemopreventive and therapeutic agent since it inhibits the Nox/PTP-κ/EGFR axis which is the primary mechanism by which it exerts strong chemopreventive activity in skin carcinogenesis [27].
Vanillic acid (VA) is a bioactive compound derived from natural sources such as vegetables, fruits, herbs, whole grains, juices, beers, and wines. It possesses various pharmacological activities such as antioxidants, and anti-proliferative, and it has been shown to block pro-inflammatory cytokines and suppress inflammatory cascades. Due to its phenolic nature, vanillic acid exerts strong inhibitory effects on variable signal transduction pathways that include the Janus kinase (JAK)/signal transducer and activator of transcription (STAT), adenosine monophosphate-activated protein kinase (AMPK), Nod-like receptor family protein (NLRP), nuclear factor kappa B (NF-κB). Target of rapamycin (mTOR), and mitogen-activated signaling proteins (MAPK). These actions display diverse therapeutic potential, including cardioprotective and liver protective. Vanillic acid scavenges free radicals, boosts antioxidant status, lowers levels of lipid peroxidation, and improves mitochondrial function as a result of decreased ROS generation. As a result, it lowers cardiac dysfunction, especially that caused by cardiotoxicity (CTX) and myocardial ischaemia–reperfusion injury (MIRI) [28, 29]. Additionally, vanillic acid has been found to possess protective effects on the liver by reducing serum levels of transaminases, inflammatory cytokines, and collagen accumulation, thereby preventing liver fibrosis. Furthermore, practically every metabolic abnormality associated with non-alcoholic fatty acid disease (NAFLD), including hepatic steatosis, inflammation, and hepatic damage has been reported to be targeted by VA [30]. In addition, vanillic acid has been found to have a beneficial effect on diabetic nephropathic rats. This effect may be linked to its potent ability to sequester free radicals, as well as its ability to down-regulate NF-κB, TNF-α, and COX-2, while also up-regulating Nrf-2 proteins in renal tissue [31]. Moreover, through the reduction of ROS formation, stabilization of mitochondrial membrane potential, restriction of mPTP opening, regulation of caspase-3 activity, and eventually suppression of cardiomyocyte apoptosis, VA protects H9c2 cells against H/R-induced damage [32]. Interestingly, VA treatment has been associated with longevity due to its ability to increase thermotolerance, reduce protein aggregation, improve motility, and extend the life span by almost 50%, an extent that is hardly achieved with a natural compound. This reveals the therapeutical properties of VA as a novel geroprotector [33].
Protocatechuic acid (PCA) is one of the main metabolites of complex polyphenols such as anthocyanins, proanthocyanins, and procyanidins which widely exist in our daily diet as they are normally found in high concentrations in vegetables, fruits, and herbs. A growing body of evidence supports the concept that PCA can exert a variety of biological antioxidant, anti-inflammatory, antibacterial, antiviral as well as antihyperglycemic, neuroprotective antiosteoporotic, analgesia, and anti-aging effects by acting on different molecular targets. Moreover, as PCA suppresses
A phenolic plant hormone, salicylic acid (SA), is essential to defensive signaling, controlling growth, development, blooming, photosynthesis, and antioxidant enzymes in response to environmental changes. Challenging conditions of the environment caused by biotic and abiotic stress factors can mediate ROS overaccumulation that in turn results in oxidative stress. Therefore, a proper balance is essential to maintain redox homeostasis in plants, and salicylic acid is known as a chief regulator of ROS. The interplay of SA and ROS requires finetuning as, under some environmental circumstances, SA functions as an antioxidant (ROS scavenging) and pro-oxidant (ROS buildup), playing a dual role. Modulation of ROS metabolism by SA is tightly connected with its ability to directly bind both main H2O2-detoxifying enzymes the CAT and ascorbate peroxidase (APX) and thereby inhibit their activities. Similarly, pre-treatment with SA results in catalase 2 (CAT2) and glutathione S-transferases (GSTs) inhibition, the key players in plant defenses against pathogens. In addition, exogenously applied SA has been reported to affect antioxidant enzymes under different heavy metal stresses, like copper (Cu), lead (Pb), cadmium (Cd), and nickel (Ni) resulting in reduced lipid peroxidation that in turn leads to increased growth and photosynthesis. Furthermore, by regulating antioxidant enzymes such as GST, GPX, GR, MDHAR, DHAR, and GS as well as by boosting Assada and Halliwell to preserve ROS homeostasis, SA can reduce salt-derived oxidative stress and thereby increase biomass, chlorophyll content, and photosynthesis. Moreover, systemic acquired resistance (SAR) and the hypersensitive response (HR) are established by SA. This is supported by a strong and intricate network of SA that includes cascades of mitogen-activated protein kinases (MAPK), ROS, calcium ions (Ca2+), nitric oxide (NO), and non-expressor of pathogenesis related protein-1 (NPR1) [38, 39, 40, 41].
Ferulic and caffeic acid. Ferulic acid is highly abundant in a variety of foods of plant origin such as cereals, fruits including apples, oranges, and bananas, and vegetables including potatoes, tomatoes, and cabbage. Due to its antioxidant properties, ferulic acid protects macromolecules such as DNA and lipids from oxidative damage, which makes it a potential candidate for anti-cancer, anti-diabetic, anti-aging, and anti-inflammatory treatments. Studies on rats revealed the nephroprotective and antioxidant effects of ferulic acid and its treatment in diabetic rats inhibited peroxidation of lipids. Caffeic acid, abundantly found in a variety of mushrooms, tea, and coffee, has been shown to possess antiviral, antioxidant, anti-cancer, and anti-inflammatory properties. In addition, caffeic acid treatment due to its antioxidant activity, by direct free radical scavenging, protects against ischemia-reperfusion injury and shows hepatoprotective ability against oxytetracycline (OXT)-induced toxicity, anti-lipid peroxidation activity, and cardioprotective activity. Rats concomitantly treated with ferulic and caffeic acid had increased antioxidant activity and increased excretion of neutral and acidic sterols (p < 0.05). This resulted in lower hepatic and plasma cholesterol levels as well as increased excretion of fecal sterols and decreased absorption of dietary cholesterol. Furthermore, the iron chelating capacity of ferulic acid and caffeic acid in the brain tissues of iron-overloaded mice has been evaluated and revealed that such treatment significantly decreased iron content in both brain and serum samples. Moreover, the iron-induced decrease of the catalase activity was restored to that of the control group upon ferulic and caffeic acid administration. Thus, ferulic acid and caffeic acid might be possible natural iron chelators for brain iron overload therapy. Additionally, mice treatment with caffeic acid and ferulic acid attenuated the effects of iron overload on the rat serum biochemical parameters [42, 43, 44]. Strikingly, ferulic and caffeic acid supplementation have shown positive effects toward prevention of multiple aspects of the metabolic syndrome and liver steatosis in an obese mouse model [45], multifunctional properties against Alzheimer’s disease (AD) [46], and the anti-cancer activity has been demonstrated by their ability to mediate cytotoxicity toward human breast cancer cell lines that in the future could be profitably applied for chemopreventive and/or chemotherapeutic purposes [47].
2.1.3 Flavonoids
Flavonoids belong to a class of naturally occurring polyphenolic compounds comprising the most common group of plant polyphenols. They are responsible for different colors of leaves, fruit, and flowers and provide much of the flavor to fruits and vegetables. To date, more than 5000 diverse types of flavonoids have been characterized. Six subclasses of flavonoids have been categorized to date that include flavones (e.g., tangeritin, apigenin, chrysin, luteolin, etc.), flavonols (e.g., quercetin, kaempferol, myricetin, etc.), flavanones (e.g., naringenin, eriodictyol, and hesperidin), catechins or flavanols (e.g., catechin, epicatechin, epicatechin gallate, gallocatechin, etc.), anthocyanidins (e.g., cyanidin, delphinidin, malvidin, pelargonidin, etc.), and isoflavones (e.g., glycitein, genistein, and daidzein). Structural variations have been described among the individual classes based on the arrangement and number of hydroxyl groups, and glycosylation or alkylation of these groups. Most of the flavonoids present in plants are attached to sugars (glycosides), although they might be occasionally found as aglycones, too. Ingestion of dietary polyphenolics leads to their digestion, resulting in their appearance in the circulatory system not as the parent compounds, but as phase II metabolites. In the gastrointestinal tract, the parent compounds and their metabolites are degraded giving rise principally to small phenolic acid and aromatic catabolites that are absorbed into the circulatory system [53, 54]. The potent health benefit of polyphenols and their metabolites and catabolites on human chronic diseases exerts from their striking antioxidant and free radical scavenging activities. The most prominent antioxidant mode of action of flavonoids is the direct scavenging of ROS through the breakdown of several free radical chain reactions. In general, flavonoids, due to their highly reactive hydroxyl group, are oxidized by radicals producing flavonoid radicals. This, in turn, results in a generation of more stable, less-reactive radicals. The antioxidant effects of flavonoids may be attributed to their ability to inhibit the chain reaction of lipid oxidation. Moreover, such pronounced antioxidant property of flavonoids leads to the anti-inflammatory, anticarcinogenic, anti-mutagenic, chemoprotective, and antimicrobial effects of those phytochemicals. In addition, flavonoids inhibit the synthesis and activities of pro-inflammatory mediators such as cytokines, eicosanoids, adhesion molecules, and C-reactive protein, exerting anti-inflammatory effects. Molecular activities of flavonoids include inhibiting transcription factors like NF-κB and activating protein-1 (AP-1). They also activate nuclear factor erythroid 2-related factor 2 (Nrf2) [55, 56]. These effects of nutritive and dietary polyphenols thus result in their dual role as cytoprotective and cytotoxic agents. They possess the ability to protect the normal healthy cells and cellular components, while they destroy or are toxic to the premalignant and neoplastic or malignant cells. Flavonoids can be found in a variety of plants. Some examples of beneficial compounds found in various fruits and vegetables include flavanol, flavonoid, and quercetin which are highly abundant in onion, broccoli, and apple. Catechin can be found in high amounts in different teas and fruits, while naringenin is considered the predominant flavanone in grapefruit. Glycitein, genistein, and daidzein are considered as the major isoflavanones in soybean. In addition, cyanogen glycosides are highly present in berries such as raspberry, blackberry, and black currant. It is noteworthy that the consumption of vegetables and fruits rich in antioxidant phytochemicals has proven to increase the antioxidant capacity of serum/plasma, thereby promoting the health-protective properties of those edibles. Altogether, the well characterized health-protection properties of flavonoids predetermine them to be utilized in future experiments or trials as attractive candidates for disease therapy [8, 57, 58, 59].
2.1.4 Coumarins
Coumarins are fused six-membered oxygen-containing benzoheterocycles that join two synthetically useful rings: α-pyrone and benzene. Higher plants such as Rutaceae and Umbelliferae contain coumarins; however, coumarins can also be found highly abundand in essential oils such as lavender, cassia leaf, and cinnamon bark oil. Coumarin compounds are bioactive substances possessing remarkable biological properties, that include anti-cancer, antioxidant, antimicrobial, antibacterial, antifungal, anti-HIV, antihypertension, anti-coagulant, antiviral, anti-inflammatory, analgesics, anti-diabetic, or anti-depressive effects. The anti-cancer activity of coumarin and its derivatives has been described to be directed against numerous types of cancers such as prostate, renal, breast, laryngeal, lung, colon, CNS, leukemia, and malignant melanoma [60, 61]. Moreover, coumarins except for the cancer treatment support have the potential to neutralize the side effects associated with radiotherapy. The anti-cancer effect of coumarin-based agents like coumarin-triazole, coumarin-chalcone, coumarin-thiosemicarbazone derivatives, coumarin-metal complexes, furanocoumarin, pyranocoumarin, pyrone-substituted coumarin, and their derivatives have been found more potent than that of simple coumarin. Due to the great potential of coumarin scaffold it is extensively used in drug design and development. This scaffold is frequently used for designing small molecules with various biological activities. Coumarin nucleus possesses six positions available for substituents. The selection of functional groups suitable for anti-cancer activity is facilitated by three of those positions (C-3, C-4, and C-7), while the remaining positions (C-5, C-6, and C-8) are either never studied or are hardly ever used [62, 63]. Medicinal chemists and drug designers pay much attention to both natural and synthetic coumarin-based compounds for the sake of their diverse pharmacological and biological properties. Numerous therapeutic drugs containing coumarin scaffolds have become commercially used pharmacological agents extensively utilized in the clinic, such as Armillarisin (Antibiotic), Novobiocin (Antibiotic), Warfarin (Anti-Coagulant), Phenprocoumon (Anti-coagulant), or hymecromone (choleretic and antispasmodic). In addition, as coumarins can act on numerous receptors or signal transduction pathways that play a role as induction factors of cancer, they can serve as multi-targeted therapy against cancer development and progression which turns out to be more efficient as compared to the single-targeted therapy. Notably, it has been demonstrated that different coumarin subtypes and their derivatives can prevent the growth and spread of innumerable cancer cells by a variety of mechanisms such as cell cycle arrest, induction of apoptosis, carbonic anhydrase inhibition, inhibition of topoisomerase, kinase and Hsp90 inhibition, monocarboxylate transporter inhibition, sulfatase inhibition, inhibition of angiogenesis and DNA synthesis inhibition, inhibition of thioredoxin reductase, microtubule polymerization, ROS generation, regulation of STAT-3 and NF-κB factor, and expression of PI3- and MAP kinases [64].
2.1.5 Carotenoids
Carotenoids, a group of nearly 600 compounds, constitute a ubiquitous group of isoprenoids, lipid-soluble pigments, produced in photoautotrophic plants, that are structurally similar to vitamin A. Provitamin A activity possesses approximately 50 of these compounds. It has been reported vitamin A, carotenoids, and provitamin A carotenoids can be effective antioxidants as they are very efficient in electron transfer reactions acting as physical quenchers of singlet oxygen, scavengers of other ROS, and stabilizers of peroxyl radicals by the hydrophobic chain of polyene units. In general, the longer the polyene chain, the greater the peroxyl radical stabilizing ability. It is quite clear that carotenoids can inhibit the propagation of radical-initiated lipid peroxidation, supporting their definition of antioxidants [65, 66, 67]. They interact synergistically with other antioxidants; mixtures of carotenoids are more effective than single compounds. The antioxidant potential of carotenoids is of particular significance to human health as an altered balance of antioxidants-ROS mediates oxidative stress, which is a critical factor in the pathogenesis of various chronic diseases. Regular intake of carotenoids has been associated with risk reduction for several chronic diseases, including the reduced incidence of obesity, type 2 diabetes, certain types of cancer, and even lower total mortality [68, 69]. Data coming from epidemiological studies and clinical trials have confirmed that carotenoids (β-carotene, α-carotene, lycopene, β-cryptoxanthin, zeaxanthin, crocin/crocetin, curcumin, and lutein) present in human serum were able to act as anticarcinogenic agents. Interestingly, carotenoids and retinoids have been shown to exert similar biological activities resulting in the inhibition of malignant tumor growth and the induction of apoptosis. Cancer chemoprevention derived by dietary carotenoids acts through PI3K/AKT/mTOR, and ERK-MAPK pathways and induces apoptotic processes through PPARs, IFNs, and RAR/RXR, which are the p53 intermediates in cancer signaling and play a role in the immune system and cellular differentiation. Moreover, carotenoids can stimulate immune system by supporting the B- and T-lymphocytes proliferation, activating the macrophages and cytotoxic T-cells, promoting the effector T-cell function, and the production of cytokines. Supplementation with carotenoids can thus influence a large number of downstream targets that can affect cell growth and modulate gene expression and immune responses [70, 71, 72]. Additionally, human studies have shown that carotenoids can protect the skin against photooxidative damage, and lutein, and zeaxanthin may be protective in eye diseases due to their ability to absorb damaging blue light that enters the eye. Epidemiological studies have demonstrated a positive correlation between an effective carotenoid intake with a breast, cervical, ovarian, colorectal cancers reduction and cardiovascular and eye diseases mitigation, indicating that carotenoids act in a dose- and time-dependent manner. The primary sources of carotenoids include a variety of fruits and vegetables, such for example the major sources of lycopene are tomato and tomato products [73, 74, 75]. Interestingly, astaxanthin, a natural carotenoid with superior antioxidant activity compared to other carotenoids, is known to exhibit a plethora of pharmacological effects. The protective effect of astaxanthin has been described on age-related diseases of multiple organs such as neurodegenerative diseases (such as Alzheimer’s disease or stroke); bone-related diseases (like osteoarthritis or osteoporosis); cancers; cardiovascular disorders (such as myocardial infarction, hypertension, or atherosclerosis), complications associated with diabetes; eye, gastric, kidney, or liver disorders; and pulmonary, muscle, or skin disorders [76]. The specific structure of carotenoids, especially the conjugated double-bond system, determines many of the fundamental properties of these molecules. Carotenoid’s structure affects their incorporation into biological membranes, as well as their interactions with ROS. In addition, the interaction of carotenoids with other co-antioxidants, primarily vitamins E and C significantly enhances their effectivity as antioxidants. However, carotenoids may lose their antioxidant effectivity at high concentrations or at high partial pressures of oxygen. Thus, the regulatory mechanisms of antioxidant and anti-inflammatory properties among carotenoids should be taken into consideration during the development of more effective therapeutic strategies to prevent chronic diseases [75, 77].
2.1.6 Vitamins and minerals
Micronutrients such as vitamins and minerals must meet their required daily intake as they act as immunomodulators and protect the host immune response and are thus essential for a healthy life. Vitamins are organic compounds, of which vitamin B1 was identified first, approximately 110 years ago, as an anti-beriberi factor. Thirteen groups of vitamins have been identified, nine water-soluble and four fat-soluble. Vitamin insufficiency is characterized by a specific deficiency symptom, such as peripheral neuropathy, dermatitis, blindness, rickets, and bleeding. The primary role of vitamins is to function as cofactors for functional proteins, such as enzymes and transcription factors. The importance of micronutrients is tightly connected to their involvement in various intracellular biological processes, biochemical signaling pathways, and synthesis of macromolecules, such as collagens, or small molecules that are necessary to maintain homeostasis (serotonin, adrenaline). Deficiencies in plasma concentrations of vitamins and minerals can lead to altered immune system function, contributing to unfavorable immunological conditions. The most prominent micronutrients are vitamins A, B, C, D, and E, riboflavin, β-carotene, selenium, zinc, and iron [78, 79]. Although beneficial effects of vitamins and minerals due to their rich bioactive properties are undoubtable, their over-supplementation may be associated with no protective effect or even harm [80, 81, 82, 83, 84, 85, 86]. However, numerous studies show health-promoting activities of vitamins and minerals exerting anti-cancer, anti-aging, immune-promoting effects, stroke prevention, preventing various medical conditions such as sepsis, ischemia, lifestyle-related diseases, etc. [78, 79, 87, 88, 89, 90, 91].
2.2 Natural antioxidants of animal origin
Meat and meat products are rich not only in essential nutrients but also possess a high nutritional value as they are rich in bioactive components that are either not found in other groups of food products or are found only in limited amounts. These compounds include primarily taurine, l-carnitine, choline, alpha-lipoic acid, conjugated linoleic acid, glutathione, creatine, coenzyme Q10, and bioactive peptides. Their antioxidant and health-promoting properties connected with their lipid-lowering, antihypertensive, anti-inflammatory, and immunomodulatory activities have been described. Bioactive substances (di- and tripeptides such as carnosine, anserine, glutathione, and ophidine) found in meat, poultry, and fish also exhibit a broad spectrum of health-promoting effects. They have been found to significantly improve lipid parameters, contribute to arterial blood pressure decrease, through inhibition of pro-inflammatory cytokines production reduce inflammatory conditions, promote detoxification, exhibit antibacterial and antimicrobial properties, reduce fat mass, and contribute to the organism protection from oxidative stress. Moreover, they participate in carbohydrate and lipid metabolism and are involved in energy generation in mitochondria [92].
Poultry eggs are considered one of the best sources of bioactive peptides producing pepsin, trypsin, chymotrypsin, and proteases produced peptides with antioxidant activities. Among the egg white proteins, ovalbumin, ovotransferrin, lysozyme, ovomucin, and ovomucoid are used to produce peptides with antioxidant activity. Structurally similar carnosine, anserine, and ophidine mediate free radicals scavenging as they inhibit lipid oxidation catalyzed by ionic iron, hydrogen peroxide-activated hemoglobin, or singlet oxygen. Glutathione is known to act as an antioxidant by neutralizing ROS and preventing oxidant-mediated cell death. However, these health-promoting activities depend on the dose of the bioactive compounds, and their amount present in meat depends on breed, age, gender, and breeding program [93, 94].
l-Carnosine (beta-alanyl-l-histidine) is a water-soluble endogenous dipeptide composed of β-alanine and l-histidine requiring carnosine synthetase and ATP for its biosynthesis. It can be found in the brain, kidneys, and skeletal muscles of fish, birds, and mammals. Studies have demonstrated that l-carnosine is capable of sequestering reactive oxygen and nitrogen species, thus exhibiting considerable antioxidant properties. Moreover, due to its ability to form complexes with metal ions (such as copper, iron, zinc, or cobalt), l-carnosine protects cells from injury. In addition, l-carnosine in combination with α-tocopherol exhibits a synergistic action [95].
l-Carnitine, a water-soluble quaternary amine, is synthesized in mammalian kidneys and the liver from lysine and methionine in the presence of vitamin C, iron, vitamin B6, and niacin. It is a conditionally essential nutrient for humans and animals as approximately only 25% of carnitine intake is synthesized in the body while the remaining 75% must be supplied with the diet. l-Carnitine plays a fundamental role in the generation of energy as it is involved in the activated long-chain fatty acids transport from the cytoplasm across the inner mitochondrial membrane, thus stimulating β-oxidation which plays an important role in human physiology. l-Carnitine through its antioxidant and anti-inflammatory properties plays a major part in protecting cellular membranes, preventing fatty acid accumulation and elimination of toxic metabolites [96].
Coenzyme Q10 also referred to as ubiquinone, is a fat-soluble vitamin-like compound. It can be found throughout the body, with its highest amount detected in muscles, the spleen, pancreas, heart, liver, kidneys, and the brain. The presence of vitamins B2, B6, B12, folic, and pantothenic acids lead to enhanced coenzyme production. The importance of ubiquinone is connected with the production of high-energy ATP molecules. Variable harmful conditions are associated with coenzyme Q10 deficiency, such as osteoporosis, fibromyalgia, cardiovascular diseases, neurodegenerative diseases, diabetes, periodontitis, nephropathies, or male infertility. Due to its antioxidant properties, it protects DNA against oxidative damage, while it also exhibits the capacity to regenerate other antioxidants, such as tocopherol or ascorbic acid, and prevents membrane lipids from peroxidation. It has been also suggested that ubiquinol (the reduced form of ubiquinone) participates in scavenging free radicals formed as a result of the metabolism of certain xenobiotics (e.g., anthracycline antibiotics) [97].
3. Synthetic antioxidants
One of the major disadvantage of using natural antioxidants as food additives is their insufficiency to cover the needs of the food industry due to extraction complications and limited stability [98]. The reason of increasing need of antioxidant supplementation in food arises from the oxidative damage caused by free radicals that lead to food spoilage, causing an unpleasant odor, loss of taste, and damaged tissues. Thus, as one of the most important aims of food technology is safety and taste, color, texture protection, appearance, nutritional value, and shelf life, synthetic antioxidants represent an advantageous alternative to natural antioxidants. Synthetic antioxidants cover a diverse range of raw materials, providing flexibility in their production, are cost-effective with minimal possible side effects [99]. Well-known and commonly used in the food industry are synthetic phenolic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tertiary butylhydroquinone (TBHQ), or propyl gallate (PG) [100]. The role of synthetic antioxidants in food stability is very important. They interact with peroxides generated by food during storage, and thereby help to protect food from spoilage under specific conditions, contributing to improved quality, stability and extended shelf life. When used correctly, they provide consumers with safer food and make economic aspects more efficient for producers [101]. However, the use of antioxidants in foods must be limited by the regulatory laws because consumption of synthetic antioxidants has to be controlled due to their toxic effects and carcinogenicity. Moreover, the inappropriate use of dietary supplements may result in antioxidative stress. Overdoses of antioxidant supplements cause negative effects as both, antioxidative and oxidative stresses lead to the imbalance of the antioxidant status of the organism causing damaging alterations to the cells that might result in carcinogenesis and aging [102, 103].
Antioxidant activity of butylated hydroxytoluene (BHT) is achieved by various mechanisms leading to the ROS generation suppression or to the free radicals scavenging. BHT is capable to scavenge reactive species that induce peroxidation, as it possesses metal chelating activity, particularly iron ions that catalyze oxidative processes leading to the release of hydroxyl radicals which in turn chelate hydroperoxides by Fenton reactions. Moreover, BHT scavenges O2•− mediated peroxides formation, assists in hindering the chain reactions of autoxidation, and reduces the local levels of O2. By the EU law, BHT (E 321) is permissible to be administered in fats and oils, both alone or in combination with other antioxidants, at maximum levels of up to 100 mg/kg expressed as fat [104].
Butylated hydroxyanisole (BHA) is one of the phenolic antioxidants synthetized from the isomers 3-tert-butyl-4-hydroxyanisole (3-BHA) and 2-tert-butyl-4-hydroxyanisole (2-BHA) and can be used as food additive to prevent oxidation. Moreover, analyses performed with BHA have revealed that it can protect animals from chemically induced toxicity. In contrast to its beneficial effects, BHA has been shown to exhibit cytotoxic and carcinogenic effects. However, it has been demonstrated on a variety model system that BHA is not mutagenic in Salmonella with or without S9 metabolic activation, nor is BHA mutagenic in mammalian test systems in rat liver cells or Chinese hamster ovary cells. BHA belongs to many synthetic antioxidants widely used in different products including food, food packaging, pharmaceuticals, and cosmetics. Thus, due to its use as an antioxidant, exposure of BHA to human occurs not only from food but also in pharmaceuticals and cosmetic products [101, 105].
The maximum estimated daily intake of BHA from personal care products in Canada is 0.06–0.07 mg/kg/day [106].
Tertiary butylhydroquinone (TBHQ) is a synthetic antioxidant commonly used to enhance the effect of insulin
4. Conclusions
Alterations to the redox balance of the organism result in oxidative stress leading to oxidative damage of biologically significant macromolecules that is a predominant cause of the development of chronic and age-related diseases. Substances possessing bioactive properties become the leading choice to treat or prevent such health-disturbing processes. The correlation between human health and the consumption of healthy and functional foods is confirmed by the development of medical sciences, dietetics, and nutrigenomics. With increasing awareness, consumers considerably prefer food with an added value containing either naturally occurring bioactive compounds, or enriched by natural bioactive ingredients, or enriched by synthetic antioxidants. Food fortification with biologically active substances becomes a powerful tool for increasing food quality. Although, due to concerns over the adverse health effects of some synthetic antioxidants, consumption of natural products is largely preferred. The use of plant extracts as antioxidants is becoming increasingly popular and is widely accepted by consumers. As food additives derived from natural raw materials are considered safe, plant extracts are a preferred source of antioxidants. Thus, growing interest in food with added value will prompt scientists to accelerate the research and technology to develop better ways of producing foodstuffs and food supplements containing higher amounts of health-promoting compounds. Enrichment of food products with antioxidants such as polyphenols will beneficially influence not only their oxidative stability but also, due to their enhanced intake to the human organism, they may reduce the incidence of degenerative diseases. Thus, the future research directions in the field of antioxidants and their applications will include characterization of novel substances with bioactive properties of either natural or synthetic origin that have great potential in health maintenance or improvement. In recent times, there has been a significant focus on thoroughly analyzing the combined effect of various types of antioxidants with the belief of synergistic positive action of tested molecules. Moreover, researchers are actively exploring the possibility of developing nano-antioxidants and nanoparticle carriers for antioxidants that can effectively increase their utilization by the body. By doing so, it is hoped that the potential health benefits of antioxidants can be effectively enhanced. In addition, the application of antioxidants into the food and medical healthcare products is also a matter of intensive science. This research area focuses on innovation and improvement of extraction, preservation, and processing technologies to maintain health-promoting properties of antioxidant molecules in the final products. These advancements are aimed to possess significant potential to improve the quality and effectiveness of healthcare products and contribute to better overall health outcomes.
Acknowledgments
This work was financially supported by the Slovak Research and Development Agency under the contract number APVV-22-0294, the SUA grant agency project 12-GASPU-2021, and the project Vega 1/0583/23.
References
- 1.
Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of Medicinal Chemistry. 2015; 97 :55-74. DOI: 10.1016/j.ejmech.2015.04.040 - 2.
Ďúranová H, Šimora V, Ďurišová Ľ, Olexiková L, Kovár M, Požgajová M. Modifications in ultrastructural characteristics and redox status of plants under environmental stress: A review. Plants (Basel). 2023; 12 (8):1666. DOI: 10.3390/plants12081666 - 3.
Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-Biological Interactions. 2014; 224 :164-175. DOI: 10.1016/j.cbi.2014.10.016 - 4.
Jomova K, Raptova R, Alomar SY, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Archives of Toxicology. 2023; 97 (10):2499-2574. DOI: 10.1007/s00204-023-03562-9 - 5.
Berger MM. Can oxidative damage be treated nutritionally? Clinical Nutrition. 2005; 24 (2):172-183. DOI: 10.1016/j.clnu.2004.10.003 - 6.
Liang J, Gao Y, Feng Z, Zhang B, Na Z, Li D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biology. 2023; 62 :102659. DOI: 10.1016/j.redox.2023.102659 - 7.
Gielecińska A, Kciuk M, Mujwar S, et al. Substances of natural origin in medicine: Plants vs. cancer. Cells. 2023; 12 (7):1-34. DOI: 10.3390/cells12070986 - 8.
Chaudhary P, Janmeda P, Docea AO, et al. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Frontiers in Chemistry. 2023; 11 :1158198. DOI: 10.3389/fchem.2023.1158198 - 9.
Chagas M d SS, Behrens MD, Moragas-Tellis CJ, GXM P, Silva AR, Gonçalves-de-Albuquerque CF. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxidative Medicine and Cellular Longevity. 2022; 2022 :e9966750. DOI: 10.1155/2022/9966750 - 10.
Van Hung P. Phenolic compounds of cereals and their antioxidant capacity. Critical Reviews in Food Science and Nutrition. 2016; 56 (1):25-35. DOI: 10.1080/10408398.2012.708909 - 11.
Duan L, Zhang C, Zhao Y, Chang Y, Guo L. Comparison of bioactive phenolic compounds and antioxidant activities of different parts of Taraxacum mongolicum . Molecules. 2020;25 (14):3260. DOI: 10.3390/molecules25143260 - 12.
Amarowicz R, Pegg RB. The potential protective effects of phenolic compounds against low-density lipoprotein oxidation. Current Pharmaceutical Design. 2017; 23 (19):2754-2766. DOI: 10.2174/1381612823666170329142936 - 13.
Saoudi MM, Bouajila J, Alouani K. Phenolic compounds of Rumex roseus L. extracts and their effect as antioxidant and cytotoxic activities. BioMed Research International. 2021;2021 :e2029507. DOI: 10.1155/2021/2029507 - 14.
Gonçalves S, Moreira E, Grosso C, Andrade PB, Valentão P, Romano A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. Journal of Food Science and Technology. 2017; 54 (1):219-227. DOI: 10.1007/s13197-016-2453-z - 15.
Lopes M, Sanches-Silva A, Castilho M, Cavaleiro C, Ramos F. Halophytes as source of bioactive phenolic compounds and their potential applications. Critical Reviews in Food Science and Nutrition. 2023; 63 (8):1078-1101. DOI: 10.1080/10408398.2021.1959295 - 16.
Sehrawat R, Rathee P, Akkol EK, et al. Phenolic acids—Versatile natural moiety with numerous biological applications. Current Topics in Medicinal Chemistry; 22 (18):1472-1484 - 17.
Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports. 2019; 24 :e00370. DOI: 10.1016/j.btre.2019.e00370 - 18.
Hassani S, Ghanbari F, Lotfi M, et al. How gallic acid regulates molecular signaling: Role in cancer drug resistance. Medical Oncology. 2023; 40 (11):308. DOI: 10.1007/s12032-023-02178-4 - 19.
Ow YY, Stupans I. Gallic acid and gallic acid derivatives: Effects on drug metabolizing enzymes. Current Drug Metabolism. 2003; 4 (3):241-248. DOI: 10.2174/1389200033489479 - 20.
Tuli HS, Mistry H, Kaur G, et al. Gallic acid: A dietary polyphenol that exhibits anti-neoplastic activities by modulating multiple oncogenic targets. Anti-Cancer Agents in Medicinal Chemistry. 2022; 22 (3):499-514. DOI: 10.2174/1871520621666211119085834 - 21.
Verma S, Singh A, Mishra A. Gallic acid: Molecular rival of cancer. Environmental Toxicology and Pharmacology. 2013; 35 (3):473-485. DOI: 10.1016/j.etap.2013.02.011 - 22.
Jiang Y, Pei J, Zheng Y, Miao YJ, Duan BZ, Huang LF. Gallic acid: A potential anti-cancer agent. Chinese Journal of Integrative Medicine. 2022; 28 (7):661-671. DOI: 10.1007/s11655-021-3345-2 - 23.
Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Suresh KC. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomedicine & Pharmacotherapy. 2018; 108 :547-557. DOI: 10.1016/j.biopha.2018.09.069 - 24.
Ogut E, Armagan K, Gül Z. The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metabolic Brain Disease. 2022; 37 (4):859-880. DOI: 10.1007/s11011-022-00960-3 - 25.
Sabahi Z, Khoshnoud MJ, Hosseini S, Khoshraftar F, Rashedinia M. Syringic acid attenuates cardiomyopathy in Streptozotocin-induced diabetic rats. Advances in Pharmacological and Pharmaceutial Sciences. 2021; 2021 :5018092. DOI: 10.1155/2021/5018092 - 26.
Liu G, Zhang BF, Hu Q , Liu XP, Chen J. Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3β signaling pathway. Biochemical and Biophysical Research Communications. 2020; 531 (2):242-249. DOI: 10.1016/j.bbrc.2020.07.047 - 27.
Ha SJ, Lee J, Park J, et al. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochemical Pharmacology. 2018; 154 :435-445. DOI: 10.1016/j.bcp.2018.06.007 - 28.
Lashgari NA, Roudsari NM, Momtaz S, Abdolghaffari AH, Atkin SL, Sahebkar A. Regulatory mechanisms of vanillic acid in cardiovascular diseases: A review. Current Medicinal Chemistry. 2023; 30 (22):2562-2576. DOI: 10.2174/0929867329666220831152608 - 29.
Yalameha B, Nejabati HR, Nouri M. Cardioprotective potential of vanillic acid. Clinical and Experimental Pharmacology & Physiology. 2023; 50 (3):193-204. DOI: 10.1111/1440-1681.13736 - 30.
Shekari S, Khonsha F, Rahmati-Yamchi M, Nejabati HR, Mota A. Vanillic acid and non-alcoholic fatty liver disease: A focus on AMPK in adipose and liver tissues. Current Pharmaceutical Design. 2021; 27 (46):4686-4692. DOI: 10.2174/1381612827666210701145438 - 31.
Singh B, Kumar A, Singh H, Kaur S, Arora S, Singh B. Protective effect of vanillic acid against diabetes and diabetic nephropathy by attenuating oxidative stress and upregulation of NF-κB, TNF-α and COX-2 proteins in rats. Phytotherapy Research. 2022; 36 (3):1338-1352. DOI: 10.1002/ptr.7392 - 32.
Yao X, Jiao S, Qin M, Hu W, Yi B, Liu D. Vanillic acid alleviates acute myocardial hypoxia/reoxygenation injury by inhibiting oxidative stress. Oxidative Medicine and Cellular Longevity. 2020; 2020 :8348035. DOI: 10.1155/2020/8348035 - 33.
Osorio-Paz I, Valle-Jiménez X, Brunauer R, Alavez S. Vanillic acid improves stress resistance and substantially extends life span in Caenorhabditis elegans . The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2023;78 (7):1100-1107. DOI: 10.1093/gerona/glad086 - 34.
Masella R, Santangelo C, D’Archivio M, Li Volti G, Giovannini C, Galvano F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Current Medicinal Chemistry. 2012; 19 (18):2901-2917. DOI: 10.2174/092986712800672102 - 35.
Song J, He Y, Luo C, et al. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacological Research. 2020; 161 :105109. DOI: 10.1016/j.phrs.2020.105109 - 36.
Bai L, Han X, Kee HJ, et al. Protocatechuic acid prevents isoproterenol-induced heart failure in mice by downregulating kynurenine-3-monooxygenase. Journal of Cellular and Molecular Medicine. 2023; 27 (16):2290-2307. DOI: 10.1111/jcmm.17869 - 37.
Wang Q , Ren X, Wu J, et al. Protocatechuic acid protects mice from influenza A virus infection. European Journal of Clinical Microbiology & Infectious Diseases. 2022; 41 (4):589-596. DOI: 10.1007/s10096-022-04401-y - 38.
Saleem M, Fariduddin Q , Castroverde CDM. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiology and Biochemistry. 2021; 168 :381-397. DOI: 10.1016/j.plaphy.2021.10.011 - 39.
Zaid A, Mohammad F, Wani SH, Siddique KMH. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicology and Environmental Safety. 2019; 180 :575-587. DOI: 10.1016/j.ecoenv.2019.05.042 - 40.
Sharma A, Sidhu GPS, Araniti F, et al. The role of salicylic acid in plants exposed to heavy metals. Molecules. 2020; 25 (3):540. DOI: 10.3390/molecules25030540 - 41.
Guo B, Liu C, Liang Y, Li N, Fu Q. Salicylic acid signals plant defence against cadmium toxicity. International Journal of Molecular Sciences. 2019; 20 (12):2960. DOI: 10.3390/ijms20122960 - 42.
AAlikhani M, Khalili M, Jahanshahi M. The natural iron chelators’ ferulic acid and caffeic acid rescue mice’s brains from side effects of iron overload. Frontiers in Neurology. 2022; 13 :951725. DOI: 10.3389/fneur.2022.951725 - 43.
Yeh YH, Lee YT, Hsieh HS, Hwang DF. Dietary caffeic acid, ferulic acid and coumaric acid supplements on cholesterol metabolism and antioxidant activity in rats. Journal of Food and Drug Analysis. 2020; 17 (2):4. DOI: 10.38212/2224-6614.2292 - 44.
Figueredo KC, Guex CG, Graiczik J, et al. Caffeic acid and ferulic acid can improve toxicological damage caused by iron overload mediated by carbonic anhydrase inhibition. Drug and Chemical Toxicology. Published online November 29. 2022:144-155. DOI: 10.1080/01480545.2022.2152043 - 45.
Bocco BM, Fernandes GW, Lorena FB, et al. Combined treatment with caffeic and ferulic acid from Baccharis uncinella C. DC. (Asteraceae) protects against metabolic syndrome in mice. Brazilian Journal of Medical and Biological Research. 2016;49 (3):e5003. DOI: 10.1590/1414-431X20155003 - 46.
He XX, Yang XH, Ou RY, et al. Synthesis and evaluation of multifunctional ferulic and caffeic acid dimers for Alzheimer’s disease. Natural Product Research. 2017; 31 (6):734-737. DOI: 10.1080/14786419.2016.1219862 - 47.
Serafim TL, Carvalho FS, Marques MPM, et al. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chemical Research in Toxicology. 2011; 24 (5):763-774. DOI: 10.1021/tx200126r - 48.
Ferreira PS, Victorelli FD, Fonseca-Santos B, Chorilli M. A review of analytical methods for p-coumaric acid in plant-based products, beverages, and biological matrices. Critical Reviews in Analytical Chemistry. 2019; 49 (1):21-31. DOI: 10.1080/10408347.2018.1459173 - 49.
Pei K, Ou J, Huang J, Ou S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. Journal of the Science of Food and Agriculture. 2016; 96 (9):2952-2962. DOI: 10.1002/jsfa.7578 - 50.
Yue Y, Shen P, Xu Y, Park Y. p-Coumaric acid improves oxidative and osmosis stress responses in Caenorhabditis elegans . Journal of the Science of Food and Agriculture. 2019;99 (3):1190-1197. DOI: 10.1002/jsfa.9288 - 51.
Mani A, Kushwaha K, Khurana N, Gupta J. p-Coumaric acid attenuates high-fat diet-induced oxidative stress and nephropathy in diabetic rats. Journal of Animal Physiology and Animal Nutrition (Berl). 2022; 106 (4):872-880. DOI: 10.1111/jpn.13645 - 52.
Ayazoglu Demir E, Mentese A, Kucuk H, Turkmen Alemdar N, Demir S. p-Coumaric acid alleviates cisplatin-induced ovarian toxicity in rats. The Journal of Obstetrics and Gynaecology Research. 2022; 48 (2):411-419. DOI: 10.1111/jog.15119 - 53.
Ross JA, Kasum CM. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annual Review of Nutrition. 2002; 22 (1):19-34. DOI: 10.1146/annurev.nutr.22.111401.144957 - 54.
Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants & Redox Signaling. 2013; 18 (14):1818-1892. DOI: 10.1089/ars.2012.4581 - 55.
George BP, Chandran R, Abrahamse H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants. 2021; 10 (9):1455. DOI: 10.3390/antiox10091455 - 56.
Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proceedings of the Nutrition Society. 2010; 69 (3):273-278. DOI: 10.1017/S002966511000162X - 57.
Dias MC, Pinto DCGA, Silva AMS. Plant flavonoids: Chemical characteristics and biological activity. Molecules. 2021; 26 (17):5377. DOI: 10.3390/molecules26175377 - 58.
Galleano M, Verstraeten SV, Oteiza PI, Fraga CG. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Archives of Biochemistry and Biophysics. 2010; 501 (1):23-30. DOI: 10.1016/j.abb.2010.04.005 - 59.
Slika H, Mansour H, Wehbe N, et al. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomedicine and Pharmacotherapy. 2022; 146 :112442. DOI: 10.1016/j.biopha.2021.112442 - 60.
Bhattarai N, Kumbhar AA, Pokharel YR, Yadav PN. Anticancer potential of coumarin and its derivatives. Mini Reviews in Medicinal Chemistry. 2021; 21 (19):2996-3029. DOI: 10.2174/1389557521666210405160323 - 61.
Xia D, Liu H, Cheng X, Maraswami M, Chen Y, Lv X. Recent developments of coumarin-based hybrids in drug discovery. Current Topics in Medicinal Chemistry. 2022; 22 (4):269-283. DOI: 10.2174/1568026622666220105105450 - 62.
Patil SA, Kandathil V, Sobha A, et al. Comprehensive review on medicinal applications of coumarin-derived imine-metal complexes. Molecules. 2022; 27 (16):5220. DOI: 10.3390/molecules27165220 - 63.
Kaur M, Kohli S, Sandhu S, Bansal Y, Bansal G. Coumarin: A promising scaffold for anticancer agents. Anti-Cancer Agents in Medicinal Chemistry. 2015; 15 (8):1032-1048. DOI: 10.2174/1871520615666150101125503 - 64.
Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorganic Chemistry. 2020; 103 :104163. DOI: 10.1016/j.bioorg.2020.104163 - 65.
Bast A, Haenen GR, van den Berg R, van den Berg H. Antioxidant effects of carotenoids. International Journal for Vitamin and Nutrition Research. 1998; 68 (6):399-403 - 66.
Krinsky NI. The antioxidant and biological properties of the carotenoids. Annals of the New York Academy of Sciences. 1998; 854 :443-447. DOI: 10.1111/j.1749-6632.1998.tb09923.x - 67.
Palace VP, Khaper N, Qin Q , Singal PK. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radical Biology & Medicine. 1999; 26 (5-6):746-761. DOI: 10.1016/s0891-5849(98)00266-4 - 68.
Vrdoljak N. Carotenoids and carcinogenesis: Exploring the antioxidant and cell signaling roles of carotenoids in the prevention of cancer. Critical Reviews in Oncogenesis. 2022; 27 (3):1-13. DOI: 10.1615/CritRevOncog.2022045331 - 69.
Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014; 6 (2):466-488. DOI: 10.3390/nu6020466 - 70.
Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: Biochemistry, pharmacology and treatment. British Journal of Pharmacology. 2017; 174 (11):1290-1324. DOI: 10.1111/bph.13625 - 71.
Varghese R, Efferth T, Ramamoorthy S. Carotenoids for lung cancer chemoprevention and chemotherapy: Promises and controversies. Phytomedicine. 2023; 116 :154850. DOI: 10.1016/j.phymed.2023.154850 - 72.
Bohn T, Balbuena E, Ulus H, et al. Carotenoids in health as studied by omics-related endpoints. Advances in Nutrition. 2023; 14 (6):1538-1578. DOI: 10.1016/j.advnut.2023.09.002 - 73.
Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Molecular Aspects of Medicine. 2005; 26 (6):459-516. DOI: 10.1016/j.mam.2005.10.001 - 74.
Stahl W, Sies H. Antioxidant activity of carotenoids. Molecular Aspects of Medicine. 2003; 24 (6):345-351. DOI: 10.1016/s0098-2997(03)00030-x - 75.
Ba W, Xu W, Deng Z, Zhang B, Zheng L, Li H. The antioxidant and anti-inflammatory effects of the main carotenoids from tomatoes via Nrf2 and NF-κB signaling pathways. Nutrients. 2023; 15 (21):4652. DOI: 10.3390/nu15214652 - 76.
Alugoju P, Krishna Swamy VKD, Anthikapalli NVA, Tencomnao T. Health benefits of astaxanthin against age-related diseases of multiple organs: A comprehensive review. Critical Reviews in Food Science and Nutrition. 2023; 63 (31):10709-10774. DOI: 10.1080/10408398.2022.2084600 - 77.
Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Archives of Biochemistry and Biophysics. 2001; 385 (1):20-27. DOI: 10.1006/abbi.2000.2149 - 78.
Shiozawa K, Agista AZ, Ohsaki Y, Shirakawa H. Emergence of novel functions of vitamins for the prevention of life-style related diseases. Journal of Nutritional Science and Vitaminology (Tokyo). 2022; 68 (Supplement):S8-S10. DOI: 10.3177/jnsv.68.S8 - 79.
Mitra S, Paul S, Roy S, et al. Exploring the immune-boosting functions of vitamins and minerals as nutritional food bioactive compounds: A comprehensive review. Molecules. 2022; 27 (2):555. DOI: 10.3390/molecules27020555 - 80.
US Preventive Services Task Force, Mangione CM, Barry MJ, et al. Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US preventive services task force recommendation statement. Journal of the American Medical Association. 2022; 327 (23):2326-2333. DOI: 10.1001/jama.2022.8970 - 81.
Donkena KV, Karnes RJ, Young CYF. Vitamins and prostate cancer risk. Molecules. 2010; 15 (3):1762-1783. DOI: 10.3390/molecules15031762 - 82.
Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. The New England Journal of Medicine. 2019; 380 (1):33-44. DOI: 10.1056/NEJMoa1809944 - 83.
Aguilera-Méndez A, Boone-Villa D, Nieto-Aguilar R, Villafaña-Rauda S, Molina AS, Sobrevilla JV. Role of vitamins in the metabolic syndrome and cardiovascular disease. Pflügers Archiv. 2022; 474 (1):117-140. DOI: 10.1007/s00424-021-02619-x - 84.
Koekkoek WACK, van Zanten ARH. Antioxidant vitamins and trace elements in critical illness. Nutrition in Clinical Practice. 2016; 31 (4):457-474. DOI: 10.1177/0884533616653832 - 85.
Asplund K. Antioxidant vitamins in the prevention of cardiovascular disease: A systematic review. Journal of Internal Medicine. 2002; 251 (5):372-392. DOI: 10.1046/j.1365-2796.2002.00973.x - 86.
Hasanain B, Mooradian AD. Antioxidant vitamins and their influence in diabetes mellitus. Current Diabetes Reports. 2002; 2 (5):448-456. DOI: 10.1007/s11892-002-0110-6 - 87.
Bjørklund G, Peana M, Dadar M, et al. The role of B vitamins in stroke prevention. Critical Reviews in Food Science and Nutrition. 2022; 62 (20):5462-5475. DOI: 10.1080/10408398.2021.1885341 - 88.
Pedroza-García KA, Careaga-Cárdenas G, Díaz-Galindo C, Quintanar JL, Hernández-Jasso I, Ramírez-Orozco RE. Bioactive role of vitamins as a key modulator of oxidative stress, cellular damage and comorbidities associated with spinal cord injury (SCI). Nutritional Neuroscience. 2023; 26 (11):1120-1137. DOI: 10.1080/1028415X.2022.2133842 - 89.
Yan F, Zhao Q , Li Y, et al. The role of oxidative stress in ovarian aging: A review. Journal of Ovarian Research. 2022; 15 :100. DOI: 10.1186/s13048-022-01032-x - 90.
Fagbohun OF, Gillies CR, Murphy KPJ, Rupasinghe HPV. Role of antioxidant vitamins and other micronutrients on regulations of specific genes and signaling pathways in the prevention and treatment of cancer. International Journal of Molecular Sciences. 2023; 24 (7):6092. DOI: 10.3390/ijms24076092 - 91.
Jain A, Tiwari A, Verma A, Jain SK. Vitamins for cancer prevention and treatment: An insight. Current Molecular Medicine; 17 (5):321-340 - 92.
Kulczyński B, Sidor A, Gramza-Michałowska A. Characteristics of selected antioxidative and bioactive compounds in meat and animal origin products. Antioxidants. 2019; 8 (9):335. DOI: 10.3390/antiox8090335 - 93.
Bhat ZF, Kumar S, Bhat HF. Bioactive peptides of animal origin: A review. Journal of Food Science and Technology. 2015; 52 (9):5377-5392. DOI: 10.1007/s13197-015-1731-5 - 94.
Abeyrathne EDNS, Nam K, Huang X, Ahn DU. Plant- and animal-based antioxidants’ structure, efficacy, mechanisms, and applications: A review. Antioxidants (Basel). 2022; 11 (5):1025. DOI: 10.3390/antiox11051025 - 95.
Budzeń S, Rymaszewska J. The biological role of carnosine and its possible applications in medicine. Advances in Clinical and Experimental Medicine. 2013; 22 (5):739-744 - 96.
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutrition and Metabolism. 2010; 7 (1):30. DOI: 10.1186/1743-7075-7-30 - 97.
Boreková M, Hojerová J, Koprda V, Bauerová K. Nourishing and health benefits of coenzyme Q10. Czech Journal of Food Sciences. 2008; 26 (4):229-241. DOI: 10.17221/1122-CJFS - 98.
Saad B, Sing Y, Nawi M, et al. Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chemistry. 2007; 105 (1):389-394. DOI: 10.1016/j.foodchem.2006.12.025 - 99.
Kim JM, Choi SH, Shin GH, et al. Method validation and measurement uncertainty for the simultaneous determination of synthetic phenolic antioxidants in edible oils commonly consumed in Korea. Food Chemistry. 2016; 213 :19-25. DOI: 10.1016/j.foodchem.2016.06.053 - 100.
Rodil R, Quintana JB, Cela R. Oxidation of synthetic phenolic antioxidants during water chlorination. Journal of Hazardous Materials. 2012; 199-200 :73-81. DOI: 10.1016/j.jhazmat.2011.10.058 - 101.
Xu X, Liu A, Hu S, et al. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chemistry. 2021; 353 :129488. DOI: 10.1016/j.foodchem.2021.129488 - 102.
Hassanpour SH, Doroudi A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna Journal of Phytomedicine. 2023; 13 (4):354-376. DOI: 10.22038/AJP.2023.21774 - 103.
Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Medicine and Cellular Longevity. 2013; 2013 :e956792. DOI: 10.1155/2013/956792 - 104.
Blundell R, Shah MA, Azzopardi JI, Iqbal S, Rasul A, Shah GM. Chapter 3.3—Butylated hydroxytoluene. In: Nabavi SM, Silva AS, editors. Antioxidants Effects in Health. United States: Elsevier Inc.; 2022. pp. 195-200. DOI: 10.1016/B978-0-12-819096-8.00033-1 - 105.
Felter SP, Zhang X, Thompson C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regulatory Toxicology and Pharmacology. 2021; 121 :104887. DOI: 10.1016/j.yrtph.2021.104887 - 106.
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the re-evaluation of butylated hydroxyanisole—BHA (E 320) as a food additive. EFSA Journal. 2011; 9 (10):2392. DOI: 10.2903/j.efsa.2011.2392 - 107.
Zhu TT, Zhu CN, Qiu Y, et al. Tertiary butylhydroquinone alleviated liver steatosis and increased cell survival via β-arrestin-2/PI3K/AKT pathway. Iranian Journal of Basic Medical Sciences. 2021; 24 (10):1428-1436. DOI: 10.22038/ijbms.2021.58156.12924 - 108.
Song H, Xu Y, Yang X, Rong X, Wang Y, Wei N. Tertiary butylhydroquinone alleviates gestational diabetes mellitus in C57BL/KsJ-Lep db/+ mice by suppression of oxidative stress. Journal of Cellular Biochemistry. 2019; 120 (9):15310-15319. DOI: 10.1002/jcb.28798