Abstract
An in situ X-ray photoelectron spectroscopy (XPS) investigation has revealed that silver metal clusters (Ag-NCs) can enhance the redox property of cerium oxide (CeO2) at relatively lower temperatures by oxidizing Ag-NCs to Ag2+ and Ag3+ states. Strong metal support interaction (SMSI) effect at the interface is indicated by a specific interaction seen in high-resolution transmission electron microscopy (HRTEM) images of CeO2/Ag-NCs, confirming Ag-NC encapsulation by deposited CeO2 layer after heating. Through chemisorption processes, the SMSI effect aids in the release of oxygen from the ceria surface by making a bond of Ag, O, and Ce. Consequently, employing silver as a supporting novel metal improves the redox behaviour of CeO2 at nearly 100°C. The band gap of cerium is modified because of this interaction as shown by UV-vis spectroscopy, influencing the electronic charge transport property of ceria. The current-voltage (I-V) characteristics in silver cluster supported ceria thin film verify the significant increase in current under visible light illumination as compared to the current in dark conditions. This renders that Ag-NCs supported CeO2 is suitable for photocatalysis and the capacitance-voltage (C-V) characteristic confirms the enhanced storage capacity of Ag-NCs /CeO2-based metal-oxide-semiconductor (MOS) devices qualifies it for use as non-volatile memory (NVM) devices.
Keywords
- thin film of cerium oxide
- silver cluster supported ceria
- low temperature redox reaction
- response under visible light
- NVM memory device
1. Introduction
Cerium metal is one of the most interesting element in Lanthanum group of periodic table as it shows different physical properties in oxidized form. It is well known that cerium metal ([Xe]
The most important nature of cerium oxide is that its oxygen adsorption and desorption property based on the experimental environment. Researchers were trying to go in a conclusion of that procedure by many ways but not succeed till now. Most of researchers are believe that cerium oxide changes its oxidation state by following Mars-van Krevelen (MvK) mechanism when one oxygen ion remove from the surface of ceria then two of neighboring
Introducing metals, the redox reaction temperature improved and occur at relatively lower temperature has been studied by many researchers [8, 9, 10, 11, 12]. Mostly the novel metals give the better result in that case compare to other metals. The growth condition of the sample determines the interaction between ceria and the corresponding metals. Many research on different structure of supported metals as single atom [13], cluster of metals [14], doping with ceria [15] has been studied for getting better redox reaction at relatively lower temperature. In general cerium oxide having higher oxygen storage and release capacity when it present in mixed valance state (presence of
The interaction and the corresponding structural change of cerium oxide with the supported metals are different in case of bulk as compare to the thin film of ceria. As it is already known that the formation of a thin film of a material from bulk can change the dimension of the material i.e. the material completely changes from 3D to 2D structure in nature. As a result the physical properties of the 2D structure is different from 3D one which affects direct on the electron transport of the material. Therefore, people are growing their interest on controlling the redox reaction of cerium oxide based thin films to make it applicable in form of devices. Considering the supported metal, as single atom and cluster or nanoparticles of a metal shows different properties. In some metals the cluster formation of metal changes its electronic structure.
Considering this situation, we have investigated the interaction between novel metal (silver Ag) cluster with cerium oxide thin film by varying temperature from 25 to
2. Experimental details
We have prepared the cerium oxide thin film by rf magnetron sputtering technique using 99.999% pure cerium oxide target. The
To form a MOS structure of this thin film combination we have again deposited cerium oxide capping layer of thickness 40 nm on Si/
3. Experimental results and discussions
The effect of heating on pristine ceria and silver cluster supported ceria was verified by performing the DSC study is presented in Figure 1(a). In case of pristine ceria thin film the endothermic peak appeared at 150 and
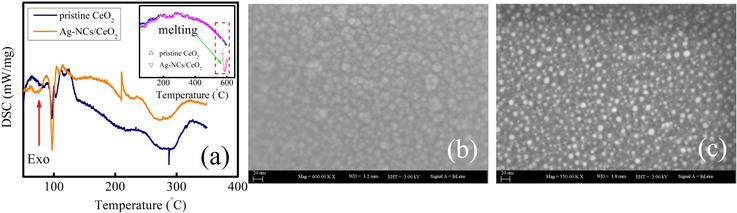
Figure 1.
(a) Is the DSC curve of pristine ceria and silver cluster supported ceria thin film, (b) is the SEM image of as deposited ceria thin film and (c) is the SEM image after depositing Ag-clusters on ceria.
GIXRD and TEM study has been performed at room temperature and after annealing under vacuum at
Based on the DSC study the in-situ XPS of both the samples has been performed and presented in Figures 2–5. From the XPS curve of Cerium 3d in absence of presence of silver metal clusters it is clearly observe that the improvement of the redox property of cerium oxide in silver cluster supported cerium oxide thin film. There is no effect of temperature on the redox property of ceria in case of pristine ceria whereas an improved redox property at relatively lower temperature as
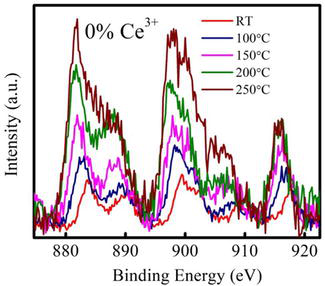
Figure 2.
Ce3d XPS spectra with temperature for pristine ceria thin film.
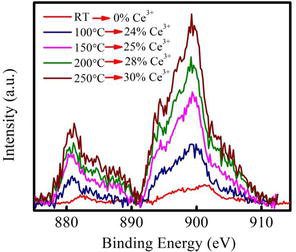
Figure 3.
Ce3d XPS spectra with temperature for Ag-NCs/ceria thin film.
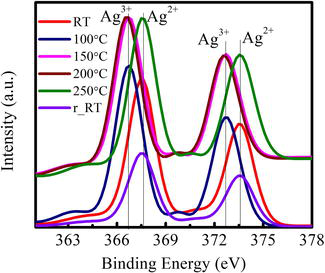
Figure 4.
Ag3d XPS spectra with temperature for Ag-NCs/ceria thin film.
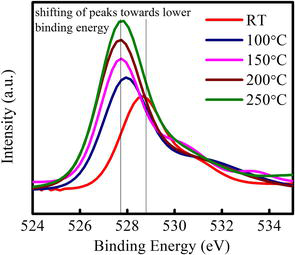
Figure 5.
O1S XPS spectra with temperature for Ag-NCs/ceria thin film.
The summary of the experiment is that the presence of silver affects the redox property of ceria in form of thin film after heat treatment. And the required temperature is relatively low in that case which can make this combination useful for application in low temperature catalytic activity of ceria.
3.1 Effect of light on novel metals
Over the past few decades the use of metal nanoparticles (NPs) to utilize light absorption grabbing the attention. As absorption of light is an interesting property of metal NPs and by introducing this NPs within oxides it can be used as a photocatalysis which is known as metal-induced photocatalysis (MIP) [20]. The effect of light absorptions of metal NPs under illumination of visible light is one of the challenging work in photocatalyst study. The localized surface plasmon resonance (LSPR) and the interband transition mainly the reason for the generation of MIP process in metal NPs [21]. Mostly for novel metal NPs the LSPR absorption occur near the visible light region. The metal oxides mostly
3.2 Application in optoelectronics
A schematic diagram of MOS structure of Ag-NCs/
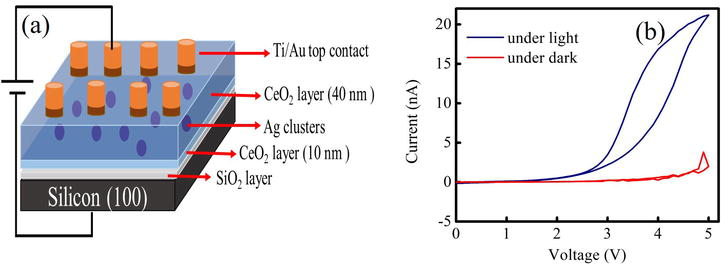
Figure 6.
(a) Is the schematic diagram of MOS based structure, (b) is the I-V curve of Ag cluster supported cerium oxide thin film under dark and illumination of visible light.
3.3 Application as non-volatile memory devices
The novel metal nanoparticle embedded high -
Figures 7 and 8 are representing the C-V characteristics of Ag-NCs/
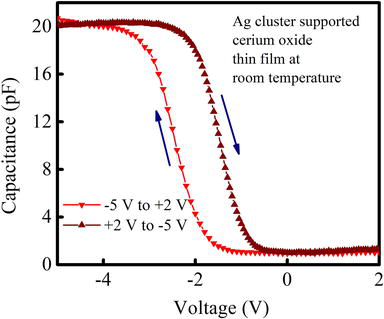
Figure 7.
High frequency C-V curve of as deposited Ag cluster supported cerium oxide thin film.
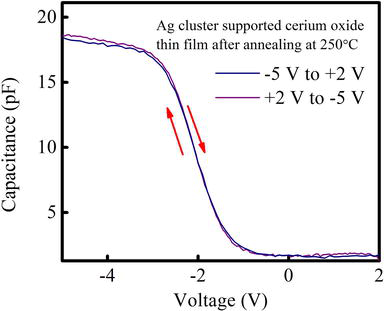
Figure 8.
High frequency C-V curve of Ag cluster supported cerium oxide thin film after heating at
4. Conclusions
We have studied the effect of interaction of silver metal in form of clusters of selected size with cerium oxide during heating and the corresponding change of electrical properties. Interaction of Ag-NCs with cerium oxide in form of thin film results the improvement of reduction temperature (at nearly
Acknowledgments
Authors wish to acknowledge Mr. Debraj Dey for helping in XPS and SEM measurement and Ms. Dimitra Das, Department of Physics, Jadavpur University, Kolkata for UV-Vis diffuse reflectance measurement. The first author wishes to acknowledge the University Grant Commission (UGC), Govt of India for financial help.
Abbreviations
differential scanning calorimetry | |
grazing incidence X-ray diffraction | |
X-ray photoelectron spectroscopy | |
non-volatile memory | |
strong metal-support interaction | |
metal-oxide-semiconductor | |
ultra high vaccum | |
transmission electron microscopy | |
high resolution transmission electron microscopy | |
localized surface plasmon resonance |
References
- 1.
Wu Y, Lin J, Ma G, Xu Y, Zhang J, Samart C, et al. Ni nanocatalysts supported on mesoporous Al2 O3-CeO2 for CO2 methanation at low temperature. RSC Advances. 2020; 10 (4):2067-2072 - 2.
Ramshanker N, Ganapathi KL, Bhat MS, Mohan S. Rf sputtered CeO2 thin films-based oxygen sensors. IEEE Sensors Journal. 2019; 19 (22):10821-10828 - 3.
Rehman S, Kim H, Khan MF, Hur J-H, Lee AD, Kee Kim D. Tuning of ionic mobility to improve the resistive switching behavior of Zn-doped CeO2. Scientific Reports. 2019; 9 :19387 - 4.
Suresh R, Ponnuswamy V, Sankar C, Manickam M, Mariappan R. Influence of Co concentration on the structural, optical, morphological and photo-diode properties of cerium oxide thin films. Ceramics International. 2016; 42 :12715-12725 - 5.
Matolin V, Sedlacek L, Matolinova I, Sutara F, Skala T, Smid B, et al. Photoemission spectroscopy study of Cu/CeO2 systems: Cu/CeO2 nanosized catalyst and CeO2(111)/Cu(111) inverse model catalyst. The Journal of Physical Chemistry C. 2008; 112 :3751-3758 - 6.
Henderson MA, Perkins C, Engelhard M, Thevuthasan S, Peden C. Redox properties of water on the oxidized and reduced surfaces of CeO2(111). Surface Science. 2003; 526 :1-18 - 7.
Kullgren J. Oxygen Vacancy Chemistry in Ceria. Uppsala: Uppsala University, Structural Chemistry; 2012. p. 896 - 8.
Schierbaum K. Ordered ultra-thin cerium oxide overlayers on Pt(111) single crystal surfaces studied by LEED and XPS. Surface Science. 1998; 399 :29 - 9.
Berner U, Schierbaum K. Cerium oxides and cerium-platinum surface alloys on Pt(111) single-crystal surfaces studied by scanning tunneling microscopy. Physical Review B. 2002; 65 :235404 - 10.
Eck S, Castellarin-Cudia C, Surnev S, Ramsey MG, Netzer FP. Growth and thermal properties of ultrathin cerium oxide layers on Rh(111). Surface Science. 2002; 520 :173 - 11.
Xiao W, Guo Q, Wang EG. Transformation of CeO2(111) to Ce2O3(0001) films. Chemical Physics Letters. 2003; 368 :527 - 12.
Zhao X, Ma S, Hrbek J, Rodriguez JA. Reaction of water with Ce-Au(111) and CeOx/Au(111) surfaces: Photoemission and STM studies. Surface Science. 2007; 601 :2445 - 13.
Liu J-C, Wang Y-G, Li J. Toward rational design of oxide-supported single-atom catalysts: Atomic dispersion of gold on Ceria. Journal of the American Chemical Society. 2017; 139 :6190-6199 - 14.
Lin F, Hoang DT, Tsung C-K, Huang W, Lo SH-Y, Wood JB, et al. Catalytic properties of Pt cluster-decorated CeO2 nanostructures. 2011; 4 :61-71 - 15.
Khalakhan I, Dubau M, Haviar S, Lavkova J, Matolinova I, Potin V, et al. Growth of nano-porous Pt-doped cerium oxide thin films on glassy carbon substrate. Ceramics International. 2013; 39 :3765-3769 - 16.
Tauster SJ, Fung SC, Garten RL. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. Journal of the American Chemical Society. 1978; 100 :170 - 17.
Miki T, Ogawa T, Haneda M, Kakuta N, Ueno A, Tateishi S, et al. Enhanced oxygen storage capacity of cerium oxides in CeO2/La2O3/Al2O3 containing precious metals. ChemInform. 1990; 21 :6464-6467 - 18.
Kern W. Cleaning solutions based on hydrogen peroxide for use in silicon semiconductor technology. RCA Review. 1970; 31 :187-206 - 19.
Paul M, Satpati B, Chakraborty S. Interaction of silver nano-clusters with ceria thin-films: An in situ temperature dependent x-ray photoelectron spectroscopy study. Journal of Alloys and Compounds. 2022; 911 :164956 - 20.
Bumajdad A, Madkour M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Physical Chemistry Chemical Physics. 2014; 16 :7146-7158 - 21.
Liu L, Zhang X, Yang L, Ren L, Wang D, Ye J. Metal nanoparticles induced photocatalysis. National Science Review. 2017; 4 :761-780 - 22.
Murugadoss G, Kumar DD, Kumar MR, Venkatesh N, Sakthivel P. Silver decorated CeO2 nanoparticles for rapid photocatalytic degradation of textile rose Bengal dye. Scientific Reports. 2021; 11 :1080 - 23.
Naqi M, Kwon N, Jung SH, Pujar P, Cho HW, Cho YI, et al. High-performance non-volatile InGaZnO based flash memory device embedded with a monolayer Au nanoparticles. Nanomateals. 2021; 11 :1101 - 24.
Yun M, Mueller DW, Hossain M, Misra V, Gangopadhyay S. Sub-2 nm size-tunable high-density Pt nanoparticle embedded nonvolatile memory. IEEE Electron Device Letters. 2009; 30 :1362-1364 - 25.
Mukherjee S, Zheng H, Gangopadhyay K, Gangopadhyay S. Influence of Pt nanoparticle induced defects and surface coverage in determining asymmetric programming/erasing signatures for nanocrystal embedded nonvolatile memory applications. Advanced Materials Interfaces. 2016; 3 :1600436 - 26.
Biswas D, Mondal S, Rakshit A, Bose A, Bhattacharyya S, Chakraborty S. Size and density controlled Ag nanocluster embedded MOS structure for memory applications. Materials Science in Semiconductor Processing. 2017; 63 :1369-8001