Receptor dispersion, mechanism, and effects in the brain.
Abstract
Serotonin affects immunological regulation, hemostasis, vasoconstriction, gut motility, and is linked to several diseases. During peristalsis, serotonin (5-HT) is released from the gut mucosa and is largely generated by enterochromaffin cells (ECs) rather than gut microbes. Gut bacteria can stimulate the production of 5-HT. Serotonin in the blood that is retained within the platelets contributes to the production of clots and platelet aggregation. It binds to receptors such as 5HT2A, producing platelet aggregation and neuronal excitement. It regulates vasoconstriction via 5HT1D in cranial blood arteries. Atherosclerosis, thrombosis, and hypertension are some cardiovascular conditions liked to serotonin dysregulation. Serotonin imbalances in the gut influence gut motility and absorption, leading to conditions such as irritable bowel syndrome (IBS). 5-HT receptor subsets (5-HT1, 5HT2B, 5-HT3, 5-HT4, and 5-HT7) in gut are promising therapeutic targets. Serotonin in the Central Nervous System (CNS) controls a variety of behavioral and cognitive activities. 5-HTRs, including 5-HT1A and 5-HT2A, can have conflicting effects on pyramidal neuron firing. The chapter comprehends 5HTRs’ involvement in the blood, gut, and brain, emphasizing its significance in modulating a variety of biological activities. Further investigation must be conducted to better comprehend the complexity of serotonin signaling to develop innovative treatment techniques that target serotonin receptor networking.
Keywords
- serotonin
- vasoconstriction
- gut brain axis
- 5-HTReceptors
- brain disorders
- platelets.
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) plays important roles in humans’ central nervous system and the other peripheral systems. In the central nervous system, it acts as a neurotransmitter, controlling brain functions such as autonomic neural activity, stress response, body temperature, sleep, mood, and appetite. In its role as a peripheral hormone, serotonin is unique in controlling the functions of several organs. In the gastrointestinal tract it is important for regulating motor and secretory functions. Apart from intestinal motility, energy metabolism is also regulated by both central and peripheral serotonin signaling. It also has fundamental effects on hemostasis, vascular tone, heart rate, respiratory drive, cell growth and immunity. Serotonin regulates almost all immune cells in response to inflammation, following the activation of platelets [1]. Serotonin (5-HT) is a neurotransmitter involved with the regulation of numerous behavioral and biological functions in the body, playing a role in both psychological processes in the central nervous system (CNS) as well as peripheral tissues such as the bone and gut [2]. Serotonin is a monoamine neurotransmitter that plays a role in several complex biological functions [3, 4]. Its common abbreviation is 5-HT because of its chemical name: 5-hydroxytryptamine [5]. The most clinically relevant function of serotonin is in psychiatric disorders; most commonly, its absence appears to be related to depression, anxiety, and mania [6, 7]. The interplay of the 5-HT system with several other classical neurotransmitter systems makes the wide range of brain processes mediated by 5-HT neurotransmission in the CNS more complicated. 5-HT exerts its effects on regulating the neurotransmitter release of these neurons by activating serotonergic receptors on cholinergic, dopaminergic, GABAergic, or glutamatergic neurons [8, 9] . Additionally, 5-HT neurons also engage in co transmission, which is the release of several classical neurotransmitters by a single neuron. Glutamate and perhaps other amino acids are co-transmitters produced by 5-HT neurons, according to research published in [9]. Intense research is being done to better understand the regulation and functional ramifications of this neuronal co-transmission [10].
2. Synthesis, activation and degradation of 5HT in gut
The gastrointestinal (GI) tract, platelets, and the serotoninergic neuronal network of the central nervous system are the primary locations of serotonin (5-hydroxytryptamine, or 5-HT). In addition to being a neurotransmitter, serotonin is a peripheral hormone [1].
Ninety percent of the serotonin in the body is produced by epithelial enteroendocrine (EE) cells, which include enterochromaffin cells (EC) as one of its kinds. Since they serve as sensory transducers and facilitate transepithelial movement, EC cells are distributed throughout the enteric epithelium that runs from the stomach to the colon. This mechanosensitivity is again monitored by 5HTRs. These cells store their 5-HT in secretion granules at the base of the cell, and they have a microvillus border that penetrates into the gut lumen; from where it makes its way to the connective tissue, passing through lamina propria, to gain access to the 5HTRs on the nerve endings, since no nerve ending crosses through the lamina propria [11]. Once synthesized, Vesicular Monoamine Transporter 1 (VMAT1) package and release it from the basal border of EC cell into interstitial space of the mucosa by not only Luminal distention but also by chemical stimuli, which activates of 5HTR in both submucosal and myenteric plexuses on Intrinsic Primary Afferent Neurons (IPANs). The actions of 5-HT are halted by the uptake of serotonin reuptake transporter (SERT), which is expressed by intestinal epithelial cells, platelets, and enteric neurons, into surrounding epithelial cells. It is followed by the intracellular breakdown of 5-hydroxyindoleacetic acid (5-HIAA) by monoamine oxidase (MAO) [12]. Serotonin is released into the intestinal lumen and the lamina propria, which is home to T lymphocytes, dendritic cells, and other immune cells (Figure 1) [13].
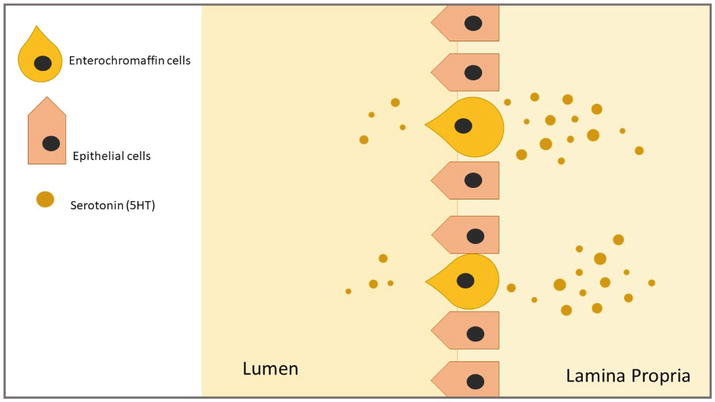
Figure 1.
Serotonin produced in EC cells, with the bulk being released into the lamina propria and a minor quantity into the gut lumen.
3. Serotonin receptor networks in brain
Drug reinforcement, stress sensitivity, mood, anxiety, and aggressiveness are all modulated by 5-HT1B receptors. A number of studies have found that decreased 5-HT1B heteroreceptor activation may promote impulsive behavior. The 5-HT1A receptor is a prominent inhibitory G-protein coupled receptor subtype identified in the auto- and heteroreceptor populations of the nervous system. It functions by interacting with Gi/Go proteins, which control a number of intracellular signaling cascades, including cAMP inhibition, calcium channel inactivation, and potassium channel activation [14]. 5-HT5A and 5-HT6 receptors are serotonin proteins present in the human brain that control neurotransmitter release as well as physiological functions such as learning and memory. The HTR5A gene encodes them, and they have been associated with neurological and mental disorders [15].
4. Localization OF 5HT1A, 5HT1B, 5HT6, 5HT5A
4.1 5HT1A
Neurons in the raphe nuclei generate tryptophan hydroxylase 2 (TPH2), which forms the 5-HT system in the brain. Among the 14 5-HT receptor genes, the 5-HT1A receptor is of particular interest because it is abundant in corticolimbic regions implicated in mood and emotion, such as the hippocampal and cortical pyramidal neurons, as well as interneurons of the prefrontal cortex, medial septum, amygdala, hypothalamus, and other regions. On 5-HT neurons, the 5-HT1A receptor is the major somatodendritic auto receptor [16], functioning as a “brake” to lower overall 5-HT system activity and is thought to delay antidepressant response, where it operates as a “brake” to inhibit overall 5-HT system activity and is thought to delay antidepressant response [17]. As a result, processes that control 5-HT1A auto receptor levels are likely to set the tone for the entire 5-HT system [18].
4.2 5HT1B
The basal ganglia, striatum, and frontal cortex are the primary sites of 5-HT1B receptor expression. They are found on presynaptic 5-HT terminals as inhibitory autoreceptors of 5-HT release, it also acts on other nerve terminals as heteroreceptors that govern the release of neurotransmitters such as acetylcholine, glutamate, dopamine, norepinephrine, and gamma-aminobutyric acid [19]. It was recently revealed that the interaction of 5-HT1B receptors with the protein p11 regulates their cellular location. Mice with p11 overexpression and hence have increased 5-HT1B receptor activity have a pattern of antidepressant. p11 mutant mice, on the other hand, display a depression-like phenotype and a reduced responsiveness to antidepressant therapies [20].
4.3 5HT5A
Humans have a protein called HTR5A. It belongs to the 5-hydroxytryptamine receptor protein family. It is a receptor having seven membrane domains that a negative correlation to adenylate cyclase and opens potassium channels when activated [21]. This has been found in the human forebrain, cerebellum, and spinal cord and is encoded by the HTR5A gene [22, 23, 24]. It is present in the basal ganglia and the frontal cortex, where it acts as a terminal auto-receptor or heteroreceptor, modulating neurotransmitter release [25].
4.4 5HT6
This is another type found in the human brain [26]. It is a type of protein that regulates how the brain develops. It accomplishes this by interacting with other proteins that regulate how neurons travel and connect to one another. This contributes to the formation of brain circuits critical for memory and behavior. [27] 5-HT6 mRNA is only found in neurons, and it is particularly concentrated near the primary cilia which are 1–5 m long membrane extensions of the cell that are linked to the ciliary basal body. Cilia are important signaling components across the central nervous system. They are found in neurons and are particularly concentrated in the primary cilia. They have also been discovered in numerous locations of the rat brain in connection with both neuronal dendrites and cilia. The subcellular location of 5-HT6 receptors influences their signaling and pathological functions [28].
5. Serotonin receptor dispersion, mechanism, and potential brain impacts
See Table 1.
| Receptor Type | Location | Role | Route | Action |
|---|---|---|---|---|
| 5-HT1A | hippocampal, Prefrontal cortex pyramidal neurons and interneurons, medial septum, amygdala, and hypothalamus | cognitive function, mood, and emotional states. | Inhibition of ACII | Inhibitory |
| 5-HT1B | basal ganglia, striatum and frontal cortex | mood, memory, | Inhibition of ACII | Inhibitory |
| 5-HT5A | hippocampus and neocortex | learning, memory, and mood regulation. | Inhibition of AC | Inhibitory |
| 5-HT6 | cortex and hippocampus | Stress, thinking, retention, and personality | Activation of AC | Stimulatory |
Table 1.
6. Pathological implications of serotonin receptors in the brain
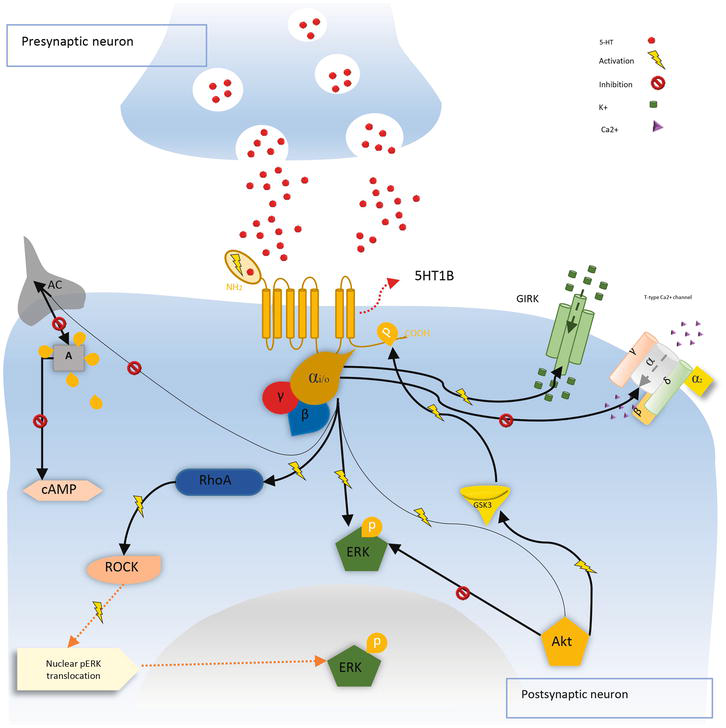
Figure 2.
The activation of 5-HT1B inhibits adenylate cyclase (AC), decreasing ATP conversion to cyclic adenosine Monophosphatase (cAMP) which activates protein kinase a (PKA), the stimulation of these specific receptors reduces Ca2+ and increases K+ conductance. A chain reaction of kinases governs the extracellular signal-regulated kinase (ERK) transfer after 5-HT1B activation. Furthermore, kinase B protein (AKT) is activated, resulting in the stimulation of glycogen synthase kinase 3 (GSK3), which is implicated in the phosphorylation and control of 5-HT1B receptor function.
The
The
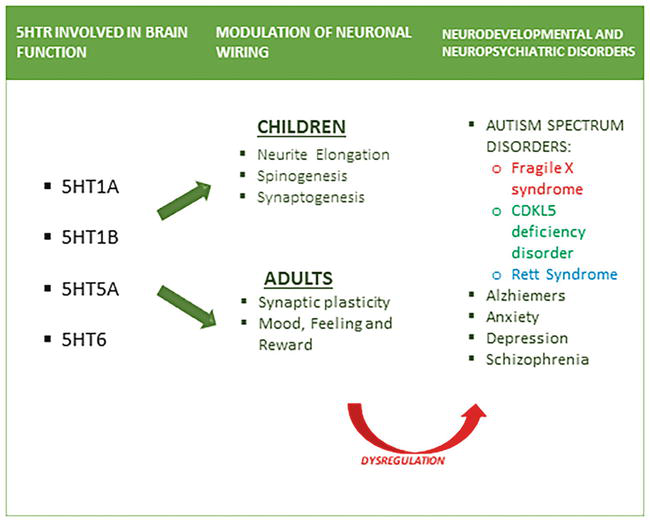
Figure 3.
Implications of dysregulation of 5HTR in brain functions in adults and children.
7. Therapeutic target of serotonin receptors
The 5-HT6R represents one of the newest receptors for serotonin to be discovered and has attracted a lot of interest following studies that showed it has pro-cognition features. 5HTR5A receptor proteins assist in regulating how the brain functions, such as how it recalls things and how it reacts to particular stimuli. They also aid in the circulatory control of the body. Agonists and antagonists are medicines that can influence how the 5-HT5A receptor works. Serotonin uptake inhibitors are medications that can help the brain recall things better [36]. This protein aids in the transmission across the nervous system and is involved in conditions like depressive illness and pain [37].
It participates in a number of physiological processes, including, memory and learning, and is connected to several neurological and psychological illnesses Drugs that inhibit this receptor may be used to treat memory issues or Alzheimer’s disease, 5-HT6R has been a good target for treating memory deterioration, owing to its modulatory role in cholinergic and glutamatergic systems. Blocking the 5-HT6R improves Brain functions in adult rodents, alters age-associated and pharmacologically induced impairments, and can assist in the repair of brain damage induced by early life experiences. This could lead to the creation of unique treatments for mental health issues Neuronal plasticity may benefit from 5-HT6R inhibition as well. Numerous 5-HT6R inhibitors are now being explored in clinical trials in response to these findings [38, 39].
8. Serotinin receptor network in blood
Enterochromaffin cells produce serotonin (5-HT), which is absorbed by platelets in the circulation through the serotonin transporter. Upon secretion by platelets, serotonin triggers both platelet aggregation and vasoconstriction at bleeding sites. Through receptors on the plasma membranes of vascular smooth muscle tissues, blood vessels, and neurons, this signaling process takes place [27].
Smooth muscle cell surface receptors like 5-HT1B and 5-HT2A are the main conduits for the vasoconstrictive actions of serotonin. However, 5-HT1B receptors are the main pathway via intracranial arteries express vasoconstriction. Additionally, the brain hosts the 5-HT1D receptor subtype, concentrated in regions linked to migraine headaches and pain modulation [40]. Activating 5-HT1D receptors can induce vasoconstriction in specific brain areas, offering potential relevance for migraine treatment.
Additionally, serotonin acts as a vasodilator by inducing endothelial cells to release nitric oxide. Different from the actions of 5-HT2A receptors, 5-HT1B and 5-HT1D transmitters primarily control this response [41]. The precise distribution of 5-HT receptors in various vascular smooth muscles, nearby vessel tissue, and the complex regulatory system controlling vascular tone, which includes parasympathetic nerves, determine whether serotonin has a vasoconstrictor or vasodilator effect; see Table 2.
| Serotonin receptors | Location | Main function | Potential effect |
|---|---|---|---|
| 5-HT2A | smooth muscle cells of blood vessel, Platelet | Regulating vascular tone and blood flow | Platelet Aggregation, Contraction |
| 5-HT1B | Cranial Blood Vessel | Smooth muscle contraction. | Autoreceptor, Vasoconstriction |
| 5-HT1D | Presynaptic Neuron | Constriction of intracranial blood vessel smooth muscle | Vasoconstriction |
Table 2.
Receptors distribution, mechanism of action, and potential effects in blood.
The management of serotonin receptors and their activities in the blood is a complicated and closely regulated process. Similar to any other physiological system, abnormalities in serotonin signaling can significantly affect a number of physiological processes and may contribute to the development of particular disorders.
8.1 5-HT2A
5-HT2A receptors (stands for 5- hydroxytryptamine) belongs to the Gg-coupled protein receptor (GCPR) which perform their role in several intracellular pathways. The central nervous system (CNS) has the highest quantity of 5-HT2A mRNA and protein, while blood cells, lymphocytes, and smooth muscle cells in the vascular system all express 5-HT2A. The main roles of 5-HT2A in the vascular system is inflammation and wound repair. For instance, serotonin is released when the endothelium of a blood artery is injured, and smooth muscle cells in the resistance vasculature are activated to produce vasoconstriction [42].
8.2 Molecular mechanisms and functions of 5-HT2A receptors
Vascular smooth muscle cells have a 5-HT2A receptor on their surface, which serotonin binds to. The 5-HT2A receptor’s structure experiences a conformational shift that enables it to exchange guanosine triphosphate (GTP) for guanosine diphosphate (GDP), enabling interaction with a particular class of G protein known as Gq. Phospholipase C [43] is activated by the Gq protein, and it cleaves phosphatidylinositol 4,5-bisphosphate (PIP2), a membrane phospholipid, into its secondary carriers: imidazol trisphosphate (IP3) and diacylglycerol (DAG).
When IP3 attaches to its receptor on the membrane of the endoplasmic reticulum, it diffuses into the cytoplasm and releases calcium ion (Ca2+) from intracellular storage. A higher amount of calcium in the cytoplasm activates signaling molecules and calcium-dependent kinases, which phosphorylates and activates myosin light chain kinase (MLCK). Vascular cells of smooth muscle contract as a result of a series of processes that are started when active MLCK phosphorylates the light chains of myosin (MLC). Vasoconstriction and an increase in vascular resistance are the results of this contraction [44].
When blood vessels constrict due to 5-HT2A receptor activation, the reduced blood flow and increased shear stress can lead to molecules that are released from the injured endothelium and the surrounding tissues. One of these molecules is adenosine diphosphate (ADP), which is stored in platelet granules [28, 45]. Activated platelets produce ADP and serotonin, which attach to the appropriate receptors on the platelets, triggering platelet activation. Platelets become sticky and undergo shape change. The surface of carbohydrate IIb/IIIa receptors on platelets become visible upon activation. Blood-circulating fibrinogen molecules have the ability to attach to nearby platelets’ activated glycoprotein IIb/IIIa receptors. However binding of fibrinogen bridges adjacent tissues and platelets, causing them to adhere to each other and form aggregates or clumps. This process is known as platelet aggregation. The platelets that have accumulated create a temporary obstruction at the location of the vessel damage. This obstruction helps to prevent excessive bleeding and initiate the process of blood clotting [45].
8.3 5-HT1B
5-HT1B receptors are found in large quantities all over the body. The 5-HT1B receptors are found on serotonergic neurons in the brain and spinal cord, where they control the release of 5-HT from the end of the nerve itself (autoreceptors); [46] because these receptors are primarily translocated to the terminals of axons, there is an anatomical inconsistency between the location of competent 5-HT1B receptor protein and mRNA. It has been discovered that 5-HT1B autoreceptors decrease 5-HT production and release while increasing reuptake through the serotonin transporter. Selective 5-HT1B (/1D) agents, also known as triptans, are utilized as antimigraine medications because 5-HT1B receptors are also present on the smooth muscles membrane of arteries and control 5-HT-induced vasoconstriction across all blood vessels [28]. Additionally, 5-HT1B receptors may facilitate constriction of blood in intra- and extracranial arteries. Although 5-HT1D and 5-HT1B receptors were formerly believed to be the rat equivalents of each other, it is now known that these two receptors are found in every mammalian species investigated and have different geographical distributions. The primary function of the closely related 5-HT1B and 5-HT1D transmitter subtypes is to suppress the production of neurotransmitters like as norepinephrine, dopamine, and serotonin. Depending on the cell types expressing these subtypes, this can have an impact on mood, pain perception, and other neurological functions [47].
8.4 5-HT1D
The central nervous system (CNS), specifically areas of the brain like the cerebral cortex, the hypothalamus and trigeminal nerve pathways, is home to an additional variant of the serotonin receptor, 5-HT1D. Human migraine neurons and trigeminal nerves have been shown to have both 5-HT1B and 5-HT1D receptors; however, only 5-HT1D receptor have been found in trigeminal nerves that bulge externally to the dural endothelium and centrally to the spinal cord trigeminal nuclei [48]. Thus, the 5-HT1D receptors are localized centrally to block the transmission of pain signals from blood vessels to brainstem sensory neurons, as well as superficially to restrict stimulated sensory nerves and prevent the production of vasoactive neuropeptides [41]. It is especially pertinent to processes pertaining to discomfort adjustment and migraine headaches because of its distribution.
9. 5HT1D/1B mediated vasoconstriction
The interactive relation between the receptors of 5-HT1B and 5-HT1D with the context of vasoconstriction involves a presynaptic modulation of the vasoconstrictive response through the inhibition of serotonin release. These receptors, which belong to the family of the G-protein coupled receptor (GPCR) family, carry signals from external ligands to intracellular signaling pathways, including hormones and antidepressants. On serotonergic terminals of the brain, the 5-HT1D receptor is primarily found initially [43]. Upon activation, it exerts inhibitory control over serotonin release into the synaptic cleft, thereby indirectly influencing vasoconstriction mediated by the 5-HT1B receptor subtype. Upon serotonin release from serotonergic nerve terminals, it binds to postsynaptic 5-HT1B receptors on smooth muscle cells a conformational change occurs, which causes a G-protein connected to the ligand (usually a Gq/11 protein) to become activated. Smooth muscles Vasoconstriction can be the activated by Gq/11 proteins that are already defined in section. Simultaneously, a portion of the released serotonin binds to initially 5-HT1D receptors located on the same serotonergic nerve terminals. This binding event activates a signaling cascade, leading to the attenuation of further serotonin release from the nerve terminal. The 5-HT1D receptor experiences a conformational change upon serotonin binding, this results in the stimulation of a class of proteins called Gi/o-type G-proteins, starting transmission cascades downstream [49]. Guanosine diphosphate (GDP) linked to the alpha subunit of Gi/o-type G-proteins is exchanged for guanosine triphosphate (GTP) when serotonin binding activates the 5-HT1D receptor. Gi/o-type G-protein activation results in the dissociation of the alpha subunit (5-HT1Dα) from the beta-gamma fractions (5-HT1Dβγ), which in turn modifies cellular signaling pathways. Gi/o-type G-proteins are triggered by the 5-HT1D receptor to inhibit adenylyl cyclase and reduce the quantity of cyclic AMP (cAMP) generated from ATP (also known as adenosine triphosphate). Consequently, the cAMP-dependent protein kinase (PKA) expression is reduced [50], which results in less phosphorylation of targeted prostate specific antigen. Based on the connective tissue and cell type, the activation of Gi/o-type G-proteins can also open or shut a few ion channels. As a result, the membrane potential and calcium levels fluctuate, changing the release of neurotransmitters and the electrical activity of neurons. The reduced release of serotonin, driven by 5-HT1D receptor activation, results in a diminished availability of serotonin in the synaptic cleft. Therefore, there is a decreased binding of serotonin to postsynaptic receptors, including the receptors of 5-HT1B on the cells of smooth muscle.
The 5-HT1D receptor functions as an indirect regulator of vasoconstriction refers by the receptor of 5-HT1B to modulating serotonin composition ratio which is released from serotonergic nerve terminals Figure 4
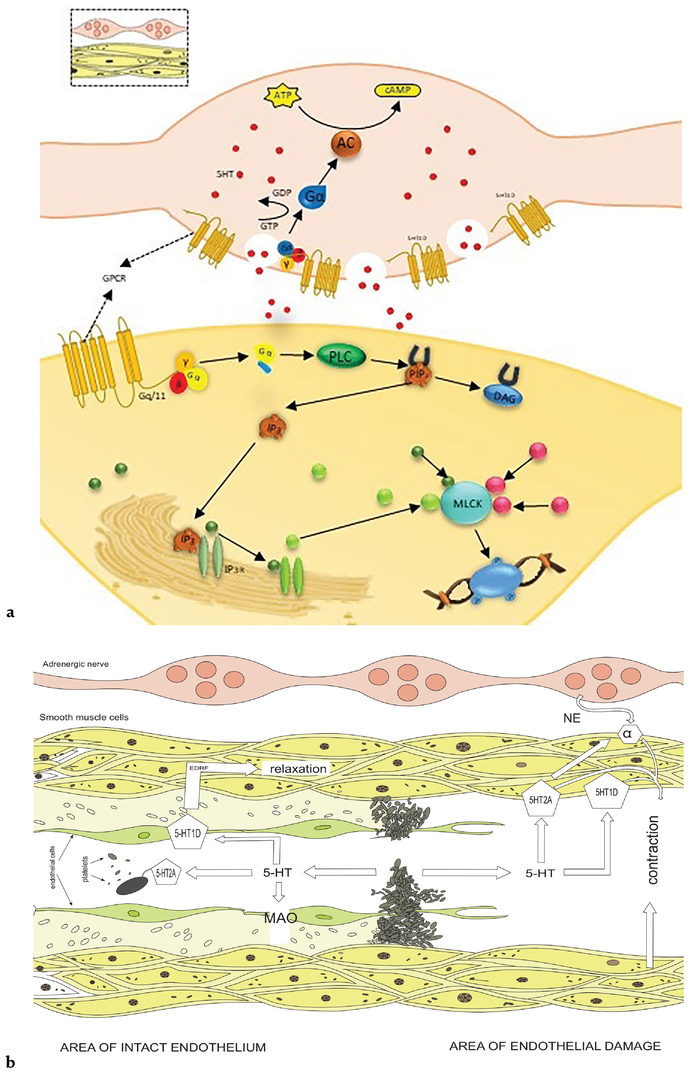
Figure 4.
(a) Mechanism of 5HT1B/ID mediated vasoconstriction. 5-HT1D receptor functions as an indirect regulator of vasoconstriction mediated by the 5-HT1B receptor by modulating the amount of serotonin released from serotonergic nerve terminals. Through this inhibitory control, the 5-HT1D receptor fine-tunes the overall vasoconstrictive effect of serotonin on smooth muscle cells, which in turn influences vascular tone and blood vessel diameter. (b) Mechanistic action of vasocontraction and dilation depicting the communication between nerve terminals and smooth muscle cells.
10. 5HT1D/1B mediated vasodilation
The free serotonin in the plasma in the systemic circulation is derived from production by chromaffin cells (primarily in the gastrointestinal tract) and overflow into the venous blood from serotonergic neuroeffector junctions (primarily in the brain). The majority of the monoamine is quickly absorbed by platelets (where it is stored in dense granules) and endothelial cells (where it is primarily metabolized by monoamine oxidase (MAO)) thanks to the action of the serotonin transporter (SERT). This ensures a relatively low plasma level of serotonin under physiological conditions [23].
On the other hand, a significant rise in the local concentration of serotonin is unavoidable whenever platelets congregate close to the blood vessel wall and release their dense granules [27]. Additionally, if the monoamine diffuses to the adventitia, it may potentially impact the activity of the sympathetic nerve endings. This activation of serotonergic receptors can occur in both endothelial and vascular smooth muscle cells.
The release of serotonin (5-HT) from aggregating platelets induces more platelet aggregation; at this point unaggregated platelets readily absorb and remove it from the plasma. The monoamine is also taken up by the endothelial cells if the endothelium is intact, and monoamine oxidase (MAO) breaks it down there [51]. Lastly, the endothelium functions as a physical barrier that prevents serotonin-producing vasoconstrictor platelet products from entering the smooth muscle. The endothelium-derived relaxing factors (EDRFs; primarily nitric oxide) Figure 4
In addition, nitric oxide will be released into the lumen to prevent platelets from adhering to the endothelium and to work in concert with prostacyclin to prevent additional platelet aggregation. These various endothelium functions are essential in preventing blood clotting and vasospastic episodes in blood vessels with a healthy intima [41].
When endothelial cells are eliminated (for example, through trauma), the endothelium’s protective function is lost locally. Serotonin then binds to the 5-HT2A receptors in the vascular smooth muscle cells, causing contraction via both direct activation of the cells and an increase in their sensitivity to other vasoconstrictors (such as norepinephrine (NE) acting on α1-adrenoceptors (α)). This vasoconstriction then occurs, which plays a part in the vascular phase of hemostasis [43, 52].
11. Pathological implications of 5-HT1D, 5-HT1B AND 5-HT2A
Disturbances in serotonin receptors 5-HT1D, 5-HT1B, and 5-HT2A have been implicated in various pathological conditions affecting the blood. Dysregulation of these receptors can lead to transformed vascular tone, platelet function, and overall hemostasis In the case of 5-HT1D and 5-HT1B receptors, their activation often results in vasoconstriction, which can lead to an increased risk of hypertension and impaired blood flow. This heightened vasoconstrictive response can contribute to conditions such as migraine, where aberrant 5-HT1D and 5-HT1B receptor activity may trigger intense vasoconstriction and subsequent dilation, leading to the characteristic headache [53]. Additionally, disturbances in 5-HT1B receptors have been associated with an increased susceptibility to thrombotic events, as these receptors play a role in platelet aggregation [54]. When overstimulated, 5-HT1B receptors can lead to excessive platelet activation and adhesion, potentially resulting in the formation of pathological blood clots, contributing to conditions such as ischemic stroke or deep vein thrombosis. Similarly, disruptions in 5-HT2A receptor function can impact platelet aggregation and vasoconstriction. Dysfunctional 5-HT2A receptors may play a role in the development of atherosclerosis [52] and other cardiovascular diseases where abnormal platelet function and endothelial dysfunction are key pathological factors. Therefore, understanding the intricate role of these serotonin receptors in blood-related processes is crucial for unraveling the underlying mechanisms of various cardiovascular pathologies and informing potential therapeutic strategies [55].
12. Therapeutic target of 5-HT1D, 5-HT1B AND 5-HT2A
There is still much to learn about the pathogenesis of migraines. Nonetheless, it is thought that dilation of the extracranial, dural, and/or pial arteries plays a key role in the development of migraine headaches. Because of triptans’ effectiveness on 5-HT1B receptors, these selective agonists of 5-HT1B/1D receptors were created as cranial vasoconstrictors. The first commercial triptan preparation, sumatriptan, caused vasoconstriction in both human isolated middle meningeal and temporal arteries. However, the selective 5-HT1B receptor antagonist SB224289.40 prevented this effect. On the other hand, sumatriptan-induced vasoconstriction of these arteries was not significantly affected by BRL15572, a selective antagonist of the 5-HT1D receptor [56].
NO-releasing Triptans e.g., Zolmitriptan-NONOate a chemical compound, a newer class of medications designed to combine the vasoconstrictive effects of triptans with the vasodilatory effects of nitric oxide (NO). These compounds act as antagonists at 5-HT1B/1D receptors while simultaneously releasing NO, which counteracts vasoconstriction and promotes vasodilation. This dual mechanism aims to provide more balanced and potentially better-tolerated relief for migraine sufferers. Serotonin (5-HT) itself acts as an endogenous agonist at the 5-HT2A receptor [57]. When released from platelets and other sources, serotonin can bind to 5-HT2A receptors on platelets and potentially contribute to platelet aggregation. While the exact mechanisms are complex and involve multiple receptor subtypes, the activation of 5-HT2A receptors may participate in platelet aggregation and thrombus formation. Ketanserin is a medication that acts as an antagonist at the 5-HT2A receptor. By blocking the binding of serotonin to these receptors, Ketanserin may inhibit or reduce the platelet activation and aggregation response mediated by 5-HT2A receptor [58]. Ketanserin’s antagonistic activity at 5-HT2A receptors has been explored for its potential therapeutic effects, including its influence on platelet function and vascular health.
13. Serotonin receptor network in bowel
The main source of 5-HT in the body is the gut and receptors for 5-HT in the gut are the key to regulate various physiological and processes such as colonic motility, secretion, visceral sensitivity and inflammatory/immune response. Such receptors that are localized in the gut are; 5HTR2B, 5HTR3, 5HTR4 and 5HTR7 Figure 5. The diverse effects are achieved through their activation, while their localization achieves specific actions [59].
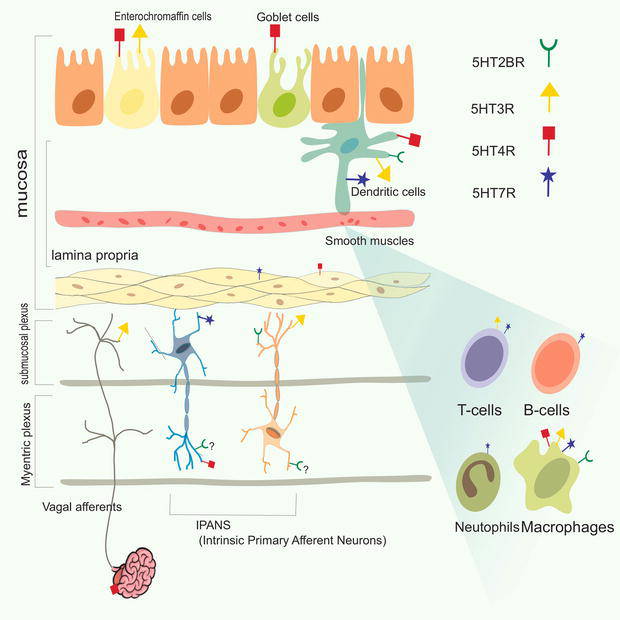
Figure 5.
Serotonin receptors presentation on various layers of the GUT with the cells involved.
The enterochromaffin cells are the whole source of serotonin production and storage which also express 5HTRs. Together with these 5HTRs, it functions as a neurotransmitter in a bi-directional manner which facilitates the gut-brain axis. This interaction of 5HT with 5HTRs allow continuous information exchange between organs in the GI tract through neural pathways and immune signals where their components also express these receptors [60].
14. 5HTR mediated serotonin functions in GIT
5HT- Serotonin plays a diverse role in modulating various metabolic functions in the body by binding with certain receptors. A vast 5-HT signaling system functions for the gastrointestinal tract (GIT). 5HT, be it synthesized by the EC cells or the gut microbiota, [61] it performs an array of functions as in Table 3.
| 5HT2B | 5HT3 | 5HT4 | 5HT7 | |
|---|---|---|---|---|
| Locations [62] | Stomach fundus, smooth muscle of the small intestine. Human colon, on longitudinal smooth muscle and the myenteric nerves | Peripheral neuron, CNS | All over the body, Smooth muscles in GIT and neurons of the nervous system in the intestine. | Smooth muscle of vascular and nonvascular tissues including – stomach, ilium, colon, with less representation seen in liver, kidney, spleen and central nervous system. |
| Agonists | α-Me-serotine BW723C86 Iodoamphetamine-2,5-dimethoxy-4, Quipazine BW-723C86 RO600175 WAY-161503 | MKC-733 2-Me-5-HT CPBG Pumosetrag | Tegaserod Prucalopride Cisapride | 5-CT 8-OH DPAT |
| Antagonists | SB200646 SB204741 SB206553 RS 127445 Methysergide Ritanserin | Ondansetron Alosetron Cilansetron | GR113808 GR125487 Piboserod | Methiotepin Metergoline SB258719 SB269970 SB656104 |
| Effectors | Gq/11 | Ion channel in Ligand gates | GS | GS |
| In the gut distribution | Longitudinal smooth muscle | Enteric neurons, EC cells | In the enterocytes, neurons of intestine, and smooth muscles of the enterocytes | In the enterocytes, neurons of intestine, and smooth muscles. |
| On stimulation- Functional response | Contraction | Increased secretion and transmitter release | Increased transmitter release, and secretion. Smooth muscles relaxation | Smooth muscles relaxation |
| Immune cells | Macrophages and Dendritic cells (DCs) | T and B cells, DCs, and Macrophages, | Macrophages, DCs, | Neutrophils, T and B cells, DCs, and Macrophages, |
Table 3.
Major serotonin/5-hydroxytryptamine/ 5-HT receptor in the bowel -types and subtypes and their location, distribution, functional response, related immune cells and effector agonists and antagonists.
It triggers mucosal peristaltic reflex through interactions with 5-HTR4, 5HT2, 5HT3, 5HT4, and 5HT7 subtypes, impacting gut motor function. It drives propulsive and segmentation motility via 5HT3 and 5HT4. Additionally, 5-HT induces relaxation via 5HT7 and 5HT4 receptors on smooth muscle cells. It enhances epithelial secretion, fat absorption, and vasodilation, while retarding gastric emptying through the receptors 5-HT3. It activates afferent vagal nerve endings, that lead to discomfort and nausea. 5-HT also elicits neurogenic secretory responses through 5-HT2, 5-HT3, and 5-HT4 receptors, supporting content neutralization. It enhances bile acid synthesis and influences glucogenesis affects glycogen synthesis. In the pancreas, it governs insulin secretion and postprandial pancreatic protein release via 5HT3 and 5HT2 receptors. Furthermore, serotonin molecules also act for pro-inflammatory response, contributing to gastrointestinal pathophysiology, engaging immune cells and by activating serotonin receptors on DCs in the lamina propria [63, 64]. Even if all kind of serotonin receptor have potentiality of activation by the serotonin, as referenced in the Table 3 above, there is differences in anatomical location, specific distribution in enteric nervous system, harmony for synthetically similar kind of molecules and signal-transduction mechanisms, that make each subtype of serotonin receptor a probable curing and healing target [65].
14.1 5-HT2B
There are three members in the serotonin receptor family of 5-HT2, i.e. 5-HT2A, B and C subtype receptors. 5-HT2B receptor have its importance coupled with the family of G-protein-related receptor. These are heavily expressed in the gastrointestinal tract, as well as in the liver, kidney, and heart. The 5-HT2B receptors in the GI tract the are found in the fundus of stomach, small intestinal smooth muscles, also expressed in intestinal neurons and as well as in the muscles. They are highly expressed in human colon, with predominant localization on longitudinal smooth muscle and the myenteric nerves that control its motility [66]. It Influences smooth muscle rhythmic contraction to propel food forward via peristalsis and secretions production. In interstitial cells of Cajal (ICC), 5-HT2B receptor modulates motility. That expression of 5-HT2B receptors in the ICC network also, operate as a GI “pacemaker” that suggests its role in the smooth muscle contraction. Network of ICC if dysregulated may lead to sluggish intestinal motility [67]. They may also serve as morphogenic agents during the development of enteric neurons by binding with endogenous 5HT in embryonic days, their agonist’s increases the enteric neurons differentiation in vitro [62, 67].
14.2 5HT3
The 5-HT3 receptor stands out among all known serotonin receptor subtypes in that it belongs to the ligand-gated ion channel superfamily and is homomeric in nature. This receptor superfamily is comprised of up of five subunits, each featuring four transmembrane segments and a large extracellular N-terminal region. They are expressed on excitable neurons of the gut, notably intrinsic afferent nerves that radiate into the mucosa, interneurons, inhibitory and excitatory motor neurons, ICCs, smooth-muscle cells, and the enterocytes [68]. 5-HT3 stimulation triggers both intrinsic and extrinsic afferent neurons as well as a modest number of excitatory postsynaptic potentials (EPSPs). Neurons in the myenteric plexus, which function as primary afferent neurons and project to the mucosa, undergo stimulation directly by 5-HT presented to the mucosa, where the response is exclusively mediated by 5HT3. They have the capacity to regulate motility, intestinal secretion, visceral sensitivity, and the emetic pathway. 5-HT3 receptor antagonists are believed to produce anti-nausea effects through vagal afferents in the stomach, and they have been observed to be helpful in treating the acute stages of chemotherapy and radiation therapy-induced emesis.
Although the anti-diarrheal pathway action of 5HT3 antagonists remains to be determined, there are suggestions that these antagonists likely inhibit 5HT3 receptors located on intrinsic and extrinsic afferent nerve fibers in the mucosa and neurons that contribute to fast EPSPs that interact with 5-HT, all of which, when put together, would alleviate diarrhea by decreasing propulsive motility and secretion locally within the gut.
Contrasting with the anti-diarrheal therapy that 5HT3 has to offer, it has also been used to treat constipation. The benefit is availed by exploiting its potential to induce propelling motion and secretory actions in the intestine, since it desensitizes quickly, the emphasis is mostly on partial agonists [68].
14.3 5HT4
5HT4 serotonin receptors is one of the three major groups of receptors including 5HT4, 5HT6 and 5HT7 which are coupled with the G protein. They are found in the whole body profoundly seen in the GIT smooth muscles and neurons of the nervous system of the intestine. That’s why also known as the “second brain of the gut”. Both 5HT3 and 5HT4 receptors are also seen in the enterochromaffin cells (EC), myenteric neurons within the plexuses of submucosa of the enteric nervous system (ENS), sensory neurons seen intrinsically and extrinsically [62].
A single gene is the source of a functioning 5-HT4 receptor protein. There are different isoforms due to alternative splicing of their genes, leading to variations in their structure and function. These isoforms can have unique roles in various physiological processes and may respond differently to ligands or signaling pathways [69] .
5HT4 receptor excitation by the serotonin plays crucial role in the GIT for gut motility regulation and facilitating release of neurotransmitters- acetylcholine and tachykinins, which increases gut motility in reflex and relaxes gut smooth muscles at certain areas. 5-HT4 receptors contribute physiologically for the regulation of propulsive motility along with promotion of normal motility through trophic actions. In the muscularis externa, 5-HT4 synergists act on pre-synaptic nerve terminals, that augment the production of of acetylcholine which result reflex of naturally occurring activity rather generation of neurotransmission [70]. It has been seen on infusion 5-HT4 agonists into the lumen or surface application to the mucosa that increase propulsive motility in peristaltic/ motility reflex in ex vivo motility assays. That suggests 5-HT4 receptors excessive number in the mucosa [71].
Further, 5-HT4 receptor has been demonstrated in the colonic epithelial cells which releases 5-HT on activation and also expresses the discharge of mucus from the goblet cells. Augmentation of the peristaltic reflex pathways is seen by pre-synaptic 5HT4 receptors activation on nerve ends to increase the acetylcholine production. Fluorescent evaluation of the promoter of the 5-HT4 gene in a strain of mice evaluated. That shows increased fluorescent protein, green in color which reveals that essentially all the colonic cells of rat and human expresses 5-HT4 receptor. Furthermore, 5-HT4 agonists activated 5-HT release from EC cells can be blocked by 5-HT4 receptor antagonists on mucosal application. That can also affect Cl- secretion by intestinal cells and mucus flow by goblet cells. For constipation therapy 5-HT4 receptors in epithelium could be pursued as a secure and applicable measure [72].
14.4 5HT7
5-HT7 is a member of the 5-HT4, 5-HT6, and 5-HT7 receptor subfamily, which is one of the three main classes of serotonin receptors that fall under the G protein-coupled receptor subfamily. They are presented in ileum, colon and stomach with limited presentation in the liver, spleen and kidneys and the CNS [68, 73]. It functions to relax human colonic smooth muscle, may inhibit peristalsis, and influence neuronal signaling of the abdominal pain to the central nervous system and peripheral tissues. Inhibiting 5-HT7 receptors has been theorized to exacerbate colitis presumably through elevating the degree of threshold pressure inducing intestinal peristalsis and lowering intestinal wall compliance. There are currently no clinically available selective 5-HT7 receptor ligands, however selective antagonists suited for in vivo administration are being researched [64, 68, 74, 75].
15. Serotonin and gut disorders
Although there are many disorders associated with serotonin deficiency, but here we sticking to the Inflammatory Bowel Syndrome abbreviated as IBS to stay within the scope of our discussion related to serotonin and colon at this point. IBS is a chronic gastrointestinal condition that affects 9–23% of the world’s population. It is characterized by a wide range of symptoms, including discomfort in the stomach and changes in bowel movement pattern, although there is no structural damage to the gut involves as per known, thus, it is not to be confused with Inflammatory Bowel Disease (IBD) which presents with damage to the anatomical structures within the gut [71]. Based on the symptoms presenting i.e. diarrhea, constipation, both, or neither IBS is classified into four types: IBS-D, IBS-C, IBS-M, and IBS-U. IBS-D is characterized by diarrhea, IBS-C by constipation, and the third by mixed symptoms of both constipation and diarrhea. While IBS-U stays undefined [64].
One way serotonin might correlate with IBS is, Intestinal serotonin deficiency is found to lead to the weakening of the intestinal lining accompanied with constipation and increased serotonin level within the gut which is presumably promoted by the reduced SERT expression in IBS patients. SERT-P polymorphism has been found to be correlated with IBS-C subtypes in Indian populations. Abnormal serotonergic functions are also caused by prolonged shunting of tryptophan in pathway of kynurenine [66, 76].
Although mucosal 5-HT and TpH1 mRNA (which are linked to serotonin synthesis) are produced at lower levels in IBS-D patients, the gut’s basic and enhanced release of serotonin (5HT) remains unchanged. This runs counter to the elevated serotonin levels shown in blood tests without platelets from people with IBS-D following meals [62]. Therapeutic agents that concentrate on the regulation of 5-HT activity in IBS include agonists and antagonists of 5-HT4 and 5-HT3 respectively. Because they minimize visceral sensitivity, 5-HT3 receptor antagonists have been used to treat diarrhea and abdominal pain, which are frequently encountered in IBS. This is very likely given that they act at 5-HT3 receptors on intrinsic neurons, which stimulate propulsive motility, and extrinsic sensory neurons, which signal pain and discomfort, unfortunately, these benefits are availed on the expense of severe side effects, thus the most of the antagonists (such as aldosterone) are tightly regulated. Certain antagonists, for instance Ondansetron, facilitate in reducing the frequency, urgency, transit duration, and consistency of stools, but the efficacy is reduced through the progression of disease. Agonists of this particular receptor is used to treat constipation predominant IBS [74]. The 5HT3R and 5HT4 antagonists repressed engine action of colon, while the 5HTR2B antagonists significantly affected colonic engine movement. 5-HT2B/C-selective antagonists SB206553, methysergide, and ritanserin impeded the proliferation of neurons in vitro by serotonin [75, 77].
5-HT4 agonists, like teaser, have proven to be both efficacious and safe in IBS presented with constipation; both acute and chronic. Unlike some other medications, they do not stimulate pain-sensing nerves or initiate muscle contractions in the gut directly. Instead, they work by leveraging natural stimuli to activate the body’s reflexes. By elevating the production of certain chemical messengers that aid in gut movement (prokinetic pathways), these medications strengthen and support the natural digestive processes [78, 79].
5HT7 receptors regulates nociception, smooth muscle relaxation and is believed to have a role in the pathogenic mechanisms that underlie the visceral paresthesia correlated with IBS, which adds to the rationale to its therapeutic potential. Elevated 5HT7 expression has been observed in the hippocampus, hypothalamus, and intestine (ileum and colon) of IBS groups of rodent models as compared to controls, which might explain comorbid depression and anxiety disorders in IBS patients, as 5HT7 receptors have previously been associated with depression (Table 4) [80].
| 5-HTR | Location | Main function | Potential effects | Pathological implications | Role in therapeutics |
|---|---|---|---|---|---|
| 5-HT1A | hippocampus, medial septum, amygdala, hypothalamus, cortical pyramidal neurons, and interneurons of prefrontal cortex | Inhibitory | cognitive function, mood and emotional states. | Anxiety disorders, bipolar disorder, schizophrenia, and severe depressive disorder | Frequent target for manage depression, Schizophrenia, bipolar disorder and anxiety. |
| 5-HT1B | basal ganglia, striatum, frontal cortex and Cranial Blood Vessel | Inhibits neurotransmitter release | mood, memory, Smooth muscle contraction. Causes both vasoconstriction and vasodilation | Hypertension, migraine and thrombotic events. | 5-HT1B agonists (triptans), in the pharmacotherapy of migraines |
| 5-HT1D | Presynaptic Neuron | Inhibits neurotransmitter release | Inhibitory, Vasoconstriction Constriction of intracranial blood vessel smooth muscle | Hypertension, migraine | Same agonists as 5HT1B |
| 5-HT2A | smooth muscle cells of blood vessel, Platelet | Regulating vascular tone and blood flow | Platelet Aggregation, Contraction | cardiovascular diseases, atheroscelerosis | Antagonists are used as antihypertensive and anticoagulants. Agonists are being explored to treat mental disorders and drug abuse. |
| 5-HT2B | the GIT, liver, kidney, and heart | peristalsis and secretions production | Contraction | Disruption might affect neuronal differentiation | -HT2B-selective antagonists impedes differentiation of neurons in vitro by 5-HT, affects colonic engine movement. |
| 5-HT3 | Peripheral neuron, CNS | ↑ transmitter release, ↑secretion | Gut motility and visceral sensitivity | IBS | Both antagonists and agonists are used in management of IBS |
| 5-HT4 | Enteric neurons, smooth muscles of GIT | ↑Transmitter release, ↑ secretion | Relaxation, regular and propulsive motility | IBS | Agonists show more promising management of IBS-C/M |
| 5-HT5A | hippocampus and neocortex | Not fully understood | learning, memory, and mood regulation. | Schizophrenia, unipolar depression and other psychiatric disorders | Therapies targeted to this receptor might show promising effects in improving memory and blood flow. |
| 5-HT6 | cortex and hippocampus | Neuronal wiring and brain development | cognition, learning, memory, mood | Alzheimer’s disease (AD), anxiety and cognitive decline | target for treating cognitive decline, antagonists might help in the reversal of age and pharmacological related impairment, repair of brain damage and neuronal plasticity. |
| 5-HT7 | Vascular and nonvascular smooth muscle like | Neural signaling of pain to peripheral tissues | Relaxation, immune activation & inflammatory response. | Visceral paresthesia, co-morbid anxiety and depression in IBS, may aggravate colitis | Potential target to address nociception and comorbid anxiety in IBS |
Table 4.
Location, main function, potential effects and pathological implications of 5HTR.
16. Conclusion and future direction
There is a plethora of literatures available regarding serotonin and its role in the gut brain axis, despite that many questions are still left unanswered that warrants future research. There is no clinical use deduced for 5HT2B receptors and the research still remains in its infancy. 5HT7 receptors presents promising therapeutic potential since it has been found to affect both gut and brain, development of drugs targeting this specific receptor may prove to be effective to simultaneously treat the gut-induced brain disorders and vice versa, possibly without misbalancing the gut-brain equilibrium a seen with SSRIs and SNRIs. Apart from that, role of Neuronal 5HT and its synthesis by TPH2 is yet another area to be explored to investigate the complexity of ENS in relation to CNS. In conclusion, the intricate network of serotonin receptors within the bloodstream constitutes a complex interplay of 5-HTR2A, 5-HTR1B, and 5-HTR1D, guide’s complicated processes involving platelet aggregation, vascular tone, and endothelial function. The vasoconstrictive effects of 5-HTR1B and 5-HTR2A expressed on smooth muscle cells are crucial for hemostasis and wound repair, while 5-HT1D receptors modulate serotonin release, impacting overall vascular constriction. Dysregulation of these receptors is implicated in migraine, hypertension, and thrombosis. Notably, the therapeutic benefits of these receptors is evident in development of triptans, designed to alleviate migraines by balancing vasoconstrictive and vasodilatory responses. Additionally, NO-releasing triptans and antagonists like ketanserin offer novel approaches to mitigate platelet activation and promote vascular health. As our understanding of these receptors deepens, future directions should focus on unraveling their intricate mechanisms, elucidating their roles in various diseases, and advancing targeted therapeutic strategies. This evolving knowledge holds the promise of shedding light on new avenues for maintaining vascular equilibrium and addressing a range of cardiovascular disorders.
References
- 1.
Kanova M, Kohout P. Serotonin—Its synthesis and roles in the healthy and the critically ill. International Journal of Molecular Sciences. 2021; 22 (9):4837 - 2.
Wu H, Denna TH, Storkersen JN, Gerriets VA. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacological Research. 2019; 140 :100-114 - 3.
David D, Gardier A. Les bases de pharmacologie fondamentale du système sérotoninergique: application à la réponse antidépressive. L'Encéphale. 2016; 42 (3):255-263 - 4.
Smith C, Smith M, Cunningham R, Davis S. Recent advances in Antiemetics: New formulations of 5-HT: 3: Receptor antagonists in adults. Cancer Nursing. 2020; 43 (4):E217-EE28 - 5.
Conde K, Fang S, Xu Y. Unraveling the serotonin saga: From discovery to weight regulation and beyond-a comprehensive scientific review. Cell & Bioscience. 2023; 13 (1):1-11 - 6.
Coleman JA, Yang D, Zhao Z, Wen P-C, Yoshioka C, Tajkhorshid E, et al. Serotonin transporter–ibogaine complexes illuminate mechanisms of inhibition and transport. Nature. 2019; 569 (7754):141-145 - 7.
Kitson SL. 5-hydroxytryptamine (5-HT) receptor ligands. Current pharmaceutical design. 2007; 13 (25):2621-2637 - 8.
De Deurwaerdère P, Di Giovanni G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Progress in Neurobiology. 2017; 151 :175-236 - 9.
Seyedabadi M, Fakhfouri G, Ramezani V, Mehr SE, Rahimian R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Experimental brain research. 2014; 232 :723-738 - 10.
Svensson E, Apergis-Schoute J, Burnstock G, Nusbaum MP, Parker D, Schiöth HB. General principles of neuronal co-transmission: Insights from multiple model systems. Frontiers in neural circuits. 2019; 12 :117 - 11.
Gershon M. Serotonin receptors and transporters—Roles in normal and abnormal gastrointestinal motility. Alimentary pharmacology & therapeutics. 2004; 20 :3-14 - 12.
Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT2B receptor in the development of enteric neurons. Journal of Neuroscience. 2000; 20 (1):294-305 - 13.
Pergolizzi S, Rizzo G, Favaloro A, Alesci A, Pallio S, Melita G, et al. Expression of VAChT and 5-HT in ulcerative colitis dendritic cells. Acta histochemica. 2021; 123 (4):151715 - 14.
Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang J-M, et al. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. National Academy of Sciences of the United States of America. 1993; 90 (18):8547-8551 - 15.
Chagraoui A, Thibaut F, Skiba M, Thuillez C, Bourin M. 5-HT2C receptors in psychiatric disorders: A review. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2016; 66 :120-135 - 16.
Hjorth S, Bengtsson HJ, Milano S. Raphe 5-HT1A autoreceptors, but not postsynaptic 5-HT1A receptors or β-adrenoceptors, restrain the citalopram-induced increase in extracellular 5-hydroxytryptamine in vivo. European journal of pharmacology. 1996; 316 (1):43-47 - 17.
Frey BN, Rosa-Neto P, Lubarsky S, Diksic M. Correlation between serotonin synthesis and 5-HT1A receptor binding in the living human brain: A combined α-[11C] MT and [18F] MPPF positron emission tomography study. NeuroImage. 2008; 42 (2):850-857 - 18.
Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience & Biobehavioral Reviews. 2005; 29 (4-5):547-569 - 19.
Doly S, Fischer J, Brisorgueil MJ, Vergé D, Conrath M. 5-HT5A receptor localization in the rat spinal cord suggests a role in nociception and control of pelvic floor musculature. Journal of Comparative Neurology. 2004; 476 (4):316-329 - 20.
Costa-Mallen P, Checkoway H, Fishel M, Cohen AW, Smith-Weller T, Franklin GM, et al. The EcoRV genetic polymorphism of human monoamine oxidase type a is not associated with Parkinson's disease and does not modify the effect of smoking on Parkinson's disease. Neuroscience Letters. 2000; 278 (1-2):33-36 - 21.
Dorszewska J, Prendecki M, Oczkowska A, Rozycka A, Lianeri M, Kozubski W. Polymorphism of the COMT, MAO, DAT, NET and 5-HTT genes, and biogenic amines in Parkinson’s disease. Current Genomics. 2013; 14 (8):518-533 - 22.
Horjales-Araujo E, Demontis D, Lund EK, Vase L, Finnerup NB, Børglum AD, et al. Emotional modulation of muscle pain is associated with polymorphisms in the serotonin transporter gene. PAIN®. 2013; 154 (8):1469-1476 - 23.
Vilaró M, Cortés R, Mengod G, Hoyer D. Distribution of 5-HT receptors in the central nervous system: An update. In: Handbook of Behavioral Neuroscience. Vol. 31. Australia NZ: Elsevier; 2020. pp. 121-146 - 24.
Garcia-Alloza M, Hirst W, Chen C, Lasheras B, Francis P, Ramirez M. Differential involvement of 5-HT1B/1D and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology. 2004; 29 (2):410-416 - 25.
Benhamú B, Martín-Fontecha M, Vázquez-Villa H, Pardo L, López-Rodríguez ML. Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer’s disease. Journal of Medicinal Chemistry. 2014; 57 (17):7160-7181 - 26.
Matthys A, Haegeman G, Van Craenenbroeck K, Vanhoenacker P. Role of the 5-HT7 receptor in the central nervous system: From current status to future perspectives. Molecular Neurobiology. 2011; 43 :228-253 - 27.
Vanhoutte PM. Serotonin: A forgotten signal from the blood. In: Handbook of Behavioral Neuroscience. Vol. 31. Australia NZ: Elsevier; 2020. pp. 393-409 - 28.
Moerland M, Kemme M, Dijkmans A, Bergougnan L, Burggraaf J. Modulation of vasoactivity and platelet aggregation by selective 5-HT receptor antagonism in humans. Journal of Cardiovascular Pharmacology. 2011; 58 (6):575-580 - 29.
Hu L, Wang B, Zhang Y. Serotonin 5-HT6 receptors affect cognition in a mouse model of Alzheimer’s disease by regulating cilia function. Alzheimer’s research & therapy. 2017; 9 (1):1-17 - 30.
Yamazaki M, Okabe M, Yamamoto N, Yarimizu J, Harada K, Yamazaki M. Novel 5-HT5A Receptor Antagonists Ameliorate Scopolamine-Induced Working Memory Deficit. USA: Oxford Academic; 2015 - 31.
Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, et al. The serotonin 5-HT 7 receptors: Two decades of research. Experimental brain research. 2013; 230 :555-568 - 32.
Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radical Biology and Medicine. 2012; 52 (9):1970-1986 - 33.
Thomas DR. 5-ht5A receptors as a therapeutic target. Pharmacology & therapeutics. 2006; 111 (3):707-714 - 34.
Sagi Y, Medrihan L, George K, Barney M, McCabe KA, Greengard P. Emergence of 5-HT5A signaling in parvalbumin neurons mediates delayed antidepressant action. Molecular Psychiatry. 2020; 25 (6):1191-1201 - 35.
Ramírez MJ. 5-HT 6 receptors and Alzheimer’s disease. Alzheimer’s research & therapy. 2013; 5 :1-8 - 36.
Richerson GB. Serotonin: The anti-SuddenDeathAmine? Serotonin & SUDEP. Epilepsy currents. 2013; 13 (5):241-244 - 37.
Jeske NA, Berg KA, Cousins JC, Ferro ES, Clarke WP, Glucksman MJ, et al. Modulation of bradykinin signaling by EP24. 15 and EP24. 16 in cultured trigeminal ganglia. Journal of Neurochemistry. 2006; 97 (1):13-21 - 38.
Bédard C, Wallman M-J, Pourcher E, Gould PV, Parent A, Parent M. Serotonin and dopamine striatal innervation in Parkinson’s disease and Huntington’s chorea. Parkinsonism & related disorders. 2011; 17 (8):593-598 - 39.
Blattner KM, Canney DJ, Pippin DA, Blass BE. Pharmacology and therapeutic potential of the 5-HT7 receptor. ACS Chemical Neuroscience. 2018; 10 (1):89-119 - 40.
Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacological Reviews. 2012; 64 (2):359-388 - 41.
Gamoh S, Hisa H, Yamamoto R. 5-hydroxytryptamine receptors as targets for drug therapies of vascular-related diseases. Biological and pharmaceutical bulletin. 2013; 36 (9):1410-1415 - 42.
Zhang G, Stackman RW. The role of serotonin 5-HT2A receptors in memory and cognition. Frontiers in Pharmacology. 2015; 6 :1-17 - 43.
Longmore J, Maguire J, MacLeod A, Street L, Schofield W, Hill R. Comparison of the vasoconstrictor effects of the selective 5-HT1D-receptor agonist L-775,606 with the mixed 5-HT1B/1D-receptor agonist sumatriptan and 5-HT in human isolated coronary artery. British journal of clinical pharmacology. 2000; 49 (2):126-131 - 44.
Pletscher A. The 5-hydroxy-tryptamine system of blood platelets: Physiology and pathophysiology. International Journal of Cardiology. 1987; 14 (2):177-188 - 45.
Nishihira K, Yamashita A, Tanaka N, Moriguchi-Goto S, Imamura T, Ishida T, et al. Serotonin induces vasoconstriction of smooth muscle cell-rich neointima through 5-hydroxytryptamine2A receptor in rabbit femoral arteries. Journal of Thrombosis and Haemostasis. 2008; 6 (7):1207-1214 - 46.
Blessing WW, Seaman B. 5-Hydroxytryptamine2A receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience. 2003; 117 (4):939-948 - 47.
Williams MS. Platelets and depression in cardiovascular disease: A brief review of the current literature. World Journal of Psychiatry. 2012; 2 (6):114 - 48.
Muller CP, Cunningham KA. Handbook of the Behavioral Neurobiology of Serotonin. Australia NZ: Academic Press; 2020 - 49.
Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5-HT1B/1D receptor agonists. Archives of Neurology. 2002; 59 (7):1084-1088 - 50.
Classey J, Bartsch T, Goadsby P. Distribution of 5-HT1B, 5-HT1D and 5-HT1F receptor expression in rat trigeminal and dorsal root ganglia neurons: Relevance to the selective anti-migraine effect of triptans. Brain Research. 2010; 1361 :76-85 - 51.
Blackburn TP. 5 Actions of Drugs on the Brain and CNS Disorders. Pharmacology for Chemists: Drug Discovery in Context. USA: Oxford University Press; 2017. p. 130 - 52.
Goadsby P, Classey J. Evidence for serotonin (5-HT) 1B, 5-HT1D and 5-HT1F receptor inhibitory effects on trigeminal neurons with craniovascular input. Neuroscience. 2003; 122 (2):491-498 - 53.
de Vries P. 5-Hydroxytryptamine Receptors Mediating Carotid and Systemic Haemodynamic Effects: The Relation to Acute Antimigraine Therapy. Rotterdam, The Netherlands; 1999 - 54.
Mendelson SD. The current status of the platelet 5-HT2A receptor in depression. Journal of Affective Disorders. 2000; 57 (1-3):13-24 - 55.
Moghaddam RA, Jazaeri F, Abdollahi A, Mohammadjafari R, Dehpour AR, Bakhtiarian A. Evaluation of isolated vascular response to 5HT1A, 5HT1B1D & 5HT2A Receptors Agonist & Antagonist in chronic Endotoxemic rats. Drug Research. 2019; 69 (06):352-360 - 56.
Schytz HW, Hargreaves R, Ashina M. Challenges in developing drugs for primary headaches. Progress in Neurobiology. 2017; 152 :70-88 - 57.
Tiger M, Varnäs K, Okubo Y, Lundberg J. The 5-HT(1B) receptor - a potential target for antidepressant treatment. Psychopharmacology. 2018; 235 (5):1317-1334 - 58.
Voronova IP, Khramova GM, Kulikova EA, Petrovskii DV, Bazovkina DV, Kulikov AV. 5-HT2A receptors control body temperature in mice during LPS-induced inflammation via regulation of NO production. Pharmacological Research. 2016; 103 :123-131 - 59.
Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterology and Motility. 2015; 27 (7):899-905 - 60.
Bayliss WM, Starling EH. The movements and innervation of the small intestine. The Journal of Physiology. 1901; 26 (3-4):125-138 - 61.
Bruta K, Vanshika BK, Bhawana. The role of serotonin and diet in the prevalence of irritable bowel syndrome: A systematic review. Translational Medicine Communications. 2021; 6 (1):1-9 - 62.
Barnes NM, Neumaier JF. Neuronal 5-HT receptors and SERT. Tocris Bioscience. 2011; 34 :1-16 - 63.
Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. The Journal of Clinical Investigation. 2016; 126 (6):2221-2235 - 64.
De Ponti F. Pharmacology of serotonin: What a clinician should know. Gut. 2004; 53 (10):1520-1535 - 65.
Raote I, Bhattacharya A, Panicker MM. In: Chattopadhyay A, editor. Serotonin Receptors in Neurobiology. Frontiers in Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis; 2007 - 66.
Gardier AM, Malagié I, Trillat A, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: Recent findings from in vivo microdialysis studies. Fundamental & Clinical Pharmacology. 1996; 10 :16-27 - 67.
Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT2B receptor in the development of enteric neurons. The Journal of Neuroscience. 2000; 20 (1):294-305 - 68.
Sbahi H, Cash BD. Chronic constipation: A review of current literature. Current Gastroenterology Reports. 2015; 17 (12):47 - 69.
Morita H, Mochiki E, Takahashi N, Kawamura K, Watanabe A, Sutou T, et al. Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on gastrointestinal motor activity in dogs. World Journal of Gastroenterology. 2013; 19 (39):6604-6612 - 70.
Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: Experimental evidence and therapeutic relevance. Handbook of Experimental Pharmacology. 2017; 239 :319-342 - 71.
Takahashi K, Khwaja IG, Schreyer JR, Bulmer D, Peiris M, Terai S, et al. Post-inflammatory abdominal pain in patients with inflammatory bowel disease during remission: A comprehensive review. Crohns Colitis 360. 2021; 3 (4):otab073 - 72.
Leclere PG, Prins NH, Schuurkes JA, Lefebvre RA. 5-HT4 receptors located on cholinergic nerves in human colon circular muscle. Neurogastroenterology and Motility. 2005; 17 (3):366-375 - 73.
Brookes S, Chen N, Humenick A, Spencer NJ, Costa M. Extrinsic sensory innervation of the gut: structure and function. The Enteric Nervous System: 30 Years Later. 2016; 891 :63-69 - 74.
Chang W-Y, Yang Y-T, She M-P, Tu C-H, Lee T-C, Wu M-S, et al. 5-HT7 receptor-dependent intestinal neurite outgrowth contributes to visceral hypersensitivity in irritable bowel syndrome. Laboratory Investigation. 2022; 102 (9):1023-1037 - 75.
Grider JR. Desensitization of the peristaltic reflex induced by mucosal stimulation with the selective 5-HT4 agonist tegaserod. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2006; 290 (2):G319-G327 - 76.
Malinova TS, Dijkstra CD, de Vries HE. Serotonin: A mediator of the gut–brain axis in multiple sclerosis. Multiple Sclerosis Journal. 2018; 24 (9):1144-1150 - 77.
Guzel T, Mirowska-Guzel D. The role of serotonin neurotransmission in gastrointestinal tract and pharmacotherapy. Molecules. 2022; 27 (5):1680 - 78.
Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010; 139 (1):249-258 - 79.
Fayyaz M, Lackner JM. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Therapeutics and Clinical Risk Management. 2008; 4 (1):41-48 - 80.
Kim JJ, Khan WI. 5-HT7 receptor signaling: Improved therapeutic strategy in gut disorders. Frontiers in Behavioral Neuroscience. 2014; 8 :396