Comparative rates of immunosuppressant nonadherence across different transplant recipient types.
Abstract
This chapter investigates the relationship between blood groups and the immune system in the background of organ transplantation. It explores how blood group compatibility plays a serious role in determining the success of organ transplants and mitigating the risk of rejection. The chapter focuses on the underlying mechanisms that affect graft acceptance or rejection by looking at the immunological importance of blood types. It explores the complex interactions between antibodies, antigens, and the immune response and emphasizes how variations in blood type antigens may trigger the immunological reactions and affect the success of transplants. Furthermore, the clinical implications of blood group matching in organ transplantation are also highlighted in this chapter. It also discusses emerging advancements in immunogenetics and immunosuppressive therapies that aim to overcome the immunological challenges associated with blood group disparities. Overall. The chapter serves as a valuable resource for healthcare professionals and researchers to facilitate improved matching strategies and enhanced outcomes in the field of transplantation medicine.
Keywords
- blood groups
- immune system
- organ transplantation
- blood group compatibility
- risk of rejection
- blood types
1. Introduction
The study aims to examine the influence of blood groups on the outcomes of organ transplantation [1]. By examining how different blood groups interact with the immune system, this research seeks to uncover the potential impact on graft acceptance, rejection rates, and overall success of transplantation procedures. Through a comprehensive understanding of these immunological interactions, the study aims to provide insights that could guide improved strategies for organ compatibility assessment and enhance transplantation outcomes [2]. Organ transplantation stands as a pinnacle of modern medical achievement, offering a transformative solution for individuals facing life-threatening organ failure. This complex procedure involves replacing a malfunctioning or injured organ with a vigorous one from a donor, often providing patients with renewed vitality and extended life. The significance of organ transplantation lies in its ability to save lives, restore function, and enhance the quality of life for recipients who might otherwise be confined to a life of medical intervention. As medical knowledge and surgical techniques continue to advance, organ transplantation has evolved from an experimental endeavor to a well-established medical practice, making a profound impact on the lives of countless individuals and their families. The significance of organ transplantation reaches far beyond medical treatment. It embodies the potential to transform a patient’s existence from one of suffering and limitations to a life of renewed vitality [3]. Transplant recipients often experience not only physical relief but also a newfound sense of purpose as they resume everyday activities, pursue careers, and engage in hobbies that were once distant dreams. This restoration of functionality has a cascading effect on their mental and emotional well-being, illustrating how organ transplantation is more than just a medical procedure; it’s a gateway to a second chance at life.
The impact of organ transplantation extends beyond individual patients, influencing society and healthcare systems [4]. While the procedure comes with initial costs, the long-term benefits are compelling. Successful transplants reduce the need for ongoing treatments like dialysis or extended hospital stays, relieving the burden on healthcare resources. Furthermore, recipients’ return to active lives contributes to a stronger workforce and reduced strain on social services. This underscores how organ transplantation is not only a medical triumph but also an avenue toward more efficient and sustainable healthcare practices [5]. However, the field of organ transplantation is not without its challenges. Ethical considerations surrounding organ procurement, distribution, and the use of living donors require careful navigation. As medical science evolves, research continues to explore regenerative medicine, innovative transplantation techniques, and personalized approaches. These endeavors offer hope for overcoming existing limitations, addressing organ shortages, and ultimately improving the success rates and accessibility of organ transplantation. Organ transplantation serves as a testament to human ingenuity, medical progress, and the unwavering pursuit of life-enhancing solutions in the realm of modern medicine. Moreover, blood groups play a pivotal role in determining the compatibility of organ transplantation [6]. These groups, categorized as A, B, AB, and O, are well-defined by explicit antigens existing on the surface of red blood cells. Blood group compatibility is crucial in transplantation to mitigate potential immunological challenges. When an organ is transplanted, the immune system of the donor can identify the antigens on the donor’s cells as distant, activating an immune reaction that may lead to graft rejection.
The compatibility of blood groups among the donor and recipient is fundamental to the success of an organ transplant [7]. Mismatched blood groups can evoke an immune response categorized by the production of antibodies alongside the foreign antigens. This response can lead to hyperacute rejection, a speedy and stark form of rejection that happens within minutes to hours afterwards transplantation. The antibodies can activate complement proteins, causing extensive damage to the transplanted organ’s blood vessels and ultimately resulting in organ failure. Incompatibility in blood groups can also lead to acute and chronic rejection over time, as the immune system continuously targets the foreign antigens [8]. Understanding the immunological challenges posed by blood group disparities is essential to devising effective transplant strategies. Immunosuppressive therapies are employed to dampen the immune response and prevent rejection. However, managing immunosuppression while maintaining a functioning immune system poses a delicate balance, as excessive suppression can increase susceptibility to infections and other complications. Additionally, advancements in transplant techniques, such as cross matching and tissue typing, have been crucial in assessing compatibility and minimizing the risk of rejection. The intricate interplay between blood group compatibility and the immune response highlights the necessity of meticulous donor-recipient matching and underscores the ongoing research efforts to refine transplantation procedures and enhance long-term graft survival.
The key contributions of this paper is given as follows:
The research builds light on how different blood groups impact the compatibility between donors and recipients in organ transplantation.
By elucidating the complex immunological interactions involved, it provides valuable insights for refining compatibility assessments, ultimately leading to better organ matching and reduced risk of rejection.
The research tailors immunosuppressive strategies and innovative desensitization techniques that mitigate the risk of immune responses, particularly those stemming from blood group incompatibilities.
The research prepares healthcare professionals with crucial information to make informed decisions regarding organ allocation and transplantation priority.
The research underscores the importance of ongoing investigations into blood group immunology, stimulating further research and innovation in the field of organ transplantation.
2. Blood groups and compatibility
The ABO and RhD blood group systems are two of the most well-known and clinically significant blood group systems that play a crucial role in blood compatibility, organ transplantation, and pregnancy [9]. These systems are strong-minded by particular antigens that are existing on the surface of red blood cells.
2.1 ABO and RhD blood group system
The ABO blood group system orders blood into four main groups: A, B, AB, and O, grounded on the occurrence or nonappearance of two antigens: A and B antigens [10]. These antigens are glycoproteins located on the surface of red blood cells. The A antigen is contemporary in blood type A, the B antigen is present in blood type B, both antigens are existing in blood type AB, and neither antigen is present in blood type O. Additionally, individuals possess antibodies compared to the antigens they lack. For example, a person with blood type A has anti-B antibodies, and a person with blood type B has anti-A antibodies. Blood type AB individuals do not produce these antibodies, while blood type O entities yield both anti-A and anti-B antibodies. This antigen-antibody interaction is important in blood transfusions to prevent adverse reactions. It also produces a risk known as Graft rejection. And it is caused due to ABO incompatibility in organ transplantation. Moreover, it potentially leading to graft failure and adverse patient outcomes. ABO-incompatible transplants can trigger severe immune responses, such as hyperacute rejection, driven by preformed antibodies targeting non-self ABO antigens. These immune reactions result in rapid damage to the transplanted organ, compromising its function and often necessitating emergency retransplantation.
2.2 RhD blood group system
The RhD blood group system, often referred to as the Rh factor or Rh antigen, focuses on the occurrence or nonappearance of the RhD antigen on the surface of red blood cells [11]. Individuals having this antigen are Rh-positive (e.g., A+, B+), while those lacking the antigen are Rh-negative (e.g., A−, B−). The RhD system is particularly important during pregnancy. An Rh-negative mother booming an Rh-positive fetus may progress antibodies alongside the RhD antigen if their blood mixes, potentially leading to hemolytic disease of the newborn (HDN) in subsequent pregnancies. To prevent HDN, Rh-negative pregnant women may receive Rh immunoglobulin (RhIg) to prevent antibody formation. These blood group systems hold critical clinical significance in blood transfusions and organ transplantation. ABO compatibility is essential to prevent adverse reactions during blood transfusions, while Rh compatibility is crucial for preventing sensitization in cases of Rh-negative individuals receiving Rh-positive blood. In organ transplantation, matching blood groups between donors and recipients is a vital consideration to minimize the risk of graft rejection due to immune responses against unmatched antigens.
Incompatibility in the Rh system can lead to the production of anti-Rh antibodies in Rh-negative individuals if they are exposed to Rh-positive blood. This is particularly relevant during pregnancy when an Rh-negative mother transfers an Rh-positive fetus. Figure 1 depicts the ABO-RhD diagram.
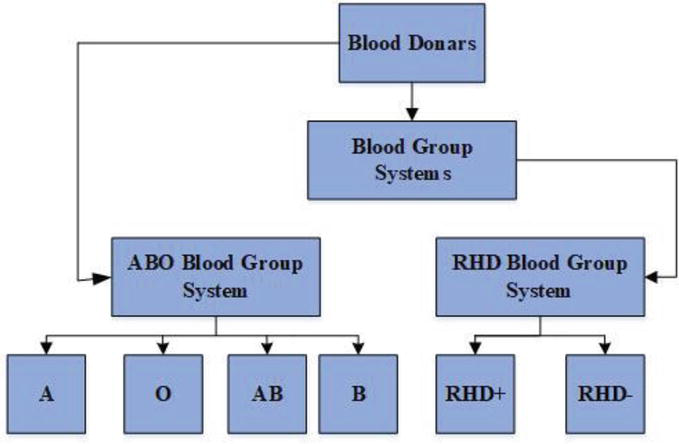
Figure 1.
ABO-RHD diagram.
2.3 Supplementary blood group systems
In addition to the ABO and RhD blood group systems, there are several other important blood group antigen systems that play a crucial role in organ transplantation, immune responses, and overall compatibility [12]. Two notable systems are the Human Leukocyte Antigen (HLA) system and the Major Histocompatibility Complex (MHC) play an important role in transplant immunology.
2.3.1 Human leukocyte antigen (HLA) system
The HLA system, also known as the human MHC (Major Histocompatibility Complex), is a extremely diverse group of DNAs positioned on chromosome 6. HLA molecules play a pivotal role in the immune system by presenting antigens to T cells, which regulate immune responses [13]. HLA particles are established on the surface of various cells, including immune cells and transplantable tissues. Two main classes of HLA are: HLA class I (HLA-A, HLA-B, and HLA-C) and HLA class II (HLA-DR, HLA-DP, and HLA-DQ). In organ transplantation, HLA compatibility is crucial for graft survival. Mismatched HLA antigens can trigger immune responses, leading to acute or chronic rejection of the transplanted organ. The immune system distinguishes the foreign HLA antigens as non-self and can mount an immune response against the transplanted tissue. To enhance organ transplant success, HLA matching between the donor and recipient is carefully considered. Figure 2 depicts the diagrammatic representation of HLA.
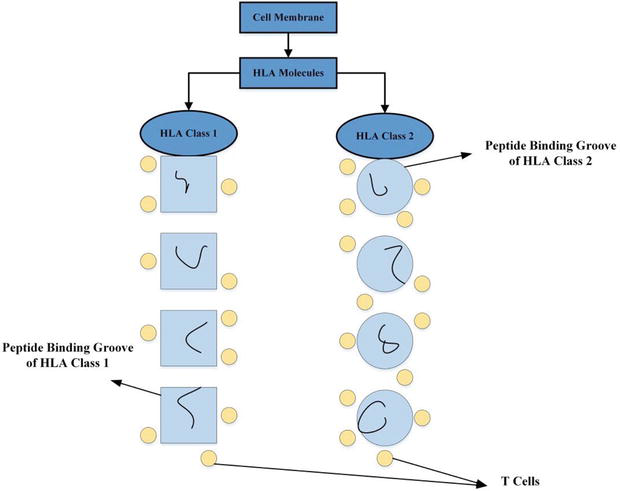
Figure 2.
Diagrammatic representation of HLA.
2.3.2 Major histocompatibility complex (MHC)
The Major Histocompatibility Complex (MHC), is also acknowledged as the Human Leukocyte Antigen (HLA) structure in humans and also it is also considered as a cluster of genetic factor that scramble cell surface proteins responsible for presenting antigens to the immune system [14]. The MHC is a dangerous constituent of the immune response and plays a central part in immune recognition, self-versus-non-self-discrimination, and immune system regulation. The MHC is separated into two main sessions: MHC class I and MHC class II.
2.3.2.1 MHC class I
MHC class I particles are initiated on the surface of nearly all nucleated cells in the body [15]. They play a crucial role in bestowing antigens consequent from within the cell, such as viral or intracellular bacterial proteins, to cytotoxic T cells (CD8+ T cells). This presentation helps the immune system identify and destroy infected or abnormal cells. MHC class I particles comprise of a transmembrane heavy chain, light chain and a small protein fragment called a peptide, which is derived from the internal antigens.
2.3.2.2 MHC class II
MHC class II particles are primarily created on the exterior of antigen-presenting cells, like dendritic cells, macrophages, and B cells [16]. These molecules present antigens consequents from extracellular pathogens, like bacteria and fungi, to helper T cells (CD4+ T cells). This interaction is crucial for activating and regulating immune responses. MHC class II molecules also consist of a transmembrane heavy chain and a peptide fragment, but they interact with different types of T cells compared to MHC class I. Figure 3 depicts the diagrammatic representation of MHC.
Figure 3.
Diagrammatic representation of MHC.
2.4 Cross matching
Cross matching is a pivotal pre-transplantation laboratory test that evaluates the compatibility between a recipient’s immune system and a potential organ donor. It encompasses both direct and indirect methods to recognize antibodies in the recipient’s serum that could respond against antigens on the donor’s tissue. In direct cross matching, the recipient’s antibodies are tested against the donor’s cells, while in indirect cross matching, recipient cell is verified in contrast to donor serum. A positive cross match indicates potential incompatibility, signaling a risk of immune-mediated rejection and hyperacute rejection [17]. Strategies like desensitization, plasmapheresis, intravenous immunoglobulin (IVIG), and immunosuppressive medications can be employed to mitigate the risk in cases of positive cross matches. By preventing adverse immune reactions, cross matching enhances the success of organ transplantation and contributes to improved recipient outcomes. In blood transfusions, non-ABO antigens, such as those from the Rh and other blood group systems, can trigger immune responses if the recipient’s immune system identifies these antigens as foreign. This can lead to hemolysis, transfusion reactions, and other adverse effects. By minimizing immune responses against these antigens, blood transfusions can be carried out safely and effectively, ensuring that the transfused blood components are compatible with the recipient’s immune system. Minimizing immune responses against non-ABO antigens is of paramount importance in various medical contexts, particularly in blood transfusions and organ transplantation. While ABO compatibility is a critical consideration, the immune system’s response to non-ABO antigens, particularly those were associated to the HLA system, can significantly impact the success of these medical procedures. Moreover, minimizing immune responses against non-ABO antigens is essential to avoid complications in treatments like bone marrow and stem cell transplantation. Mismatched HLA antigens can lead to graft-versus-host disease (GVHD), where immune cells from the transplanted tissue destroy the recipient’s tissues.
3. Immunological basis of blood group compatibility
Blood group compatibility is founded on the complicated interaction among the immune system and the distinct antigens present on the surface of red blood cells [18]. The major blood group systems, such as ABO and Rh, are key determinants of compatibility in blood transfusions and organ transplants. The ABO system, for instance, is governed by the occurrence or nonappearance of A and B antigens, which are formed by glycosyltransferases encoded by the ABO gene. These antigens act as markers that are recognized by the immune system as either self or non-self. Incompatible blood transfusions can trigger an immune response as a consequence of the immune system’s recognition of non-self-antigens. Individuals possess naturally occurring antibodies compared to the ABO antigens they lack. For instance, individuals with blood type A have anti-B antibodies, while those with blood type B have anti-A antibodies. In type O individuals, who lack both A and B antigens, both anti-A and anti-B antibodies are present. Upon exposure to non-self-antigens, these antibodies can drag to the distant antigens on transfused red blood cells, inducing agglutination and potential blockages within blood vessels, leading to serious complications. The immune basis of blood group compatibility extends to the Rh system as well. The Rh antigen, also recognized as the D antigen, is a critical determinant in Rh-positive and Rh-negative blood types. Rh-negative individuals can create antibodies in contrast to the Rh antigen if exposed to Rh-positive blood. This becomes particularly relevant for pregnant Rh-negative women bearing an Rh-positive fetus, as maternal antibodies can cross the placenta, potentially leading to hemolytic disease of the newborn.
3.1 Hyperacute rejection by anti-A and anti-B antibodies
Hyperacute rejection is a rapid and severe immune response that occurs in organ transplantation when the recipient’s preformed antibodies react against antigens on the transplanted organ’s vascular endothelium [19]. The presence of anti-A and anti-B antibodies can contribute to hyperacute rejection. When an organ from a donor with a different ABO blood type is transplanted into a recipient, the ABO antigens present on the surface of the transplanted organ’s blood vessels can trigger an immediate immune response. This happens because the recipient’s immune system identifies these ABO antigens as distant and targets them with the preformed antibodies that are specific to the antigens they lack. For example, if a blood type A individual obtains an organ from a blood type B donor, the recipient’s immune system contains preformed anti-B antibodies. These antibodies can quickly drag to the B antigens on the transplanted organ’s blood vessels. This binding triggers a series of events, including activation of complement proteins and recruitment of immune cells, which leads to inflammation, blood clot formation, and damage to the blood vessels and the organ itself. This process can occur within minutes to hours after transplantation, resulting in the failure of the transplanted organ.
The exploration of the immunological significance of blood groups in transplantation has yielded valuable insights, yet several promising areas for future research remain. Firstly, investigating the underlying mechanisms that drive hyperacute rejection due to ABO incompatibility could lead to more targeted interventions. Understanding the factors influencing the rapid activation of complement proteins and immune cells upon encountering non-self ABO antigens could potentially open avenues for developing therapies that mitigate this severe form of rejection. Also, delving into the complexities of the immune response against non-ABO antigens, such as those within the HLA system, offers an opportunity to enhance transplantation outcomes further. Research could focus on deciphering the intricacies of T cell recognition and activation in response to mismatched HLA antigens. This could lead to the development of personalized immunosuppressive strategies that dampen the immune response while preserving the overall immune function, thereby improving long-term graft survival.
Moreover, advancements in immunogenetics and molecular techniques also present exciting avenues. Investigating genetic variations that influence immune responses to blood group antigens could yield insights into individual susceptibility to rejection. This could pave the way for precision medicine approaches that tailor transplant protocols based on a recipient’s genetic profile, enhancing compatibility and minimizing adverse reactions. Therefore, exploring the role of non-ABO antigens beyond direct graft rejection is an emerging research area. Investigating how these antigens influence the immune microenvironment post-transplantation and their potential impact on long-term graft health could lead to more comprehensive strategies for immunomodulation and graft preservation.
3.2 Character of preformed antibodies and supplement stimulation in graft damage
Preformed antibodies and supplement stimulation play critical roles in graft damage, particularly in the background of organ transplantation and autoimmune diseases [20]. These mechanisms contribute to hyperacute rejection in organ transplantation and can exacerbate tissue damage in autoimmune conditions. Preformed antibodies are immune molecules that have been previously generated in response to antigens encountered in previous infections or sensitization events. In the context of organ transplantation, preformed antibodies can identify distant antigens on the transplanted organ’s surface. This acknowledgment generates a rapid and powerful immune response that can lead to graft damage. In hyperacute rejection, preformed antibodies specifically target the antigens present on the transplanted organ, initiating a cascade of immune reactions that include complement activation. Complement activation is a series of enzymatic reactions that involve a group of proteins called the supplement system. The supplement system can be stimulated through the classical, lectin, or alternate pathways. When preformed antibodies bind to antigens on the graft, they can trigger the classical conduit of supplement activation. This pointers to the sequential cleavage of complement proteins, ultimately resulting in the development of membrane attack complexes (MACs) that puncture the cell membranes of the target tissue or graft cells. This leads to cell lysis, inflammation, and tissue damage.
4. Impact on transplantation success
However, the extent of non-adherence to immunosuppressants varies among different categories of transplant recipients [21]. The findings reveal that kidney recipients exhibit the highest non-adherence prevalence, with a rate of 38 cases of100 patient per years (PPY). This rate surpasses that detected in heart recipients by more than two-fold and exceeds liver recipients by over five-fold. The lower rates in the latter groups could stem from the diverse practices across programs, possibly implementing more rigorous psychosocial criteria during the transplant candidate selection process compared to kidney candidates. Alternatively, the higher stakes of graft loss may contribute: while kidney recipients can resume dialysis, heart or liver recipients often face more limited options for life extension. Nevertheless, even the rates of nonadherence among heart and liver recipients, which stand at 15 to 20 cases per 100 PPY and 8 cases per 100 PPY respectively, could raise clinical concerns. These rates are potentially deemed unacceptable. Notably, all these rates surpass the incidence of tobacco use, a potent contributor to post transplant morbidity and mortality. Even at its comparatively lower level of 5 to 6 patients per 100 PPY, tobacco use still significantly impacts patient outcomes after transplantation. Table 1 depicts about the type of transplant recipient, rate, impact and its significance.
| Transplant recipient type | Non-adherence rate | Impact | Significance |
|---|---|---|---|
| Kidney | 38 | Fewer demanding psychosocial standards and graft loss effects | High percentage of nonadherence which could affect graft survival. |
| Heart | 15–20 | Psychosocial requirements, effects of graft loss | Despite minimal nonadherence rates patient results are nevertheless affected. |
| Liver | 8 | High psychosocial standards and serious graft loss effects | High rates of nonadherence, which impact graft survival. |
| Tobacco Use | 5–6 | Impactful but relatively lower | Use of tobacco increases posttransplant morbidity and mortality. |
Table 1.
5. Strategies to improve organ transplantation
The research examines a multifaceted exploration of key approaches aimed at elevating the success and effectiveness of organ transplantation procedures [22]. This critical discourse underscores the paramount importance of optimizing various facets of the transplantation process to enhance patient outcomes and the longevity of graft survival. The monarchy of immunosuppression management, show a vital role in suppressing the recipient’s immune response to prevent graft rejection. Advancements in personalized immunosuppressive regimens, often combining multiple medications with distinct mechanisms of action, strive to strike a delicate balance between minimizing the risk of rejection and avoiding over-immunosuppression-related complications. Additionally, targeted therapies that focus on specific immune pathways hold promise in mitigating the adverse effects of immunosuppression while maintaining graft tolerance. Moreover, the research navigates the advanced tissue typing and cross matching techniques enable a more nuanced evaluation of compatibility between donor and recipient, reducing the risk of immune-mediated graft rejection. Thus this encapsulates a holistic perspective on the dynamic approaches that converge to create a transformative impact on the success of organ transplantation. By synergizing innovations in immunosuppression, organ preservation, and compatibility optimization, this section underscores the unyielding commitment of the medical community to elevate transplantation outcomes, ultimately enhancing the quality of life for recipients and advancing the frontiers of transplantation medicine.
5.1 Advancements in immunosuppressive drugs
Advancements in immunosuppressive drugs have played a pivotal role in mitigating immune reactions against non-ABO antigens in the perspective of organ transplantation [23]. These drugs are designed to moderate the recipient’s immune response, thus dropping the hazard of graft rejection and improving the long-term existence of transplanted organs. One key category of immunosuppressive drugs includes calcineurin inhibitors such as cyclosporine and tacrolimus. These drugs work by inhibiting the activity of calcineurin, a crucial enzyme in T cell activation. By dampening T cell responses, these drugs help prevent the immune system from recognizing the transplanted organ as foreign. This mechanism is particularly relevant in reducing immune reactions against non-ABO antigens, as T cells are major contributors to immune-mediated graft rejection. Another class of immunosuppressants comprises mTOR inhibitors like sirolimus and everolimus. These drugs target the mammalian target of rapamycin (mTOR) path, which normalizes cell proliferation and immune responses. By inhibiting mTOR, these drugs impede T cell activation and proliferation, further reducing the immune system’s ability to mount a strong response against non-ABO antigens. Moreover, Corticosteroids like prednisone, have been used for decades as immunosuppressive agents. They exert broad anti-inflammatory effects and can suppress various components of the immune response, including T cell activity and antibody production. While their use is related with a range of side effects, comprising metabolic disturbances and increased infection risk, corticosteroids remain an essential tool in managing immune reactions after transplantation.
Consequently, the emergence of targeted biologic agents has ushered in a new era of immunosuppression. Moreover, monoclonal antibodies like basiliximab and alemtuzumab specifically target certain immune cell surface markers, effectively depleting or suppressing the activity of these cells. These agents offer a more precise means of immune suppression, allowing for better control over immune reactions against non-ABO antigens. Thus, advancements in immunosuppressive drugs have enabled clinicians to tailor treatment regimens to individual patient needs, optimizing the balance between immune suppression and the risk of infections or other complications. By specifically targeting pathways involved in immune responses, these drugs help minimize the risk of immune reactions against non-ABO antigens, thus enhancing graft survival and improving the overall success of organ transplantation [24]. However, the challenge lies in maintaining a delicate equilibrium between suppressing the immune response and ensuring the recipient’s ability to fight infections and other challenges. Ongoing research continues to refine immunosuppressive strategies and develop novel agents to further enhance transplantation outcomes.
The allocation of scarce donor organs is a deeply challenging and ethically charged endeavor, as it involves balancing equitable access to life-saving treatments with the imperative to maximize positive outcomes for recipients. Blood group compatibility, particularly in ABO and RhD systems, is a fundamental criterion in organ allocation, aimed at minimizing the risk of graft rejection and improving transplantation success rates. However, this approach raises ethical dilemmas, as prioritizing recipients with compatible blood groups can inadvertently disadvantage those with less common or rare blood types. This can result in longer wait times for some patients and potentially compromise their health and quality of life. One ethical concern pertains to fairness and distributive justice. Allocating organs primarily based on blood group compatibility might disproportionately benefit individuals with more prevalent blood types, leaving those with rarer blood types at a disadvantage. This challenge is especially relevant when considering the global diversity of blood groups and the varying prevalence of different types within different populations.
Additionally, the ethical discourse extends to the consideration of medical urgency and the principle of utility. Balancing medical urgency against blood group compatibility raises questions about the ethical imperative to save lives versus the importance of minimizing post-transplant complications. Furthermore, the ethical complexities intersect with the broader context of organ trafficking, commercialization, and illegal organ trade. As the ultimatum for organs exceeds the obtainable source, there is a risk that the focus on blood group compatibility could exacerbate the incentive for illegal organ transactions and exploitation, compromising the ethical foundations of organ allocation.
These ethical challenges should navigate transplant societies, ethicists, and policymakers must carefully deliberate on allocation criteria that balance compatibility, medical need, fairness, and justice. It is imperative to uphold transparency in organ allocation processes, engage in public discourse, and consider innovative approaches that ensure equitable access to transplantation for all individuals, regardless of their blood group compatibility. Ultimately, addressing these ethical concerns requires a delicate balance between optimizing transplantation outcomes and upholding principles of justice, autonomy, and human dignity in the allocation of life-saving organs.
Moreover, transplantation techniques and strategies evolve to enhance compatibility and minimize graft rejection, the broader societal landscape experiences various positive transformations. Improved transplantation outcomes contribute to a significant improvement in the overall quality of life for transplant recipients. As graft rejection rates decrease due to enhanced compatibility measures, recipients experience longer graft survival, reduced need for re-transplantation, and decreased rates of post-transplant complications. This leads to improved physical health, reduced dependency on medical interventions, and a greater ability to engage in daily activities, work, and social interactions. Transplant recipients often regain a sense of normalcy and the ability to actively participate in society, contributing to a higher quality of life and increased overall well-being. On a societal level, the positive impact extends to healthcare systems and resource allocation. Successful transplantation outcomes reduce the burden on healthcare resources by decreasing the need for prolonged hospital stays, ongoing medical interventions, and expensive treatments associated with graft failure or complications. This optimization of healthcare utilization frees up resources that can be redirected toward other medical needs, ultimately enhancing the efficiency and sustainability of healthcare systems. Furthermore, improved transplantation outcomes have a positive ripple effect on the workforce and economy. Successful transplant recipients who return to work and productive activities contribute to a more robust labor force and increased economic productivity. This, in turn, generates a positive economic impact over augmented tax profits, condensed dependence on social welfare platforms, and improved economic growth. Beyond the immediate health and economic benefits, the societal impact extends to public perception and awareness of transplantation. Positive outcomes and reduced graft rejection rates reinforce public confidence in transplantation as a viable and life-transforming medical solution. This may lead to increased organ donation rates, as the public witnesses the tangible benefits of their organ donations in the form of successful transplant stories.
Moreover, the societal impact of improved transplantation outcomes and reduced graft rejection rates is multifaceted and profound. From individual recipients experiencing a better quality of life to healthcare systems benefiting from optimized resource allocation, and from an enhanced labor force to heightened public trust in transplantation, these advancements catalyze a positive cycle of improved well-being and societal well-being. As research and innovation continue, the potential for even greater societal benefits through transplantation remains a promising avenue for medical progress and societal advancement.
The focus must be directed toward deciphering the underlying mechanisms that intricately influence the acceptance or rejection of grafts by unveiling the immunological significance of blood types. Through an exploration of the complex interplay between antibodies, antigens, and the immune response, the chapter underscores how even subtle variations in blood type antigens can incite profound immunological reactions, ultimately shaping the success of transplantation outcomes. The clinical implications of blood group matching in the context of organ transplantation are underscored, as this investigation sheds light on the profound influence of blood compatibility on graft survival. In addition, the discourse extends to the horizon of emerging advancements in immunogenetics and immunosuppressive therapies, offering a promising arsenal to surmount the challenges posed by blood group disparities. In sum, this chapter constitutes an invaluable resource for healthcare practitioners and researchers, providing strategic insights that can propel the refinement of matching strategies and elevate the overall efficacy of transplantation practices in the dynamic landscape of medical science.
The recognition of non-ABO antigens, from the Rh and other blood group systems, poses a potential risk for immune responses during blood transfusions and organ transplantation. These immune reactions can result in hemolysis, transfusion reactions, and other adverse effects that compromise patient safety. While ABO compatibility remains a fundamental consideration, addressing non-ABO antigens, especially within the HLA system, holds equal significance in ensuring the success of these medical interventions. The importance of minimizing immune responses against these antigens cannot be overstated, as it directly influences the compatibility and acceptance of transplanted organs. Furthermore, this principle extends to treatments involving bone marrow and stem cell transplantation, where preventing immune reactions against non-ABO antigens is pivotal in preventing harmful graft-versus-host responses. By meticulously addressing and managing non-ABO antigen compatibility, healthcare professionals strive to enhance patient outcomes, reduce complications, and optimize the effectiveness of transfusions and transplantation therapies. Further study in this area has the possibility to enhance patient satisfaction, reduce graft rejection rates, and enhance transplantation procedures. Researchers may contribute to the creation of novel medicines, individualized methods, and a greater comprehension of the immune system’s function in transplantation by unraveling the complex immunological connections caused by blood group antigens.
References
- 1.
Amoroso A et al. HLA and AB0 polymorphisms may influence SARS-CoV-2 infection and COVID-19 severity. Transplantation. 2021; 105 (1):193-200 - 2.
Gotlieb N et al. The promise of machine learning applications in solid organ transplantation. NPJ Digital Medicine. 2022; 5 (1):89. DOI: 10.1038/s41746-022-00637-2 - 3.
McCormack D. The times and spaces of transplantation: Queercrip histories as futurities. Medical Humanities. 2021; 47 (4):397-406 - 4.
Angelico R, Trapani S, Manzia TM, Lombardini L, Tisone G, Cardillo M. The COVID-19 outbreak in Italy: Initial implications for organ transplantation programs. American Journal of Transplantation. 2020; 20 (7):1780-1784. DOI: 10.1111/ajt.15904 - 5.
Jain S, Ahlstrom D. Technology legitimacy and the legitimacy of technology: The case of chronic kidney disease therapies. Journal of Engineering and Technology Management. 2021; 62 :101653. DOI: 10.1016/j.jengtecman.2021.101653 - 6.
Pendu JL, Breiman A, Rocher J, Dion M, Ruvoën-Clouet N. ABO blood types and COVID-19: Spurious, anecdotal, or truly important relationships? A reasoned review of available data. Viruses. 2021; 13 (2):160 - 7.
Ukirde V, Usulumarty D, Maru H, Billa V. Are weak blood groups important to look for in kidney transplantation? A case report on interchanging blood groups. Indian Journal of Transplantation. 2020; 14 (4):355. DOI: 10.4103/ijot.ijot_92_20 - 8.
Callemeyn J, Lamarthée B, Koenig A, Koshy P, Thaunat O, Naesens M. Allorecognition and the spectrum of kidney transplant rejection. Kidney International. 2022; 101 (4):692-710. DOI: 10.1016/j.kint.2021.11.029 - 9.
Gao H, Misevic G, LIBO J, Medicine Biotechnology Co., Ltd. Jiangyin, Jiangsu 214400, China. Microchip technology applications for blood group analysis. Blood and Genomics. 2020; 4 (2):83-95. DOI: 10.46701/BG.2020022020109 - 10.
Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Annals of Internal Medicine. 2020; 173 (4):262-267 - 11.
Opara ER. Review on knowledge of issues of rhesus incompatibility among nigerians. Opera. 2023; 3 (2) - 12.
Altayar M et al. Prevalence and association of transfusion transmitted infections with ABO and Rh blood groups among blood donors in the Western region of Saudi Arabia: A 7-year retrospective analysis. Medicina. 2022; 58 (7):857. DOI: 10.3390/medicina58070857 - 13.
Arnaiz-Villena A et al. Evolution and molecular interactions of major histocompatibility complex (MHC)-G, −E and -F genes. Cellular and Molecular Life Sciences. 2022; 79 (8):464. DOI: 10.1007/s00018-022-04491-z - 14.
Tersigni C et al. Role of human leukocyte antigens at the feto-maternal interface in normal and pathological pregnancy: An update. International Journal of Molecular Sciences. 2020; 21 (13):4756. DOI: 10.3390/ijms21134756 - 15.
Robinson RA, McMurran C, McCully ML, Cole DK. Engineering soluble T-cell receptors for therapy. The FEBS Journal. 2021; 288 (21):6159-6173. DOI: 10.1111/febs.15780 - 16.
Accolla RS, Ramia E, Tedeschi A, Forlani G. CIITA-driven MHC class II expressing tumor cells as antigen presenting cell performers: Toward the construction of an optimal anti-tumor vaccine. Frontiers in Immunology. 2019; 10 :1806. DOI: 10.3389/fimmu.2019.01806 - 17.
Lemieux W, Mohamma-dhassanzadeh H, Klement W, Daniel C, Sapir-Pichhadze R. Matchmaker, matchmaker make me a match: Opportunities and challenges in optimizing compatibility of HLA eplets in transplantation. International Journal of Immunogenetics. 2021; 48 (2):135-144. DOI: 10.1111/iji.12525 - 18.
Stowell CP, Stowell SR. Biologic roles of the ABH and Lewis histo-blood group antigens part I: Infection and immunity. Vox Sanguinis. 2019; 114 (5):426-442. DOI: 10.1111/vox.12787 - 19.
Kummer L et al. Vascular Signaling in allogenic solid organ transplantation – The role of endothelial cells. Frontiers in Physiology. 2020; 11 :443. DOI: 10.3389/fphys.2020.00443 - 20.
Santarsiero D, Aiello S. The complement system in kidney transplantation. Cell. 2023; 12 (5):791 - 21.
Kostalova B, Mala-Ladova K, Sulkova SD, Denhaerynck K, De Geest S, Maly J. Comparison of different methods to assess tacrolimus concentration intra-patient variability as potential marker of medication non-adherence. Frontiers in Pharmacology. 2022; 13 :973564. DOI: 10.3389/fphar.2022.973564 - 22.
Beheshtizadeh N et al. Vascular endothelial growth factor (VEGF) delivery approaches in regenerative medicine. Biomedicine & Pharmacotherapy. 2023; 166 :115301. DOI: 10.1016/j.biopha.2023.115301 - 23.
Hong H, Duque MA, Mana AFA, Wu Y. Noninfectious transfusion-associated adverse events. Annals of Blood. 2022; 7 :19-19. DOI: 10.21037/aob-21-83 - 24.
Cheng C-H et al. ABO-incompatible liver transplantation: State of art and future perspectives. Current Pharmaceutical Design. 2020; 26 (28):3406-3417. DOI: 10.2174/1381612826666200506094539