Integration methods for the insertion of transgenes in zebrafish embryos to study regulatory function of enhancer elements.
Abstract
Enhancers are genetically encoded elements that are critical for controlling gene expression. Despite the importance of enhancers in development, normal biological function, and disease, they have been historically overlooked and remain understudied. To understand how enhancers function, appropriate molecular tools are required that can capture the temporal and spatial function of enhancers within appropriate biological contexts. Zebrafish are an excellent in vivo model for the study of enhancer elements and the genetic variants that alter their function. Because zebrafish larvae are transparent, transgenes encoding enhancers tagged by fluorescent reporters can be visualized in the tissues and developmental stages where the enhancers function. Transgenesis of enhancers can be achieved using various plasmid technologies and transgene integration methods. Here, we describe the history and most recent developments in the zebrafish enhancer assay, from vector designs to various transgene integration techniques. We go on to describe how the application of these assays have been integral for our understanding of genetic variants found in humans and within enhancers that can contribute to both human Mendelian and complex polygenic disease.
Keywords
- enhancer
- non-coding
- transgenesis
- genome-wide association studies
- disease
1. Introduction
Phenotypic diversity between the cells and tissues of an individual organism is immense, but differences in the protein coding sequences of genes do not fully explain this diversity. Instead, genomic regulatory elements, that reside outside of genes in the non-coding regions of the genome, are largely responsible for this diversity by controlling gene expression [1]. This regulation of gene expression typically involves two types of genomic elements: the gene promoter and the enhancer [2]. The term enhancer reflects its function; the ability to enhance or modify the transcription of a gene from its promoter [3]. Enhancers are defined as pieces of DNA, spanning 100 base pairs in length to a few kb in length, that encode instructions that transcription factors recognize to promote gene transcription (Figure 1) [4]. Importantly, this regulation of gene expression involves the coordination of chromatin looping events that bring the enhancer, bound by transcription factors, and gene promoters into close spatial proximity. The configuration of these physical interactions, such as single enhancer: gene pairs (Figure 1A), single enhancer: multiple genes, or multiple enhancers: multiple genes (Figure 1B) are important for the tissue specificity, developmental timing, and strength of the transcriptional control of genes [5]. This fine tuning of gene expression drives multicellular development, lineage commitment, and ultimately leads to the phenotypic differences that we observe between cells, tissues, individuals and animal species.
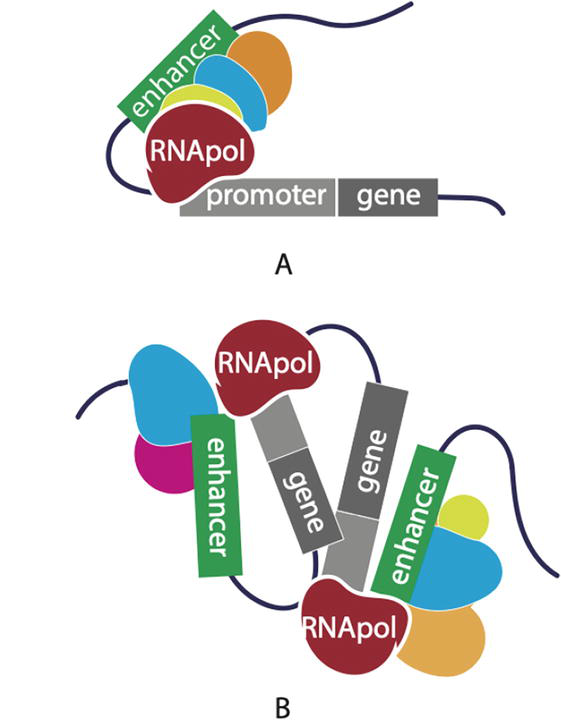
Figure 1.
Enhancer mediated regulation of gene expression. A single (A) or multiple enhancer(s) (green) (B) loops to a gene promoter (gray) bound by transcription factors and/or chromatin binding proteins and recruits RNA polymerase to drive gene expression.
2. The study of enhancers for human health
The human genome is predicted to have 1 million enhancers, although this is likely an underestimate [6]. Given this, and their role in gene expression, it is not surprising that the disruption of enhancers has been implicated time and time again as the cause of genetic trait differences, Mendelian disease, and predisposition to common diseases. The function of enhancers can be altered in a number of ways; deletions, insertions that separate the target gene from the influence of the enhancer, and single nucleotide changes in the enhancer that either ablate transcription factor binding, or introduce novel or additional binding sites. These alterations in the enhancer code can affect the regulation of gene expression, either by loss of function resulting in diminished gene expression, or gain of function resulting in overexpression of the target gene. A prime example of this, and commonly overlooked as being controlled by the non-coding genome, is the blue eyed human trait. The blue eye trait follows a predictable Mendelian inheritance pattern, which indicates that it is caused by a single genetic change. The causal genetic variant, found in roughly 18% of people with European ancestry, is located in the intron of
In comparison to the blue eye genetic variant, only a small handful of genetic variants responsible for Mendelian disease that disrupt enhancers have been identified. This is likely due to the historical focus on protein-coding genes, and difficulty in unraveling molecular mechanisms of regulatory genetics. However, genetic variation within non-coding regulatory elements can have profound biological impacts that are equivalent to coding variants. For example, the duffy mutation that impacts susceptibility to malaria [9], and non-coding variants at
The recent developments in sequencing technologies, and the reduction in cost, enables us to expand our search to the non-coding genome. The explosion of genome-wide association studies (GWAS), indicates that >90% of the genetic variants underpinning complex human diseases and traits fall in the non-coding genome and overlap significant regions of regulatory activity [10]. Researchers are now interrogating enhancers for discovery of disease and disease susceptibility genetic variants, particularly where variants in the protein-coding genes cannot be identified. Recent examples are emerging of therapeutics that target non-coding elements. For example, an erythroid-specific enhancer that was initially discovered via GWAS has been successfully targeted in gene therapy for sickle cell disease [12]. Importantly because regulatory control by enhancers is often tissue-specific, therapeutic targeting of these non-coding elements raises the possibility that there will be fewer side effects due to systemic off-target effects. Moreover, the sheer number of non-coding elements in the genome offers a wealth of drug targets to exploit. Therefore, identifying and characterizing non-coding disease-causing genetic variants has remarkable therapeutic promise for the treatment of Mendelian and complex disease.
The conserved characteristics of enhancers can be exploited for their discovery. Active enhancers have distinct hallmarks in the chromatin landscape. They are generally depleted of nucleosomes, and have characteristic histone modifications (
The wealth of information gleaned from these datasets and repositories indicate that our efforts should now be focussed on the functional validation of these computationally and biochemically predicted enhancer elements, and how the genetic variants within them alter their activity. The gold standard to date for functional validation has utilized reporter assays (
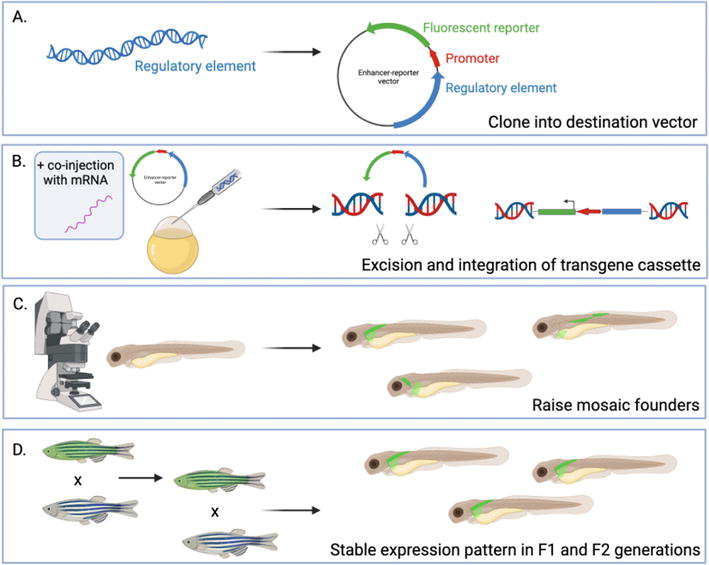
Figure 2.
Reporter assay strategy in zebrafish. (A) a regulatory element is cloned into a destination vector. Vector design varies, but at minimum includes a promoter and a fluorescent reporter. (B) the reporter assay vector is injected into fertilized zebrafish eggs with, or without, the co-injection of additional integration technologies. The transgene is excised from the donor plasmid vector and integrated into the zebrafish genome. (C) Injected fish are screened for reporter fluorescence by confocal microscopy. Individual fish that express the reporter are raised. (D) F0 founders are outcrossed with wild-type fish to establish F1 and F2 transgenic lines, where stable reporter expression patterns emerge. Created using
3. Zebrafish as an ideal model for enhancer function and application of reporter assays
Zebrafish have been an important in vivo model for the study of enhancers and gene regulation, and studies consistently show that human regulatory elements can be effectively studied using this model [24, 25, 26, 27, 28, 29]. Zebrafish are ideal for the study of enhancers for a number of reasons. For example, humans and zebrafish are only ~420 million years diverged [30]. This equates to >70% of protein-coding genes at the sequence level being conserved between humans and zebrafish. These genes include a large suite of transcriptional machinery, such as transcription factors, which via enhancers function to regulate gene expression [31, 32]. HNF4α is an excellent example of the conservation of transcriptional machinery between humans and zebrafish. HNF4α is a key transcriptional regulator that controls cell-specific gene expression in development, and is conserved between humans and zebrafish [33]. The function of this transcription factor in development is so tightly constrained by evolutionary mechanisms that there are no amino acid differences in the DNA binding domain between zebrafish and humans [34]. In both humans and zebrafish, the conserved DNA binding domain of HNF4α functions to recognize the HNF4α motif that is encoded in the DNA sequence. The ligand binding domain of HNF4α responds to cell-specific cues to drive HNF4α transcriptional regulation [34]. Therefore, zebrafish HNF4α maintains the ability to recognize human HNF4α motifs encoded by human DNA sequence [35, 36].
While a large proportion of the protein-coding genome is conserved, the zebrafish genome lacks many enhancers identifiable by sequence conservation in other species, including humans [37, 38]. However, because the transcriptional machinery involved in gene regulation is conserved, the genetic regulatory mechanisms also persist [39]. For example, enhancer elements within the RET locus exist in both humans and zebrafish, which act analogously to control RET expression. Although the enhancer elements have evolved so that the sequence is no longer orthologous, their function, recruitment of the same transcription factors, is preserved [40].
Additional features of zebrafish, including ease of transgenic manipulation [41] and larval transparency, make it possible to directly observe enhancer activity in vivo [42]. For example, layering imaging of regulatory element expression with fluorescently-labeled cell markers allows for the assessment of tissue-specific enhancer activity over developmental time. Additionally, by comparing tissues where the enhancer drives expression to those where a gene is expressed (
In zebrafish, a typical in vivo reporter assay (outlined in Figure 2) involves the construction and insertion of a plasmid vector. This vector contains a reporter protein placed downstream of a minimal promoter and a predicted enhancer sequence to be tested (Figure 2A). Insertion of the transgene into the zebrafish genome is mediated via endonuclease, retrovirus, transposon, or recombinase-based integration (Figure 2B) [44, 45]. Expression of the reporter protein in zebrafish larvae is used as a readout of enhancer activity because the reporter gene should only be expressed if the minimal promoter is driven by regulatory activity in the vicinity of the enhancer element.
In the context of human health and disease, alterations to enhancer activity by genetic variants within them can also be assessed in zebrafish reporter assays [19]. Several consequences of variants can be tested. For example, a complete loss of expression in a tissue may indicate the disruption of a key transcription factor binding site within the enhancer, affecting the formation of a transcription factor complex required for regulatory activity [46]. Modification of the strength of expression similarly may indicate a change in transcription factor binding affinity [19]. A novel expression pattern may indicate ‘gain of function’, where the DNA sequence change is such that a transcription factor binds more tightly, or a new transcription factor binding site is introduced [19]. Finally, no change in fluorescence strength or location may indicate that the variant has no functional effect on the enhancer’s activity, or may play some other role that is not captured in the context of this assay, for example in the organization of chromatin in the nucleus [47].
Importantly, there are several factors to consider when interpreting the results of a reporter assay in zebrafish. First, transgenic F0 fish will display mosaic expression of the transgene. This is because not all of the cells of the injected embryos will receive the transgene. However, a proportion of embryos will incorporate the transgene into their gametes, and their F1 progeny (stable lines) will either have the transgene in all cells, or none at all. Additionally, a phenomenon termed ‘position effect’ exists. The position effect is caused by the transgene being integrated into various genomic positions, which can in turn be influenced by the genomic context with which it is inserted [48, 49, 50]. The phenomenon often causes fluorescent expression to be identified that is not driven by the non-coding sequence of interest, but by the random insertion of the transgene into a region where strong regulatory activity exists. This can result in variable ectopic transgene expression, silencing of the transgene, or mutagenic effects if it is inserted within a coding region of the genome. To reduce the potential for bias arising from the over-interpretation of inaccurate fluorescent patterns and overcome mosaicism, multiple independent stable lines (F1 or F2 generations) of zebrafish should be screened for reproducible expression patterns, which may indicate the most likely expression pattern of the putative enhancer’s activity.
Before the advent of superior transgenesis techniques in the early 2000s, the method employed to generate transgenic zebrafish involved the injection of vast quantities of plasmid DNA (~106 plasmid copies) in the hope that some of this DNA would be transcribed and then integrated into the genome to be inherited in subsequent generations [51]. This approach has very low rates of integration and germline transmission, and many different integration techniques have subsequently been developed that have dramatically improved these rates. For example, I-Scel mediated integration, first described in 2002 in medaka fish [45]. This method involves the co-injection of the meganuclease I-SceI, a rare-cutting endonuclease isolated from yeast, alongside a vector containing a transgene flanked by corresponding I-SceI recognition sites. The meganuclease excises the transgene and provides recombinogenic ends to the DNA that facilitate integration into the genome. In comparison to a non-mediated transgenesis approach, this method reduces the amount of mosaicism present in the F0 fish and increases germline transmission rates (Table 1) [45].
| Technique | Method | Merits | Limitations |
|---|---|---|---|
| Large plasmid quantity | Inject large quantities of plasmid DNA containing a transgene of interest (~106 plasmid copies) into zebrafish embryos, where some of this DNA is likely to be transcribed and then integrated into the genome. | Low rates of genomic integration Low rates of germline transmission High mosaicism present Position effects Tandem insertions leads to silencing | |
| Endonuclease | Endonuclease mRNA is co-injected with a vector containing a transgene flanked by corresponding endonuclease cutting sites. Once excised from the vector, the transgene is left with recombinogenic ends that facilitate integration into the zebrafish genome. | Reduced F0 mosaicism Increased germline transmission | Position effects Tandem transgene insertions |
| Retrovirus | Vector is injected into zebrafish embryos that contain the transgene and a modified viral sequence that has removed proteins required to replicate the virus but retained those that promote its integration into the genome. | High integration rate High rate of germline transmission | Variable copy number integration Position effects Multicopy insertions |
| Transposase | Transposase mRNA is co-injected into single cell zebrafish embryo with a vector containing a transgene flanked by non-autonomous transposon sequences recognized by the transposase which catalyzes the transposition of the transgene into the genome. | High integration rate High rate of germline transmission Plenty of published vector technologies for use in reporter assays No tandem insertions | Position effects Multicopy insertions Transgenes can remobilize if you inject with Tol2 |
| Recombinase | Transgenic fish are generated with recombinase ‘landing sites’. Injected into embryos of the progeny of these fish is the recombinase mRNA and a vector containing a recombinase site. The enzyme initiates the recombination of the transgene into the genome at the ‘landing site’. | Very high integration rate Very high rate of germline transmission Single-insertion Targeted integration limits position effects Reduced F0 mosaicism Transgene insertion can be permanent (can inject these with phiC31 + vector) | Application is not widespread in zebrafish Specialized zebrafish transgenic lines required Current lack of understanding of good genomic integration positions and stable lines with mapped landing sites |
Table 1.
The use of a retrovirus-mediated system has also been explored within the zebrafish model for the detection of putative enhancer sequences [52]. A retroviral vector system contains viral sequences that promote reverse transcription from RNA to DNA and integration into the genome, but do not encode the viral proteins required to replicate and produce additional virus. Instead of viral proteins, reporter genes and non-coding sequences can be engineered within the vector [53]. The use of these engineered retroviral vectors in zebrafish reporter assays enables the integration of a transgene into the genome and its inheritance through the germline. However, variation in reporter expression is caused by variable copy number integration and position effects (Table 1).
Over the past two decades, two dominant approaches have emerged for the integration of transgenes into the zebrafish genome that aim to combat the issues described above. The first, transposons, mediate the random integration of a transgene into a genome while the second, recombinase enzymes, mediate site-directed integration. These two methods will be discussed further in sections 3.1 and 3.2 and are outlined in Table 1.
3.1 Transposon-based integration
Transposons are genetic elements that can move from one area in the genome to another. Some elements are autonomous and encode a functional transposase protein that can catalyze the transposition of DNA [54], and other elements are non-autonomous, and do not encode a functional transposase but maintain essential sequences that can be transposed in the presence of transposase [55, 56]. An autonomous Tol2 mobile element, belonging to the hAT transposon family [57], was discovered in the medaka fish [58]. In zebrafish, where this element is naturally absent, a two-component transposition system utilizing the Tol2 transposon to integrate a transgene of interest has been developed [59]. This system requires both a plasmid containing a nonautonomous Tol2 element lacking part of the transposase gene, and the mRNA of the Tol2 transposon. These components are co-injected into a single-cell zebrafish embryo, where the Tol2 transposon catalyzes the transposition of the nonautonomous element from the plasmid into the zebrafish genome (Figure 2) [60, 61, 62]. This technology has been adapted to study predicted enhancer elements by designing a vector with an enhancer-promoter-reporter cassette in-between two nonautonomous Tol2 elements [63]. Fish injected using this system are analyzed for their fluorescent expression patterns indicative of enhancer activity, and these fish are then raised to adulthood and used to create transgenic lines. The rate of transgene integration is very high, particularly when compared to simply injecting just plasmid DNA, and this technology has been successfully applied to study a range of human enhancers. However, a large caveat to transposon-mediated transgenesis is that the transgene is inserted randomly and predominantly into active genomic regions, which causes position effects [63, 64]. Many groups have utilized this Tol2 transposon-based system to functionally investigate enhancers in zebrafish through the use of specifically designed vectors.
Some vector designs containing Tol2 sequences are of a relatively simple design; a green fluorescent protein (GFP) reporter, a minimal promoter, and a Gateway cassette for easy recombination of DNA of interest [17]. While these designs are successfully used to generate transgenic fish, there are additional components that can be added for ease of use, and that have improved our ability to assess enhancer activity in zebrafish. For example, the more simple vector designs lack an internal positive control, which makes it difficult to determine the success of a genome integration event or the passage of this integration down the germline. This is particularly challenging when the spatiotemporal nature of enhancer activity is considered, whereby an enhancer may only drive expression of the reporter at a specific developmental stage and/or a tissue that may not be visually obvious.
The Zebrafish Enhancer Detector (ZED) vector was designed to address the need for a positive control for transgenesis and additionally, inconsistent fluorescent expression caused by the position effect phenomenon (Figure 3) [65]. The ZED vector contains a positive control cassette which includes a Cardiac Actin promoter and red fluorescent protein (RFP). This cassette drives RFP expression in the somites of the fish and therefore, can be used as an obvious indicator of the success of vector integration into the genome. In comparison, the enhancer detection cassette contains the green fluorescent protein (GFP) as a reporter for activity so that visualization of the success of integration and potential enhancer activity can be visualized on different light channels. The ZED vector decreases position effects by flanking the reporter cassette with insulator sequences so that the cassette is shielded from position effects. Furthermore, this vector contains an improved minimal promoter to drive GFP which was selected for optimal enhancer activity detection, and two excision cassettes. The excision cassettes allow for the genetic deletion of the transgene, should a researcher want to confirm that the fluorescent activity is dependent specifically on the enhancer element.
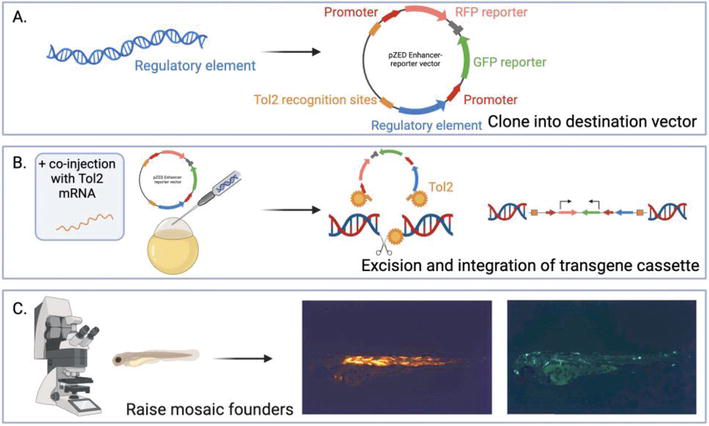
Figure 3.
Zebrafish enhancer detection (ZED) vector system. (A) a regulatory element is cloned into the ZED vector which contains a positive control cassette, consisting of a cardiac actin promoter and RFP, and a reporter cassette, consisting of a gateway entry site upstream of a minimal promoter and GFP. (B) the vector is co-injected into single-cell fertilized zebrafish eggs alongside Tol2 mRNA. The Tol2 transposase is transcribed into an enzyme that binds to the recognition sites on the vector and excises the transgene, and then binds to a sequence in the genome where it mediates the transgene’s integration. (C) Confocal microscopy is used to screen injected embryos for RFP (integration control), and GFP (regulatory element activity) expression. Zebrafish photos are original work. Created using
The ZED vector’s key improvements allow for the efficient and rapid generation of transgenic zebrafish that contain an internal transgenesis control and reduced position effects. However, limitations to this system remain. For example, while position effects have been reduced, they unfortunately still remain which can interfere with the interpretation of fluorescent signals. Another issue with this system, is while RFP expression in somites makes for an obvious and effective control, the expression of this protein can be so strong and so abundant throughout the fish that the RFP can bleed through other fluorescent light channels (as seen in Figure 3C) and it is hard to analyze the location of specific GFP expression in the fish. Despite the short-comings of this particular vector, various improved transgenesis reporter fluorescent markers continue to be designed and incorporated into reporter assays for example, cryaa: venus is a cerulean fluorescent marker of transgenesis expressed only in the lens on the fish [66].
Another example of an assay that applies the Tol2-mediated transgenesis is the dual-color reporter-based assay, used for the analysis of variants within enhancer elements in parallel (Figure 4) [19]. In this assay, two Tol2 constructs bearing the alternate alleles of a genetic variant within an enhancer are recombined with different fluorescent reporter cassettes in a multi-gateway reaction. These two plasmids are then co-injected with Tol2 mRNA into zebrafish embryos. Developing fish are then screened for reporter fluorescence and the positive fish are outcrossed to generate stable dual-reporter-expressing lines. Using confocal microscopy, tissues where the activity of two enhancers overlap are observed as yellow, while independent activities are green or red. This system allows for the direct comparison of the spatial and temporal activities of different enhancers, or different variants within the same enhancer, in a single zebrafish [19]. This assay significantly reduces the number of zebrafish normally required in a reporter assay. A limitation is that this system cannot control for the position of insertion sites, and the zebrafish tend to display variation in expression patterns due to positional effects. Additionally, the two enhancer sequences will integrate into the genome in variable numbers because they are within different plasmids, limiting the system for quantitative assessment of altered enhancer activity.
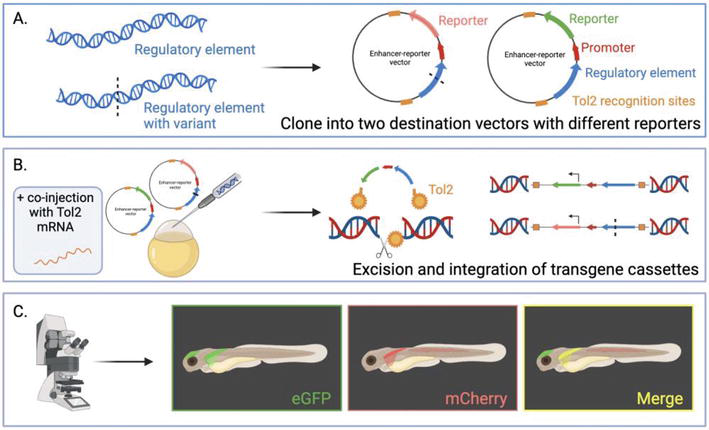
Figure 4.
Dual-color zebrafish transgenesis strategy. (A) Two regulatory elements are cloned into vectors containing a minimal promoter and two different fluorescent reporters. (B) both vectors are co-injected into single-cell fertilized zebrafish eggs alongside Tol2 mRNA. The Tol2 transposase is transcribed into an enzyme that binds to the recognition sites on the vector and excises both transgenes, and then binds to a sequence in the genome where it mediates the transgene’s integration. (C) Confocal microscopy is used to screen injected embryos for fluorescence where independent regulatory activity is observed as green or red, and overlapping activities are yellow. These mosaic F0 founders are raised to generate transgenic lines. Created using
3.2 Recombinase-based integration
In comparison to the random integration methodology of transposons such as Tol2, site-specific recombinases are enzymes that catalyze recombination events at highly specific target sequences. This method has the potential to mitigate the variability caused by the random integration of transgenes and resulting positional effects by creating an efficient and reproducible site-directed integration system, where enhancer elements and their variation can be quantitatively compared. Owing to their specificity, pre-defined recombinase sites can be established within a model organism’s genome, permitting the integration (Figure 5B) or excision (Figure 5A/6B) of transgenes at a defined position by recombinases [67, 68, 69, 70]. Notable recombinases that have been employed within the zebrafish model system include Cre, derived from bacteriophage P1, Flp, from yeast, and phiC31 recombinase, from phage phiC31. In comparison to the recombinases Cre and Flp who target loxP and FRT sites respectively, phiC31 targets two heterotypic sites termed attB and attP, resulting in the directional and irreversible integration of DNA (Figure 6D) [71, 72, 73]. PhiC31’s canonical role is the integration of a phage genome into a bacterial genome via recombination between attP (phage) and attB (bacteria) sequences. Utilizing the specificity of phiC31 recombinase to these sites, an exogenous plasmid containing a transgene with an attP (or attB) site can be injected alongside phiC31 encoding mRNA into a zebrafish embryo harboring a previously integrated attB (or attP) landing site in their genome (Figure 6). The phiC31 initiates the recombination of this transgene into the genome at these attB/attP sites [74, 75, 76, 77, 78]. The irreversible nature of this system caused by the completed attP/attB recombination, produces hybrid attL (left) and attR (right) sites which cannot be used by phiC31 as substrates. This lends itself to heightened efficiency because even in the presence of large quantities of plasmid, the recombination between the attP/attB sites cannot be reversed [71].
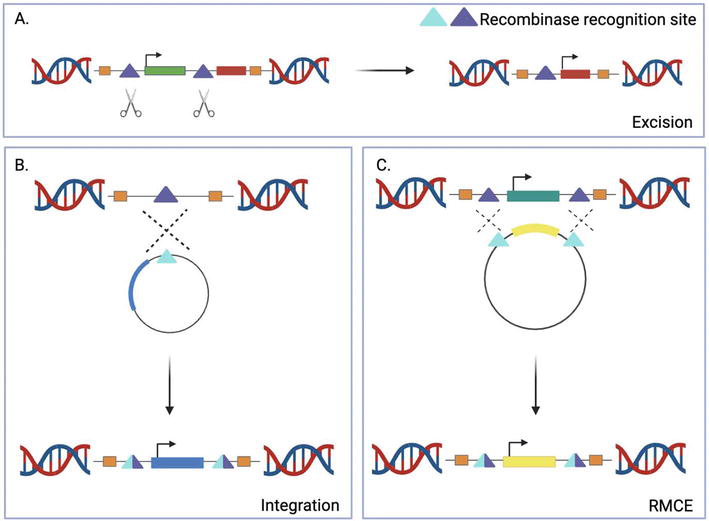
Figure 5.
Recombinase-based transgene integration strategies. (A) Excision of a previously inserted cassette, typically employed to induce regulatory activity at a specific tissue or point in development. (B) Site-directed integration of an introduced transgene in the genome. (C) Recombination-mediated cassette exchange (RMCE) between a new and previously introduced transgene. The phiC31 integrase system is effective at controlling cassette excision, integration, as well as RMCE. Created using
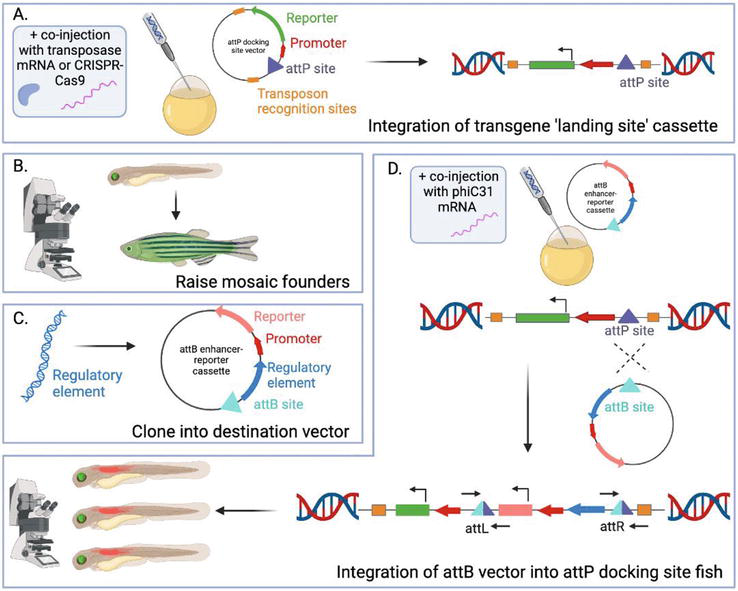
Figure 6.
Generation at attP/attB-recombinant zebrafish transgenic lines using phiC31 recombinase. (A) a vector containing GFP-tagged attP sites is co-injected with integration technologies, such as transposase mRNA, into fertilized single-cell zebrafish embryos, where the transgene is integrated into the genome. (B) Using confocal microscopy, larvae are screened for fluorescence, reflecting the integration of the landing site cassette into the zebrafish genome, and these fish are raised to generate transgenic lines. (C) a regulatory element is cloned into a vector containing an attB site and a fluorescence reporter. (D) the vector is co-injected with phiC31 mRNA into transgenic embryos containing an attP integration site. A recombination event occurs, mediated by phiC31, between the attB and attP sites, and the transgene is integrated at this landing site. Using confocal microscopy, larvae are screened for fluorescence expression. Created using
The use of phiC31 attP/attB recombination for the insertion of transgenes such as putative enhancer sequences, is routine in Drosophila work [68, 79] however, its translation into the zebrafish model has been largely limited to proof-of-principle studies. Two such studies describe a technique by which phiC31 catalyzes a recombination event in a part of a previously inserted transgene, causing the excision of a control cassette and the activation of a second coding sequence (Figure 5A) [75, 76]. In comparison to the single phiC31-mediated recombination event, recombinase-mediated cassette exchange (RMCE) involves the phiC31 integrase excising and replacing a reporter-tagged integrated transgene with another reporter labeled transgene (Figure 5C) [80]. During RMCE, a transgene containing a fluorescent reporter protein flanked by attP (or attB) sites is integrated into the zebrafish genome via Tol2-mediated transgenesis. These zebrafish are screened for germline transmission, and the fluorescent-positive embryos co-injected with phiC31 mRNA and a vector containing an enhancer sequence tagged with a different fluorescent reporter, flanked by attB (or attP) sites. PhiC31 facilitates recombination between the attP/attB sites which excise the fluorescently labeled reporter cassette and integrates the other fluorescently labeled cassette. A limitation of this approach comes down to the initial random insertion of the transgene by Tol2, as the number and position of these insertions are not controlled. Therefore, zebrafish can have multiple independent insertions and many transmit both fluorescent markers following injection, reflecting that recombination did not occur at every attP/attB site [80]. To improve upon this system, stable transgenic zebrafish lines with well characterized attP/attB sequences that support efficient phiC31-mediated recombination are required. These lines would eliminate the time and labor required to generate new attP/attB-containing transgenic acceptor lines for every experiment and additionally, would eliminate the need to screen and map each transgene insertion site.
A large amount of time and labor is dedicated to the generation of stable single-insertion attP/attB transgenic zebrafish lines. A research group in 2013 reported to have generated two such lines [78]. Tol2-mediated transgenesis was employed to generate several independent attP landing site zebrafish lines containing a full-length attP acceptor site alongside a GFP-tagged promoter. These lines were assessed for a 50% Mendelian inheritance pattern in the F2 generation, reflective of a single transgene insertion. These lines were co-injected with phiC31 mRNA and a vector containing a GFP-tagged transgenesis marker flanked by full-length attB sites, and screened for fluorescence reflecting a successful integration of the transgene. Two landing site lines consistently demonstrated reproducible attP/attB recombination, and the integration position was mapped and characterized. Reproducible attP/attB recombination was determined through a variety of experiments. One such screen tested integration in mutant backgrounds, where attP landing-site lines were crossed with fish lacking a transcription factor essential for melanocyte development. The progeny were injected with phiC31 mRNA and a vector containing the missing transcription factor, and skin pigmentation was successfully rescued [78]. Ultimately, this group was able to successfully establish, characterize, and maintain functional attP landing site fish for the site-directed generation of transgenic lines. Multiple other teams of scientists have also been generating single landing-site zebrafish [42, 77], and have demonstrated that this technique successfully limits variability of transgene activity caused by positional effects, particularly when compared to lines generated with random Tol2-mediated transgene insertions [77].
While this technique has proven successful for the integration of transgenes at a single genomic position, our understanding of the genomic positions where the rate of this integration is most successful is lacking. The use of gene editing technologies such as CRISPR-Cas9, would enable a specific locus to be targeted for attP/attB-site insertion, removing the unpredictability caused by the initial Tol2 transposon-mediated insertion. Validated integration loci have been identified in mammalian systems at the Hipp11 (h11) locus [81, 82, 83], and work has been undertaken to identify similar sites in zebrafish [84]. There is some evidence that zebrafish lines with stable transgene expression have integrated transgenes adjacent to regions marked with native histone marks, reflecting open and active chromatin [84]. In the future, we strongly encourage researchers to map the chromatin dynamics and the genomic coordinates of transgene integration sites produced by random integration. These insights will help identify features that support successful, reproducible, and inherited integration which can then be applied to generate landing site lines with targeted technologies, such as CRISPR-Cas9.
4. Application of reporter assays to understand human disease
Historically, the search for genetic variants contributing to human disease has been restricted to exons, representing only 1–2% of the human genome. In comparison, variants within non-coding regions and their contribution to disease remain poorly investigated. This is because variants within coding regions are much easier to interpret, as mutations that truncate the mRNA transcript or alter the structure of the protein intuitively result in aberrant protein function and disease phenotypes. Less clear are the potential effects of variants within non-coding enhancer elements. However, the impact of non-coding variants have become well recognized in the etiology of Mendelian and polygenic diseases, and the utilization of the zebrafish reporter assay has been an invaluable tool for our understanding of their contribution.
4.1 Mendelian disease
Complex phenotypes and diseases are influenced by thousands of genes and genetic variants that are common. Comparatively, Mendelian disorders are caused by rare and typically singular genetic variants that have predictable Mendelian inheritance patterns. For example, Cystic Fibrosis is an autosomal recessive disorder that is caused by disruptions, most commonly the p.Phe508del variant, in the cystic fibrosis transmembrane conductance regulator (
The zebrafish reporter assay can be employed to validate Mendelian genetic variants discovered in enhancers, furthering our understanding of their contribution to disease. For example, a regulatory variant was identified in a person with the cardiac features of Holt Oram disease (congenital condition of the heart and limbs) that disrupts the expression of
Another example of an enhancer that is causal of Mendelian disease, that was characterized in zebrafish, is found upstream of the
4.2 Complex polygenic disease
Complex polygenic conditions, such as type 2 diabetes and cardiovascular disease, are those where individual common genetic variants contribute only very small effects. To capture important regions of the genome that contribute to the development of polygenic disease, genome-wide association studies (GWAS) that consist of many hundreds of thousands of participants are the gold standard approach [97]. Crucially, GWAS find that <10% of associated regions of the genome have causal mechanisms implicating a protein-coding variant. Instead, the majority of the polygenic associations are outside of the coding regions, suggesting that altered gene regulation is a major contributor to complex disease. Integrated meta-analyses of genomic and epigenomic annotations have demonstrated that GWAS variants are significantly enriched in functional non-coding regions [98, 99], particularly in close proximity to enhancers [100, 101]. For example, ~60% of likely causal variants map to enhancer elements in an autoimmune disease GWAS [102]. These data suggest that a substantial proportion of genetic variants identified using GWAS contribute to disease by disrupting enhancer activity. However, studying the biological consequences of genetic variation at GWAS loci is challenging for a number of reasons. Identifying the causal gene(s) can be difficult, as candidate enhancers do not always affect nearby genes, but can have long-range interactions with promoters [103]. In addition, in most cases, the causal variant at a GWAS locus will also be associated with many other genetic variants due to linkage disequilibrium. Unfortunately, there has been very little progress in translating these loci into biology since the advent of GWAS in the early 2000s, and identifying causal variants and determining how and where non-coding variants affect target genes remains poorly understood [104].
For example, it took almost 10 years for the well-known obesity associated locus in intron 1 of
In an attempt to assign function to the
The BAC approach described above, where very large pieces of DNA are inserted, was particularly useful before we had better prediction methods for regulatory function for genetic variants. Now, with data made available by the ENCODE consortium [6] and GTEx, it is possible to overlay genetic variants with regulatory data and gene expression data (eQTL) to prioritize putative causal variants before testing in zebrafish assays. The success of this approach is highlighted by two studies [35, 36] that investigated loci with genetic associations of serum urate, a key metabolite involved in the development of the inflammatory arthritis, gout [114]. In these studies, transcription factor binding sites were overlaid with urate-associated genetic variants at the
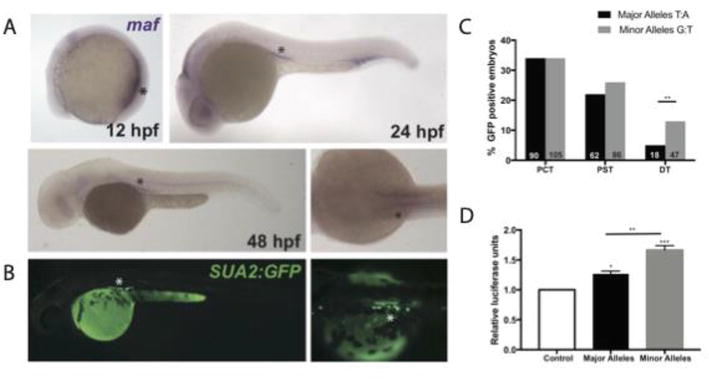
Figure 7.
Zebrafish
For the enhancer at
5. Conclusion
This chapter has highlighted the role of the zebrafish report assay to test enhancer activity in vivo and the various tools available to researchers using this model. We describe how in zebrafish, reporter assays have been applied and improved over the years to test for regulatory activity of predicted enhancer elements. In the future, recombinase landing site zebrafish lines will reduce the intensive labor required to screen transgenic fish generated using random integration technologies. These lines will also be an essential tool for the accurate in vivo quantification that both Mendelian and complex-disease variants have on enhancer function. The advent of cost-effective whole genome sequencing and large-scale GWAS has enabled us to comprehensively discover non-coding Mendelian disease variants and common variants that contribute to complex disease. It is therefore more important than ever to develop tools that are scalable and can accurately recapitulate enhancer activity in the relevant biological context, to comprehensively understand the underlying molecular and disease mechanisms.
Acknowledgments
The authors would like to thank the University of Alabama at Birmingham for funding (Underrepresented in Medicine Early Career Investigator Award award to Dr. Leask).
References
- 1.
Mattick JS. Non-coding RNAs: The architects of eukaryotic complexity. EMBO Reports. 2001; 2 (11):986-991 - 2.
Maston GA, Evans SK, Green MR. Transcriptional regulatory elements in the human genome. Annual Review of Genomics and Human Genetics. 2006; 7 :29-59 - 3.
Banerji J, Rusconi S, Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981; 27 (2 Pt 1):299-308 - 4.
Spitz F, Furlong EEM. Transcription factors: From enhancer binding to developmental control. Nature Reviews. Genetics. 2012; 13 (9):613-626 - 5.
He B, Chen C, Teng L, Tan K. Global view of enhancer–promoter interactome in human cells. National Academy of Sciences of the United States of America. 2014; 111 (21):E2191-E2199 - 6.
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012; 489 (7414):57-74 - 7.
Visser M, Kayser M, Palstra RJ. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Research. 2012; 22 (3):446-455 - 8.
Eiberg H, Troelsen J, Nielsen M, Mikkelsen A, Mengel-From J, Kjaer KW, et al. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Human Genetics. 2008; 123 (2):177-187 - 9.
McManus KF, Taravella AM, Henn BM, Bustamante CD, Sikora M, Cornejo OE. Population genetic analysis of the DARC locus (Duffy) reveals adaptation from standing variation associated with malaria resistance in humans. PLoS Genetics. 2017; 13 (3):e1006560 - 10.
Boocock J, Leask M, Okada Y, Matsuo H, Kawamura Y, Asian Genetic Epidemiology Network (AGEN) Consortium, et al. Genomic dissection of 43 serum urate-associated loci provides multiple insights into molecular mechanisms of urate control. Human Molecular Genetics. 2020; 29 (6):923-943 - 11.
Tin A, Marten J, Halperin Kuhns VL, Li Y, Wuttke M, Kirsten H, et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nature Genetics. 2019; 51 (10):1459-1474 - 12.
Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. The New England Journal of Medicine. 2021; 384 (3):252-260 - 13.
Jindal GA, Bantle AT, Solvason JJ, Grudzien JL, D’Antonio-Chronowska A, Lim F, et al. Single-nucleotide variants within heart enhancers increase binding affinity and disrupt heart development. Developmental Cell. 2023; 58 (21):2206-16.e5 - 14.
Ryan GE, Farley EK. Functional genomic approaches to elucidate the role of enhancers during development. Wiley Interdisciplinary Reviews. Systems Biology and Medicine. 2020; 12 (2):e1467 - 15.
Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in drosophila. BioTechniques. 2000; 29 (4):726, 728, 730, 732 - 16.
Barolo S, Castro B, Posakony JW. New drosophila transgenic reporters: Insulated P-element vectors expressing fast-maturing RFP. BioTechniques. 2004; 36 (3):436-440, 442 - 17.
Ishibashi M, Mechaly AS, Becker TS, Rinkwitz S. Using zebrafish transgenesis to test human genomic sequences for specific enhancer activity. Methods. 2013; 62 (3):216-225 - 18.
Bhatia S, Kleinjan DA. Disruption of long-range gene regulation in human genetic disease: A kaleidoscope of general principles, diverse mechanisms and unique phenotypic consequences. Human Genetics. 2014; 133 (7):815-845 - 19.
Bhatia S, Gordon CT, Foster RG, Melin L, Abadie V, Baujat G, et al. Functional assessment of disease-associated regulatory variants in vivo using a versatile dual colour transgenesis strategy in zebrafish. PLoS Genetics. 2015;11 (6):e1005193 - 20.
Chahal G, Tyagi S, Ramialison M. Navigating the non-coding genome in heart development and congenital heart disease. Differentiation. 2019; 107 :11-23 - 21.
Goode DK, Elgar G. Capturing the regulatory interactions of eukaryote genomes. Briefings in Functional Genomics. 2013; 12 (2):142-160 - 22.
Rainger JK, Bhatia S, Bengani H, Gautier P, Rainger J, Pearson M, et al. Disruption of SATB2 or its long-range cis-regulation by SOX9 causes a syndromic form of Pierre Robin sequence. Human Molecular Genetics. 2014; 23 (10):2569-2579 - 23.
Yuan X, Song M, Devine P, Bruneau BG, Scott IC, Wilson MD. Heart enhancers with deeply conserved regulatory activity are established early in zebrafish development. Nature Communications. 2018; 9 (1):4977 - 24.
Ghiasvand NM, Rudolph DD, Mashayekhi M, Brzezinski JA 4th, Goldman D, Glaser T. Deletion of a remote enhancer near ATOH7 disrupts retinal neurogenesis, causing NCRNA disease. Nature Neuroscience. 2011; 14 (5):578-586 - 25.
Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011; 470 (7333):279-283 - 26.
Smemo S, Campos LC, Moskowitz IP, Krieger JE, Pereira AC, Nobrega MA. Regulatory variation in a TBX5 enhancer leads to isolated congenital heart disease. Human Molecular Genetics. 2012; 21 (14):3255-3263 - 27.
Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, Mularoni L, Miguel-Escalada I, Akerman İ, et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nature Genetics. 2014; 46 (2):136-143 - 28.
Kramer ET, Godoy PM, Kaufman CK. Transcriptional profile and chromatin accessibility in zebrafish melanocytes and melanoma tumors. G3 [Internet]. 2022; 12 (1):jkab379. DOI: 10.1093/g3journal/jkab379 - 29.
Ferre-Fernández JJ, Muheisen S, Thompson S, Semina EV. CRISPR-Cas9-mediated functional dissection of the foxc1 genomic region in zebrafish identifies critical conserved cis-regulatory elements. Human Genomics. 2022; 16 (1):49 - 30.
Webb AE, Kimelman D. Analysis of early epidermal development in zebrafish. Methods in Molecular Biology. 2005; 289 :137-146 - 31.
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013; 496 (7446):498-503 - 32.
Kettleborough RNW, Busch- Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013; 496 (7446):494-497 - 33.
Bertrand S, Brunet FG, Escriva H, Parmentier G, Laudet V, Robinson-Rechavi M. Evolutionary genomics of nuclear receptors: From twenty-five ancestral genes to derived endocrine systems. Molecular Biology and Evolution. 2004; 21 (10):1923-1937 - 34.
Taniguchi H, Fujimoto A, Kono H, Furuta M, Fujita M, Nakagawa H. Loss-of-function mutations in Zn-finger DNA-binding domain of HNF4A cause aberrant transcriptional regulation in liver cancer. Oncotarget. 2018; 9 (40):26144-26156 - 35.
Ketharnathan S, Leask M, Boocock J, Phipps-Green AJ, Antony J, O’Sullivan JM, et al. A non-coding genetic variant maximally associated with serum urate levels is functionally linked to HNF4A-dependent PDZK1 expression. Human Molecular Genetics. 2018; 27 (22):3964-3973 - 36.
Leask M, Dowdle A, Salvesen H, Topless R, Fadason T, Wei W, et al. Functional urate-associated genetic variants influence expression of lincRNAs LINC01229 and MAFTRR. Frontiers in Genetics. 2018; 9 :733 - 37.
Lee AP, Kerk SY, Tan YY, Brenner S, Venkatesh B. Ancient vertebrate conserved noncoding elements have been evolving rapidly in teleost fishes. Molecular Biology and Evolution. 2011; 28 (3):1205-1215 - 38.
Ravi V, Venkatesh B. Rapidly evolving fish genomes and teleost diversity. Current Opinion in Genetics & Development. 2008; 18 (6):544-550 - 39.
Taher L, McGaughey DM, Maragh S, Aneas I, Bessling SL, Miller W, et al. Genome-wide identification of conserved regulatory function in diverged sequences. Genome Research. 2011; 21 (7):1139-1149 - 40.
Fisher S, Grice EA, Vinton RM, Bessling SL, McCallion AS. Conservation of RET regulatory function from human to zebrafish without sequence similarity. Science. 2006; 312 (5771):276-279 - 41.
Howe DG, Bradford YM, Eagle A, Fashena D, Frazer K, Kalita P, et al. The zebrafish model organism database: New support for human disease models, mutation details, gene expression phenotypes and searching. Nucleic Acids Research. 2017; 45 (D1):D758-D768 - 42.
Bhatia S, Kleinjan DJ, Uttley K, Mann A, Dellepiane N, Bickmore WA. Quantitative spatial and temporal assessment of regulatory element activity in zebrafish. Elife [Internet]. 2021; 10 :e65601. DOI: 10.7554/eLife.65601 - 43.
Taminato T, Yokota D, Araki S, Ovara H, Yamasu K, Kawamura A. Enhancer activity-based identification of functional enhancers using zebrafish embryos. Genomics. 2016; 108 (2):102-107 - 44.
Rogers WA, Williams TM. Quantitative comparison of cis-regulatory element (CRE) activities in transgenic Drosophila melanogaster. Journal of Visualized Experiments [Internet]. 2011; 58 :3395. DOI: 10.3791/3395 - 45.
Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, et al. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mechanisms of Development. 2002; 118 (1-2):91-98 - 46.
Kleinjan DJ, Coutinho P. Cis-ruption mechanisms: Disruption of cis-regulatory control as a cause of human genetic disease. Briefings in Functional Genomics & Proteomics. 2009; 8 (4):317-332 - 47.
Helmsauer K, Valieva ME, Ali S, Chamorro González R, Schöpflin R, Röefzaad C, et al. Enhancer hijacking determines extrachromosomal circular MYCN amplicon architecture in neuroblastoma. Nature Communications. 2020; 11 (1):5823 - 48.
Jaenisch R, Jähner D, Nobis P, Simon I, Löhler J, Harbers K, et al. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981; 24 (2):519-529 - 49.
Wilson C, Bellen HJ, Gehring WJ. Position effects on eukaryotic gene expression. Annual Review of Cell Biology. 1990; 6 :679-714 - 50.
Rossant J, Nutter LMJ, Gertsenstein M. Engineering the embryo. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108 (19):7659-7660 - 51.
Westerfield M, Wegner J, Jegalian BG, DeRobertis EM, Püschel AW. Specific activation of mammalian Hox promoters in mosaic transgenic zebrafish. Genes & Development. 1992; 6 (4):591-598 - 52.
Laplante M, Kikuta H, König M, Becker TS. Enhancer detection in the zebrafish using pseudotyped murine retroviruses. Methods. 2006; 39 (3):189-198 - 53.
Amsterdam A, Becker TS. Transgenes as screening tools to probe and manipulate the zebrafish genome. Developmental Dynamics. 2005; 234 (2):255-268 - 54.
Fedoroff N, Wessler S, Shure M. Isolation of the transposable maize controlling elements Ac and Ds. Cell. 1983; 35 (1):235-242 - 55.
Döring HP, Tillmann E, Starlinger P. DNA sequence of the maize transposable element dissociation. Nature. 1984; 307 (5947):127-130 - 56.
Sutton WD, Gerlach WL, Peacock WJ, Schwartz D. Molecular analysis of ds controlling element mutations at the adh1 locus of maize. Science. 1984; 223 (4642):1265-1268 - 57.
Koga A, Suzuki M, Inagaki H, Bessho Y, Hori H. Transposable element in fish. Nature. 1996; 383 (6595):30-30 - 58.
Kawakami K, Koga A, Hori H, Shima A. Excision of the tol2 transposable element of the medaka fish, oryzias latipes, in zebrafish, danio rerio. Gene. 1998; 225 (1-2):17-22 - 59.
Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo . Developmental Dynamics. 2004;231 (2):449-459 - 60.
Kawakami K, Shima A. Identification of the Tol2 transposase of the medaka fish Oryzias latipes that catalyzes excision of a nonautonomous Tol2 element in zebrafish Danio rerio. Gene. 1999; 240 (1):239-244 - 61.
Kawakami K, Shima A, Kawakami N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proceedings of the National Academy of Sciences of the United States of America. 2000; 97 (21):11403-11408 - 62.
Kawakami K. Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods in Cell Biology. 2004; 77 :201-222 - 63.
Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006; 174 (2):639-649 - 64.
Vrljicak P, Tao S, Varshney GK, Quach HNB, Joshi A, LaFave MC, et al. Genome-wide analysis of transposon and retroviral insertions reveals preferential integrations in regions of DNA flexibility. G3. 2016; 6 (4):805-817 - 65.
Bessa J, Tena JJ, de la Calle-Mustienes E, Fernández-Miñán A, Naranjo S, Fernández A, et al. Zebrafish enhancer detection (ZED) vector: A new tool to facilitate transgenesis and the functional analysis of cis-regulatory regions in zebrafish. Developmental Dynamics. 2009; 238 (9):2409-2417 - 66.
Zhong Y, Huang W, Du J, Wang Z, He J, Luo L. Improved Tol2-mediated enhancer trap identifies weakly expressed genes during liver and β cell development and regeneration in zebrafish. The Journal of Biological Chemistry. 2019; 294 (3):932-940 - 67.
Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Developmental Cell. 2004; 6 (1):7-28 - 68.
Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for drosophila using germ-line-specific φC31 integrases. National Academy of Sciences of the United States of America. 2007; 104 (9):3312-3317 - 69.
Allen BG, Weeks DL. Transgenic Xenopus laevis embryos can be generated using phiC31 integrase. Nature Methods. 2005; 2 (12):975-979 - 70.
Bateman JR, Lee AM, Wu CT. Site-specific transformation of drosophila via ϕC31 integrase-mediated cassette exchange. Genetics. 2006; 173 (2):769-777 - 71.
Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proceedings of the National Academy of Sciences of the United States of America. 2000; 97 (11):5995-6000 - 72.
Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic drosophila by using the site-specific integrase from phage φC31. Genetics. 2004; 166 (4):1775-1782 - 73.
Smith MCA, Till R, Brady K, Soultanas P, Thorpe H, Smith MCM. Synapsis and DNA cleavage in ϕC31 integrase-mediated site-specific recombination. Nucleic Acids Research. 2004; 32 (8):2607-2617 - 74.
Lister JA. Use of phage φC31 integrase as a tool for zebrafish genome manipulation. Methods in Cell Biology. 2011; 104 :195-208 - 75.
Lister JA. Transgene excision in zebrafish using the phiC31 integrase. Genesis. 2010; 48 (2):137-143 - 76.
Lu J, Maddison LA, Chen W. PhiC31 integrase induces efficient site-specific excision in zebrafish. Transgenic Research. 2011; 20 (1):183-189 - 77.
Roberts JA, Miguel-Escalada I, Slovik KJ, Walsh KT, Hadzhiev Y, Sanges R, et al. Targeted transgene integration overcomes variability of position effects in zebrafish. Development. 2014; 141 (3):715-724 - 78.
Mosimann C, Puller AC, Lawson KL, Tschopp P, Amsterdam A, Zon LI. Site-directed zebrafish transgenesis into single landing sites with the phiC31 integrase system. Developmental Dynamics. 2013; 242 (8):949-963 - 79.
Fogg PCM, Colloms S, Rosser S, Stark M, Smith MCM. New applications for phage integrases. Journal of Molecular Biology. 2014; 426 (15):2703-2716 - 80.
Hu G, Goll MG, Fisher S. ΦC31 integrase mediates efficient cassette exchange in the zebrafish germline. Developmental Dynamics. 2011; 240 (9):2101-2107 - 81.
Hippenmeyer S, Youn YH, Moon HM, Miyamichi K, Zong H, Wynshaw-Boris A, et al. Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron. 2010; 68 (4):695-709 - 82.
Tasic B, Hippenmeyer S, Wang C, Gamboa M, Zong H, Chen-Tsai Y, et al. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108 (19):7902-7907 - 83.
Zhu F, Gamboa M, Farruggio AP, Hippenmeyer S, Tasic B, Schüle B, et al. DICE, an efficient system for iterative genomic editing in human pluripotent stem cells. Nucleic Acids Research. 2014; 42 (5):e34 - 84.
Lalonde RL, Kemmler CL, Riemslagh FW, Aman AJ, Kresoja-Rakic J, Moran HR, et al. Heterogeneity and genomic loci of ubiquitous transgenic Cre reporter lines in zebrafish. Developmental Dynamics. 2022; 251 (10):1754-1773 - 85.
Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science. 1989; 245 (4922):1059-1065 - 86.
Cannavò E, Khoueiry P, Garfield DA, Geeleher P, Zichner T, Gustafson EH, et al. Shadow enhancers are pervasive features of developmental regulatory networks. Current Biology. 2016; 26 (1):38-51 - 87.
Hay D, Hughes JR, Babbs C, Davies JOJ, Graham BJ, Hanssen L, et al. Genetic dissection of the α-globin super-enhancer in vivo . Nature Genetics. 2016;48 (8):895-903 - 88.
Osterwalder M, Barozzi I, Tissières V, Fukuda-Yuzawa Y, Mannion BJ, Afzal SY, et al. Enhancer redundancy provides phenotypic robustness in mammalian development. Nature. 2018; 554 (7691):239-243 - 89.
Chong JX, Buckingham KJ, Jhangiani SN, Boehm C, Sobreira N, Smith JD, et al. The genetic basis of Mendelian phenotypes: Discoveries, challenges, and opportunities. American Journal of Human Genetics. 2015; 97 (2):199-215 - 90.
Weedon MN, Cebola I, Patch AM, Flanagan SE, De Franco E, Caswell R, et al. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nature Genetics. 2014; 46 (1):61-64 - 91.
Liberatore CM, Searcy-Schrick RD, Yutzey KE. Ventricular expression of tbx5 inhibits normal heart chamber development. Developmental Biology. 2000; 223 (1):169-180 - 92.
Takeuchi JK, Koshiba-Takeuchi K, Suzuki T, Kamimura M, Ogura K, Ogura T. Tbx5 and Tbx4 trigger limb initiation through activation of the Wnt/Fgf signaling cascade. Development. 2003; 130 (12):2729-2739 - 93.
Benko S, Fantes JA, Amiel J, Kleinjan DJ, Thomas S, Ramsay J, et al. Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nature Genetics. 2009; 41 (3):359-364 - 94.
Jakobsen LP, Ullmann R, Christensen SB, Jensen KE, Mølsted K, Henriksen KF, et al. Pierre Robin sequence may be caused by dysregulation of SOX9 and KCNJ2. Journal of Medical Genetics. 2007; 44 (6):381-386 - 95.
Pritchett J, Athwal V, Roberts N, Hanley NA, Hanley KP. Understanding the role of SOX9 in acquired diseases: Lessons from development. Trends in Molecular Medicine. 2011; 17 (3):166-174 - 96.
Gordon CT, Attanasio C, Bhatia S, Benko S, Ansari M, Tan TY, et al. Identification of novel craniofacial regulatory domains located far upstream of SOX9 and disrupted in Pierre Robin sequence. Human Mutation. 2014; 35 (8):1011-1020 - 97.
Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: Illuminating the dark road from association to function. American Journal of Human Genetics. 2013; 93 (5):779-797 - 98.
Degner JF, Pai AA, Pique-Regi R, Veyrieras JB, Gaffney DJ, Pickrell JK, et al. DNase I sensitivity QTLs are a major determinant of human expression variation. Nature. 2012; 482 (7385):390-394 - 99.
Trynka G, Sandor C, Han B, Xu H, Stranger BE, Liu XS, et al. Chromatin marks identify critical cell types for fine mapping complex trait variants. Nature Genetics. 2013; 45 (2):124-130 - 100.
Schaub MA, Boyle AP, Kundaje A, Batzoglou S, Snyder M. Linking disease associations with regulatory information in the human genome. Genome Research. 2012; 22 (9):1748-1759 - 101.
Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011; 473 (7345):43-49 - 102.
Farh KKH, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015; 518 (7539):337-343 - 103.
Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Research. 2019; 47 (D1):D1005-D1012 - 104.
Ritchie GR, Flicek P. Computational approaches to interpreting genomic sequence variation. Genome Medicine. 2014; 6 (10):87 - 105.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007; 316 (5826):889-894 - 106.
Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genetics. 2007; 3 (7):e115 - 107.
Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gómez-Marín C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014; 507 (7492):371-375 - 108.
Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, et al. FTO obesity variant circuitry and adipocyte browning in humans. The New England Journal of Medicine. 2015; 373 (10):895-907 - 109.
Rinkwitz S, Geng FS, Manning E, Suster M, Kawakami K, Becker TS. BAC transgenic zebrafish reveal hypothalamic enhancer activity around obesity associated SNP rs9939609 within the human FTO gene. Genesis. 2015; 53 (10):640-651 - 110.
Kraus P, Winata CL, Lufkin T. BAC transgenic zebrafish for transcriptional promoter and enhancer studies. Methods in Molecular Biology. 2015; 1227 :245-258 - 111.
Zhang Y, Muyrers JP, Testa G, Stewart AF. DNA cloning by homologous recombination in Escherichia coli . Nature Biotechnology. 2000;18 (12):1314-1317 - 112.
Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli . Nature Genetics. 1998;20 (2):123-128 - 113.
Pahl MC, Grant SFA, Leibel RL, Stratigopoulos G. Technologies, strategies, and cautions when deconvoluting genome-wide association signals: FTO in focus. Obesity Reviews. 2023; 24 (5):e13558 - 114.
Lin KC, Lin HY, Chou P. The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. The Journal of Rheumatology. 2000; 27 (6):1501-1505