Valorization of vine pruning residues.
Abstract
The wine sector generates high quantities of residues that are still poorly exploited as feedstock. Normally, these wastes are directly discarded into the fields or burned, thus causing environmental problems. Wine production wastes, like vine pruning and grape pomace, are available at relatively low prices and are considered prime materials for biochemical conversion into added-value products. In this context, the reutilization of these wastes is very important not only for minimizing environmental impact but also for obtaining higher profitability. The main objective of the present chapter is to address what are the possible reutilizations and valorizations of these wastes.
Keywords
- vine pruning
- grape pomace
- grape stems
- lees
- waste valorization
1. Introduction
Agro-food residues with high organic content represent environmental hazards, but at the same time are sources of added-value compounds like polyphenols, carbohydrates, proteins,
Normally, a big part of the wine waste is incinerated, a process that has high operation costs and generates hazardous gases and ashes. It can be used also for animal feed or left in fields. Wine residues are not hazardous by nature, but the fact that wine production is concentrated in a specific period of the year poses potential pollution issues and implications.
There are different studies aiming at possible solutions for valorization and further reutilization of these wastes, according to the concepts of circular economy. The main intuition of the present chapter is the systematization and recognition of the existing research carried out on wine residue valorization.
2. The winemaking
The International Organization of Vine and Wine (OIV), which represents 49 member states, accounting for about 87% of world wine production, defines wine as the beverage resulting exclusively from the partial or complete alcoholic fermentation of fresh grapes (
In 2022, according to the OIV, 7.30 × 106 ha of vines, allowed the production of 258 × 108 L of wine around the world [6]. This amount changes every year, depending on various circumstances like occasionally unfavorable climate conditions.
The two main biotechnological processes associated with wine production are alcoholic fermentation (AF), conducted by yeasts, and malolactic fermentation (MLF), by lactic acid bacteria. Alcoholic fermentation is the primary fermentation during winemaking. Throughout the AF, the fermentable sugars of the must, mainly glucose and fructose, are transformed into ethanol, carbon dioxide, and several other compounds contributing to the global taste and aroma of the wine. They belong to several chemical families: organic acids, higher alcohols, aldehydes, volatile fatty acids, ethyl esters and acetates,
Nevertheless, the process of winemaking includes several other steps before and after the fermentation processes [9, 11]. Figure 1 presents the generic processes for making white and red wines, including the fermentation stages, the processes for preparing the must and finishing the wine before bottling, and also the residues, by-products, and liquid effluents generated. Although a few products are added to the must and/or wine, several residues are rejected, either as liquid or solid wastes [12].
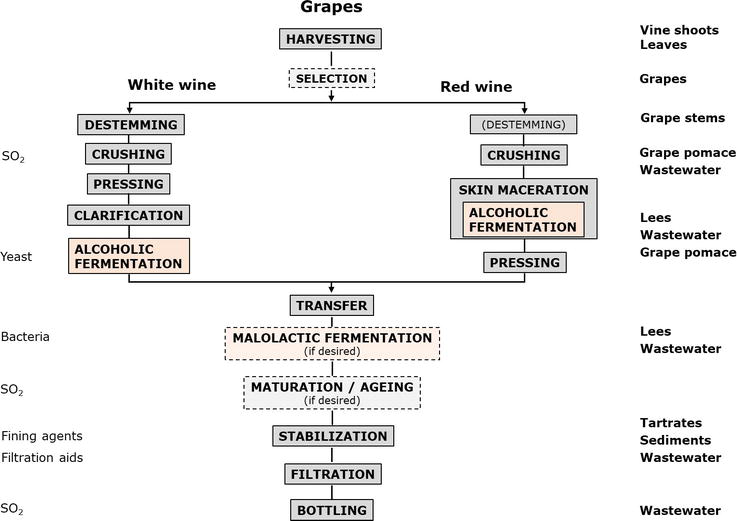
Figure 1.
Generic biotechnological process of winemaking, including waste and by-products generated.
White wine is usually produced by alcoholic fermentation of a clarified grape must. Malolactic fermentation may also occur in some wines. To obtain the most, after harvesting, and eventually selecting the bunches, the grapes should be crushed and pressed before the juice is clarified either by gravity or by dynamic processes—centrifugation, filtration, or flotation; pre-fermentative skin maceration is sometimes applied. Before alcoholic fermentation, the must usually need some specific preparation, depending on its initial composition and on the desired characteristics of the resulting wine. Sometimes the total must acidity may need adjustment of either increasing (acid addition, using for example tartaric acid) or decreasing (acidity reduction, using for example CaCO3) [13]. The addition of enzymes (pectinases) accelerates particle sedimentation and helps the clarification of the grape must; glycosidases may be used to enhance varietal flavor [14, 15]. Sulfur dioxide (SO2) is added to the grape must prevent oxidation and growth of wild yeasts and bacteria. To promote alcoholic fermentation, selected dry Saccharomyces cerevisiae yeasts are usually applied as fermentation starters, as this eases the control of the fermentation and can bring to the final product specific aroma compounds [9, 16]. A temperature of 18°C constitutes a good compromise between the fermentation rate and the final quality of the product. Recently, the use of non-
Contrarily to white wine, the fermentation of red wines is accompanied by maceration, to maximize the extraction of color (and other relevant compounds) from the grape pomace (mainly skins). The length and intensity of the macerations are very important and depend on the grape variety and the desired type of wine to be produced [9, 16]. In order to facilitate the extraction of coloring matter, temperatures around 25°C are used. Moreover, unlike white wines, red wines usually undergo malolactic fermentation and are aged (frequently in oak barrels) before bottling.
Rosé wines are made from red grapes, by alcoholic fermentation of the must that results from the immediate pressing, or that results after a slight/controlled skin maceration; the grape variety and the maceration time influence the shade of the final product [9].
3. Reutilization and valorization of winemaking residues
In the framework of the circular economy, obtaining natural components from food waste has gained interest. The disposal of winemaking residues in the environment is a serious concern and as such, in recent years, attracted much attention of the scientific community in an effort to find profitable and sustainable solutions for their reutilization and valorization. The main winemaking residues that will be addressed in the following rows are vine pruning, grape stems, grape pomace, and lees.
3.1 Vine pruning
Vine pruning is fundamental for the vineyards. It is a practice where shoots, branches, and herbaceous parts are cut to achieve good harvest and quality grapes. There are different types of grape pruning with distinctive characteristics that influence the vine and consecutively the produced grapes. Vine pruning residues (VPR) are a lignocellulosic material and even though it is known to have interesting added-value compounds with different bioactivities, this material is still economically underutilized [19]. Lignocellulosic residues are a possible sustainable substitute for fossil-based fuels, chemicals, and materials. It was concluded that VPR contains more lignin than other renewable sources like wheat straw and corn stalks. The proximal composition of VPR was found to be as follows: cellulose from 24.8 to 32.4%, hemicellulose from 9.6 to 13.8%, and lignin from 23.7 to 32.4% [20]. Moreover, VPR is a rich source of polyphenol compounds.
VPR can be used for different purposes (Table 1). For example, it was used as raw material for ethanol production by hydrothermal treatment, also known as autohydrolysis [21]. The autohydrolysis was used in two sequential stages in an attempt for the integral valorization of VPR. With the proposed method, xylooligosaccharides, phenolic compounds, and ethanol were obtained. In summary, 69 kg of value-added compounds were obtained from processing of 100 kg VPR. In the study from Gullón
| Compound | Method used | Reference |
|---|---|---|
| polyphenols | Ohmic heating | [19] |
| ethanol, xylooligosaccharides, polyphenols | autohydrolysis | [21] |
| lactic acid, furfural | Saccharification, fermentation | [22] |
| polyphenols | microwave-assisted extraction, subcritical water extraction, conventional extraction | [23] |
| polyphenols | microwave-assisted extraction | [24] |
| particleboard | direct use | [25] |
| activated carbon | physical, chemical activation | [26] |
| monomeric sugars, furfural, HMF, formic acid, lactic acid | enzymatic hydrolysis | [2] |
| solid biofuel | burning | [27] |
| oligosaccharides, polyphenol compounds | autohydrolysis followed by ethyl acetate extraction | [28] |
| Energy source | catalytic combustion | [29] |
Table 1.
A biorefinery concept was used for the production of lactic acid and furfural from VPR. Moreover, the life cycle assessment of the process was performed on the selected scenario. It was concluded that with this scenario there were significant reductions in climate change, fossil fuel depletion, freshwater ecotoxicity, and human toxicity impacts compared to their counterfactual systems [22].
VPRs are rich in polyphenol compounds and frequently are used for the extraction of these valuable compounds. Polyphenols have various biological activities like antioxidant, anti-mutagenic, anti-inflammatory, antimicrobial, and anti-carcinogenic properties [19]. The composition of the recovered phenolic compounds from VPR depends on grapevine variety, age, and growth conditions. Moreover, the extraction yield and type of extracted phenolics depend on the temperature, time, and solvent composition used in the experiments. The phenolic composition of extracts from VPR from the grape varieties Touriga Nacional and Tinta Roriz were evaluated [23]. Gallic acid, catechin, myricetin, and kaempferol were the main polyphenol compounds found in the extracts. Moreover, the extracts demonstrated high antioxidant activity and inhibitory activities against α-amylase and acetylcholinesterase enzymes, showing their potential to be used in the treatment of Alzheimer and diabetes’s diseases [23]. In another study, the main polyphenol compounds identified in the extracts made with microwave-assisted extraction were apigenin and ellagic acid [24]. The main polyphenol compounds found in the ethyl acetate extracts of VPR were vanillin, acetovanillone, guaiacylacetone, syringaldehyde, and acetosyringone. The extracts had antioxidant and antimicrobial activities against both Gram-positive and -negative bacteria [28].
In other studies, VPR is used directly, like for example in the construction of particleboards. The experimental three-layer boards where wood particles from the core part were substituted with 50% of VPR demonstrated good properties. However, the use of vine pruning particles as surface material should be excluded as it deteriorates [25]. VPR was used directly as solid biofuel inside the vineyard. The idea of the project was to use VPR as solid chips for the boilers inside of the vineyards, replacing the pine pellets that are usually used. This change in solid biofuel decreased the total CO2 emission of the vineyard, and consecutively its carbon footprint. It was calculated that the proposed process brings economic savings for the vineyard [27]. The high combustion efficiency of VPR was also obtained using catalytic combustion (Pd catalyst) in a conical spouted bed combustor [29].
VPRs were used after being transformed into activated carbon and applied in wines as a fining agent. The produced activated carbon was able to decrease the presence of unpleasant aromas, as well as to alleviate the negative effects of browning in a white wine [26].
An integrated biorefinery concept was applied with the objective of reusing all the winery waste streams. VPR were subjected to hydrothermal pretreatment followed by enzymatic hydrolysis. The obtained compounds depended on the severity of the treatment of the material and included the compounds glucan and xylan (at milder pretreatment conditions), furans (furfural, HM-hydroxymethylfurfural), and organic acids (formic, lactic) [2].
3.2 Grape stems
The grape stems (GSs), also known as grape stalks, form the skeleton of the grape bunches. GS is obtained during the destemming process. It is a lignocellulosic material with the following proximal composition: 34% lignin, 36% cellulose, 24% hemicellulose, and 6% tannins [30]. The high contents of lignin and tannins make the grape stems a challenging material for processing and reutilization [30]. Compared to grape pomace and seeds GS has fewer bioactive molecules, but it is still an important source of antioxidant compounds [31].
At the same time, it is considered that using the GS exclusively to produce ethanol is not a cost-effective and beneficial process. In this context GP was exploited as biorefinery biomass for the production of second-generation ethanol, as well as added-value compounds including cellulose, hemicellulose, lignin, and cellulose nanocrystals (Table 2) [31].
| Compound | Method used | References |
|---|---|---|
| lignin, sugars, tannins | dilute sulfuric acid, ethanol organosolv, wet oxidation, | [30] |
| ethanol, cellulose, hemicellulose, lignin, cellulose nanocrystals | acid and enzymatic hydrolyses | [31] |
| modified grape stems | heat process mercerization | [32] |
| to produce porous bricks | direct use | [33] |
| ( | photo-molecularly imprinted sorbent | [34] |
| sulfur dioxide replacement | extraction | [35] |
| corrosion inhibitor | water extraction | [36] |
| polyphenols | extraction | [37] |
| tannins | microwave extraction | [38] |
| solid substrate for fungi growth | direct use | [39] |
| as filler in biocomposites | direct use | [40] |
| succinic acid | alkaline and acid pretreatment, enzymatic hydrolysis | [41] |
| adsorbent | direct use, activated carbon | [42, 43] |
Table 2.
Examples of possible valorization of grape stem residues.
GS were treated physically (heat process) and chemically (mercerization) with the intuition to be included in biocomposites for the production of new products that can be used in industries like packaging, aerospace, automotive, and construction [32]. In this context, GS was used as a primary source in the process of making highly porous bricks. The obtained total porosity and pore dimensions enhanced the durability and thermal insulation properties of the produced bricks. With respect to the same type of clay, bricks produced with GS decreased heat transmission by around 40% [33]. Another utilization for GS in biocomposites formula is as filler in bioplastics while polybutylene succinate was the basic polymer. The obtained product demonstrated improved mechanical properties, and lower production costs [40]. GS was, also, used directly as a solid substrate for the growing of R. oryzae
The antioxidant extracts of GS were considered a promising replacement for sulfur dioxide in wine preservation. However, the authors concluded that before the implementation of this process in the wineries, additional studies should be carried out [35]. The polyphenol compounds present in the GS have the capacity to bond with the iron vacant orbitals protecting them from corrosion. In this context, the extracts of GS were used as corrosion inhibitors of mild steel in 0.5 mol/L NaCl. The GS extract applied at 400 mg/L was found to provide maximum protection of 88% [36].
The potential application of GS extracts in the cosmetic, pharmaceutical, and food industries was also evaluated. The analysis of the extracts confirmed the presence of polyphenol compounds with catechin showing the highest concentrations. Moreover, the extracts had diverse bioactivities including antioxidant, anti-inflammatory, and anti-aging [37]. GS was directly applied in the vinification of the grapes from the variety Bonarda with the objective to increase the tannin content of the resulting wine. The result demonstrated that the use of GS in the fermentation process modified the chromatic characteristics and phenolic composition, improved the color stability, and changed the volatile and polysaccharide profile of the produced Bonarda wines [38].
GS can be used as a raw material for the production of succinic acid. Succinic acid has many applications in the pharmaceutical, agricultural, food, and chemical industries. The proposed process valorization included alkaline and acid pretreatment of the stems, followed by enzymatic hydrolyses. The sugar-rich hydrolysate was further used as fermentation broth for the production of succinic acid [41].
GS contains various functional groups (tannins, tartrate, organic acids,
It is to refer to the fact that, grape stem extracts were evaluated for their multiple biological activities like anti-cancer (breast, colon, renal, and thyroid cancer cells) [44], anti-microbial (including digestive pathogens) [45], also against ultraviolet irradiation [46], antioxidant, anti-inflammatory, anti-aging, and others [37]. In this context, further studies are needed to find out and understand the full potential of this waste material and to project its further utilization.
3.3 Grape pomace
Grape pomace (also known as grape marc) is the residue after pressing the grapes. It is a mixture of grape skins, grape seeds, and some grape stems. Traditionally grape pomace is used for ethanol distillation, animal feeding, or spread in the land. The grape pomace distillation and resulting spirits are an important industrial activity in countries like France and Italy. High amounts of bioactive compounds are present in GP, especially polyphenols. Their concentration and presence depend on numerous variables like type of grape variety, type of vinification process, geographic region, and year of harvest [47].
Grape pomace (GP) was used for the extraction of oleanolic acid, a natural triterpenoid with antidiabetic properties (Table 3) [48]. Besides oleanolic acid other compounds like polyphenolic compounds, anthocyanins, minerals, and amino acids were also identified in the GP extracts. The most abundant polyphenols found in the extracts were catechin, epicatechin, caffeic acid, and quercetin. A sustainable production process of ethyl hexanoate (ester with flavor of pineapple) from GP was evaluated. GP was used for the production of hexanoic acid that was further converted to ethyl hexanoate [49]. The proposed process demonstrated to be a possible alternative to fossil-derived hexanoic acid.
| Compound | Method used | Reference |
|---|---|---|
| oleanolic acid, polyphenols, amino acids, anthocyanins | ethanol extraction | [48] |
| polyphenols, fibers, polyunsaturated fatty acids, minerals and protein | ethanol extraction | [47] |
| hexanoic acid | fermentation | [49] |
| polyphenols | surfactants extraction | [50] |
| anthocyanins | ultrasound-assisted extraction | [51] |
| anthocyanins | extraction with eutectic solvents | [52] |
| substitute for wheat flour | direct use | [53] |
| pectin | conventional, microwave-assisted, and pulsed ultrasound-assisted extraction | [54] |
| polyphenols | non-ionic surfactants extraction | [55] |
| animal feed | direct use | [56] |
| energy | pyrolysis | [57] |
| polysaccharides | extraction | [58] |
Table 3.
Valorization of grape pomace residues.
In the context of circular economy and waste reutilization, the use of green technologies is the best possible scenario. Aqueous solutions of surfactants are green substitutes for organic solvents and were used for the extraction of polyphenols from GP [50]. The main extracted polyphenols were catechin and quercetin. Catechin and quercetin were also the main polyphenol compounds found in GP extracts made with non-ionic surfactants [55]. The study was conducted with 11 different surfactant solutions and demonstrated that the structure specificities of the surfactants influence the extraction efficiency of the polyphenols.
The eco-friendly ultrasound-assisted extraction method was used to obtain anthocyanins from red GP [51]. Anthocyanins are known to have many health benefits, for this reason in our days anthocyanin supplements are an important part of the nutrition market. Anthocyanin isolation from agro-industrial wastes is attracting much attention. Besides their bioactive properties, anthocyanins are also used as natural colorants. For this purpose, anthocyanins were extracted from GP using eutectic solvents [52]. The proposed method of isolation and stabilization of the extracted anthocyanins had low environmental impacts and economic costs compared to the conventional extraction with solvents.
GP, in powder, can be used directly in the elaboration of cakes, as a substitute for wheat flour. Cakes produced with 4% GP powder showed good sensory quality with enhanced nutritional properties, once the GP powder provided the cakes with polyphenolic compounds [53].
GP was also used for the extraction of pectin applying green technologies, like microwave and ultrasound-assisted extractions. Pectin is a polysaccharide found in the plant cell walls. Normally it is extracted from apples pomace and citrus peel using organic or mineral acids. The use of different extraction methods resulted in extracted pectin with diverse structural characteristics [54]. However, it was concluded that pectin from GP has the potential to be applied in different fields including the food industry. In another study, the extracts of GP were successfully applied as antimicrobial additives in polypropylene food packaging. The obtained materials demonstrated antimicrobial activity against Gram-negative (Escherichia coli) and Gram-positive (Bacillus subtilis) bacteria [59].
GP is rich in polysaccharides, especially the GP from white grapes. GP from red grapes is only liberated after the fermentation of the juice, so it contains less pulp and residual sugars, than the white GP. However, polysaccharides may be also obtained from red GP. The most abundant polysaccharides in extracts made with hot water were arabinose, xylose, mannose, glucose, and galactose [58].
Cheese made from the milk of ewes on a diet formula that included GP had improved sensory characteristics. GP supplement mainly affected the aromatic profile of the cheese [56].
GP was also evaluated as an energy source. The proposed idea is to use the energy obtained from GP pyrolysis in the food processing plants to reduce the final cost of energy [57].
As a final remark, we can say that grape pomace is a versatile source of different compounds and products that can be used in the food industry, in the pharmaceutical and cosmetics industries, in agriculture, as well as energy sources.
3.4 Lees
Wine lees are one of the main byproducts of the wine industry. This sludge is essentially made up of dead yeast, which precipitates at the bottom tanks after the fermentation process has taken place [60, 61, 62]. According to Galanakis, 85.7 t (dry basis) of wine lees are generated for every 1000 m3 of wine produced [63].
Since lees contain large amounts of polysaccharides, lipids, proteins, and other compounds with high demand for oxygen, their deposition to the environment, causes serious pollution problems, due to the high organic content and a low pH. These characteristics also make their direct use in agricultural practices inappropriate, such as the production of compost and use for animal feed.
On the other hand, since this by-product contains a wide variety of value-added compounds, the investigation of potential recovery routes has gained relevance. In this way, the deepening of this strategy would not only avoid serious environmental problems but would also reduce the costs associated with its deposition [64]. More specifically, wine lees contain both liquid and solid fractions. The first is called vinasse and contains bark, part of the seed, and dead yeast, consisting of the spent fermentation broth. In turn, the solid fractions contain organic and inorganic salts, grain, seeds, cellulose, hemicellulose, and lignin. Both fractions have the potential to produce added value by-products, with high economic relevance [60, 61].
Thus, in order to avoid the conventional applications of this by-product (agriculture and animal feed), which are not appropriate and generate environmental problems, several approaches have been applied and several studies have been carried out in order to find effective, sustainable, and economically viable alternatives.
On an industrial scale, only ethanol and tartaric acid extraction have been applied. From the wine lees distillation process, it is possible to recover ethanol and produce distilled beverages [65]. The by-product obtained from distillation (vinasses) contains several value-added components with the potential to be extracted.
Wine lees are the major source of tartaric acid and the most valuable component that can be extracted from vinasses [66]. This acidifier is widely used in the food and beverage industry, as well as in the pharmaceutical, cosmetic, and chemical industries [67, 68]. Conventional processes for obtaining tartaric acid (precipitation, crystallization, acidification, ion exchange,
In addition to these more developed strategies, there are other promising possibilities that have been studied and tested. One of them is the biotechnological application of remaining lees as a culture medium for lactic acid bacteria [68]. Studies have shown the possibility of integrating the production of microbial substrates from this by-product, with the extraction of ethanol and tartaric acid, in order to extract the greatest number of components and make the processes viable [64, 68].
Furthermore, the cell walls of mannoproteins and β-glucans from wine lees have been explored, proving to be promising for various types of applications, namely the food industry (emulsifier, fat replacers, cholesterol reducer, antioxidant) and viticulture (foam, mouthfeel enhancer). Extraction methods can vary between physical, chemical, enzymatic methods, and a combination of these [72, 73]. In turn, Iseppi studied the development of methods for yeast glycocompounds extraction from wine lees, through physical (autoclave and ultrasonication) and enzymatic methods, demonstrating their possibility of use as winemaking additives [74].
Moreover, wine lees present a valuable opportunity as rich sources of anthocyanins and other polyphenols, showcasing the strong potential for use within the food, cosmetics, and pharmaceutical industries. This ability to foster health benefits is attributed to their well-recognized properties as antioxidants, antimicrobial agents, anti-inflammatory substances, and promoters of cardiovascular well-being [75].
Additionally, the production of biogas has been tested as a potential application of the solid fraction of wine lees. This strategy involves anaerobic co-digestion of wine lees by Escherichia coli under mesophilic and thermophilic conditions [76].
In short, wine lees have great potential for extracting value-added compounds. The investigation of the methods to be adopted must be deepened in order to optimize the extraction efficiency. The development of integrated approaches aimed at extracting various types of compounds for application in different sectors would be an asset to promote the valorization of wine lees, applying the circular economy in this sector and promoting environmental and economic sustainability.
Table 4 presents the possible wine lees valorization strategies that have been studied.
| Compound | Method used | Reference |
|---|---|---|
| ethanol | distillation | [77] |
| polyphenols | organic solvents | [75] |
| polyphenols | microfiltration | [78] |
| β-glucans | extraction using NaOH | [73] |
| microbial media | preparation for microbial media | [68] |
| polyphenols, polysaccharides | ultrafiltration and nanofiltration | [70] |
| ethanol, tartaric acid, polyphenols, microbial media | cation exchange resin | [79] |
| tartaric acid polyphenols | nanofiltration | [67] |
| ethanol, tartaric acid, polyphenols, microbial media | extraction and preparation for microbial media | [64] |
| biogas | anaerobic digestion | [76] |
| yeast glycocompounds | ultrasonication, enzymatic, autoclave | [74] |
Table 4.
Summary of the papers that studied valorization approaches for wine lees.
As final remarks about the extraction methods used to obtain valuable products, one can see that there are various methods used. The right choice of method will depend on the desired result as well as the existing conditions in each laboratory. It is evident that the simplest and the easiest method to be implemented and used is the conventional heat extraction method. Clearly, the method of extraction to be applied will depend on the nature of the desired final compounds. The best choice is that the used method is environmentally friendly and of a low cost,
4. Conclusion
Wine residues are rich sources of various compounds of interest like polyphenols, dyes, carbohydrates, organic acids, and others. As such wine residues can be a cheap alternative for the development of new products, functional foods, pharmaceuticals, and cosmetics. Regardless of the many existing studies on the valorization of wine wastes, the proposed processes have not been widely implemented in the winemaking industry so far. There is still much work to be done until wine residues gain an established recovery pathway that brings economic benefits.
Acknowledgments
Zlatina Genisheva wishes to thank you for the financial support to the project Mission2GG—To Grow Green, by PRR—Plano de Recuperação e Resiliência, enquadrado na Missão Interface.
References
- 1.
FAOStat. Food and Agriculture Organization of the United Nations. 2022. [Accessed: August 20, 2023] - 2.
Ioannidou SP, Margellou AG, Petala MD, Triantafyllidis KS. Pretreatment/fractionation and characterization of winery waste streams within an integrated biorefinery concept. Sustainable Chemistry and Pharmacy. 2022; 27 :100670. DOI: 10.1016/j.scp.2022.100670 - 3.
OIV. International Organization of Vine and Wine. State of the world vine and wine sector 2021. International Organisation of Vine and Wine. 2022; 1 :1-20. [Accessed: July 18, 2023] - 4.
OIV. Regulation EU No 1308/2013. European Union. 2013. [Accessed: July 18, 2023] - 5.
Wine MA. In: Schmidt T, editor. Encyclopedia of Microbiology. Cambridge, Massachusetts, U.S.: Academic Press; 2019. pp. 138-143. ISBN: 9780128117378 - 6.
OIV. International code of oenological practices. International Organisation of Vine and Wine. 2022; 1 :209-336. ISBN: 9782850380594 - 7.
Hirst MB, Richter CL. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. American Journal of Enology and Viticulture. 2016; 67 :361-370. DOI: 10.5344/ajev.2016 - 8.
Oliveira JM, Oliveira P, Baumes RL, Maia O. Changes in aromatic characteristics of Loureiro and Alvarinho wines during maturation. Journal of Food Composition and Analysis. 2008; 21 :695-707. DOI: 10.1016/j.jfca.2008.08.002 - 9.
Ribéreau-Gayon P, Glories Y, Maujean A, Dubourdieu D. Handbook of Enology: The Chemistry of Wine Stabilization and Treatments. Hoboken, New Jersey, U.S.: John Wiley & Sons, Ltd; 2021. DOI: 10.1002/0470010398 - 10.
Bartowsky EJ, Costello PJ, Chambers PJ. Emerging trends in the application of malolactic fermentation. Australian Journal of Grape and Wine Research. 2015; 21 :663-669. DOI: 10.1111/ajgw.12185 - 11.
Rebéreau-Gayon P, Dubourdieu D, Donèche BB, Lonvaud AA. Handbook of Enology the Microbiology of Wine and Vinifications. Hoboken, New Jersey, U.S.: John Wiley & Sons, Ltd; 2021. DOI: 10.1002/0470010363 - 12.
Brito AG, Peixoto J, Oliveira JM, Oliveira JA, Costa C, Nogueira R, et al. Brewery and winery wastewater treatment: Some focal points of design and operation. In: Oreopoulou V, Russ W, editors. Util by-Products Treat Waste Food Ind. Bonton: Springer; 2007. pp. 109-131. DOI: 10.1007/978-0-387-35766-9_7 - 13.
Vigne DLA, Vin D. International code of oenological practices. International Organisation of Vine and Wine. 2023; 1 :118-218. ISBN: 9782850380716 - 14.
Espejo F. Role of commercial enzymes in wine production: A critical review of recent research. Journal of Food Science and Technology. 2021; 58 :9-21. DOI: 10.1007/s13197-020-04489-0 - 15.
Ottone C, Romero O, Aburto C, Illanes A, Wilson L. Biocatalysis in the winemaking industry: Challenges and opportunities for immobilized enzymes. Comprehensive Reviews in Food Science and Food Safety. 2020; 19 :595-621. DOI: 10.1111/1541-4337.12538 - 16.
Grainer K, Tattersall H. Wine Production and Quality, 2nd Edition by Grainger, Keith Tattersall, Hazel. Hoboken, New Jersey, U.S.: John Wiley & Sons, Ltd; 2016. DOI: 10.1002/9781118934562 - 17.
Borren E, Tian B. The important contribution of non-saccharomyces yeasts to the aroma complexity of wine: A review. Food. 2021; 10 . DOI: 10.3390/foods10010013 - 18.
Comitini F, Capece A, Ciani M, Romano P. New insights on the use of wine yeasts. Current Opinion in Food Science. 2017; 13 :44-49. DOI: 10.1016/j.cofs.2017.02.005 - 19.
Jesus MS, Ballesteros LF, Pereira RN, Genisheva Z, Carvalho AC, Pereira-Wilson C, et al. Ohmic heating polyphenolic extracts from vine pruning residue with enhanced biological activity. Food Chemistry. 2020; 316 :126298. DOI: 10.1016/j.foodchem.2020.126298 - 20.
Zhang X, Fang Z, Zhao D, Kamal R, Wang X, Jin G, et al. Biorefinery of vineyard winter prunings for production of microbial lipids by the oleaginous yeast Cryptococcus curvatus. Waste Management. 2023; 168 :221-229. DOI: 10.1016/j.wasman.2023.06.003 - 21.
Jesus MS, Romaní A, Genisheva Z, Teixeira JA, Domingues L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. Journal of Cleaner Production. 2017; 168 . DOI: 10.1016/j.jclepro.2017.08.230 - 22.
Pachón ER, Mandade P, Gnansounou E. Conversion of vine shoots into bioethanol and chemicals: Prospective LCA of biorefinery concept. Bioresource Technology. 2020; 303 :122946. DOI: 10.1016/j.biortech.2020.122946 - 23.
Moreira MM, Barroso MF, Porto JV, Ramalhosa MJ, Švarc-Gajić J, Estevinho L, et al. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Science of the Total Environment. 2018; 634 :831-842. DOI: 10.1016/j.scitotenv.2018.04.035 - 24.
Jesus MS, Genisheva Z, Romaní A, Pereira RN, Teixeira JA, Domingues L. Bioactive compounds recovery optimization from vine pruning residues using conventional heating and microwave-assisted extraction methods. Industrial Crops and Products. 2019; 132 . DOI: 10.1016/j.indcrop.2019.01.070 - 25.
Ntalos GA, Grigoriou AH. Characterization and utilisation of vine prunings as a wood substitute for particleboard production. Industrial Crops and Products. 2002; 16 :59-68. DOI: 10.1016/S0926-6690(02)00008-0 - 26.
Calderón-Martín M, Valdés-Sánchez E, Alexandre-Franco MF, Fernández-González MC, Vilanova de la Torre M, Cuerda-Correa EM, et al. Waste valorization in winemaking industry: Vine shoots as precursors to optimize sensory features in white wine. LWT. 2022; 163 :113601. DOI: 10.1016/j.lwt.2022.113601 - 27.
Fernández-Puratich H, Hernández D, Tenreiro C. Analysis of energetic performance of vine biomass residues as an alternative fuel for Chilean wine industry. Renewable Energy. 2015; 83 :1260-1267. DOI: 10.1016/j.renene.2015.06.008 - 28.
Gullón B, Eibes G, Moreira MT, Dávila I, Labidi J, Gullón P. Antioxidant and antimicrobial activities of extracts obtained from the refining of autohydrolysis liquors of vine shoots. Industrial Crops and Products. 2017; 107 :105-113. DOI: 10.1016/j.indcrop.2017.05.034 - 29.
San José MJ, Alvarez S, López R. Catalytic combustion of vineyard pruning waste in a conical spouted bed combustor. Catalysis Today. 2018; 305 :13-18. DOI: 10.1016/j.cattod.2017.11.020 - 30.
Ping L, Brosse N, Sannigrahi P, Ragauskas A. Evaluation of grape stalks as a bioresource. Industrial Crops and Products. 2011; 33 :200-204. DOI: 10.1016/j.indcrop.2010.10.009 - 31.
Atatoprak T, Amorim MM, Ribeiro T, Pintado M, Madureira AR. Grape stalk valorization for fermentation purposes. Food Chemisty: Molecular Sciences. 2022; 4 :100067. DOI: 10.1016/j.fochms.2021.100067 - 32.
Engel JB, Luchese CL, Tessaro IC. Insights on the properties of physically and chemically treated grape stalks. Sustainable Materials and Technologies. 2022; 34 :e00506. DOI: 10.1016/j.susmat.2022.e00506 - 33.
Coletti C, Bragié E, Dalconi MC, Mazzoli C, Hein A, Maritan L. A new brick-type using grape stalks waste from wine production as pore-agent. Open Ceramics. 2023; 14 :100365. DOI: 10.1016/j.oceram.2023.100365 - 34.
Bzainia A, Dias RCS, Costa MRPFN. A simple process to purify (E)-resveratrol from grape stems with a photo-molecularly imprinted sorbent. Food and Bioproducts Processing. 2023; 142 :1-16. DOI: 10.1016/j.fbp.2023.08.01 - 35.
Nogueira DP, Jiménez-Moreno N, Esparza I, Moler JA, Ferreira-Santos P, Sagües A, et al. Evaluation of grape stems and grape stem extracts for sulfur dioxide replacement during grape wine production. Current Research in Food Science. 2023; 6 . DOI: 10.1016/j.crfs.2023.100453 - 36.
Kumari K, Ji G. Aqueous extract of grapes stems for mild steel protection in 0.5 M NaCl. Materials Today Proceedings. 2023. DOI: 10.1016/j.matpr.2023.01 [In press] - 37.
Leal C, Gouvinhas I, Santos RA, Rosa E, Silva AM, Saavedra MJ, et al. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Industrial Crops and Products. 2020; 154 :112675. DOI: 10.1016/j.indcrop.2020.112675 - 38.
Fanzone M, Coronado I, Sari S, Catania A, Gil i Cortiella M, Assof M, et al. Microwave-assisted maceration and stems addition in Bonarda grapes: Effects on wine chemical composition over two vintages. Food Research International. 2022;156:111169. DOI: 10.1016/j.foodres.2022.111169 - 39.
Groff MC, Scaglia G, Gaido M, Kassuha D, Ortiz OA, Noriega SE. Kinetic modeling of fungal biomass growth and lactic acid production in Rhizopus oryzae fermentation by using grape stalk as a solid substrate. Biocatalysis and Agricultural Biotechnology. 2022; 39 :102255. DOI: 10.1016/j.bcab.2021.102255 - 40.
Nanni A, Cancelli U, Montevecchi G, Masino F, Messori M, Antonelli A. Functionalization and use of grape stalks as poly(butylene succinate) (PBS) reinforcing fillers. Waste Management. 2021; 126 :538-548. DOI: 10.1016/j.wasman.2021.03.050 - 41.
Filippi K, Georgaka N, Alexandri M, Papapostolou H, Koutinas A. Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Industrial Crops and Products. 2021; 168 :113578. DOI: 10.1016/j.indcrop.2021.113578 - 42.
Portinho R, Zanella O, Féris LA. Grape stalk application for caffeine removal through adsorption. Journal of Environmental Management. 2017; 202 :178-187. DOI: 10.1016/j.jenvman.2017.07.033 - 43.
Davarnejad R, Afshar S, Etehadfar P. Activated carbon blended with grape stalks powder: Properties modification and its application in a dye adsorption. Arabian Journal of Chemistry. 2020; 13 :5463-5473. DOI: 10.1016/j.arabjc.2020.03.025 - 44.
Sahpazidou D, Geromichalos GD, Stagos D, Apostolou A, Haroutounian SA, Tsatsakis AM, et al. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicology Letters. 2014; 230 :218-224. DOI: 10.1016/j.toxlet.2014.01.042 - 45.
Dias C, Domínguez-Perles R, Aires A, Teixeira A, Rosa E, Barros A, et al. Phytochemistry and activity against digestive pathogens of grape (Vitis vinifera L.) stem’s (poly)phenolic extracts. LWT—Food Science and Technology. 2015; 61 :25-32. DOI: 10.1016/j.lwt.2014.11.033 - 46.
Che DN, Xie GH, Cho BO, Shin JY, Kang HJ, Il JS. Protective effects of grape stem extract against UVB-induced damage in C57BL mice skin. Journal of Photochemistry and Photobiology B: Biology. 2017; 173 :551-559. DOI: 10.1016/j.jphotobiol.2017.06.042 - 47.
Abouelenein D, Mustafa AM, Caprioli G, Ricciutelli M, Sagratini G, Vittori S. Phenolic and nutritional profiles, and antioxidant activity of grape pomaces and seeds from Lacrima di Morro d’Alba and Verdicchio varieties. Food Bioscience. 2023; 53 :102808. DOI: 10.1016/j.fbio.2023.102808 - 48.
Errichiello F, D’Amato M, Gambuti A, Moio L, Pastore A, AL-Hmadi H, et al. Oleanolic acid: A promising antidiabetic metabolite detected in Aglianico grape pomace. Journal of Functional Foods 2023;104:105548. DOI: 10.1016/j.jff.2023.105548 - 49.
D’Ambrosio V, Martinez G, Jones E, Bertin L, Pastore C. Ethyl hexanoate rich stream from grape pomace: A viable route to obtain fine chemicals from agro by-products. Separation and Purification Technology. 2023; 309 :123100. DOI: 10.1016/j.seppur.2023.123100 - 50.
Atanacković Krstonošić M, Sazdanić D, Ćirin D, Maravić N, Mikulić M, Cvejić J, et al. Aqueous solutions of non-ionic surfactant mixtures as mediums for green extraction of polyphenols from red grape pomace. Sustainable Chemistry and Pharmacy. 2023; 33 :101069. DOI: 10.1016/j.scp.2023.101069 - 51.
Zhou Z, Yang D. Economical and eco-friendly isolation of anthocyanins from grape pomace with higher efficiency. Food Chemistry: X. 2022; 15 :100419. DOI: 10.1016/j.fochx.2022.100419 - 52.
de Souza Mesquita LM, Sosa FHB, Contieri LS, Marques PR, Viganó J, Coutinho JAP, et al. Combining eutectic solvents and food-grade silica to recover and stabilize anthocyanins from grape pomace. Food Chemistry. 2023; 406 :135093. DOI: 10.1016/j.foodchem.2022.135093 - 53.
Nakov G, Brandolini A, Hidalgo A, Ivanova N, Stamatovska V, Dimov I. Effect of grape pomace powder addition on chemical, nutritional and technological properties of cakes. LWT. 2020; 134 :109950. DOI: 10.1016/j.lwt.2020.109950 - 54.
Spinei M, Oroian M. Structural, functional and physicochemical properties of pectin from grape pomace as affected by different extraction techniques. International Journal of Biological Macromolecules. 2023; 224 :739-753. DOI: 10.1016/j.ijbiomac.2022.10.162 - 55.
Sazdanić D, Atanacković Krstonošić M, Ćirin D, Cvejić J, Alamri A, Galanakis CM, et al. Non-ionic surfactants-mediated green extraction of polyphenols from red grape pomace. Journal of Applied Research on Medicinal and Aromatic Plants. 2023; 32 :100439. DOI: 10.1016/j.jarmap.2022.100439 - 56.
Bennato F, Ianni A, Bellocci M, Grotta L, Sacchetti G, Martino G. Influence of dietary grape pomace supplementation on chemical and sensory properties of ewes’ cheese. International Dairy Journal. 2023; 143 :105671. DOI: 10.1016/j.idairyj.2023.105671 - 57.
Madadian E, Rahimi J, Mohebbi M, Simakov DSA. Grape pomace as an energy source for the food industry: A thermochemical and kinetic analysis. Food and Bioproducts Processing. 2022; 132 :177-187. DOI: 10.1016/j.fbp.2022.01.006 - 58.
Dong X, Zhu C-P, Huang G-Q , Xiao J-X. Fractionation and structural characterization of polysaccharides derived from red grape pomace. Process Biochemistry. 2021; 109 :37-45. DOI: 10.1016/j.procbio.2021.06.022 - 59.
da Silva DJ, de Oliveira MM, Wang SH, Carastan DJ, Rosa DS. Designing antimicrobial polypropylene films with grape pomace extract for food packaging. Food Packaging and Shelf Life. 2022; 34 :100929. DOI: 10.1016/j.fpsl.2022.100929 - 60.
Ahmad B, Yadav V, Yadav A, Rahman MU, Yuan WZ, Li Z, et al. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Science of the Total Environment. 2020; 719 :137315. DOI: 10.1016/j.scitotenv.2020.137315 - 61.
Bharathiraja B, Iyyappan J, Jayamuthunagai J, Kumar RP, Sirohi R, Gnansounou E, et al. Critical review on bioconversion of winery wastes into value-added products. Industrial Crops and Products. 2020; 158 :112954. DOI: 10.1016/j.indcrop.2020.112954 - 62.
Ioannidou SM, Filippi K, Kookos IK, Koutinas A, Ladakis D. Techno-economic evaluation and life cycle assessment of a biorefinery using winery waste streams for the production of succinic acid and value-added co-products. Bioresource Technology. 2022; 348 :126295. DOI: 10.1016/j.biortech.2021.126295 - 63.
Galanakis CM. Handbook of Grape Processing By-Products. Cambridge, Massachusetts, U.S.: Elsevier; 2017. ISBN: 978-0-12-809870-7 - 64.
Kopsahelis N, Dimou C, Papadaki A, Xenopoulos E, Kyraleou M, Kallithraka S, et al. Refining of wine lees and cheese whey for the production of microbial oil, polyphenol-rich extracts and value-added co-products. Journal of Chemical Technology and Biotechnology. 2018; 93 :257-268. DOI: 10.1002/jctb.5348 - 65.
Khan K, Ullah MF, Shahzada K, Amin MN, Bibi T, Wahab N, et al. Effective use of micro-silica extracted from rice husk ash for the production of high-performance and sustainable cement mortar. Construction and Building Materials. 2020; 258 :119589. DOI: 10.1016/j.conbuildmat.2020.119589 - 66.
Keller M. The Science of Grapevines: Anatomy and Physiology. Cambridge, Massachusetts, U.S.: Elsevier; 2015. DOI: 10.1016/C2013-0-06797-7 - 67.
Kontogiannopoulos KN, Patsios SI, Mitrouli ST, Karabelas AJ. Tartaric acid and polyphenols recovery from winery waste lees using membrane separation processes. Journal of Chemical Technology and Biotechnology. 2017; 92 :2934-2943. DOI: 10.1002/jctb.5313 - 68.
Pérez-Bibbins B, Torrado-Agrasar A, Salgado JM, de Oliveira RP, Domínguez JM. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Management. 2015; 40 :72-81. DOI: 10.1016/j.wasman.2015.03.009 - 69.
Zhang K, Wang M, Gao C. Tartaric acid production by ion exchange resin-filling electrometathesis and its process economics. Journal of Membrane Science. 2011; 366 :266-271. DOI: 10.1016/j.memsci.2010.10.013 - 70.
Giacobbo A, Bernardes AM, de Pinho MN. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Separation and Purification Technology. 2017; 173 :49-54. DOI: 10.1016/j.seppur.2016.09.007 - 71.
Tao Y, Wu D, Zhang Q-A, Sun D-W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrasonics Sonochemistry. 2014; 21 :706-715. DOI: 10.1016/j.ultsonch.2013.09.005 - 72.
Silva Araújo VB da, Melo, A.N.F. de, Costa AG, Castro-Gomez RH, Madruga MS, de Souza EL, et al. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innovative Food Science and Emerging Technologies 2014;23:164-170. DOI: 10.1016/j.ifset.2013.12.013 - 73.
Varelas V, Tataridis P, Liouni M, Nerantzis ET. Valorization of winery spent yeast waste biomass as a new source for the production of β-Glucan. Waste and Biomass Valorization. 2016; 7 :807-817. DOI: 10.1007/s12649-016-9530-4 - 74.
De Iseppi A, Marangon M, Vincenzi S, Lomolino G, Curioni A, Divol B. A novel approach for the valorization of wine lees as a source of compounds able to modify wine properties. LWT. 2021; 136 :110274. DOI: 10.1016/j.lwt.2020.110274 - 75.
Romero-Díez R, Rodríguez-Rojo S, Cocero MJ, Duarte CMM, Matias AA, Bronze MR. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chemistry. 2018; 259 :188-195. DOI: 10.1016/j.foodchem.2018.03.119 - 76.
Da Ros C, Cavinato C, Cecchi F, Bolzonella D. Anaerobic co-digestion of winery waste and waste activated sludge: Assessment of process feasibility. Water Science and Technology. 2014; 69 :269-277. DOI: 10.2166/wst.2013.692 - 77.
Bordiga M. In: Bordiga M, editor. Valorization of Wine Making By-Products. Boca Raton, Florida, U.S.: CRC Press; 2016. DOI: 10.1201/b19423 - 78.
Giacobbo A, do Prado JM, Meneguzzi A, Bernardes AM, de Pinho MN. Microfiltration for the recovery of polyphenols from winery effluents. Separation and Purification Technology. 2015; 143 :12-18. DOI: 10.1016/j.seppur.2015.01.019 - 79.
Kontogiannopoulos KN, Patsios SI, Karabelas AJ. Tartaric acid recovery from winery lees using cation exchange resin: Optimization by response surface methodology. Separation and Purification Technology. 2016; 165 :32-41. DOI: 10.1016/j.seppur.2016.03.040