Advantages and disadvantages of various physical and chemical methods used for the treatment of Cr (VI) from wastewater [34].
Abstract
The release of high volumes of untreated effluents containing different forms of chromium into waterbodies and further use of this wastewater for ferti-irrigation purposes pose a direct threat to health of human populations consuming produces from such agricultural fields. The higher concentration of chromium above permissible limits at these sites may pose harm to flora and fauna. The conventional processes used for treatment of chromium-containing effluents have low treatment efficiency, high operational costs, and produce toxic sludge requiring safe disposal. In contrast, the approaches exploiting use of living systems, such as microbes/microbial products and microbes, may provide sustainable treatment options. The emerging advanced/novel treatment technologies based on harnessing metabolic potential of microbiome of the polluted sites have potential to achieve the efficient removal of heavy metals from polluted sites. The success of protocols developed and tested at lab scale needs to be replicated at pilot/industrial to handle high volumes with varying levels of organic co-contaminants and harsh physiological conditions. The presented chapter provides an overview of impact of high chromium levels on ecosystem and various treatment processes with advanced aspect of management of heavy metals to prevent harmful effects on the environment.
Keywords
- chromium treatment
- heavy metals
- metal-containing wastewater treatment
- toxicity
- Cr (VI)
- bioremediation
1. Introduction
Due to rapid globalization and industrialization, there is an extensive use of chromium (Cr) in various industries involved in the production of steel and chromium alloys, leather tanning, textile industries, and wood preservation for meeting the need of increasing population. Various types of Cr-containing waste are being generated
The commonly used methods for lowering/removal of Cr (VI) from effluents/wastewater are based on physical/chemical methods such as adsorption (activated carbon and renewable agricultural waste), ion exchange, membrane filtration, electrochemical methods, hydroxide precipitation, sulfide precipitation, and chelating precipitation, etc. The use of physical/chemical processes has numerous disadvantages such as recurring cost of consumables, high operation cost, and formation of toxic sludge requiring proper disposal in sanitary landfills. In light of this, there is an urgent need to develop sustainable treatment strategies, which might be less expensive than existing treatment options and do not produce toxic sludge/byproducts, which may need cost intensive handling processes. Additionally, a treatment option with possibility of recovery of metals for their reuse in process will make the treatment options more attractive for end users and will be more environmentally friendly. The treatment methods based on use of biological tools, such as plants and microorganisms, are attractive options being used. However, the accumulation of metals in plant and microbial biomass poses a problem in both extraction of metals of their safe disposal. The more appropriate options being conversion of the toxic form to less toxic form by reduction or precipitating out the metals from effluents or
This chapter outlines application of Cr (VI) in various industries, its environmental, and health impact as well as management of chromium contaminated wastewater using conventional, biological, and advanced methods. This chapter also serve as valuable source for scientist and researchers working in remediation of Cr (VI) present in industrial wastewater to protect our environment and ecosystem.
2. Chromium-based industries and pollution
Chromium finds its applications in many industries, especially in steel manufacturing, textile and dyes, leather, and chemical manufacture, such as chromated copper arsenate (CCA), used in wood preservation. Many of these industrial facilities use low-grade chromite ores for a variety of purposes such as producing pigments, polishing metal, preventing corrosion, synthesizing organic materials, tanning leather, and preserving wood as shown in Figure 1 [9]. The leather industry alone accounts for 40% of the worldwide usage. Chromium is used as a tanning agent for making leather durable and less susceptible to decomposition. Chromium is also used in the production of steel and chromium alloys to improve their hardening and corrosion resistance [10]. Chromium is used in wood preservation in the form of chromated copper arsenate (CCA) to impregnate wood products so that it can be protected from insects and termites [11]. The tetrahalides, CrF4, CrCl4, and CrBr4 of chromium are used as mordant and catalyst in dyeing processes of chrome dyes in textile industries. The manufacture/synthesis processes from abovementioned products may produce a large amount of solid wastes and effluents rich in different forms of chromium with other components with pollution potential. Large number of heavy metal contaminants, including Cr (VI),are produced from industries such as tannery processing, chromate preparation, metal preparation, production of the colors chrome and ferrochrome, and temper steel welding [12].
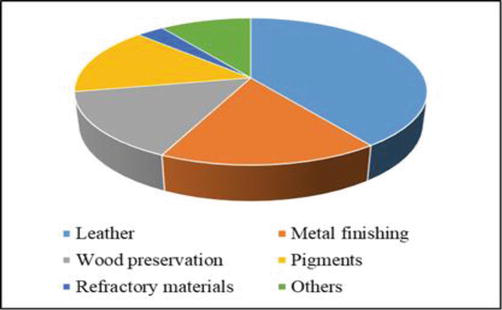
Figure 1.
Consumption of chromium in different industries.
The most important cause of chromium contamination in the environment is the release of untreated wastewater from various industries involved in either the production or utilization of chromium. Additionally, the disposal of toxic sludge from various industries and treatment plants can cause soil pollution, ultimately percolating to groundwater sources. Many of industries release varying amount of heavy metals found in their wastewater, which are released into the environment either directly or indirectly [13]. Due to the wide variations in the nature of these wastewaters depending on the type of industry, treatment of these wastewaters is a major topic of concern for achieving removal of heavy metals. In addition to this, leaching and weathering of chromite ores reserves can lead to major contamination of soil and water, causing environmental concern [14].
3. Impact of high level of chromium on environment and human health
In recent times, due to unrestricted discharge by point and nonpoint sources of varied industries, heavy metal, such as Cr (VI), caused irreparable damage to environment and animal/human health. The persistent release of Cr (VI) contaminated wastewater has led to its buildup in water and soil environment, causing adverse effects on flora and fauna. Some of these effects/impacts of Cr (VI) are discussed in the section below.
3.1 Cr (VI) impact on environment
Due to extensive use of Cr (VI) in many applications causes unprecedented damage to the environment. Recent studies have shown the accumulation of Cr (VI) beyond permissible limits in groundwater sources, wastewater effluents, and soil environments. In plants, Cr (VI) can be used for development, productivity, and metabolism [15]. However, plants exposed to higher concentrations of Cr (VI) had been reported for adverse effects on their physiology, morphology, and structural makeup. The nutrient, as well as water uptake mechanisms, may be affected, resulting in slowing down the growth and photosynthetic activity [16]. In literature, Wang et al., [17] studied that the exposure of maize plant to 300 mg/l of Cr (VI) as K2Cr2O7 can cause the alterations in leave morphology of maize plant. Pandey et al., [18] reported inhibition of electron transport in photosystem-I and photosystem-II in presence of high concentration of Cr (VI) in which plants system. Also, the higher concentrations of Cr (VI) can cause oxidative damage to DNA and membrane lipids, as well as chlorosis and necrosis in plants [19].
The accumulation of Cr (VI) in soil may affect the overall productivity of agricultural crops as it had been reported that 94% reduction in stem growth was evident in plants of 32 species exposed to 1000 mg/kg plant weight as compared to control plants without exposure to any Cr (VI) [20]. The Cr (VI) bioaccumulation had been reported in fish, aquatic plants, algae, and invertebrates leading to hazardous and toxic effects on overall growth and development [21]. It was reported that accumulation of Cr (VI) in aquatic system cause increase in Cr (VI) level in higher trophic levels of food chains due to the phenomenon of bioaccumulation. This can lead to more harmful consequences in terms of toxicity in higher trophic level of food chain. More importantly, the potential risk of Cr (VI) has to studied in more depth in order to evaluate its concern regarding wildlife and environmental damages [22].
3.2 Cr (VI) impact on human health
Cr (VI) is reported to be highly soluble in water as compared to Cr (III) form, which can lead to its absolute presence in water sources. Lower concentrations of Cr (VI) are necessary for number of bodily functions such as lowering blood cholesterol level by decreasing low-density lipoproteins (LDLs), maintaining blood sugar level and as chromium picolinate used in supplements for gaining muscle mass [23, 24]. However, at higher concentrations, it can lead to cytotoxic and genotoxic effects on animal cells. Accidental breathing of Cr can cause irritation in the lungs and nose. Further, it can lead to lung cancer, kidney dysfunction, asthma, ulcers, and diarrhea [25]. Acute exposure can lead to choking, wheezing, and shortness of breath, whereas chronic exposure can lead to development of ulcers and perforations in respiratory, gastrointestinal, bronchitis, septum parts, etc. In literature, Cr (VI) can cause damage to blood cells, causing kidney and liver failure and also can lead to toxicity in bloodstream due to its oxidative nature [26]. In light of the serious effects of environment and overall health of flora and fauna, including human beings due to high levels of different forms of chromium, there is an urgent need to develop environmentally friendly and sustainable treatment systems for wastes/effluents having their high levels. Hence, due to severe environmental damage and its harmful health effects, we should design protocols for the treatment of effluents containing Cr (VI) as a pollutant.
4. Conventional treatment technologies
Cr (VI) can be removed from wastewater using variety of methods, including physical/chemical and biological methods. Various physical/chemical methods, which can be used for the treatment of Cr (VI) containing wastewater are described below.
4.1 Physical methods
Various physical methods, such as adsorption, ion exchange, membrane filtration, and electrochemical removal, can be used for the removal of Cr (VI) from wastewater.
4.1.1 Adsorption
Adsorption method is a surface deposition based on phase transfer process to remove chromium from contaminated water. It is based on the principle of purification or sorption of metal onto the pores of the sorbent, which reduces the concentration of toxic metal ions from contaminated wastewater [27]. Adsorption process is simple to carry out and cost-effective for the treatment of wastewaters. For adsorption, various types of adsorbent materials can be used, such as activated carbon and agricultural waste, as mentioned below [28].
4.1.1.1 Activated carbon
The most widely used adsorbent materials for the removal of Cr (VI) from wastewater is activated carbon. Different materials, such as coconut shell, wood, and sawdust, can be used for making activated carbon [27]. Activated carbon can be classified into two forms, that is. powdered activated carbon (PAC) and granular activated carbon (GAC) [29]. Commercially available activated carbon includes: Filtrasorb-100, F-400 (commercially activated carbon) and GA-3 [30, 31]. The advantage of using activated carbon includes low price, high surface area (500 to 1500 m2/g), and easy operation as compared to conventional methods [27]. The high adsorption capacity of activated carbon depends on pH and temperature during the adsorption process. An increase in temperature was reported to decrease the adsorption capacity for the removal of chromium, whereas a low pH was reported to increase the adsorption capacity of activated carbon [32]. Moreover, activated carbon needs to be chemically activated to enhance its adsorption capacity and should be regenerated after each treatment cycle [27].
4.1.1.2 Use of agricultural waste
Agricultural waste or its byproducts can be a good option to remove Cr (VI) from wastewaters, for example, pine needles, cactus [33], timber, grain crops, peanut shells, hazelnut shell, bark, and tea leaves [34]. There are various mechanisms used in bio-sorption, such as chemisorption or complexation, with pores present on its surface due to adsorption and ion exchange [35]. The advantage of this method is that the adsorbent material is derived from low-cost agricultural waste, which can be used for the recovery of metals such, as Cr (VI) [27]. However, the main disadvantage of this technique is that plant material cannot be directly used without chemical pretreatments (using acid or base), for example, treatment of orange peels with calcium chloride (CaCl2) and sodium hydroxide (NaOH), can result in increased adsorption capacity because of the transformation of methyl esters as inhibiting groups to carboxylate ligands. As a result, the metal binding capability was greatly enhanced. The metal uptake can be increased by increasing number of binding sites, better ion-exchange ability, and formation of new functional groups. The application of these chemical pretreatments might increase the cost of treatment hindering its use for long-term treatment [34, 35, 36].
4.1.2 Ion exchange-based removal
Ion exchange methods are based on removal of chromium using a support having resins for ion exchange. The support materials used in ion exchange columns can include Dowex 2X4 [37], Ambersep 132 [38], solvent-impregnated resin with quaternary ammonium salt (aliquat 336), polyacrylate anion exchanger having strong base functional groups Amberlite IRA 458, and Amberlite IRA 958 [39]. The Cr (VI) containing wastewater was made to pass through resin bed under pressure, where chromium is removed by ion-exchange mechanism. When resin capacity is exhausted due to binding of chromium, the column is backwashed to remove trapped solids and then it can be regenerated using HCl [40]. Ion exchange method requires low maintenance. However, it is expensive, susceptible to fouling, and readily clogged by organic materials and other compounds found in wastewater. In general, pretreatment is required before using ion exchange resin on wastewater that contains significant quantities of suspended particles and salts, thereby limiting its application for the treatment of industrial wastewater having Cr (VI) as a pollutant [39]. The use of acid for the regeneration of ion exchange columns makes this process more expensive. Additionally, the release of this acid after the regeneration process from ion exchangers can cause secondary pollution [9, 40].
4.1.3 Membrane filtration
The fundamental component of membrane processes is the use of a semipermeable membrane to divide a solution into two distinct streams. The use of different membranes for the treatment of wastewater containing Cr (VI) determines the separation selectivity. There are many types of membranes, which can be used for the removal of Cr (VI) from wastewater such as polymeric, inorganic, and liquid membranes. The inorganic membranes are very costly, but they are chemically and thermally stable [27]. The polymer-based membrane filters made up of polyethersulphone with carboxymethyl sulphonate (CMC) as complexing agent was found to achieve 99.5% efficiency of 10 mg/l of Cr (VI). However, these polymer membranes generally face biofouling problem [41]. Liquid membranes have high diffusion rates, but they instable due membrane expansion and liquid loss during the removal of Cr (VI) [27]. The main disadvantage of using membrane filtration is its high cost and recovery process for the removal of Cr (VI) is not efficient.
4.1.4 Electrochemical methods
It is also referred to as electro-dissolution or electrocoagulation method. The combination of electrochemical and chemical reactions makes this method of choice to be used for the treatment Cr (VI) present in solid, liquid, and gaseous matters [42]. Electrocoagulation process uses two different electrodes namely: anode (sacrificial electrode) that is usually aluminum or iron and a cathode (liberates hydrogen gas) as shown in Figure 2 [43]. Electrochemical removal process has two stages: [1] Reduction of Cr (VI) to Cr (III) and [2] Precipitation as hydrated chromium complex. The electro-dissolution method involves dissolution of iron (anode) under strong acidic condition followed by reduction of Cr (VI) as shown in reactions of Figure 2 [44]. It is widely used in electroplating industry to treat industrial wastewater, but its efficiency is affected by the presence of other ion, especially salt ions and change in pH.
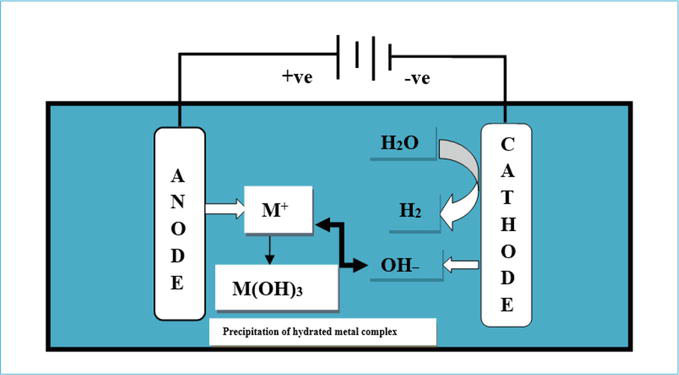
Figure 2.
Electrocoagulation of chromium.
Reaction mechanisms of chromium reduction in electrochemical cell are as follows:
4.2 Chemical precipitation methods
Chemical precipitation is used as a common method for the removal of chromium from industrial wastewater. Chemical precipitation is a relatively simple process in which different chemicals react with heavy metals to form insoluble precipitates. These precipitates can be separated from the water by sedimentation or filtration, followed by its decantation for appropriate discharge [45]. The main disadvantages of chemical precipitation include high operational and maintenance cost, energy input, manual oversight, and generation of large sludge [34]. Chemical treatment of wastewater containing heavy metals includes different methods as described below.
4.2.1 Hydroxide precipitation
Hydroxide precipitation is the most commonly used method among chemical precipitation. It is based on the principle that metal hydroxide complex, which can be formed by the addition of Ca (OH)2 or NaOH in wastewater containing heavy metals. These metal hydroxide complexes can be removed by the process of sedimentation or flocculation [46]. In literature, it was reported that the addition of 4% Ca (OH)2 achieved complete removal of 30 mg/l of Cr (VI) at a pH of 8.3 [47]. The advantage of using hydroxide precipitation is that it is simple and low-cost method used for the removal of heavy metals from industrial effluents. The disadvantages of hydroxide precipitation include generation of large amount of sludge due to amphoteric nature of hydroxides, and it can create problem in maintaining pH of the wastewater, which can inhibit metal hydroxide precipitation.
4.2.2 Sulfide precipitation
Sulfide precipitation is used for the removal of heavy metals from wastewater by the addition of iron sulfide or pyrite [48]. Sulfide precipitation is non-amphoteric in nature, so it does not require maintenance of pH for the removal of heavy metal. The main disadvantage of this process includes evolution of H2S fumes and formation of colloidal precipitates that cause separation problems [34].
4.2.3 Chemical precipitation with other methods
Chemical precipitation (i.e., hydroxide and sulfide precipitation) can be used with other methods, such as nano-filtration or with ion exchange method in order to overcome some of the limitations of hydroxide or sulfide precipitation method. Sulfide precipitation can be used with nano-filtration for the removal of heavy metals. Ion exchange method can be used in combination with chemical precipitation for the removal of heavy metals. But the combination of these methods can be expensive as acid used in regeneration of ion exchange columns can cause secondary pollution [41].
4.2.4 Chelating precipitation
This method uses chelating precipitation for the removal of heavy metals from wastewater. N-bis-(dithiocarboxy) piperazine (BDP) and 1,3,5- hexahydrotriazinedithiocarbamate (HTDC) were used for the removal of complex heavy metal from wastewater containing Ni2+, Cr6+ and Cu2+ [41]. Both BDP and HTDC can effectively lower the concentration of different heavy metals in wastewater to less than 0.5 mg/l. In literature, it was reported that the use of potassium ethyl xanthate (KEX) for the complete removal of 1000 mg/l of Cu2+ from wastewater [49]. All the above mentioned methods are conventionally used for the removal of Cr (VI) as large volumes can be treated in short time; however, they have several disadvantages. The use of physicochemical methods requires specific conditions for proper treatment and can be affected by interferences of other pollutants, which actually reduce their efficiency for proper removal of Cr (VI). Moreover, generation of higher quantities of sludge, high operational costs, and secondary pollution caused due to the release of chemicals/acids used in the treatment process make them environmentally unsustainable [50]. The various advantages and disadvantages of various physical/chemical methods are given in Table 1.
| Technique | Method | Advantages | Disadvantages |
|---|---|---|---|
| Physical methods | Adsorption |
|
|
| Ion exchange method |
|
| |
| Use of agricultural waste |
|
| |
| Membrane filtration |
|
| |
| Electrochemical methods |
|
| |
| Chemical precipitation | Hydroxide precipitation |
|
|
| Sulfide removal |
|
| |
| Combination of methods |
|
| |
| Chelating precipitation |
|
|
Table 1.
5. Bioremediation: an eco-friendly approach
The biological treatment for achieving removal/reduction of chromium from wastewater provides an environmentally sustainable alternative to conventional treatment system. The use of microorganisms, such as bacteria, fungi, and yeast to reduce the toxic hexavalent chromium at polluted site to relatively less-toxic trivalent chromium, is an attractive option. Different microbial species, in mixed or pure cultures, are being reported for wide array of enzymes, which can achieve reduction of Cr (VI) [8]. These microbes belong to both aerobic and anaerobic domains and can work effectively at interface of such environment, thus providing multitude of options for natural bioremediation processes. Different groups of microorganisms can be used for the removal of Cr (VI) from wastewater, and these include the following.
5.1 Bacteria
Bacteria are diverse group of microorganisms, which can grow/divide rapidly and possess array of different enzymes. As per various literature reports, bacterial cells and their enzymes can reduce Cr (VI) to Cr (III), which is a significantly less hazardous metal [51]. Cr (VI) is mutagenic and toxic to bacteria, but it was observed that gram-positive bacteria are more resistant to Cr (VI) than gram-negative bacteria because of their thick cell wall. In literature, it was reported that 10–12 mg/l of Cr (VI), inhibited most soil bacteria in liquid media, such as
The reduction of Cr (VI) can be carried by either pure culture or by mixed bacterial culture. There are various reports for the treatment of Cr (VI) by bacterial isolates but only few of them can perform under both aerobic and anaerobic conditions [54]. Most of the studies for the reduction of Cr (VI) were carried out using pure microbial culture. In literature, various bacterial isolates were reported for the reduction of Cr (VI), for example,
However, the efficiency of pure cultures under field-scale applications can be affected by their survival under extreme environmental conditions and establishing themselves in competition with other predominating bacterial species already adapted to such conditions [58]. Hence, mixed bacterial consortium might have a better chance to survive during field-scale studies as compared to pure culture [59]. The metabolic versatility of different groups of microorganisms can complement each other to achieve better cleanup efficiency as compared to that achieved by individual pure cultures [60]. Bacteria can use different mechanisms by which it can achieve removal of heavy metals such as bioaccumulation, bio-sorption, bio-leaching, bio-transformation based detoxification, or
5.2 Fungi
Fungi, mostly yeasts isolated from different chromium-contaminated sites, had been reported for their resistance to high levels of chromium (VI). This metal resistance was attributed to the presence of cell wall and production of different enzymes, which carry out extracellular and intracellular precipitation, redox reactions, and active intake into the cells [62]. In literature, Xu et al. [63] isolated
5.3 Algae
The photosynthetic organisms, such as cyanobacteria or algae, have a ubiquitous distribution, and there are reports regarding their tolerance or resistance of algae to Cr (VI) [67]. The resistance to Cr (VI) in algae was attributed to presence of polysaccharides, proteins, or lipids in their cell wall, which can act as binding agent of heavy metals as it contains carboxyl, sulfate, amino, and hydroxyl groups. Some studies suggested sequestration of Cr (VI) by its complexation with organic compounds in algal exudates/biomass [68]. Megharaj et al. [69] reported suppression of algal growth when it was exposed to 40 mg/kg of Cr (VI). Gupta and Rastogi [68] isolated
5.4 Cyanobacteria
Cyanobacteria also known as blue-green algae have been reported for affinity to heavy metals due to their higher extracellular mucilage content [72]. Garnham and Green [73] reported the accumulation of 10 nmoles of Cr (VI)/gm dry weight by unicellular non-nitrogen-fixing cyanobacterium
| Group | Culture name | Site of isolation | Reduction efficiency | Reference |
|---|---|---|---|---|
| Bacteria | Chromium-contaminated soil | 95% removal of 130 mg/l Cr (VI) | [75] | |
| Soil sample | 43.6% reduction of 500 mg/l Cr (VI) | [76] | ||
| Soil sample | 87.6% reduction of 500 mg/l Cr (VI) | [76] | ||
| Sediment sample | Complete reduction of 100 mg/l Cr (VI) | [77] | ||
| Mine tailings | 82% reduction of 100 mg/l Cr (VI) | [78] | ||
| Coalmine lake | Complete reduction of 200 mg/l Cr (VI) | [55] | ||
| Geothermal region | 93.71% reduction of 20 mg/l Cr (VI) | [79] | ||
| Soil under the chrome slag of a chromate plant | 100% reduction of 200 mg/l of Cr (VI) | [80] | ||
| Fungi | Tannery effluent | Complete removal of 10 mg/l Cr (VI) | [81] | |
| Soil and sludge sample | 73% reduction of 9.86 mg/l Cr (VI) | [82] | ||
| Soil sample | Able to reduce 2.7 mg/l of Cr (VI) | [83] | ||
| Soil sample | Able to reduce 8.35 mg/l of Cr (VI) | [83] | ||
| Air contaminated with industrial vapors | Complete reduction of 200 mg/l Cr (VI) | [64] | ||
| Electroplating water | 99.4% reduction of 50 mg/l Cr (VI) | [66] | ||
| Algae | National chemical laboratory | 98% bio-sorption of 5 mg/l Cr (VI) | [84] | |
| Tannery wastewater | Reduce 3.22 mg/l of Cr (VI) | [70] | ||
| Cyanobacteria | Metal contaminated soil | Complete bio-sorption of 20 mg/l Cr (VI) | [85] | |
| Metal contaminated soil | Complete bio-sorption of 20 mg/l Cr (VI) | [85] | ||
| Wetland | 50% removal of 15 mg/l Cr (VI) | [72] |
Table 2.
Bio-reduction of chromium by different groups of organisms.
6. Advanced removal methods
Recently, advanced approaches are developed with the goal of enhancing effectiveness and sustainability for the elimination of Cr (VI) from wastewater. These methods are essential for addressing the serious environmental and health issues associated with heavy metal pollution. A few of them are mentioned below.
6.1 Using nanotechnology approach
The field of nanotechnology can be used for the removal of heavy metals from industrial wastewaters. It is a very efficient method for the removal of heavy metals from wastewaters due to their stability and small size, large surface area, and easily accessible pore space [86]. In addition to this, due to their small size, they possess efficient Brownian motion properties, making them suspended in wastewater for removal of heavy metals, such as Cr (VI). The use of nanoparticles in conjunction with other adsorbent materials, such as ion exchange resin and zerovalent ions, can increase the bioremediation potential for the removal of heavy metals, such as Cr (VI), present in wastewaters [87, 88]. Currently, various types of nanomaterials can be used for the uptake of heavy metals, such as Cr (VI), present in wastewater such as metal-organic framework (MOFs), metal oxides, carbon materials, and chitosan. Nithiya et al. [89] reported 55.71 mg/g removal of Cr (VI) based on adsorption phenomenon using chitosan/silicagel-based nano-composites. Recently, carbon nano-tubes (CNTs) can be used for the removal of heavy metals from wastewater due to their unique hollow structure, outside surface area, and high activity due to fast transport of water. Further, the surface modification of CNTs with groups, such as hydroxyl and carboxylic acid, can increase solubility of CNTs in wastewater due to high hydrogen bonding capacity, ion exchange, and increasing functionalization using nanoparticles increasing affinity toward heavy metals [90, 91]. In literature, Lyu et al. [88] reported adsorption of 130 mg/g of Cr (VI) using a biochar-supported nanoscale iron sulfide (FeS) composite (CMC-FeS@biochar) having three components
6.2 Combined treatment technologies
Combined treatment methods include the use of two techniques to achieve the efficient removal of heavy metals, such as Cr (VI). The removal of chromium from wastewater various approaches such as chemical precipitation, adsorption, membrane filtration, biological treatment, electrocoagulation, and advanced oxidation processes [86, 92]. These methods can be used alone or in combination to effectively remove chromium depending on the specific characteristics of the wastewater and regulatory requirements. Certain systems are designed not solely for the purpose of chromium removal, but also with the intention of recycle it for reuse. Pilot-scale testing is typically done to determine the most suitable treatment approach for a given wastewater stream [13]. As electrochemical and biological treatment can be used in culmination for the removal of heavy metals. In literature, Suganthi et al. [93] reported the removal of color and chemical oxygen demand (COD) present in tannery wastewater by using hybrid membrane bioreactor having activated sludge and electrocoagulation. Hou et al. [94] reported 51.3% removal of overall Cr and 48.1% of hexavalent chromium using electrochemically operated bio-sorption framework followed by adsorption using
6.3 Use of synthetic biology tools for improving microbial potential
Synthetic biology tools use combination of molecular biology tools combining systems and cell biology to design/synthesize genes, enzymes, or transcription factors to redesign metabolic pathways for achieving targeted studies. Some of the methods, that is, microbiome engineering and synthetic biology are used nowadays to design synthetic microbes, specifically for achieving bioremediation are described in detail below.
6.3.1 Microbiome engineering
The interaction between microbial population and heavy metals is the basis of bioremediation of metal contaminated environments. The presence of diverse autochthonous microbial population can help in mitigation of heavy metals [96]. Microbiome includes different groups of micro-organisms capable to achieving bioremediation of heavy metals. The knowledge regarding microbiome of polluted environments and synthetic biology can pave ways for the removal of heavy metals from complex wastewater environments [97]. Nowadays, molecular biological tools (MBTs) can be used to identify contaminant degrading capabilities of microbiome present at polluted sites and further designing bioremediation protocols. The microbiome present in soil or wastewater environment can replenish the microenvironments using different methods such as immobilization, reduction of heavy metals to less toxic forms, and binding to cellular entities [98]. However, these activities depend upon efficiency of cultures present, nutrient sources, and physical and environmental factors. The activity of these autochthonous population can be increased by alteration in any of these factors. Also, manipulations of genes can increase siderophore, metallothionine production, and exo-polymer production increasing mitigation of heavy metals. In addition to this, efficient indigenous microbial culture can be used to achieve the desired bioremediation of wastewaters and soil environment [99].
6.3.2 Synthetic biology
The field of synthetic biology allows scientists to design and construct genetically engineered microorganisms capable of achieving reduction Cr (VI) to less toxic Cr (III). The use of interdisciplinary fields, such as biochemistry, genetic, and systems biology can plays a pivotal role in enhancing the efficiency of bioremediation processes using micro-organisms [100]. The field of biochemistry apprises various mechanisms by which microorganisms bind, sequester, or transform heavy metals and also focuses on the characteristics of various biomolecules, such as peptides or synthesis of metal-chelating compounds to achieve metal-binding proteins [92]. It can unravel various enzymes responsible for achieving reduction of heavy metals, such as Cr (VI). The knowledge regarding metabolic pathways and enzymes can be used to construct engineered microbes with enhanced abilities to reduce heavy metals to less toxic forms [101]. To achieve this, various genetic techniques such as gene knockout, overexpression or introduction of genes coding for metal-binding proteins, transporters proteins, efflux pumps, or enzymes can be employed to modify microorganisms for increased biotransformation capabilities [92, 100, 101, 102]. In literature, Xue et al. [103] engineered
In recent times, systems biology can help develop biosensors providing real-time monitoring to detect the presence of heavy metals in wastewater or polluted environments [105]. In literature, Adekunle et al. [106] described the use of floating microbial fuel cells (MFCs) as biosensors for the detecting the presence of copper (Cu) as low as 35–40 μg/L and other heavy metals present in aquatic environments. Systems biology can provide holistic understanding of complex biological processes, including microbial communities that can play an important role in bioremediation of heavy metals. It includes the identification of microbial species, their functions, and understanding complex interplay/interactions among themselves and environmental factors to optimize bioremediation processes [107]. Also, mathematical modeling and simulation can predict the behavior of microbial communities in response to changing environmental conditions to design and optimization of bioremediation strategies. By integrating experimental data with mathematical models, researchers can develop efficient strategies to remove chromium from wastewater. Systems biology can also be used to monitor the impact of chromium bioremediation on the surrounding environment [108]. The ongoing development of innovative technologies and interdisciplinary collaboration will continue to drive progress in the field of bioremediation.
7. Conclusion
Chromium is widely used in several industrial processes as an important component, which leads to its pervasive presence in wastewaters of industrial origin. Further, the release of these wastewaters into rivers and drains leads to heavy metal presence in water resources causing harm to inhabiting flora and fauna. This leads to increase in the presence of heavy metals pollution in the environment (water/soil). The environment, human health, and the ecosphere are all at risk when excessive levels of heavy metals are released into the air, water, and soil. This chapter discusses pollution related to Cr (VI), and its harmful effects on environment and human health. We have also highlighted the treatment/removal of Cr (VI) using various conventional techniques, having many drawbacks; in contrast, biological treatment offers better alternative for the treatment of Cr (VI) from industrial wastewaters. In bioremediation aspect, different microorganisms, such as bacteria, fungi, algae, and cyanobacteria, can use a variety of mechanisms, such as metal sequestration or reduction for the removal of Cr (VI). In addition to this, advanced removal methods, including nanotechnology, combined treatment technologies, and synthetic biology tools, such as microbiome engineering and systems biology, can be used as one of the efficient and sustainable method for the removal of heavy metals from wastewater. Hence, the removal of Cr (VI) from wastewater is an important need of time in order to prevent harmful effects on environment and human health.
References
- 1.
Kumar M, Saini HS. Reduction of hexavalent chromium (VI) by indigenous alkaliphilic and halotolerant microbacterium sp. M5: Comparative studies under growth and nongrowth conditions. Journal of Applied Microbiology. 2019;127 :1057-1068. DOI: 10.1111/jam.14366 - 2.
Irshad MA, Nawaz R, Wojciechowska E, Mohsin M, Nawrot N, Nasim I, et al. Application of nanomaterials for cadmium adsorption for sustainable treatment of wastewater: A review. Water, Air, & Soil Pollution. 2023; 234 :54. DOI: 10.1007/s11270-023-06064-7 - 3.
Bao Z, Feng H, Tu W, Li L, Li Q. Method and mechanism of chromium removal from soil: A systematic review. Environmental Science and Pollution Research. 2022; 29 :35501-35517. DOI: 10.1007/s11356-022-19452-z - 4.
Majumder S, Raghuvanshi S, Gupta S. Application of a hybrid biofilter column for the removal of Cr (VI) from aqueous solution using an indigenous bacterial strain Pseudomonas taiwanensis . Bioremediation Journal. 2016;20 :10-23. DOI: 10.1080/10889868.2015.1113923 - 5.
Chiu A, Shi XL, Lee WKP, Hill R, Wakeman TP, Katz A, et al. Review of chromium (VI) apoptosis, cell-cycle-arrest, and carcinogenesis. Journal of Environmental Science and Health. 2010; 28 :188-230. DOI: 10.1080/10590501.2010.504980 - 6.
Vikrant K, Kumar V, Vellingiri K, Kim KH. Nanomaterials for the abatement of cadmium (II) ions from water/wastewater. Nano Research. 2019; 12 :1489-1507. DOI: 10.1007/s12274-019-2309-8 - 7.
Chang J, Deng S, Liang Y, Chen J. Cr (VI) removal performance from aqueous solution by pseudomonas sp. strain DC-B3 isolated from mine soil: Characterization of both Cr (VI) bio-reduction and total Cr biosorption processes. Environmental Science and Pollution Research. 2019;26 :28135-28145. DOI: 10.1007/s11356-019-06017-w - 8.
Pradhan D, Sukla LB, Sawyer M, Rahman PK. Recent bio-reduction of hexavalent chromium in wastewater treatment: A review. Journal of Industrial and Engineering Chemistry. 2017; 55 :1-20. DOI: 10.1016/j.jiec.2017.06.040 - 9.
Bharagava RN, Mishra S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicology and Environmental Safety. 2018;147 :102-109. DOI: 10.1016/j.ecoenv.2017.08.040 - 10.
Robins KJ, Hooks DO, Rehm BHA, Ackerley DF. Escherichia coli NemA is an efficient chromate reductase that can be biologically immobilized to provide a cell free system for remediation of hexavalent chromium. PLoS One. 2013;8 :1-8. DOI: 10.1371/journal.pone.0059200 - 11.
Radivojevic S, Cooper PA. Extraction of hexavalent chromium from chromated copper arsenate treated wood under alkaline conditions. Environmental Science and Technology. 2008; 42 :3739-3744. DOI: 10.1021/es702885f - 12.
Kamaludeen SPB, Megharaj M, Juhasz AL, Sethunathan N, Naidu R. Chromium-microorganism interactions in soils: Remediation implications. Reviews of Environmental Contamination and Toxicology. 2003; 178 :93-164. DOI: doi.org/10.1007/0-387-21728-2_4 - 13.
Nur-E-Alam M, Mia MAS, Ahmad F, Rahman MM. An overview of chromium removal techniques from tannery effluent. Applied Water Science. 2020; 10 :205. DOI: 10.1007/s13201-020-01286-0 - 14.
Das P, Mishra S. Hexavalent chromium [Cr (VI)]: Yellow water pollution and its remediation. Sarovar Saurabh ENVIS Newsl Wetl Ecosyst. 2009; 5 :1-8 - 15.
Stambulska UY, Bayliak MM, Lushchak VI. Chromium (VI) toxicity in legume plants: Modulation effects of rhizobial symbiosis. BioMed Research International. 2018; 2018 :8031213. DOI: 10.1155/2018/8031213 - 16.
Plaza SM, ND L. The safety and efficacy of high-dose chromium. Alternative Medicine Review. 2002; 7 :218-235 - 17.
Wang ZX, Chen JQ , Chai LY, Yang ZH, Huang SH, Zheng Y. Environmental impact and site-specific human health risks of chromium in the vicinity of a ferro-alloy manufactory, China. Journal of Hazardous Materials. 2011; 190 :980-985. DOI: 10.1016/j.jhazmat.2011.04.039 - 18.
Pandey V, Dikshit V, Shyam R. Hexavalent chromium induced inhibition of photosynthetic electron transport in isolated spinach chloroplasts. In: Photosynthesis. London: IntechOpen; 2013 - 19.
Tumolo M, Ancona V, De Paola D, Losacco D, Campanale C, Massarelli C, et al. Chromium pollution in European water, sources, health risk, and remediation strategies: An overview. International Journal of Environmental Research and Public Health. 2020; 17 :5438. DOI: 10.3390/ijerph17155438 - 20.
Lukina AO, Boutin C, Rowland O, Carpenter DJ. Evaluating trivalent chromium toxicity on wild terrestrial and wetland plants. Chemosphere. 2016; 162 :355-364. DOI: 10.1016/j.chemosphere.2016.07.055 - 21.
Muthukumaravel K, Rajaraman P. A study on the toxicity of chromium on the histology of gill and liver of freshwater fish Labeo rohita . Journal of Pure and Applied Zoology. 2013;1 :122-126 - 22.
Speer RM, Wise SS, Croom-Perez TJ, Aboueissa AM, Martin-Bras M, Barandiaran M, et al. A comparison of particulate hexavalent chromium cytotoxicity and genotoxicity in human and leatherback sea turtle lung cells from a one environmental health perspective. Toxicology and Applied Pharmacology. 2019; 376 :70-81. DOI: 10.1016/j.taap.2019.05.013 - 23.
Cabral-Pinto MMS, Inácio M, Neves O, Almeida AA, Pinto E, Oliveiros B, et al. Human health risk assessment due to agricultural activities and crop consumption in the surroundings of an industrial area. Exposure and Health. 2020; 12 :629-640. DOI: 10.1007/s12403-019-00323-x - 24.
Mohan D, Rajput S, Singh VK, Steele PH, Pittman CU Jr. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. Journal of Hazardous Materials. 2011; 188 :319-333. DOI: 10.1016/j.jhazmat.2011.01.127 - 25.
Mohanty K, Jha M, Meikap BC, Biswas MN. Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. Chemical Engineering Science. 2005;60 :3049-3059. DOI: 10.1016/j.ces.2004.12.049 - 26.
Lewicki S, Zdanowski R, Krzyzowska M, Lewicka A, Debski B, Niemcewicz M, et al. The role of chromium III in the organism and its possible use in diabetes and obesity treatment. Annals of Agricultural and Environmental Medicine. 2014; 21 :331-335 - 27.
Owlad M, Aroua MK, Daud WAW, Baroutian S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water, Air, and Soil Pollution. 2009; 200 :59-77. DOI: 10.1007/s11270-008-9893-7 - 28.
Sujatha S, Sivarethinamohan R. A critical review of Cr (VI) ion effect on mankind and its amputation through adsorption by activated carbon. Materials Today: Proceedings. 2021; 37 :1158-1162. DOI: 10.1016/j.matpr.2020.06.351 - 29.
Narayanan NV, Ganesan M. Use of adsorption using granular activated carbon (GAC) for the enhancement of removal of chromium from synthetic wastewater by electrocoagulation. Journal of Hazardous Materials. 2009; 161 :575-580. DOI: 10.1016/j.jhazmat.2008.03.113 - 30.
Hamadi NK, Chen XD, Farid MM, Lu MGQ. Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chemical Engineering Journal. 2001; 4 :95-105. DOI: 10.1016/S1385-8947(01)00194-2 - 31.
Hu MJ, Wei YL, Wang YW, Lee JF. Immobilization of chromium (VI) with debris of aquatic plants. Bulletin of Environmental Contamination and Toxicology. 2003; 71 :840-847. DOI: 10.1007/s00128-003-0212-0 - 32.
Loghavi MM, Mohammadi-Manesh H, Eqra R. Material for lithium-ion batteries. Journal of Solid State Electrochemistry. 2019; 23 :2569-2578 - 33.
Dakiky M, Khamis M, Manassra A, Me’reb M. Selective adsorption of Cr (VI) in industrial adsorbents. Advances in Environmental Research. 2002; 6 :533-540 - 34.
Xia S, Song Z, Jeyakumar P, Shaheen SM, Rinklebe J, Ok YS, et al. A critical review on bioremediation technologies for Cr (VI)-contaminated soils and wastewater. Critical Reviews in Environmental Science and Technology. 2019; 49 :1027-1078. DOI: 10.1080/10643389.2018.1564526 - 35.
Gardea-Torresdey JL, Peralta-Videa JR, Montes M, Rosa GDL, Corral-Diaz B. Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: Impact on plant growth and uptake of nutritional elements. Bioresource Technology. 2004; 92 :229-235. DOI: 10.1016/j.biortech.2003.10.002 - 36.
Nguyen TLT, Hermansen JE, Mogensen L. Environmental performance of crop residues as an energy source for electricity production: The case of wheat straw in Denmark. Applied Energy. 2013; 104 :633-641. DOI: 10.1016/j.apenergy.2012.11.057 - 37.
Sapari N, Idris A, Hamid NHA. Total removal of heavy metal from mixed plating rinse wastewater. Desalination. 1996; 106 :419-422 - 38.
Lin SH, Kiang CD. Chromic acid recovery from waste acid solution by an ion exchange process: Equilibrium and column ion exchange modelling. Chemical Engineering Journal. 2003; 92 :193-199. DOI: 10.1016/S1385-8947(02)00140-7 - 39.
Kabay N, Arda M, Saha B, Streat M. Removal of Cr (VI) by solvent impregnated resins (SIR) containing aliquat 336. Reactive and Functional Polymers. 2003; 54 :103-115. DOI: 10.1016/S1381-5148(02)00186-4 - 40.
Jachuła J, Hubicki Z. Removal of Cr (VI) and As (V) ions from aqueous solutions by polyacrylate and polystyrene anion exchange resins. Applied Water Science. 2013; 3 :653-664. DOI: 10.1007/s13201-013-0110-5 - 41.
Fu F, Wang Q. Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management. 2010; 92 :407-418. DOI: 10.1016/j.jenvman.2010.11.011 - 42.
Lugo-Lugo V, Barrera-Díaz C, Ureña-Núñez F, Bilyeu B, Linares-Hernández I. Bio-sorption of Cr (III) and Fe (III) in single and binary systems onto pretreated orange peel. Journal of Environmental Management. 2012; 112 :120-127. DOI: 10.1016/j.jenvman.2012.07.009 - 43.
Emamjomeh MM, Sivakumar M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. Journal of Environmental Management. 2009; 90 :1663-1679. DOI: 10.1016/j.jenvman.2008.12.011 - 44.
Moussa DT, El-Naas MH, Nasser M, Al-Marri MJ. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. Journal of Environmental Management. 2017; 186 :24-41. DOI: 10.1016/j.jenvman.2016.10.032 - 45.
Ku Y, Jung IL. Photocatalytic reduction of Cr (VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Research. 2001; 35 :135-142. DOI: 10.1016/S0043-1354(00)00098-1 - 46.
Chen QY, Tyrer M, Hills CD, Yang XM, Carey P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Management. 2009; 29 :390-403. DOI: 10.1016/j.wasman.2008.01.019 - 47.
Mirbagheri SA, Hosseini SN. Pilot plant investigation on petrochemical wastewater treatment for the removal of copper and chromium with the objective of reuse. Desalination. 2005; 171 :85-93. DOI: 10.1016/j.desal.2004.03.022 - 48.
Özverdi A, Erdem M. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. Journal of Hazardous Materials. 2006; 137 :626-632. DOI: 10.1016/j.jhazmat.2006.02.051 - 49.
Chang YK, Chang JE, Lin TT, Hsu YM. Integrated copper-containing wastewater treatment using xanthate process. Journal of Hazardous Materials. 2002; 94 :89-99. DOI: 10.1016/S0304-3894(02)00060-2 - 50.
Su X, Kushima A, Halliday C, Zhou J, Li J, Alan T. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nature Communications. 2018; 9 :4701. DOI: 10.1038/s41467-018-07159-0 - 51.
Thatoi H, Das S, Mishra J, Rath BP, Das N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. Journal of Environmental Management. 2014; 146 :383-399. DOI: 10.1016/j.jenvman.2014.07.014 - 52.
Kang CH, Kwon YJ, So JS. Bioremediation of heavy metals by using bacterial mixtures. Ecological Engineering. 2016; 89 :64-69. DOI: 10.1016/j.ecoleng.2016.01.023 - 53.
Kang C, Wu P, Li L, Yu L, Ruan YLB, Gong B, et al. Cr (VI) reduction and Cr (III) immobilization by resting cells of Pseudomonas aeruginosa CCTCC AB93066: Spectroscopic, microscopic, and mass balance analysis. Environmental Science and Pollution Research. 2017;24 :5949-5963. DOI: 10.1007/s11356-016-8356-8 - 54.
Xu L, Luo M, Jiang C, Wei X, Kong P, Liang X, et al. In vitro reduction of hexavalent chromium by cytoplasmic fractions of Pannonibacter phragmitetus LSSE-09 under aerobic and anaerobic conditions. Applied Biochemistry and Biotechnology. 2012;166 :933-941. DOI: 10.1007/s12010-011-9481-y - 55.
Banerjee S, Misra A, Chaudhury S, Dam B. A bacillus strain TCL isolated from Jharia coalmine with remarkable stress responses, chromium reduction capability and bioremediation potential. Journal of Hazardous Materials. 2019;367 :215-223. DOI: 10.1016/j.jhazmat.2018.12.038 - 56.
Zeng Q , Hu Y, Yang Y, Hu L, Zhong H, He Z. Cell envelop is the key site for Cr (VI) reduction by Oceanobacillus oncorhynchi W4, a newly isolated Cr (VI) reducing bacterium. Journal of Hazardous Materials. 2019;368 :149-155. DOI: 10.1016/j.jhazmat.2019.01.031 - 57.
Sevak P, Pushkar B, Mazumdar S. Mechanistic evaluation of chromium bioremediation in Acinetobacter junii strain b2w: A proteomic approach. Journal of Environmental Management. 2019;328 :116978. DOI: 10.1016/j.jenvman.2022.116978 - 58.
Ma L, Xu J, Chen N, Li M, Feng C. Microbial reduction fate of chromium (Cr) in aqueous solution by mixed bacterial consortium. Ecotoxicology and Environmental Safety. 2019; 170 :763-770. DOI: 10.1016/j.ecoenv.2018.12.041 - 59.
Tekerlekopoulou AG, Tsiflikiotou M, Akritidou L, Viennas A, Tsiamis G, Pavlou S, et al. Modelling of biological Cr (VI) removal in draw-fill reactors using microorganisms in suspended and attached growth systems. Water Research. 2013; 47 :623-636. DOI: 10.1016/j.watres.2012.10.034 - 60.
Desai C, Jain K, Patel B, Madamwar D. Efficacy of bacterial consortium-AIE2 for contemporaneous Cr (VI) and azo dye bioremediation in batch and continuous bioreactor systems, monitoring steady-state bacterial dynamics using qPCR assays. Biodegradation. 2009; 20 :813-826. DOI: 10.1007/s10532-009-9269-8 - 61.
Singh R, Kumar A, Kirrolia A, Kumar R, Yadav N, Bishnoi NR, et al. Removal of sulphate, COD and Cr (VI) in simulated and real wastewater by sulphate reducing bacteria enrichment in small bioreactor and FTIR study. Bioresource Technology. 2010; 102 :677-682. DOI: 10.1016/j.biortech.2010.08.041 - 62.
Cárdenas-González JF, Acosta-Rodríguez I. Hexavalent chromium removal by a Paecilomyces sp. fungal strain isolated from environment. Bioinorganic Chemistry and Applications. 2010; 2010 :676243. DOI: 10.1155/2010/676243 - 63.
Xu X, Xia L, Chen W, Huang Q. Detoxification of hexavalent chromate by growing Paecilomyces lilacinus XLA. Environmental Pollution. 2017;225 :47-54. DOI: 10.1016/j.envpol.2017.03.039 - 64.
Long B, Ye B, Liu Q , Zhang S, Ye J, Zou L, et al. Characterization of Penicillium oxalicum SL2 isolated from indoor air and its application to the removal of hexavalent chromium. PLoS One. 2018;13 :0191484. DOI: 10.1371/journal.pone.0191484 - 65.
Igiehon NO, Babalola OO. Fungal bio-sorption potential of chromium in Norkrans liquid medium by shake flask technique. Journal of Basic Microbiology. 2019; 59 :62-73. DOI: 10.1002/jobm.201800011 - 66.
Kumar V, Dwivedi SK. Hexavalent chromium stress response, reduction capability and bioremediation potential of Trichoderma sp. isolated from electroplating wastewater. Ecotoxicology and Environmental Safety. 2019;185 :1-12. DOI: 10.1016/j.ecoenv.2019.109734 - 67.
Cui Y, Masud A, Aich N, Atkinson JD. Phenol and Cr (VI) removal using materials derived from harmful algal bloom biomass: Characterization and performance assessment for a biosorbent, a porous carbon, and Fe/C composites. Journal of Hazardous Materials. 2019; 368 :477-486. DOI: 10.1016/j.jhazmat.2019.01.075 - 68.
Gupta VK, Rastogi A. Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. Journal of Hazardous Materials. 2009;163 :396-402. DOI: 10.1016/j.jhazmat.2008.06.104 - 69.
Megharaj M, Avudainayagam S, Naidu R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Current Microbiology. 2003; 47 :0051-0054. DOI: 10.1007/s00284-002-3889-0 - 70.
Das C, Naseera K, Ram A, Meena RM, Ramaiah N. Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris . Journal of Applied Phycology. 2017;29 :235-243. DOI: 10.1007/s10811-016-0910-8 - 71.
Costa IG, Terra NM, Cardoso VL, Batista FR, Reis MH. Photoreduction of chromium (VI) in microstructured ceramic hollow fibers impregnated with titanium dioxide and coated with green algae Chlorella vulgaris . Journal of Hazardous Materials. 2019;379 :1-10. DOI: 10.1016/j.jhazmat.2019.120837 - 72.
Sen S, Dutta S, Guhathakurata S, Chakrabarty J, Nandi S, Dutta A. Removal of Cr (VI) using a cyanobacterial consortium and assessment of biofuel production. International Biodeterioration & Biodegradation. 2017; 119 :211-224. DOI: 10.1016/j.ibiod.2016.10.050 - 73.
Garnham GW, Green M. Chromate (VI) uptake by and interactions with cyanobacteria. Journal of Industrial Microbiology. 1995; 14 :247-251 - 74.
Parveen S, Khattar JIS, Singh DP. The cyanobacterium Synechocystis sp. PUPCCC 62: A potential candidate for biotransformation of Cr (VI) to Cr (III) in the presence of sulphate. Environmental Science and Pollution Research. 2015;22 :10661-10668. DOI: 10.1007/s11356-015-4260-x - 75.
Long D, Tang X, Cai K, Chen G, Chen L, Duan D, et al. Cr (VI) reduction by a potent novel alkaliphilic halotolerant strain Pseudochrobactrum saccharolyticum LY10. Journal of Hazardous Materials. 2013;256 :24-32. DOI: 10.1016/j.jhazmat.2013.04.020 - 76.
Ge S, Ge S, Zhou M, Dong X. Bioremediation of hexavalent chromate using permeabilized Brevibacterium sp. andStenotrophomonas sp. cells. Journal of Environmental Management. 2015;157 :54-59. DOI: 10.1016/j.jenvman.2015.04.011 - 77.
Ran ZHAO, Bi WANG, Cai QT, Li XX, Min LIU, Dong HU, et al. Bioremediation of hexavalent chromium pollution by Sporosarcina saromensis M52 isolated from offshore sediments in Xiamen, China. Biomedical and Environmental Sciences. 2016;29 :127-136. DOI: 10.3967/bes2016.014 - 78.
Nayak AK, Panda SS, Basu A, Dhal NK. Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain andVetiveria zizanioides L. International journal of Phytoremediation. 2018;20 :682-691. DOI: 10.1080/15226514.2017.1413332 - 79.
Yang W, Hong W, Huang Y, Li S, Li M, Zhong H, et al. Exploration on the Cr (VI) resistance mechanism of a novel thermophilic Cr (VI)-reducing bacteria Anoxybacillus flavithermus ABF1 isolated from Tengchong geothermal region, China. Environmental Microbiology Reports. 2022;14 :795-803. DOI: 10.1111/1758-2229.13070 - 80.
Gu Y, Chen X, Liu L, Wang S, Yu X, Jia Z, et al. Cr (VI)-bioremediation mechanism of a novel strain bacillus paramycoides Cr6 with the powerful ability to remove Cr (VI) from contaminated water. Journal of Hazardous Materials. 2023;455 :131519. DOI: 10.1016/j.jhazmat.2023.131519 - 81.
Guria MK, Guha AK, Bhattacharyya M. A green chemical approach for biotransformation of Cr (VI) to Cr (III), utilizing Fusarium sp. MMT1 and consequent structural alteration of cell morphology. Journal of environmental. Chemical Engineering. 2014;2 :424-433. DOI: 10.1016/j.jece.2014.01.016 - 82.
Sharma S, Malaviya P. Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecological Engineering. 2016; 91 :419-425. DOI: 10.1016/j.ecoleng.2016.03.005 - 83.
Chang F, Tian C, Liu S, Ni J. Discrepant hexavalent chromium tolerance and detoxification by two strains of Trichoderma asperellum with high homology. Chemical Engineering Journal. 2016;298 :75-81. DOI: 10.1016/j.cej.2016.04.023 - 84.
Mane PC, Bhosle AB. Bioremoval of some metals by living algae spirogyra sp. andSpirullina sp. from aqueous solution. International. Journal of Environmental Research. 2012;6 :571-576 - 85.
Anjana K, Kaushik A, Kiran B, Nisha R. Bio-sorption of Cr (VI) by immobilized biomass of two indigenous strains of cyanobacteria isolated from metal contaminated soil. Journal of Hazardous Materials. 2017; 148 :383-386. DOI: 10.1016/j.jhazmat.2007.02.051 - 86.
Mitra S, Sarkar A, Sen S. Removal of chromium from industrial effluents using nanotechnology: A review. Nanotechnology for Environmental Engineering. 2017; 2 :1-14. DOI: 10.1007/s41204-017-0022-y - 87.
Yu F, Wang L, Ma H, Pan Y. Zeolitic imidazolate framework-8 modified active carbon fiber as an efficient cathode in electro-Fenton for tetracycline degradation. Separation and Purification Technology. 2020; 237 :116342. DOI: 10.1016/j.seppur.2019.116342 - 88.
Lyu H, Tang J, Huang Y, Gai L, Zeng EY, Liber K, et al. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chemical Engineering Journal. 2017; 322 :516-524. DOI: 10.1016/j.cej.2017.04.058 - 89.
Nithya R, Gomathi T, Sudha PN, Venkatesan J, Anil S, Kim SK. Removal of Cr(VI) from aqueous solution using chitosan-g-poly(butyl acrylate)/silica gel nanocomposite. International Journal of Biological Macromolecules. 2016; 87 :545-554. DOI: 10.1016/j.ijbiomac.2016.02.076 - 90.
Jin Q , Dai M, Zhan X, Wang S, He Z. Carbon nanotubes and graphene composites used in Cr (VI) detection techniques: A review. Journal of Alloys and Compounds. 2022; 922 :166268 - 91.
Wang D, Wu C, Zong Z, Ye J, Wu Q , Li R, et al. Carbon nanotubes-based fuel cell for Cr (VI) removal and electricity generation. Langmuir. 2022; 38 :9021-9029. DOI: 10.1021/acs.langmuir.2c01472 - 92.
Igiri BE, Okoduwa SI, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. Journal of Toxicology. 2018; 2018 (1-16):2568038 DOI: 10.1155/2018/2568038 - 93.
Suganthi V, Mahalakshmi M, Balasubramanian B. Development of hybrid membrane bioreactor for tannery effluent treatment. Desalination. 2013; 309 :231-236. DOI: 10.1016/j.desal.2012.10.014 - 94.
Hou Y, Liu H, Zhao X, Qu J, Chen JP. Combination of electroreduction with biosorption for enhancement for removal of hexavalent chromium. Journal of Colloid and Interface Science. 2012; 385 :147-153. DOI: 10.1016/j.jcis.2012.05.056 - 95.
Moradi M, Moussavi G. Enhanced treatment of tannery wastewater using the electrocoagulation process combined with UVC/VUV photoreactor: Parametric and mechanistic evaluation. Chemical Engineering Journal. 2019; 358 :1038-1046. DOI: 10.1016/j.cej.2018.10.069 - 96.
Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environmental Monitoring and Assessment. 2019; 191 :1-21. DOI: 10.1007/s10661-019-7528-7 - 97.
Jiang S, Tang J, Rahimi S, Mijakovic I, Wei Y. Efficient treatment of industrial wastewater with microbiome and synthetic biology. Frontiers in Environmental Science. 2022; 10 :432 - 98.
Pande V, Pandey SC, Sati D, Bhatt P, Samant M. Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Frontiers in Microbiology. 2022; 13 :824084. DOI: 10.3389/fmicb.2022.824084 - 99.
Bhati R, Sreedharan SM, Rizvi A, Khan MS, Singh R. An insight into efflux-mediated arsenic resistance and biotransformation potential of Enterobacter cloacae RSC3 from arsenic polluted area. Indian Journal of Microbiology. 2022;62 :456-467. DOI: 10.1007/s12088-022-01028-7 - 100.
Jaiswal S, Singh DK, Shukla P. Gene editing and systems biology tools for pesticide bioremediation: A review. Frontiers in Microbiology. 2019; 10 :87. DOI: 10.3389/fmicb.2019.00087 - 101.
Ojuederie OB, Babalola OO. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. International Journal of Environmental Research and Public Health. 2017; 14 (12):1504. DOI: 10.3390/ijerph14121504 - 102.
Jung J, Lee SJ. Biochemical and biodiversity insights into heavy metal ion-responsive transcription regulators for synthetic biological heavy metal sensors. Journal of Microbiology and Biotechnology. 2019; 29 (10):1522-1542. DOI: 10.4014/jmb.1908.08002 - 103.
Xue Y, Qiu T, Sun Z, Liu F, Yu B. Mercury bioremediation by engineered pseudomonas putida KT2440 with adaptationally optimized biosecurity circuit. Environmental Microbiology. 2022;24 (7):3022-3036. DOI: 10.1111/1462-2920.16038 - 104.
Naz M, Benavides-Mendoza A, Tariq M, Zhou J, Wang J, Qi S, et al. CRISPR/Cas9 technology as an innovative approach to enhancing the phytoremediation: Concepts and implications. Journal of Environmental Management. 2022; 323 :116296. DOI: 10.1016/j.jenvman.2022.116296 - 105.
Dangi AK, Sharma B, Hill RT, Shukla P. Bioremediation through microbes: Systems biology and metabolic engineering approach. Critical Reviews in Biotechnology. 2019; 39 (1):79-98. DOI: 10.1080/07388551.2018.1500997 - 106.
Adekunle A, Rickwood C, Tartakovsky B. Online monitoring of heavy metal–related toxicity using flow-through and floating microbial fuel cell biosensors. Environmental Monitoring and Assessment. 2020; 192 :1-12. DOI: 10.1007/s10661-019-7850-0 - 107.
Rylott EL, Bruce NC. How synthetic biology can help bioremediation. Current Opinion in Chemical Biology. 2020; 58 :86-95. DOI: 10.1016/j.cbpa.2020.07.004 - 108.
Basu A, Ali SS, Hossain SS, Asif M. A review of the dynamic mathematical modeling of heavy metal removal with the biosorption process. PRO. 2022; 10 (6):1154. DOI: 10.3390/pr10061154