Abstract
Progressive spinal deformity is a significant comorbidity associated with MMC. It leads to loss of truncal height and causes sitting, breathing, eating, and urination difficulties due to increased chest and abdominal pressures. Spinal deformities associated with MMC can be divided into 2 major groups: predominantly neuromuscular kyphoscoliosis or lordoscoliosis and severe rigid kyphosis or sharp-angled kyphosis. Kyphoscoliosis is a common finding in patients with thoracolumbar myelodysplasia, whereas lordoscoliosis is more common in patients with cauda equina and conus medullaris dysplasia. Early surgical correction improves body balance and quality of life and helps reduce the aggressiveness of surgical intervention. The dual growing rod technique is safe and effective in cases of moderate neuromuscular kyphoscoliosis or lordoscoliosis at an early age. Kyphectomy is a challenging procedure with high rates of complications, especially skin problems, but in patients with significant rigid kyphosis, there are no viable alternative procedures.
Keywords
- myelomeningocele
- myeloschisis
- neural tube defects
- MMC-related kyphosis
- post MMC syndrome
- caudal regression
- myelocele
- post MMS-related kyphosis
- caudal regression syndrome
1. Introduction
Spinal deformities are frequently observed in myelomeningocele (MMC) patients. Scoliosis, for instance, manifests in approximately 94% of thoracolumbar MMC cases and encompasses around 52% of cases overall. The prevalence of kyphosis stands at 10–20%, while lumbar hyperlordosis occurs in 1.5% of cases.
Progressive spinal deformity stands out as a significant comorbidity associated with MMC. It leads to a reduction in trunk height, giving rise to challenges in sitting, breathing, eating, and urination due to heightened chest and abdominal pressures. These deformities are rooted in neuromuscular disruptions and vertebral anomalies. Spinal deformities linked with MMC can be classified into two major categories: primarily neuromuscular kyphoscoliosis or lordoscoliosis, and severe rigid kyphosis or sharply angled kyphosis. Kyphoscoliosis commonly presents in patients with thoracolumbar myelodysplasia, whereas lordoscoliosis is more prevalent among those with cauda equina and conus medullaris dysplasia.
Bracing for patients with MMC and spinal deformities often proves ineffective, potentially leading to rib deformation, diminished respiratory capacity, and neuropathic skin ulcers. In contrast, early surgical correction holds the promise of enhancing body balance, quality of life, and reducing the need for more aggressive surgical interventions.
The dual growing rod technique emerges as both safe and effective for cases involving moderate neuromuscular kyphoscoliosis or lordoscoliosis at an early age, although it is accompanied by a notable incidence of rod fractures. On the other hand, kyphectomy presents as a demanding procedure with elevated complication rates, particularly skin-related issues. However, for patients grappling with significant rigid kyphosis, viable alternative procedures remain scarce.
In the following account, we share our experiences working with patients harboring diverse types of MMC-related spinal deformities. This account centers on the prompt correction of deformities via the dual growing rod technique and instrumented fusion, sometimes involving vertebrectomy. Notably, only a handful of studies have outlined successful treatments for MMC-related spinal deformities and their long-term outcomes.
2. General information
The term “spinal dysraphia” or “spinal dysraphia syndrome” and its accompanying anatomical characteristics were initially introduced by the German pathologist F.D. von Recklinghausen in 1886. This groundbreaking terminology comprehensively encompassed all variations of both closed and open neural tube defects (NTDs). Fast-forward to the year 2000, when P. Tortori-Donati et al. further elucidated and systematically categorized the primary forms of spinal dysgraphia [1]. Newer classifications of NTDs refer to the basics of embryonic development in an attempt to separate these abnormalities depending on the stage of their occurrence (gastrulation, primary neurulation, junctional neurulation, and secondary neurulation) [2].
NTDs occur on average in 1 out of every 1000 newborns [3]. Among these cases, open forms are observed in an average of 4.7 out of every 100,000 newborns [4, 5].
Myelomeningocele (MMC), also referred to as an open neural tube defect (NTD), stands as the most prevalent form of neural tube abnormalities. An additional variant within the realm of open NTDs is myeloschisis. Myeloschisis, alongside the classical thoracolumbar and lumbar MMCs, arises during the primary neurulation phase due to disrupted apposition, fusion, and neuroectodermal disjunction that transpires in the third to fourth week of embryonic development. A segment of MMC cases, specifically those presenting as sacral forms below the S1 level, has recently been associated with disorders of secondary neurulation. This attribution stems from distinct clinical symptoms observed in these patients, setting them apart from the primary group [5]. MMC and myeloschisis represent the predominant presentation within the spectrum of NTDs, accounting for approximately 80% of all cases. Estimates place the prevalence of this type of NTDs between 3.4 and 4.6 cases per 10,000 live births, underscoring its significance in the realm of congenital anomalies [4, 6].
Eubanks J.D. and Cheruvu V.K. discuss the prevalence, which stands at 12% of the population. The majority of these cases manifest as asymptomatic forms, often detected incidentally during MRI and CT scans in patients [3]. The group of so-called closed NTSs is diverse and extensive, encompassing presumed gastulation defects (such as split cord malformations and neuroenteric cysts and tracts), primary neurulation defects (type 1 spinal lipomas, limited dorsal myeloschisis), junctional neurulation defects (junctional neural tube defect, type 2 spinal lipomas, and segmental spinal dysgeneses), and early and late secondary neurulation defects (type 3 and 4 lipomas, retained conus medullaris, sacral meningocele, dermal sinuses) [7]. While this embryonic classification certainly sparks debates and discussions, it presents a logical structure and holds practical utility.
All spinal deformities in patients with NTDs contribute to issues concerning respiration, digestion, and urination due to heightened intra-abdominal pressure and elevated position of the diaphragmatic dome.
One facet of comprehensive rehabilitation for patients affected by severe forms of spinal dysraphia involves managing and treating the manifestations of post-MMC syndrome. This syndrome embodies an array of pathological effects stemming from spinal lesions, materializing as deformities of varying forms, intensities, and intricate courses. As a result, patients exhibit distinct prognoses, necessitate tailored social support, and call for personalized surgical interventions. Our endeavor has been to outline the fundamental principles for managing such treatments.
3. General terminology
See Table 1.
| Vertebral syndrome | A compilation of clinical and radiological symptoms that define the anatomical condition (structure, shape) and functional alterations in the spine, spinal canal, and spinal cord |
| Vertebrogenic syndrome | A compilation of intricate clinical and neurological (motor, sensory, visceral, autonomic), positional (postural), and radiological symptoms, originating from pathogenetic alterations in the anatomy and functionality of the spine and spinal cord |
| Consequences of MMC and mieloshysis (post-myelomeningocele syndrome, post-MMC syndrome) | A compilation of comprehensive clinical (orthopedic, neurological, adaptive) and radiological symptoms that depict the aftermath of surgical interventions for diverse forms of ONTDs |
| Dysraphic syndrome (Synonym: Bremer syndrome, spinal dysraphia syndrome, dysraphic complex (status), dysraphic myelodysplasia, dysraphia, Fuchs myelodysraphia) | The overarching term for developmental anomalies distinguished by the absence of fusion among anatomical structures along the body’s midline |
| Spinal dysraphia (vertebral component of Bremer’s syndrome) | A term employed to describe the incomplete fusion or absence of fusion of midline structures, including vertebrae, spinal canal, and spinal cord |
Table 1.
4. Nosological groups according to ICD-11
LA02 Spina bifida.
LA02.0 Spina bifida cystica.
LA02.00 Myelomeningocele with hydrocephalus.
LA02.01 Myelomeningocele without hydrocephalus.
LA02.02 Myelocystocele.
LA02.0Y Other specified spina bifida cystica.
LA02.0Z Spina bifida cystica, unspecified.
LA02.1 Spina bifida aperta.
LA02.Y Other specified spina bifida.
LA02.Z Spina bifida, unspecified.
LA07 Structural developmental anomalies of the neurenteric canal, spinal cord or vertebral column.
LA07.0 Primary tethered cord syndrome.
LA07.1 Diastematomyelia.
LA07.2 Amyelia.
LA07.3 Primary syringomyelia or hydromyelia.
LA07.4 Arnold-Chiari malformation type I.
LB73.0 Occult spinal dysraphism.
LA07.Y Other specified structural developmental anomalies of the neurenteric canal, spinal cord, or vertebral column.
LA07.Z Structural developmental anomalies of the neurenteric canal, spinal cord, or vertebral column, unspecified (Table 2).
| Orthopedic |
|
| Neurological and neurosurgical |
|
| X-ray |
|
| Other clinical manifestations |
|
5. Clinical and radiation features of vertebral syndrome in ONTDs
Absence of posterior vertebral structures (spina bifida) [8, 11, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34]
Broadened vertebral canal
Flattening of vertebral bodies
Reduced bone density
Hypoplasia of the sacrum and pelvis or sacral agenesis
Scar tissue changes at the apex of the deformity
Soft tissue deficiency, leading to pressure ulcers at the apex of the deformity
Predominance of combined kyphosis due to the interplay of congenital anomalies and neurogenic factors
Pelvic rotation
Tethered spinal cord and myelodysplasia (paresis)
Resistance to conservative treatment methods
6. Spinal deformities in closed NTDs
The prevalence of congenital vertebral anomalies or scoliosis varies according to the type of closed NTD. For instance, in cases of split cord malformations (SCM), disturbances in spinal segmentation (like hemivertebra, butterfly vertebra, and unsegmented bar) are frequently encountered (Figure 1) [35, 36]. This occurrence may be attributed to the likelihood that these anomalies originate during the early phases of embryonic development.
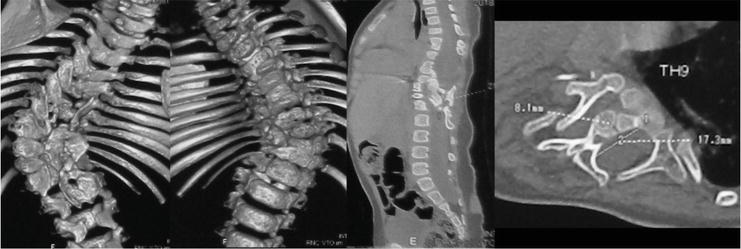
Figure 1.
CT scan of the thoracic and lumbar spine of a 17-month-old child with multiply segmentation disorders, split cord malformation type 1 at the level of Th11-L1.
Segmental spinal dysgenesis falls under disruptions of the junctional neurulation stage and is characterized by localized spinal cord thinning, with the underlying conus medullaris and segmental vertebral agenesis in the thoracolumbar transition area—typically without associated anomalies (Figure 2). In spinal cord lipomas, vertebral segmentation irregularities are rarely encountered, usually presenting with spina bifida only. Notably, the Сurrarino syndrome holds a distinct position, where developmental anomalies of the caudal spinal cord region (such as filum terminalis lipoma) coincide with agenesis of the lower sacrum (Figure 3).
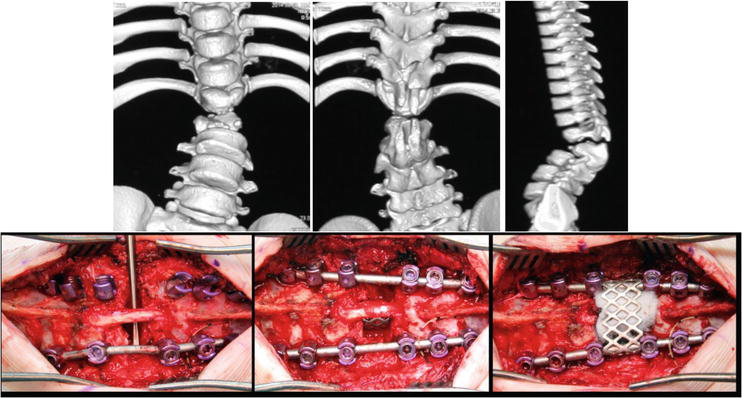
Figure 2.
CT scan and surgical treatment of the spine of a 6-year-old child with segmental spinal dysgenesis.
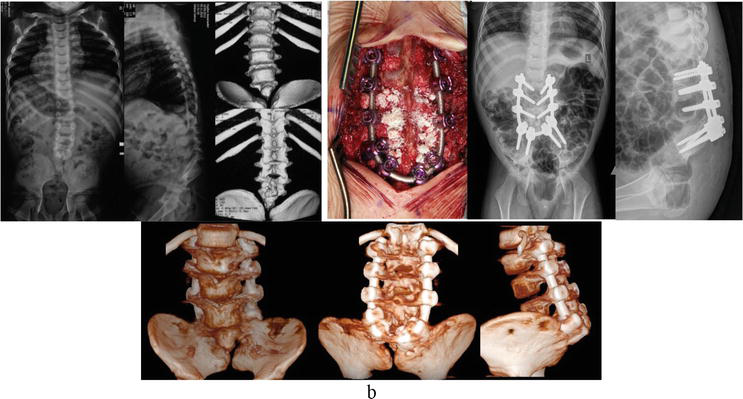
Figure 3.
Surgical management for lumbo-sacral agenesis.
7. Spinal deformities in open NTDS
Spinal deformities resulting in ONTDs are not uncommon; however, the degree of deformity aligns with both the extent of the dysraphic level and the concurrent spinal anomalies. In the cervical spine, open NTDs are situated in 2–5% of cases; within the thoracic region, the prevalence is around 2–3%; in the lumbar region, it accounts for 25%; and in the lumbosacral area, the majority, comprising 65–70%, of open NTDs are localized [1, 4, 8, 9]. The neurological level of the lesion stands as a pivotal factor that impacts mobility, functional-motor capacity, the development of secondary orthopedic complications, their treatment, overall outcome, and eventual prognosis.
Spinal deformities are prevalent among individuals with open NTDs. Scoliosis and lordoscoliosis are observed in 52% of cases and in 94% of patients with thoracolumbar open NTDs. The occurrence of kyphosis stands at 20%, while lumbar hyperlordosis is encountered in 1.5% of cases [6, 11, 14, 21, 26, 37]. These deformities can be instigated by a denervation (paralytic) component, vertebral anomalies, or frequently, a combination of both [15, 21, 22, 29, 37, 38, 39, 40, 41, 42]. Spinal deformities linked to open neural tube defects (NTDs) can be categorized into two major groups: predominantly neuromuscular kypho- or lordoscoliosis (Figure 4a and b), and distinct rigid kyphosis or acute-angled kyphosis (Figure 4c). The kyphoscoliotic variant of deformity is more prevalent among patients with thoracolumbar myelodysplasia, while lordoscoliosis is commonly observed in lower (lumbar and sacral) ONTDs.
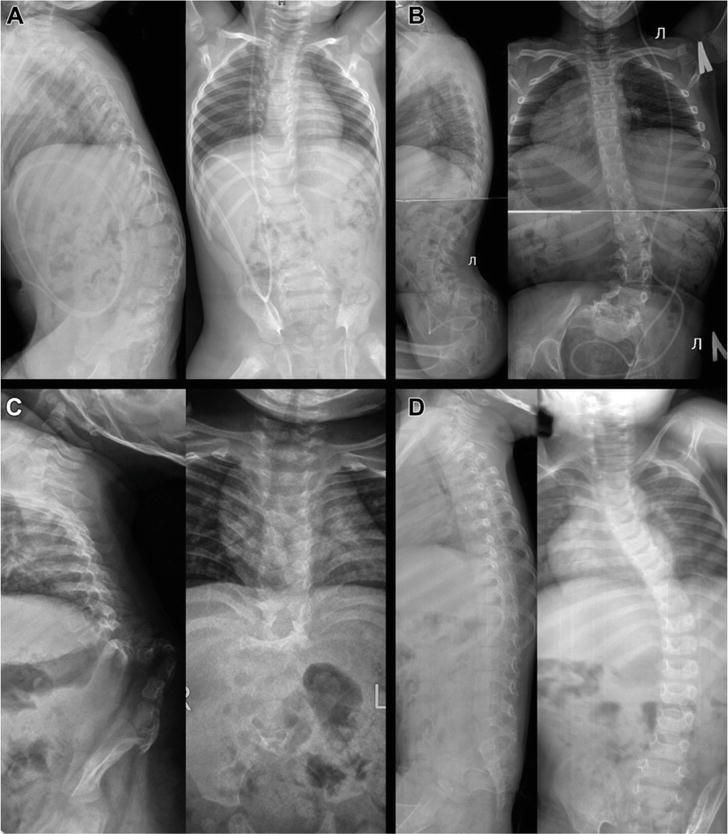
Figure 4.
Lateral and anteroposterior radiographs of four patients showcasing diverse types of spinal deformities associated with myelomeningocele: (A) kyphoscoliosis; (B) lordoscoliosis; (C) pronounced lumbar kyphosis; (D) scoliosis and “flatback” spine.
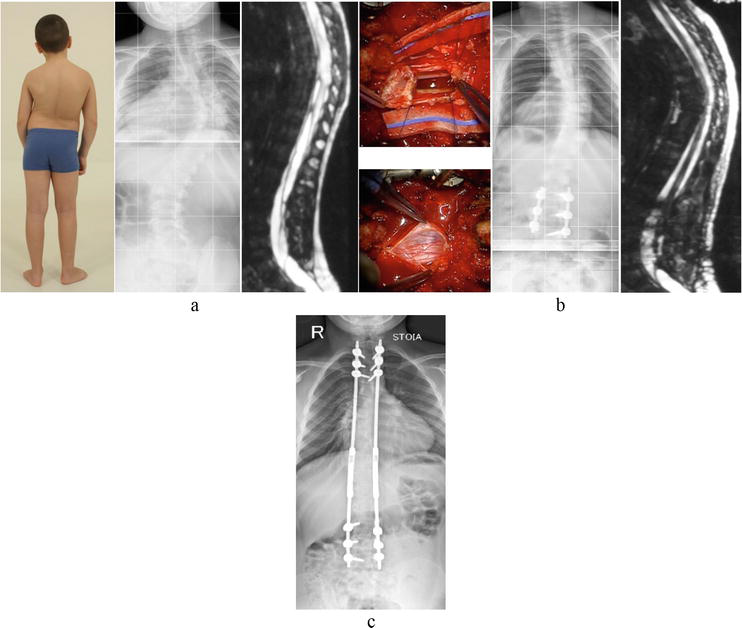
Figure 5.
Staged approach to treating progressive scoliosis in a 9-years-old boy with retained conus medullaris, tethered spinal cord syndrome, syringomyelia, and lower limb paraparesis: (a) visual depiction, spinal radiography, and MRI, T2-weighted sagittal scan prior to surgery. (b) Intraoperative wound photograph, spine radiography, and MRI, T2-weighted sagittal scan after spinal cord untethering, and placement of phantom screws. (c) Follow-up X-ray of the spine during the second treatment phase – Multistage deformity correction.
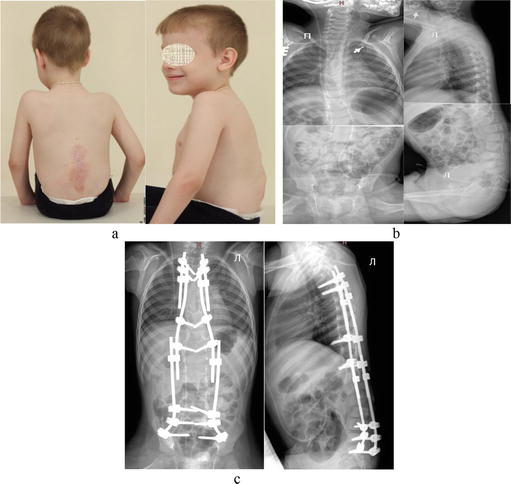
Figure 6.
Illustrations of treatment for mobile spinal deformities in patient with post-MMC deformity. (a) Depiction of a 5-year-old child’s appearance; (b) spine X-ray before surgery; (c) spine X-ray after the implantation of dual growing rod system.
Progression of the deformity is a characteristic feature of open NTDs, resulting in a decrease in body height, compromised upright posture, and challenges with respiration, digestion, and urination due to heightened intra-abdominal pressure and elevated position of the diaphragmatic dome [18].
Almost always, with kyphotic deformity, the vertebrae at the apex of the deformity are wedge-shaped, and in severe forms of acute-angled kyphosis with ulcer at the apex, there may be a complete absence of one or two vertebrae.
Neuromuscular deformities affecting the hips, ankles, and feet in patients with open NTDs commonly present as contractures, subluxations, or dislocations. These deformities can contribute to, or exacerbate, pelvic tilt and spinal misalignment. Furthermore, patients with open NTDs frequently experience torsional deformities in the lower extremities, impacting the femur and/or lower legs.
Objectives of surgical intervention for spinal deformities in open NTDs [42, 43, 44].
Rectification of deformity and reestablishment of trunk equilibrium
Restoration of upright stance capability
Enhancement of lung capacity (SAL) and abdominal space
Diminished stress on the spinal cord in fixed spinal cord syndrome (through kyphosis correction with shortening osteotomy)
Amplification of manual dexterity
Addressing and thwarting pressure ulcers at the apex
Augmenting urinary passage by alleviating intra-abdominal pressure
Advancing patient care
Elevating the quality of life for patients and their caregivers
Extending life expectancy
SeeTable 3.
| Characteristics of spinal deformity | Features of orthopedic correction |
|---|---|
| Mobile scoliosis, lordoscoliosis, and kyphoscoliosis without asymmetrical vertebral segmentation disorders (Figures 5 and 6) [21] | Growth-friendly systems (dual growing rods, VEPTR) for Risser 0–3 [16, 21, 22, 23, 24, 38] Deformity correction with Schwab I-II osteotomy for Risser 4 Closely monitor fixed spinal cord syndrome |
| Rigid deformities with congenital component—hemivertebra, asymmetrical butterfly vertebrae, asymmetrical unsegmented bar, acute-angle kyphosis, no depend on Risser (Figure 7) [21] | Vertebrectomy Schwab III-VI + short posterior spine fusion [15, 21, 22, 23, 24, 30, 37] Indirect treatment of spinal cord fixation by shortening the length of the spinal column |
| Deformities complicated by infected bedsores (usually at the apex of kyphosis) (Figure 8) [21, 36, 45] | Two-stage surgical treatment: Stage 1—external temporary fixation (halo-pelvic, transpedicular-pelvic) for stage distraction; Stage 2—kyphectomy with posterior spine fusion [21, 36, 45]. |
Table 3.
Options for surgical correction of open NTDs-related deformities, taking into account the characteristics of the pathology of the spine.
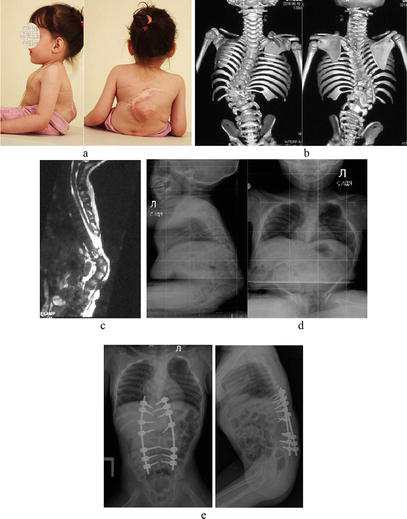
Figure 7.
Correction of a rigid spinal deformity in an 18-month-old child with post-myeloshysis severe kyphosis. (a) Child’s preoperative appearance, (b) CT scan, and (c) MRI before surgery; (d) intraoperative photographs; (e) spinal X-ray after surgery.
Important Note: Employing spinal-pelvic fixation for correcting post-open NTDs deformities enhances spinal stability. Nevertheless, in younger children, it could potentially correlate with the incomplete maturation of supporting bone structures due to their diminutive size, spatial arrangement, and strength. Consequently, the decision to extend spinal-pelvic fixation may be deferred from the primary intervention to a subsequent phase during ongoing child monitoring (refer to the “Complications and Prevention” section for further details).
8. Constraints on the surgical correction of spinal deformities within the context of spinal dysraphia syndrome
The primary objectives of surgical rehabilitation for patients with spinal deformities stemming from spinal dysraphia syndrome encompass the enhancement of the child’s quality of life, facilitation of social integration, and the enablement of proper care. Consequently, surgical intervention may be contraindicated under the following circumstances:
Severe, decompensated comorbidities, including genetic disorders and congenital malformations that impose significant limitations on the anticipated lifespan.
Concurrent brain pathology coupled with profound cognitive impairment and/or recurrent episodes of convulsive seizures.
Ongoing infectious processes.
See Table 4.
| Complication | Frequency (%) | Prevention methods | Treatment |
|---|---|---|---|
| Wound failure | 10 | Precise closure of the wound involves delicately aligning the edges without imposing tension, and whenever feasible, considering scar excision | If conservative treatment is ineffective and there is a risk of construct exposure, soft tissue reconstruction involving a plastic surgeon is considered |
| Implant-related complications (more often—dislocation of hooks and screws) | 15 | Thorough preoperative strategizing. Thorough evaluation of the dimensions of the underlying bones relevant to their respective structural components. Comprehensive evaluation of the strength of supporting bones, with particular emphasis on the caudal elements such as the sacrum and pelvis. Strategizing for pivotal surgical interventions | Re-do surgery |
| Surgical site infection | 5 | Preoperative rehabilitation of foci of chronic infection (primarily urinary). Active drainage of the wound, evacuation of hematoma and serous contents. Adequate antibiotic therapy. Prevention of bedsores. | Early re-do surgery |
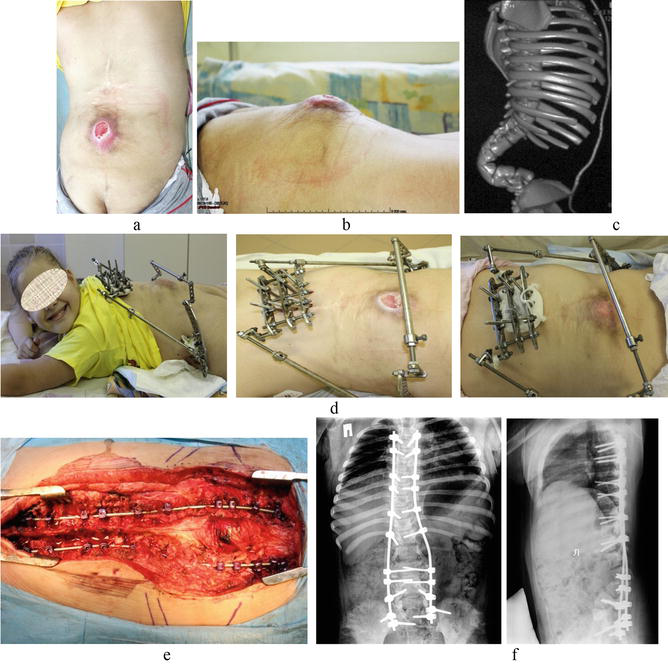
Figure 8.
Staged surgical treatment of post-myeloshysis spinal deformity complicated by recurrent bedsore in a 9-year-old child. Appearance of the child (a, b) and CT scan of the spine (d) before the surgical treatment; (c) appearance at the stages of treatment in the external fixation frame; (e) intraoperative photos; (f) X-rays of the spine after kyphectomy with posterior spinal-pelvic fixation.
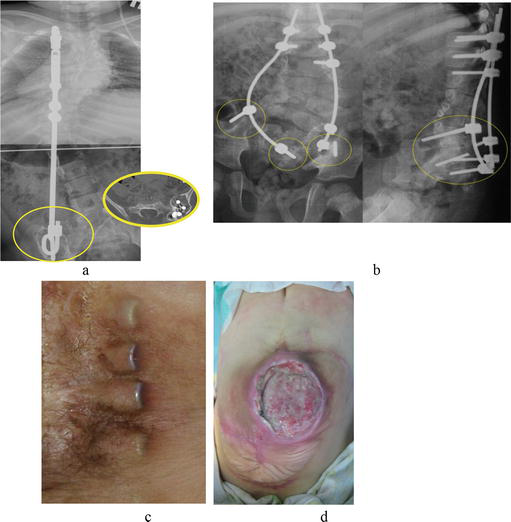
Figure 9.
Complication variants: (a, b) instability of the construction, bone resorbtion; (c) emergence of an “internal pressure sore” due to tissue deficiency; and (d) infection of a pressure sore at the apex of kyphosis.
9. Treatment of segmental spinal dysgenesis
Segmental Spinal Dysgenesis (SSD) is a rare congenital spinal anomaly characterized by localized malformations in the spine’s development. It primarily affects the thoracolumbar region and is often accompanied by neurological deficits. SSD involves incomplete formation or absence of vertebral structures, often leading to varying degrees of spinal cord tethering.
The etiology of SSD remains unclear, with theories encompassing vascular disruption, mechanical stress, and teratogenic influences during embryogenesis. Clinical manifestations vary widely, including spinal deformities, neurological deficits, and musculoskeletal abnormalities. SSD can be diagnosed through radiographic imaging and MRI scans, which reveal vertebral abnormalities and associated spinal cord changes [51].
Surgical intervention is often considered to address neurological deficits and prevent deformity progression. However, SSD poses challenges due to the complex anatomical alterations. Long-term outcomes vary, and multidisciplinary management involving orthopedic surgeons, neurosurgeons, and rehabilitation specialists is crucial for optimizing patient care.
The main principle of treatment of SSD involves the removal of rudimentary vertebral elements at the level of dysgenesis, spinal mobilization, posterior instrumental fixation, and anterior spondylodesis (Figure 2) [52].
10. Surgical treatment of sacral agenesis
Sacral agenesis is a rare congenital anomaly characterized by the incomplete or absent development of the sacrum, a crucial component of the spine and pelvic structure. This condition arises during embryonic development, often leading to a range of musculoskeletal, neurological, and genitourinary complications. Sacral agenesis may present as a spectrum of severity, with partial agenesis involving varying degrees of sacral vertebrae absence.
Clinically significant forms of sacral agenesis include lumbo-sacral agenesis (Figure 3), complete sacral agenesis, and hemisacrum. Lower sacral agenesis (below S1) typically does not require surgical treatment.
11. Conclusions
Progressive spinal deformities are prevalent among patients with open NTDs. The underlying causes of these deformities stem from disturbances in neuromuscular functions and abnormalities in vertebral development. Spinal deformities linked with open NTDs can be broadly categorized into two main groups: predominantly neuromuscular kyphoscoliosis or lordoscoliosis, and severe rigid kyphosis or sharp-angled kyphosis. While kyphoscoliosis is frequently observed in patients with thoracolumbar myelodysplasia, lordoscoliosis is more prevalent in those with low form of MMC.
Timely surgical correction plays a pivotal role in restoring body balance and improving the overall quality of life, thereby mitigating the need for aggressive surgical interventions. The dual growing rod technique offers a safe and effective option for moderate neuromuscular kyphoscoliosis or lordoscoliosis in young patients. Kyphectomy, though challenging and linked to significant complication rates, remains a vital intervention for patients with substantial rigid kyphosis when alternative procedures are limited.
Surgical indications span a spectrum of therapeutic, social, and functional factors. These include preventing and treating pressure sores at the apex of kyphosis, facilitating child care, and ensuring stable positioning during verticalization. The intricacies of spinal pathology within the realm of spinal dysraphia syndrome necessitate personalized surgical planning and the collaboration of a multidisciplinary team. Individualized correction methods, often planned in stages, need to factor in the elevated risk of complications stemming from the diverse range of spinal anomalies.
References
- 1.
Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: A review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology. 2000; 42 :471-491 - 2.
Morota N, Sakamoto H. Surgery for spina bifida occulta: Spinal lipoma and tethered spinal cord. Child’s Nervous System. 2023; 10 :2847-2864. DOI: 10.1007/s00381-023-06024-w - 3.
Cohen E et al. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012; 130 (6):e1463-e1470 - 4.
Eubanks JD, Cheruvu VK. Prevelance of sacral spina bifida occulta and its relationship to age, sex, race, and the sacral table angle: An anatomic osteologic study of three thousand one hundred specimens. Spine. 2009; 15 :1539-1543 - 5.
Lee JY, Kim JW, Shim Y, Kim SP, Kim KH, Yang J, et al. Myelomeningocele as an anomaly of secondary neurulation. Child’s Nervous System. 2022; 38 (11):2091-2099. DOI: 10.1007/s00381-022-05591-8. Epub 2022 Jul 12. Erratum in: Childs Nerv Syst. 2023 Feb;39(2):561-562 - 6.
Müller EB, Nordwall A. Prevalence of scoliosis in children with myelomeningocele in Western Sweden. Spine (Phila Pa 1976). 1992; 17 :1097-1102 - 7.
Blount JP, George TM, Koueik J, Iskandar BJ. Concepts in the neurosurgical care of patients with spinal neural tube defects: An embryologic approach. Birth Defects Research. 2019; 111 (19):1564-1576. DOI: 10.1002/bdr2.1588. Epub 2019 Oct 2 - 8.
Lindseth RE. Myelomeningocele spine. The Pediatric Spine: Principles and Practice. 1994; 2 :1043-1067 - 9.
Siegel MJ. Ultrassonografia Pediatrica/ Guanabara Koogan. 3rd ed. Brazil: Rio de Janeiro; 2003 - 10.
McDonnell GV, McCann JP. Issues of medical management in adults with spina bifida. Child’s Nervous System. 2000; 16 :222-227 - 11.
Trivedi J, Thomson JD, Slakey JB, Banta JV, Jones PW. Clinical and radiographic predictors of scoliosis in patients with myelomeningocele. The Journal of Bone and Joint Surgery. American Volume. 2002; 84-A :1389-1394 - 12.
Kayaba H, Hebiguchi T, Itoh Y, Yoshino H, Mizuno M, Morii M, et al. Evaluation of anorectal function in patients with tethered cord syndrome: Saline enema test and fecoflowmetry. Journal of Neurosurgery: Spine. 2003; 98 (3):251-257 - 13.
Yamada S, Won DJ, Yamada SM. Pathophysiology of tethered cord syndrome: Correlation with symptomatology. Neurosurgical Focus. 2004; 16 (2):1-5 - 14.
Duddy JC, Caird J, Connolly P. Repair of a large thoracolumbar myelomeningocele with associated lumbar kyphosis. Acta Neurochirurgica. 2013; 155 :1965-1968 - 15.
Dunn RN, Bomela LN. Kyphectomy in children with severe myelomeningocele-related kyphosis. Spine Deform. 2016; 4 :230-236 - 16.
Schroeder JE, Barzilay Y, Hasharoni A, Kaplan L. Long-term outcome of surgical correction of congenital kyphosis in patients with myelomeningocele (MMC) with segmental spino-pelvic fixation. Evidence-Based Spine-Care Journal. 2011; 2 :17-22 - 17.
Eysel P et al. Development of scoliosis in myelomeningocele. Differences in the history caused by idiopathic pattern. Neurosurgical Review. 1993; 16 (4):301-306 - 18.
Guille JT et al. Congenital and developmental deformities of the spine in children with myelomeningocele. Journal of the American Academy of Orthopaedic Surgeons. 2006; 14 (5):294-302 - 19.
Carstens C et al. Development of pathological lumbar kyphosis in myelomeningocele. Bone & Joint Journal. 1996; 78 (6):945-950 - 20.
Park TS et al. Progressive spasticity and scoliosis in children with myelomeningocele: Radiological investigation and surgical treatment. Journal of Neurosurgery. 1985; 62 (3):367-375 - 21.
Ryabykh SO, Pavlova OM, Savin DM, Burcev AV, Gubin AV. Surgical management of myelomeningocelerelated spinal deformities. World Neurosurgery. 2018; 112 :e431-e441. DOI: 10.1016/j.wneu.2018.01.058 - 22.
Ko AL, Song K, Ellenbogen RG, Avellino AM. Retrospective review of multilevel spinal fusion combined with spinal cord transection for treatment of kyphoscoliosis in pediatric myelomeningocele patients. Spine (Phila Pa 1976). 2007; 32 :2493-2501 - 23.
Parisini P et al. Surgical treatment of scoliosis in myelomeningocele. Studies in Health Technology and Informatics. 2001; 91 :442-447 - 24.
Tachdijian M. Myelomeningocele/scoliosis. In: Pediatric Orthopaedics. Philadelphia: WB Saunders; 1990. pp. 1843-1848 - 25.
Müller EB, Nordwall A, Odén A. Progression of scoliosis in children with myelomeningocele. Spine. 1994; 19 (2):147-150 - 26.
Hudgins RJ, Gilreath CL. Tethered spinal cord following repair of myelomeningocele. Neurosurgical Focus. 2004; 16 (2):1-4 - 27.
Herman JM et al. Analysis of 153 patients with myelomeningocele or spinal lipoma reoperated upon for a tethered cord. Pediatric Neurosurgery. 1993; 19 (5):243-249 - 28.
Sarwark JF et al. Tethered cord syndrome in low motor level children with myelomeningocele. Pediatric Neurosurgery. 1996; 25 (6):295-301 - 29.
Bowman RM et al. Tethered cord release: A long-term study in 114 patients: Clinical article. Journal of Neurosurgery: Pediatrics. 2009; 3 (3):181-187 - 30.
Dias MS. Neurosurgical causes of scoliosis in patients with myelomeningocele: An evidence-based literature review. Journal of Neurosurgery: Pediatrics. 2005; 103 (1):24-35 - 31.
Samuelsson L, Skoog M. Ambulation in patients with myelomeningocele: A multivariate statistical analysis. Journal of Pediatric Orthopaedics. 1988; 8 (5):569-575 - 32.
Sharma S et al. Prevalence of complications in neuromuscular scoliosis surgery: A literature meta-analysis from the past 15 years. European Spine Journal. 2013; 22 (6):1230-1249 - 33.
Wai EK et al. Assessing physical disability in children with spina bifida and scoliosis. Journal of Pediatric Orthopaedics. 2000; 20 (6):765-770 - 34.
Wai EK et al. The relationship between function, self-perception, and spinal deformity: Implications for treatment of scoliosis in children with spina bifida. Journal of Pediatric Orthopaedics. 2005; 25 (1):64-69 - 35.
Feng F, Tan H, Li X, Chen C, Li Z, Zhang J, et al. Radiographic characteristics in congenital scoliosis associated with split cord malformation: A retrospective study of 266 surgical cases. BMC Musculoskeletal Disorders. 2017; 18 (1):420. DOI: 10.1186/s12891-017-1782-z - 36.
Sergeenko (Pavlova) OM, Savin DM, Ryabykh SO. Treatment of spinal deformity with diastematomyelia type I: One-stage, two-stage surgery and new technique (vertebral column resection through wide bony septum). Child’s Nervous System. 2022; 38 (1):163-172. DOI: 10.1007/s00381-021-05382-7 - 37.
Sato T, Yonezawa I, Onda S, Yoshikawa K, Takano H, Shimamura Y, et al. Surgical treatment for lumbar hyperlordosis after resection of a spinal lipoma associated with spina bifida: A case report. Medicine (Baltimore). 2017; 96 :e7895 - 38.
Bas CE, Preminger J, Olgun ZD, Demirkiran G, Sponseller P, Yazici M. Safety and efficacy of apical resection following growth-friendly instrumentation in myelomeningocele patients with gibbus: Growing rod versus Luque trolley. Journal of Pediatric Orthopedics. 2015; 35 :e98-e103 - 39.
Yuan N et al. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine. 2005; 30 (19):2182-2185 - 40.
Samdani AF et al. A patient with myelomeningocele: Is untethering necessary prior to scoliosis correction? Neurosurgical Focus. 2010; 29 (1):E8 - 41.
Wild A et al. Is sacral instrumentation mandatory to address pelvic obliquity in neuromuscular thoracolumbar scoliosis due to myelomeningocele? Spine. 2001; 26 (14):E325-E329 - 42.
Rodgers WB et al. Spinal deformity in myelodysplasia: Correction with posterior pedicle screw instrumentation. Spine. 1997; 22 (20):2435-2443 - 43.
Guille JT et al. The feasibility, safety, and utility of vertebral wedge osteotomies for the fusionless treatment of paralytic scoliosis. Spine. 2003; 28 (20S):S266-S274 - 44.
Cardoso M, Keating RF. Neurosurgical management of spinal dysraphism and neurogenic scoliosis. Spine. 2009; 34 (17):1775-1782 - 45.
Karami M, Akbarnia BA. Ilizarov kyphectomy technique in the management of the gibbus deformity with an open wound in meningomyelocele patients: A case report with five years of follow-up. European Spine Journal. 2022; 31 :3713-3718. DOI: 10.1007/s00586-021-07058-x - 46.
Smith JT, Novais E. Treatment of gibbus deformity associated with myelomeningocele in the young child with use of the vertical expandable prosthetic titanium rib (VEPTR). The Journal of Bone and Joint Surgery. American Volume. 2010; 92 (12):2211-2215 - 47.
Drummond DS, Moreau M, Cruess RL. The results and complications of surgery for the paralytic hip and spine in myelomeningocele. Bone & Joint Journal. 1980; 62 (1):49-53 - 48.
Hatlen T et al. Contributory factors to postoperative spinal fusion complications for children with myelomeningocele. Spine. 2010; 35 (13):1294-1299 - 49.
Sponseller PD et al. Deep wound infections after neuromuscular scoliosis surgery: A multicenter study of risk factors and treatment outcomes. Spine. 2000; 25 (19):2461-2466 - 50.
Boemers TML et al. Urological problems after surgical treatment of scoliosis in children with myelomeningocele. The Journal of urology. 1996; 155 (3):1066-1069 - 51.
Faciszewski T, Winter RB, Lonstein JE, Sane S, Erickson D. Segmental spinal dysgenesis. A disorder different from spinal agenesis. JBJS. 1995; 77 (4):530-537 - 52.
Pavlova OM, Ryabykh SO, Kozyrev DA, Gubin AV. Surgical treatment of thoracolumbar segmental spinal dysgenesis: Optimal type of fusion. World Neurosurgery. 2017; 106 :551-556. DOI: 10.1016/j.wneu.2017.07.031