Abstract
The term “exposome” encompasses all the environmental elements, both infectious and non-infectious, that an individual encounters throughout life. It refers to the collective exposure to various factors in the environment that can have an impact on human health and finally result in a disease or affect the disease course. The exposome is a term implicated in all skin diseases including psoriasis. Ranging from lifestyle habits such as diet, smoking, obesity, sunlight exposure, pre-existing diseases, and infectious agents’ exposure to patients’ unique features such as skin microbes, oxidative stress parameters, skin chemical environment, and cutaneous immune reactions, skin seems to encounter a variety of different exposures. All these exposures in turn affect and contribute in distinct ways to the pathogenesis pathways implicated in the creation of the psoriatic skin lesions and shape the disease course and progression. Also, the interaction between environmental and genetic factors is a well-established disease contributor. This chapter discusses the link between each aspect of exposome and psoriasis pathways and mechanisms as well as treatment plans taking into consideration environmental factors. Understanding the exposome–psoriasis relationship would lead to implications and targeted interventions to mitigate possible risk factors and give future directions.
Keywords
- psoriasis
- exposome
- environment
- lifestyle
- genetics
- microbioma
1. Introduction
Psoriasis is a persistent skin disease caused by immune system dysfunction and manifests with various phenotypically distinct subtypes such as plaque, guttate, pustular, or erythrodermic psoriasis. The disease, in its plaque form, which is the most frequent subtype, is characterized by well-defined, reddish, scaly plaques resulting from increased keratinocyte proliferation and proinflammatory cytokines. All forms are linked with genetic contributors, whose products are mainly involved in skin immune reactions and skin barrier formation [1].
The exposome represents all environmental exposures, from infectious and noninfectious causes that can contribute to the disease onset, making the hypothesis that everyone’s disease including psoriasis is the result of the individual history of exposures, considering the individual’s genetic susceptibilities. Apart from environmental exposures (air pollution, sunlight exposure) and lifestyle aspects (diet, exercise), exposome concept encompasses psycho-social practices while its yields such as epigenomics, transcriptomics, proteomics, and metabolomics as disease mechanisms are in the spotlight [2].
The pathogenesis of psoriasis is multifactorial, combining environmental and genetic factors and necessitating a further exploration of the concept of exposome. When individuals with a genetic predisposition encounter triggers for psoriasis, the adaptive immune system sets off a cascade of immune responses. The immunological pathways, specifically the IL-17 signaling pathway and its products, play a crucial role in driving the inflammatory cycle of psoriasis. More precisely, the myeloid dendritic cells release IL-12 and IL-23, with the IL-23 pathway being the primary driver in psoriasis pathogenesis as this cytokine supports the survival, differentiation, and activation of Th17 cells, which produce IL-17 cytokines. These cytokines, in turn, induce keratinocyte proliferation and promote the production of various psoriasis-related cytokines, chemokines, inflammatory mediators, and antimicrobial peptides. Pinpointing the psoriasis triggers and understanding their impact on specific aspects of psoriasis pathophysiology can pave the way for preventive strategies and practical application of the exposome concept [1].
Additionally, the presence of a disrupted skin barrier with impaired permeability plays a crucial role in the development of psoriasis. In susceptible individuals, skin injury can trigger psoriatic lesions, a phenomenon called Koebner phenomenon [3]. This process is likely mediated by the injury prompting keratinocytes to produce type 1 interferons, TNF-α, IL-6, and IL-36. In psoriasis, skin barrier dysfunction is also linked to the disruption of epidermal tight, gap, and adherent junction proteins. The reduced expression of these proteins likely contributes to increased transepidermal water loss and decreased hydration observed in psoriatic lesions [3, 4]. Therefore, mechanical or external exposures can contribute to the disease because of the compromised skin barrier.
Psoriasis disease is evaluated by the extent of skin involvement (body surface area (BSA)) and the severity of erythema, induration, and scaling, resulting in disease assessment scores such as the Psoriasis Area Severity Index (PASI). Treatment options include topical therapies such as vitamin D analogs (calcipotriol) or corticosteroids as well as phototherapy, systemic agents (methotrexate, ciclosporin and acitretin) and biologics such as TNF (adalimumab, etanercept, infliximab and certolizumab), IL-12/23p40 (ustekinumab), IL-23p19 (rizankizumab, guselkumab and tildrakizumab), IL-17 (ixekizumab and secukinumab), and IL-17 receptor (brodalumab) inhibitors. The combination of the proper treatment choice and the limitations of the exposome’s psoriasis modulatory factors can open new perspectives in the approach of psoriasis patients [1].
2. Inner contributors of psoriasis disease
The internal exposome pertains to individual-specific exposures within the body, encompassing genetic determinants, metabolic processes, and circulating blood biomarkers such as systematic oxidative stress parameters, hormones, and variability of skin as well as oral or gut microbiota [2].
2.1 Genetics and/or genomics of psoriasis
2.2 Oxidative stress parameters and psoriasis
2.3 Pre-existing conditions as a trigger factor of psoriasis
Also, some medications used to treat specific diseases can lead to drug-induced psoriasis. For example, beta-blockers are widely prescribed for treating and preventing various medical conditions and block the beta-adrenergic subtype 2 receptors. As a result, adenyl cyclase is no longer activated, decreasing cAMP and intracellular calcium levels. This decrease disrupts the normal regulation of cell differentiation and promotes keratinocyte proliferation, which can have adverse effects on the skin [18].
2.4 Psoriasis and hormonal impact
Thyroid hormones, specifically T3 (Triiodothyronine) and T4 (Thyroxine), trigger an elevation in epidermal growth factor (EGF) levels, resulting in epidermal hyperplasia or T3 itself promotes the proliferation of keratinocytes by T3 receptors on the skin. Stress, fast-modulation hormones, and circadian rhythm hormones will be discussed in the respective sections [21].
2.5 Metabolism profile (metabolics) in psoriasis patients
2.6 Microbiome (skin, oral, and gut) role in the development of psoriasis
The skin and gut are heavily colonized by microbial cells, which in turn train the immune cells and determine the immunology capacity of the host. The gut–skin axis through the microbiome is a concept that has been referred to as skin disorders such as atopic dermatitis. The gut microbiome of infants with atopic dermatitis (AD) is characterized by lower levels of Bacteroidetes and Bifidobacterium and higher quantities of Clostridium and Escherichia, which, in turn, boost the inflammatory state in the intestine [23]. Those alterations in the gut microbiome disrupt the immune system balance by the production of inflammatory metabolites, which are released in the circulation and can affect skin. The Western diet and use of probiotics exacerbate and improve the skin manifestations of atopic dermatitis, respectively, indicating the existence of a skin-gut interaction, possibly by the microbiome [24].
The skin-gut axis in psoriasis is not studied as deeply as in the case of atopic dermatitis. However, some structural variations have been reported, such as a decreased surface in the jejunum. This variation is responsible for differences in gut microbiome such as lower levels of Bacteroidetes and higher Firmicutes. Also, gut microbiome changes have been reported after biologic treatment such as secukinumab. Since the oral cavity is part of the gastrointestinal tract, a similar association is expected. An increased presence of oral Candida in patients with psoriasis has also been reported [23].
3. External contributors of psoriasis disease
The external contributors of exposome that promote psoriasis can be divided into general external factors (climate, biodiversity, urban environment, social, and economic elements) and specific external factors (infections, allergens, diet, tobacco, pollutants, and toxic substances) [27].
3.1 Environmental toxification and psoriasis disease
3.2 Stress and psoriasis disease
Elevated levels of cytokines have been observed in stress-related disorders, as indicated by a study involving medical students that connected psychological stress was associated with increased levels of cytokines [34]. Cytokines’ levels were also assessed in psoriatic patients exposed to psychological stress. The salivary levels of IL-1β after stress stimuli were compared between psoriasis patients and control. Interestingly, after the stressful event, the control group showed an increase in IL-1β levels, while the psoriasis group did not, indicating an impaired immune system response to adrenergic stimuli [35]. However, this observation is not in line with the cytokine-mediated psoriasis flare-up that may be induced by stress.
In addition to acute experience of stress, chronic stress as well as depression has been linked to persistently high levels of proinflammatory cytokines, notably IL-6, TNF-α, and IL-1β. IL-6 and TNF-α can alter the metabolism of neurotransmitters like norepinephrine, serotonin, and dopamine, leading to depressive symptoms. Additionally, IL-6 promotes the production of Th17 cells and along with action of TNF-α, plays a central role in the development of psoriasis lesions [31, 32]. Additionally, the reduced levels of serotonin (5-HT) lead to increased production of certain inflammatory mediators like TNF-α and IL-1β [36].
3.3 Sleep habits and psoriasis-circadian rhythm
As for the skin, the pineal gland produces melatonin, which is a crucial regulator of the circadian balance. Melatonin levels follow the circadian rhythm, peaking at night and decreasing during the day. When exposed to light, melatonin levels promptly decline due to feedback inhibition, reducing its production. Melatonin is associated with hair growth, protection against ultraviolet (UV) damage in skin cells, wound healing, and antitumor effects [39].
Also, sleep loss is associated with function of the hypothalamic–pituitary–adrenal (HPA) axis, leading to psoriasis flare-ups as indicated in the stress-exposome, with increased secretion of cortisol and proinflammatory cytokines [38].
3.4 Diet and psoriasis
Finally, the connection between obesity and psoriasis is well-established. As obesity progresses, adipocytes undergo senescence and dysfunction, altering their proteomic programming toward a proinflammatory phenotype. This shift may significantly influence the immune system’s function and serve as a critical factor in the development of various organ pathologies including the skin, as far as the skin is concerned, and cause chronic inflammation. Gut dysbiosis and microbiome dysregulation as well as lipid signaling are involved in the inflammatory process [42, 44]. Notably, individuals with a body mass index (BMI) of 35 or higher demonstrated an increase in the risk of developing psoriasis in women population [45].
3.5 Exercise and psoriasis
3.6 Sun exposure and psoriasis
3.7 Alcohol and tobacco abuse
Another abuse form that needs to be highlighted is alcohol consumption. Ethanol can affect cutaneous skin barrier as well as cutaneous immune reactions. Also, alcohol consumption is related to many other disorders such as obesity, depression, and liver disorders that can further exacerbate any skin disorder [54].
Ethanol can be detected within human skin, being secreted by eccrine glands, mainly sweat glands, or through passive diffusion, and by its metabolites can enhance the proliferation and mRNA expression of proliferation-associated genes of keratinocytes, disrupting the skin’s barrier function and increasing its permeability. Moreover, alcohol also affects lipid metabolism, affecting the lipid composition of the skin barrier. Also, the metabolism of ethanol is associated with the production of ROS. As a result, both ethanol and the produced ROS formed during ethanol metabolism generate an inflammatory environment and trigger psoriasis by regulating different signal transduction pathways and inducing the production of various proinflammatory cytokines in lymphocytes, macrophages, and keratinocytes [54].
3.8 Mechanical trigger of psoriasis lesions
Tattooing involves permanently marking the body with exogenous pigments or dyes introduced into the dermis for artistic purposes. The Koebner phenomenon, where psoriatic lesions develop at the site of skin trauma, has been documented in several case reports and one case series of patients with psoriasis who had tattoos [59].
3.9 Psoriasis and infectomics
In case of streptococcal infections and other Gram-positive organisms, the streptococcal cell wall is predominantly composed of peptidoglycan (PG), which is regarded as a potentially proinflammatory element and, therefore, another psoriasis mechanism can be observed besides superantigen action [63]. Additionally, serum anti-
4. Discussion
The exposome is a complex area of scientific research that profoundly influences health. This concept provides a comprehensive understanding of the various exposures individuals encounter during their lifetime including internal and external environmental factors that can influence the onset and progression of specific diseases. Some aspects of the exposome, particularly external contributors, can be modified, such as quitting smoking, leading to potential positive effects on the disease [2]. Also, some external contributors can ameliorate the disease, such as sunlight-induced cutaneous immunosuppression [51].
Clinical perspectives on the exposome and the integration of internal factors like genomics with external contributors like diet are crucial in personalized medicine, promising a better approach to treating patients with psoriasis. Moreover, external exposure factors can trigger disease onset directly, as seen in the case of unhealthy diets which cause systemic inflammation and trigger psoriasis mechanisms. Additionally, these external factors can modify internal exposome factors, as a high-fat diet can lead to gut dysbiosis, which can worsen psoriasis through the gut–skin axis. The interaction between internal and external exposome factors plays a significant role in the development of psoriatic disease, with combinations like exercise-induced oxidative stress and stress-induced hormonal impacts (Figure 1). The result of those combinations as well as the direct effect of external and internal contributors can lead to systemic and cutaneous inflammation, leading to psoriasis (Figure 1). Understanding this interplay is of utmost importance in comprehending the complexities of psoriasis.
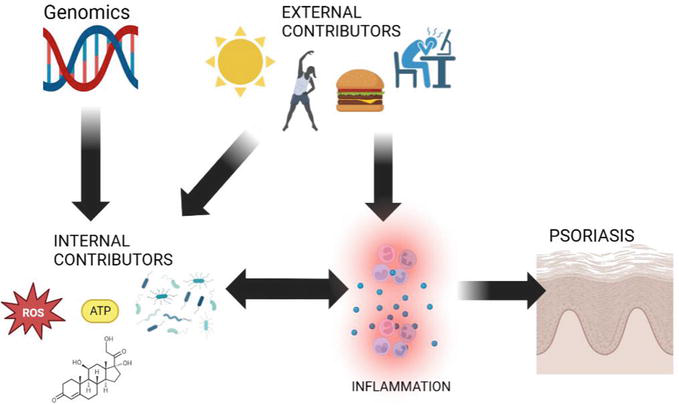
Figure 1.
The interaction between genomics, other internal contributors of exposome (oxidative stress parameters, metabolics, microbioma, hormonal impact), external contributors (sunlight, exercise, diet and stress), and main pathogenesis of psoriasis disease (skin inflammation) by single or bidirectional pathways (created by biorender.com).
However, some questions arise on whether an exposome variant is adequate for the expression of a disease phenotype or whether genomics is the indispensable inner contributor. A study showed that polymorphisms of the glutamate cysteine ligase catalytic subunit that regulates glutathione biosynthesis (GCLC) combined with tobacco smoking and alcohol abuse are significantly associated with the risk of psoriasis and related to its clinical features [69].
Also, the impact of exposomes on psoriasis disease seems to depend on the psoriasis stage. During the initiation stage, new inflammatory lesions continually emerge, while during the stationary stage, the lesions stabilize. Also, early and chronic psoriasis diseases differ in terms of immunology. In the initiation stage, the IL-23/IL-17 axis and activated DCs are the main contributors to the disease, while in chronic disease, mature dermal DCs and T cells contribute to the cytokine milieu [1]. Therefore, the result of exposome factors depends on the psoriasis stage. Also, the treatment status of psoriasis patients can defend against the psoriasis-provoking actions of some exposome factors. For example, patients under biologic treatment showed less frequent episodes of psoriasis flare-up following COVID-19 vaccination [67].
5. Conclusion
Exposome represents a multifaceted area of research that significantly impacts our understanding of psoriasis. This concept provides a comprehensive view of how various internal and external environmental factors interact to influence the onset and progression of psoriatic disease. More research in exposome in psoriasis disease is needed as its further exploration may open exciting possibilities for personalized medicine and targeted therapies.
References
- 1.
Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. International Journal of Molecular Sciences. 2019; 20 :1475 - 2.
Wild CP. The exposome: From concept to utility. International Journal of Epidemiology. 2012; 41 :24-32 - 3.
Orsmond A, Bereza-Malcolm L, Lynch T, March L, Xue M. Skin barrier dysregulation in psoriasis. International Journal of Molecular Sciences. 2021; 22 :10841 - 4.
Montero-Vilchez T, Segura-Fernández-Nogueras M-V, Pérez-Rodríguez I, Soler-Gongora M, Martinez-Lopez A, Fernández-González A, et al. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water loss and temperature as useful tools to assess disease severity. Journal of Clinical Medicine. 2021; 10 :359 - 5.
Capon F. The genetic basis of psoriasis. International Journal of Molecular Sciences. 2017; 18 :2526 - 6.
Villarreal-Martinez A, Gallerdo-Blanco H, Cerda-Flores R, Torres-Munoz I, Gomez-Flores M, Salas-Alanis J, et al. Candidate gene polymorphisms and risk of psoriasis: A pilot study. Experimental and Therapeutic Medicine. 2016; 11 :1217-1222 - 7.
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: Harms and benefits for human health. Oxidative Medicine and Cellular Longevity. 2017; 2017 :1-13 - 8.
Pleńkowska J, Gabig-Cimińska M, Mozolewski P. Oxidative stress as an important contributor to the pathogenesis of psoriasis. International Journal of Molecular Sciences. 2020; 21 :6206 - 9.
Vorobjeva N, Prikhodko A, Galkin I, Pletjushkina O, Zinovkin R, Sud’ina G, et al. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. European Journal of Cell Biology. 2017; 96 :254-265 - 10.
Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-κB: An essential transcription factor in psoriasis. Journal of Dermatological Science. 2013; 69 :89-94 - 11.
Karampinis E, Aloizou A-M, Zafiriou E, Bargiota A, Skaperda Z, Kouretas D, et al. Non-melanoma skin cancer and vitamin D: The “lost sunlight” paradox and the oxidative stress explanation. Antioxidants. 2023; 12 :1107 - 12.
Kökçam İ, Nazıroğlu M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clinica Chimica Acta. 1999; 289 :23-31 - 13.
Medovic MV, Jakovljevic VLJ, Zivkovic VI, Jeremic NS, Jeremic JN, Bolevich SB, et al. Psoriasis between autoimmunity and oxidative stress: Changes induced by different therapeutic approaches. Oxidative Medicine and Cellular Longevity. 2022; 2022 :1-17 - 14.
Katsimbri P, Korakas E, Kountouri A, Ikonomidis I, Tsougos E, Vlachos D, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: Nutrition as therapeutic tool? Antioxidants. 2021; 10 :157 - 15.
Guarneri F, Bertino L, Pioggia G, Casciaro M, Gangemi S. Therapies with antioxidant potential in psoriasis, vitiligo, and lichen planus. Antioxidants. 2021; 10 :1087 - 16.
de de Oliveira MFSP, de Rocha BO, Duarte GV. Psoriasis: Classical and emerging comorbidities. Anais Brasileiros de Dermatologia. 2015; 90 :9-20 - 17.
Daugaard C, Iversen L, Hjuler KF. Comorbidity in adult psoriasis: Considerations for the clinician. Psoriasis: Targets and Therapy. 2022; 12 :139-150 - 18.
Awad VM, Sakhamuru S, Kambampati S, Wasim S, Malik BH. Mechanisms of beta-blocker induced psoriasis, and psoriasis de novo at the cellular level. Cureus. 2020; 12 - 19.
Adachi A, Honda T. Regulatory roles of estrogens in psoriasis. Journal of Clinical Medicine. 2022; 11 :4890 - 20.
Ceovic R, Mance M, Bukvic Mokos Z, Svetec M, Kostovic K, Stulhofer BD. Psoriasis: Female skin changes in various hormonal stages throughout life—Puberty, pregnancy, and menopause. BioMed Research International. 2013; 2013 :1-6 - 21.
Sweta K, Mm F, Lenin M. The putative role of thyroid hormones and vitamin D on severity and quality of life in psoriasis. International Journal of Applied & Basic Medical Research. 2020; 10 :173 - 22.
Carmona-Cruz S, Orozco-Covarrubias L, Sáez-de-Ocariz M. The human skin microbiome in selected cutaneous diseases. Frontiers in Cellular and Infection Microbiology. 2022; 12 - 23.
De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021; 9 :353 - 24.
Hrestak D, Matijašić M, Čipčić Paljetak H, Ledić Drvar D, Ljubojević Hadžavdić S, Perić M. Skin microbiota in atopic dermatitis. International Journal of Molecular Sciences. 2022; 23 :3503 - 25.
Langan EA, Künstner A, Miodovnik M, Zillikens D, Thaçi D, Baines JF, et al. Combined culture and metagenomic analyses reveal significant shifts in the composition of the cutaneous microbiome in psoriasis. British Journal of Dermatology. 2019; 181 :1254-1264 - 26.
Chang H-W, Yan D, Singh R, Liu J, Lu X, Ucmak D, et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018; 6 :154 - 27.
Celebi, Sozener Z, Özbey Yücel Ü, Altiner S, Ozdel Oztürk B, Cerci P, Türk M, et al. The external exposome and allergies: From the perspective of the epithelial barrier hypothesis. Frontiers in Allergy. 2022; 3 - 28.
Koohgoli R, Hudson L, Naidoo K, Wilkinson S, Chavan B, Birch-Machin MA. Bad air gets under your skin. Experimental Dermatology. 2017; 26 :384-387 - 29.
Bellinato F, Adami G, Vaienti S, Benini C, Gatti D, Idolazzi L, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatology. 2022; 158 :375 - 30.
Wang T, Xia Y, Zhang X, Qiao N, Ke S, Fang Q , et al. Short-term effects of air pollutants on outpatients with psoriasis in a Chinese city with a subtropical monsoon climate. Frontiers in Public Health. 2022; 10 - 31.
Alesci A, Lauriano ER, Fumia A, Irrera N, Mastrantonio E, Vaccaro M, et al. Relationship between immune cells, depression, stress, and psoriasis: Could the use of natural products be helpful? Molecules. 2022; 27 :1953 - 32.
Rousset L, Halioua B. Stress and psoriasis. International Journal of Dermatology. 2018; 57 :1165-1172 - 33.
Tampa M, Sarbu M-I, Mitran M-I, Mitran C-I, Matei C, Georgescu S-R. The pathophysiological mechanisms and the quest for biomarkers in psoriasis, a stress-related skin disease. Disease Markers. 2018; 2018 :1-14 - 34.
Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and TH1-like response in stress-induced anxiety. Cytokine. 1998; 10 :313-318 - 35.
Mastrolonardo M, Alicino D, Zefferino R, Pasquini P, Picardi A. Effect of psychological stress on salivary interleukin-1β in psoriasis. Archives of Medical Research. 2007; 38 :206-211 - 36.
Wardhana M, Windari M, Puspasari N, Suryawati N. Role of serotonin and dopamine in psoriasis: A case-control study. Open Access Macedonian Journal of Medical Sciences. 2019; 7 :1138-1142 - 37.
Tzeng Y-M, Li I-H, Kao H-H, Shih J-H, Yeh C-B, Chen Y-H, et al. Protective effects of anti-depressants against the subsequent development of psoriasis in patients with major depressive disorder: A cohort study. Journal of Affective Disorders. 2021; 281 :590-596 - 38.
Nowowiejska J, Baran A, Flisiak I. Mutual relationship between sleep disorders, quality of life and psychosocial aspects in patients with psoriasis. Frontiers in Psychiatry. 2021; 12 - 39.
Lyons AB, Moy L, Moy R, Tung R. Circadian rhythm and the skin: A review of the literature. The Journal of Clinical and Aesthetic Dermatology. 2019; 12 :42-45 - 40.
Luengas-Martinez A, Paus R, Iqbal M, Bailey L, Ray DW, Young HS. Circadian rhythms in psoriasis and the potential of chronotherapy in psoriasis management. Experimental Dermatology. 2022; 31 :1800-1809 - 41.
Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. International Journal of Molecular Sciences. 2020; 21 :5405 - 42.
Barros G, Duran P, Vera I, Bermúdez V. Exploring the links between obesity and psoriasis: A comprehensive review. International Journal of Molecular Sciences. 2022; 23 :7499 - 43.
Shi Z, Wu X, Yu S, Huynh M, Jena PK, Nguyen M, et al. Short-term exposure to a Western diet induces Psoriasiform dermatitis by promoting accumulation of IL-17A–producing γδ T cells. Journal of Investigative Dermatology. 2020; 140 :1815-1823 - 44.
Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016; 232 :633-639 - 45.
Setty AR. Obesity, waist circumference, weight change, and the risk of psoriasis in women. Archives of Internal Medicine. 2007; 167 :1670 - 46.
Villarreal-Calderón JR, Cuéllar RX, Ramos-González MR, Rubio-Infante N, Castillo EC, Elizondo-Montemayor L, et al. Interplay between the adaptive immune system and insulin resistance in weight loss induced by bariatric surgery. Oxidative Medicine and Cellular Longevity. 2019; 2019 :1-14 - 47.
Duchnik E, Kruk J, Tuchowska A, Marchlewicz M. The impact of diet and physical activity on psoriasis: A narrative review of the current evidence. Nutrients. 2023; 15 :840 - 48.
Yeroushalmi S, Hakimi M, Chung M, Bartholomew E, Bhutani T, Liao W. Psoriasis and exercise: A review. Psoriasis (Auckland, N.Z.). 2022; 12 :189-197 - 49.
Goto H, Nakatani E, Yagi H, Moriki M, Sano Y, Miyachi Y. Late-onset development of psoriasis in Japan: A population-based cohort study. JAAD International. 2021; 2 :51-61 - 50.
Frankel HC, Han J, Li T, Qureshi AA. The association between physical activity and the risk of incident psoriasis. Archives of Dermatology. 2012; 148 :918-924 - 51.
Queirós CS, Freitas JP. Sun exposure: Beyond the risks. Dermatology Practical & Conceptual. 31 Oct 2019; 9 (4):249-252. DOI: 10.5826/dpc.0904a01 - 52.
Karampinis E, Goudouras G, Ntavari N, Bogdanos DP, Roussaki-Schulze A-V, Zafiriou E. Serum vitamin D levels can be predictive of psoriasis flares up after COVID-19 vaccination: A retrospective case control study. Frontiers in Medicine (Lausanne). 2023; 10 - 53.
Fowles J. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tobacco Control. 2003; 12 :424-430 - 54.
Szentkereszty-Kovács Z, Gáspár K, Szegedi A, Kemény L, Kovács D, Törőcsik D. Alcohol in psoriasis—From bench to bedside. International Journal of Molecular Sciences. 2021; 22 :4987 - 55.
Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nature Reviews. Cardiology. 2013; 10 :219-230 - 56.
Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: A systematic review and meta-analysis. British Journal of Dermatology. 2014; 170 :304-314 - 57.
Setty AR, Curhan G, Choi HK. Smoking and the risk of psoriasis in women: Nurses’ health study II. The American Journal of Medicine. 2007; 120 :953-959 - 58.
Malakou LS, Gargalionis AN, Piperi C, Papadavid E, Papavassiliou AG, Basdra EK. Molecular mechanisms of mechanotransduction in psoriasis. Annals of Translational Medicine. 2018; 6 :245-245 - 59.
Grodner C, Beauchet A, Fougerousse A-C, Quiles-Tsimaratos N, Perrot J-L, Barthelemy H, et al. Tattoo complications in treated and non-treated psoriatic patients. Journal of the European Academy of Dermatology and Venereology. 2020; 34 :888-896 - 60.
Teng Y, Xie W, Tao X, Liu N, Yu Y, Huang Y, et al. Infection-provoked psoriasis: Induced or aggravated (review). Experimental and Therapeutic Medicine. 2021; 21 :567 - 61.
Zhou S, Yao Z. Roles of infection in psoriasis. International Journal of Molecular Sciences. 2022; 23 :6955 - 62.
Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DYM, Schlievert PM. Staphylococcal and streptococcal Superantigen exotoxins. Clinical Microbiology Reviews. 2013; 26 :422-447 - 63.
Baker B, Laman J, Powles A, van der Fits L, Voerman J, Melief M-J, et al. Peptidoglycan and peptidoglycan-specific Th1 cells in psoriatic skin lesions. The Journal of Pathology. 2006; 209 :174-181 - 64.
Azizzadeh M, Nejad ZV, Ghorbani R, Pahlevan D. Relationship between helicobacter pylori infection and psoriasis. Annals of Saudi Medicine. 2014;34 :241-244 - 65.
Hübner AM, Tenbaum SP. Complete remission of palmoplantar psoriasis through helicobacter pylori eradication: A case report. Clinical and Experimental Dermatology. 2008; 33 :339-340 - 66.
Imafuku S, Nakayama J. Profile of patients with psoriasis associated with hepatitis C virus infection. The Journal of Dermatology. 2013; 40 :428-433 - 67.
Karampinis E, Gravani A, Gidarokosta P, Bogdanos DP, Roussaki-Schulze A-V, Zafiriou E. Pustular eruption following COVID-19 vaccination: A narrative case-based review. Vaccines (Basel). 2023; 11 :1298 - 68.
Ozaras R, Berk A, Ucar DH, Duman H, Kaya F, Mutlu H. Covid-19 and exacerbation of psoriasis. Dermatologic Therapy. 2020; 33 (4):e13632. DOI: 10.1111/dth.13632 - 69.
Efanova E, Bushueva O, Saranyuk R, Surovtseva A, Churnosov M, Solodilova M, et al. Polymorphisms of the GCLC gene are novel genetic markers for susceptibility to psoriasis associated with alcohol abuse and cigarette smoking. Life. 2023; 13 :1316