Abstract
Screening for prediabetes and type 2 diabetes risk through an informal assessment of risk factors or with an assessment tool, such as the ADA (American Diabetes Association) risk test, is recommended to guide health care professionals on whether performing a diagnostic test is appropriate. Approximately one-quarter of people with diabetes in the US and nearly half of Asian and Hispanic American people with diabetes are undiagnosed. Although screening of asymptomatic individuals to identify those with prediabetes or diabetes might seem reasonable, rigorous clinical trials to prove the effectiveness of such screening have not been conducted and are unlikely to occur. Clinical conditions, such as hypertension, hypertensive pregnancy and obesity, enhance risk. Additional considerations regarding testing for type 2 diabetes and prediabetes in asymptomatic individuals include the following: age, BMI (body mass index) and ethnicity, medication, HIV, testing interval, community screening and screening in dental practice.
Keywords
- screening
- testing
- type II diabetes
- prediabetes
- asymptomatic
1. Introduction
In 2016 diabetes was the third leading cause of years lived with disability and the seventh leading cause of death in the United States. The number of people living with type 2 diabetes mellitus increased significantly over the past decades, going from around 151 million in 2000 to 463 million in 2019. Along with this increased prevalence, the economic health cost related to type 2 diabetes mellitus experienced a staggering increase. The expenditures related to diabetes in United States were estimated at US$232 billion and US$760 billion in 2007 and 2019, respectively [1].
This significant increase in diabetes prevalence, especially in Asia, the Middle East and North Africa (MENA), is largely attributed to changes in the living environment and lifestyles that led to declines in nutritional quality and increases in sedentary behaviors. These drastic changes in lifestyle increase the prevalence of overweight and obesity, promoting the development of insulin resistance and diabetes mellitus [1].
Prior to diabetes mellitus development and pancreatic beta-cell dysfunction, a state of prediabetes commonly preceded. Prediabetes which was defined by a fasting plasma glucose concentration of 5,6–6,9 mmol/L or hemoglobin A1c concentration of 39–46 mmol/mol, was reported to affect around 86 million adults in the United States. In a 5-year period, approximately 70% of prediabetes eventually develop type 2 diabetes mellitus.
Prediabetes state has been associated with the development of macrovascular and microvascular complication, such as nephropathy, chronic kidney disease, small fiber neuropathy, diabetic retinopathy, cognitive dysfunction and also cardiovascular disease. Despite the association of prediabetes to pathophysiological changes and morbidities, most individual with prediabetes are asymptomatic and thus unaware that they have the condition [2].
Early screening for prediabetes state and subsequent early intervention has been associated with improved all-cause mortality and morbidity caused by type 2 diabetes mellitus [3]. In this brief article, we review the need for screening for prediabetes and diabetes in the asymptomatic adult population.
2. Diabetes mellitus
Diabetes mellitus is defined as abnormal plasma glucose concentration associated with glucose metabolism disorders. Diabetes mellitus is diagnosed if fasting plasma glucose (FPG) concentration is equal or greater than 7.0 mmol/L, or a 2-h plasma glucose (2hrPG) concentration is equal or greater than 11.1 mmol/L during an oral glucose tolerance test (OGTT), or hemoglobin A1c (HbA1c) concentration is equal or greater than 47 mmol/mol.
2.1 Classification of diabetes mellitus
A new proposed classification of T2DM was based on six variables, including glutamate decarboxylate (GAD) antibodies, age at diagnosis, body mass index (BMI), HbA1c concentrations and homeostatic model assessment estimates of ß-cell function (HOMA-2B) and insulin resistance (HOMA2-IR). This novel classification divided T2DM into five groups: severe autoimmune diabetes (SAID), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD) and mild age-related diabetes (MARD) [4, 5].
Cluster 1 (SAID) is characterized by early-onset disease, relatively low BMI, poor metabolic control, insulin deficiency and the presenve of GADA. Cluster 2 (SIDD) is similar to SAID but with negative GADA: low age at onset, relatively low BMI, low insulin secretion (low HOMA2-B index) and poor metabolic control. Cluster 3 (SIRD) is characterized by insulin resistance (high HOMA2-IR index) and high BMI. Cluster 4 (MOD) is characterized by obesity without insulin resistance and lastly, Cluster 5 (MARD) is similar to Cluster 4 but with older onset and milder metabolic derangements [4, 5].
This new classification identifies patients with a high risk of diabetic complications and provides information about the underlying disease mechanism. Patients in Cluster 3 (SIRD) have a higher risk for diabetic kidney disease than those in Cluster 4 and 5 patients in Cluster 2 (SIDD) have the highest risk of diabetic retinopathy. SIRD is also associated with a higher risk for diabetic ketoacidosis, diabetic retinopathy, atherosclerotic cardiovascular disease and nonalcoholic fatty liver disease [4, 5].
2.2 Prevalence of type 2 diabetes mellitus
The Global Burden of Disease (GBD) data in 2019 announced that the age-standardized global prevalence of type 2 diabetes mellitus was approximately 6.0% in men and 5.0% in women. The incidence increases with age, with the mean age of diagnosis occurring around 55 to 59 years. The highest increase in the proportion of type 2 diabetes mellitus age-standardized prevalence happens in the Middle East and North Africa (MENA) and in South Asia. Data from the International Diabetes Federation published the prevalence of people living with type 2 diabetes mellitus in 2045 will approximately increase to 700 million [1].
2.3 Risk factors for diabetes
Several factors are associated with type 2 diabetes mellitus, and they can be classified into nonmodifiable and modifiable risk factors. Nonmodifiable risk factors include age, ethnicity and genetics, while modifiable risk factors include diet, physical activity and tobacco use (Figure 1) [1].
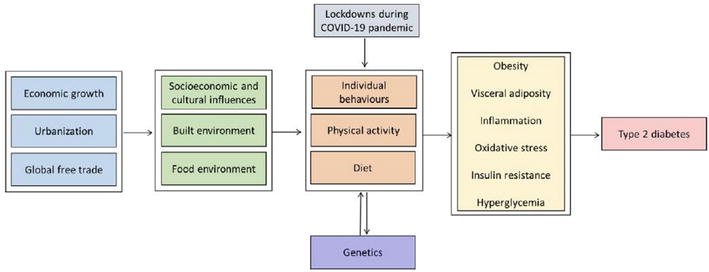
Figure 1.
Factors associated with increased prevalence of type 2 diabetes mellitus [
Before the age of 30, type 2 diabetes incidence is typically low in most populations but rises quickly and steadily as people become older. According to prospective observational research, age is often a significant risk factor, despite the control of obesity [6, 7].
The physiological reaction to meals high in saccharides can be used to gauge the quality of dietary saccharides. The ability of particular foods to raise postprandial plasma glucose concentration is ranked by the glycemic index, which reflects the quality of saccharides. The glycemic load is the cross-product of a food’s glycemic index and the number of saccharides, reflecting both the quality and quantity of the saccharides. Numerous prospective studies have assessed the relationships between glycemic index and load and risk of diabetes. These meta-analyses revealed that low glycemic index and glycemic load diets may be associated with a lower risk of developing diabetes than high glycemic index diets [6, 7].
Sedentary lifestyle choices were associated with a greater risk of type 2. According to a global assessment, physical inactivity, which the World Health Organization (2010) defines as not enough physical activity to fulfill current global standards, is responsible for 7% of type 2 diabetes mellitus burden [7].
The biggest independent risk factor for type 2 diabetes is excessive body fat. The risk is connected to high body fat, indicated by BMI or anthropometric measurements like waist circumference or skinfold thickness. A dose-dependent rise in diabetes risk is linked to clinical BMI risk categories. Longer periods of maintaining a high body weight are a significant risk factor for type 2 diabetes and the degree of overweight and obesity. The Framingham Heart Study (FHS) revealed the age-adjusted relative risk per extra 2-year duration of obesity was 1.11 for men and 1.06 for women [7].
2.4 Mechanism increasing the risk of type 2 diabetes
The main pathophysiology driving the development of type 2 diabetes is impaired insulin secretion and insulin resistance in both muscle and the liver. Type 2 diabetes is commonly preceded by long-term prediabetes, in which pancreatic beta-cell dysfunction may have occurred. Type 2 diabetes is commonly diagnosed when the pancreas cannot increase insulin secretion to compensate for the insulin resistance in the peripheral insulin-sensitive tissues. Additional mechanisms affecting insulin secretion in type 2 diabetes mellitus are increasing hormone deficiency/resistance in the gastrointestinal tract and hyperglucagonemia [8].
3. Prediabetes
Prediabetes is defined as an abnormal elevation in plasma glucose concentration, but lower than needed to be diagnosed as type 2 diabetes. Prediabetes is a glycemic state between normoglycemia and diabetes mellitus and carries an increased risk for developing type 2 diabetes mellitus [9]. Prediabetes is further classified into impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). There are currently two accepted definitions for IFG and IGT, either by American Diabetes Association (ADA) or by World Health Organization (WHO). ADA 2020 defined IFG as a fasting plasma glucose concentration of 5.6–6.9 mmol/L or hemoglobin A1c (HbA1c) concentration of 39–46 mmol/mol and IGT as a fasting plasma glucose concentration less than 6.9 mmol/L and 2-h plasma glucose concentration of 7.8–11.0 mmol/L. WHO 2006 defined IFG as fasting plasma glucose of 6.1–6.9 mmol/L and 2-h plasma glucose concentration less than 7.8 mmol/L (if measured) and IGT as 2-h plasma glucose concentration of 7.8–11.0 mmol/L [9].
3.1 Biological phenotypes of prediabetes
Both IFG and IGT phenotypes have various degrees of insulin resistance and beta-cell dysfunction; however, IGT is characterized with almost completely insulin resistant and has less than 20% beta-cell function, and thus IGT represents a worse glucose homeostasis disturbance.
These two phenotypes are diagnosed by fasting plasma glucose and 2-h plasma glucose concentrations which provide information on the fasting and postprandial states and, thus, on the pancreatic beta cells. HbA1c shares information about chronic hyperglycemia state and therefore may miss short-term acute hyperglycemia. Previous studies showed that HbA1c was weakly correlated with insulin resistance and beta-cell function, while FPG and 2hBG correlated better with insulin resistance and beta-cell function.
Genome-wide association studies (GWAS) revealed an overlap in the loci associated with FBG and 2hBG traits, such as MTNR1B, PCSK1 and DPYSL5, which are specific to FBG, and VPS13C, GCKR, GIPR and VPS13C which are specific to 2hBG. Some gene variants associated with HbA1c overlap with glycemia, including GCK, G6PC2 and MNTR1B, and others are specific to HbA1c.
3.2 Prediabetes burden
Differences in ADA’s and WHO’s criteria for prediabetes affected the number of IFG and IGT prevalence. The prevalence of IFG and IFG + IGT is estimated at 36–53% and 16–20%, respectively [9]. In 2017, the National Diabetes Statistics Report estimated around 33.9% of adult in the United States has prediabetes based on either fasting glucose or hemoglobin A1c (HbA1c) concentration, and only 103 mmol/mol adults know they have prediabetes [10].
3.3 Risk factor for prediabetes
Risk factors for prediabetes include age over 45 years, male sex, obesity, hypertension, dyslipidemia, family history of diabetes, history of gestational diabetes and Hispanic, black or Asian ethnicity [11]. According to the previous study, gaining weight results in increased insulin resistance, which may cause prediabetes. Consuming junk food with saturated fatty acids might increase weight and result in insulin resistance [12].
Changes in food habits and physical inactivity are the main causes of weight gain globally in the past decades. As mentioned before, the main independent risk factor for diabetes is excessive adiposity, which is measured by a higher body mass index (BMI). However, individuals with Asian ethnicity tend to develop diabetes at significantly lower BMIs than those with European ancestry. According to previous meta-analysis, there may be a marginally stronger association between risk of diabetes and waist size than between risk of diabetes and body mass index. Therefore, monitoring both waist circumference and BMI is crucial in clinical practice [12].
Individuals exposed to ETS (Environmental Tobacco Smoke) had an elevated risk of developing diabetes. The incidence of diabetes in prediabetic participants was also statistically affected by active smoking. The findings of this study support the notion that T2DM and smoking, whether passive or active, are related [12].
Men who drink are more likely to develop type 2 diabetes, whereas women who drink less are less likely to develop the disease. High beer intake and high booze consumption in men were associated with greater risks of prediabetes and type 2 diabetes, respectively. Women demonstrated a lower risk of prediabetes with high wine consumption and a lower risk of type 2 diabetes with medium intakes of both wine and spirits, but prediabetes risk rose with high spirits consumption. In summary, males who drink a lot are more likely to have impaired glucose regulation. The relationships are more nuanced in women, with risk decreasing with low to moderate alcohol use and rising with high consumption [12].
3.4 Progression to prediabetes
The onset of prediabetes is influenced by several factors, comprising genetics, peripheral insulin resistance, defects in insulin secretion, glucotoxicity, lipotoxicity, impaired incretin release, amylin accumulation, inflammation, oxidative stress and decreased beta cell mass, and eventually beta cell dysfunction [11].
Previous studies pointed out several predictors of transition from normal glucose regulation to prediabetes, consisting of demographic and adiposity (older age, male sex, higher body mass index, higher waist circumference, higher total and abdominal fat), glucoregulatory (upper-normal fasting plasma glucose and 2-h plasma glucose concentration, low insulin sensitivity, impaired insulin secretion and lower disposition index), plasma lipid and amino acid levels (higher triglycerides, higher LDL or lower HDL cholesterol, higher asparagine or aspartic acid, higher glutamine or glutamic acid and lower histidine) and miscellaneous factors (higher plasma pressure, lower adiponectin, higher hematocrit and higher alanine aminotransferase) [13].
Prediabetes condition is strongly associated with an increased risk of diabetes development. A previous meta-analysis showed that HbA1c concentration of 42–47 mmol/mol was associated with a 25 to 50% higher risk of incident diabetes over 5 years. The cumulative probability of progression to diabetes in patients diagnosed with HbA1c alone and IFG was 53 and 73 mmol/mol, respectively, over a 4.7-year follow-up period. In a pooled analysis, the annual incidence rate of diabetes was 3.6% in patients with HbA1c concentrations of 42–47 mmol/mol, while in patients with ADA-defined IFG, the annual rate was 3.6% [14].
Prediabetes shares a similar pathophysiology pathway as type 2 diabetes mellitus that, include insulin resistance, pancreatic beta-cell dysfunction and insulin resistance in organs. Insulin resistance will cause hyperinsulinemia in prediabetic patients, characterized by alteration of the insulin signaling pathway, reduced concentration, phosphorylation and activity of insulin receptors and decreased intracellular translocation of glucose transporter 4 (GLUT-4) [15].
Both IFG and IGT share a similar state of insulin resistance; however, the former is associated with hepatic insulin resistance, while the latter is associated with muscle insulin resistance. IFG has moderate hepatic insulin resistance and impaired early (1–30 min) insulin response exocytosis of insulin during OGTT. The intact late-phase plasma insulin response and normal to near-normal muscle sensitivity in IFG will return 2-h plasma glucose concentration to the initial fasting plasma glucose concentration. On the other hand, IGT is associated with impaired early and also late insulin (60–120 min) response during OGTT [15].
Pancreatic beta-cell dysfunction occurs along with insulin resistance in prediabetic patients. The sensitivity of pancreatic beta-cells in responding to changes in glucose concentration is impaired in prediabetes. Normally, as long as beta-cells can secrete insulin to overcome the insulin resistance, the body’s tolerance to glucose remains normal; however, as the beta-cells fail to overcome the insulin resistance, the individual will develop prediabetes state and then eventually develop type 2 diabetes mellitus [15].
Physiologically, the secretion of insulin occurs in a biphasic pattern. This biphasic pattern may aid in differentiating IFG from IGT. The first phase of insulin secretion inhibits the endogenous hepatic glucose production in postprandial period, which maintain glucose concentrations at 150–160 g/dL in the first hour of OGTT. The second half is responsible for the gradual decrease in plasma glucose concentration. IFG will show a reduction in the first phase of insulin secretion and a greater increase in glucose concentrations at 60 and 30 min, as normally, the response following glucose ingestion is late. Changes in the second phase of insulin secretion are associated with muscle insulin resistance in IGT and showed through constant concentration of plasma glucose concentrations after 60–120 min during OGTT [15].
3.5 Prediabetes screening
Screening is defined as the utilization of tests to identify unrecognized diseases among apparently healthy populations. Screening is used for early identification of disease in the population and thus leads to early intervention to reduce the individual risk of the disease. Screening for undiagnosed diabetes is not recommended in the general population as the screening may be poorly targeted and may fail to reach the intended group at risk of diabetes; therefore, screening should be done in high-risk individuals to increase the likelihood of diabetes detection and to maximize the cost-effectiveness of screening testing [3].
There are currently two widely used screening tests to identify people with prediabetes and to diagnose type 2 diabetes, CDC and ADA screening tools [11]. These include the oral glucose tolerance test and the fasting glucose concentration.
After the food ingested is broken down into sugars, glucose molecules will then be absorbed into the plasma stream and transported to the cells in the body. In response to the elevated glucose concentration, pancreatic beta cells will secrete insulin. Insulin facilitates the transportation of glucose from the plasma stream into cells. Insulin also inhibits gluconeogenesis in the liver and facilitates the storage of glucose in the form of glycogen (glycogenesis) and fats (de novo lipogenesis (DNL)), which serve as short- and long-term stores of energy, respectively [16].
Plasma glucose homeostasis is maintained at 4–6 mmol/L. This homeostasis is influenced by several factors, such as the functional capacity of pancreatic beta-cells and cellular (skeletal muscles, liver and adipose tissue) sensitivity to insulin. In diabetes mellitus, there is either insulin deficiency or insulin resistance. Dysfunction in the production or uptake of insulin may disturb the plasma glucose homeostasis [16].
The oral glucose tolerance test (OGTT) is considered the gold standard for diabetes and prediabetes diagnosis. OGTT provides a serial measurement of plasma glucose after a specific amount of glucose is given orally to evaluate the individuals. OGTT may help distinguish IFG grom IGT and is reported to have a higher sensitivity than fasting plasma glucose measurement. However, the regular use of OGTT is limited by the need for an overnight fast, a lengthy testing time, higher cost, intra-individual variability, differences in glucose absorption rate and low reproducibility [17, 18].
OGTT should be avoided in hospitalized, acutely ill or inactive patients. Before starting the test, the discontinuation of medications that affect glucose tolerance should be confirmed. The test should be done in the morning with 75-gram glucose load orally in nonpregnant adults and 15 grams in children (1.75 g/kg up to 75 grams). The glucose should be dissolved in 300 mL water and ingested within 5 min period [17, 18].
Theoretically, fasting insulin and glucose concentration could differentiate beta-cell dysfunction from insulin resistance; however, in homeostasis model assessment (HOMA), there exists a feedback loop from fasting insulin which hinders the precision and interpretation of assay results. Overnight basal plasma glucose concentrations are lower than stressed fasting glucose concentrations. As hyperglycemia results from the combination of beta-cell dysfunction and insulin resistance, the degree of hyperglycemia does not directly associate with either degree of beta-cell dysfunction or insulin resistance. Therefore, the fasting plasma insulin concentration should be measured in a 15-min period from a rested patient to avoid confounding effects of oscillatory release and stress [19].
HOMA index (Insulin * glycemia in μmol/L/22.5) has been used widely in epidemiological studies for insulin sensitivity assessment. The beta-cell function was estimated from responses to a hyperglycemic clamp and intravenous glucose tolerance test. In 2000, the QUICKY index (1/log insulin + log glycemia in mg/dL) was introduced and compared with the HOMA index for measuring insulin sensitivity. HOMA index was able to discriminate the increase in insulin resistance in diabetic patients with impaired glucose tolerance and healthy person; however, the QUICKI index was shown to be more sensitive and more straightforward in discriminating the two [19, 20].
Random plasma glucose concentration is reported to have 63% sensitivity and 87% specificity in detecting diabetes disease. Individuals with glucose concentrations indicative of diabetes should then undergo a second confirmatory test. On the other hand, fasting plasma glucose has modest sensitivity for prediabetes screening, with 56% sensitivity and 97.7% specificity [17].
Glycated hemoglobin with a 48 mmol/mol cutoff has 68.4% sensitivity and 95.9% specificity for diabetes diagnosis; however, its use in prediabetes screening is complicated by the varying definitions from ADA and the International Expert Committee (IEC). A previous meta-analysis has shown that HbA1c was neither sensitive nor specific in detecting prediabetes, with a mean sensitivity of 49% and specificity of 79% [17].
Previous studies evaluated the reproducibility of both IGT and IFG and showed that the kappa coefficient for IGT indicated a poor-to-fair agreement (0.04 to 0.56), while it indicated a moderate agreement for IFG (0.22 to 0.56). The reproducibility of IGT was reported at 33–48%, whereas IFG was 51–64%. These results suggested that the reproducibility of prediabetes defined by FPG or 2hBG (~50%) is lower than that for diabetes (> 70%). However, there is limited data on the reproducibility of HbA1c for prediabetes [14].
Studies have found biological variability of glycemic markers, which drove the ADA diagnostic recommendation to repeat glycemic marker testing (glucose or/and HbA1c) on two different occasions to minimalize a false-positive diagnosis. However, the latest study suggested that the diagnosis of diabetes based on HbA1c and FBG tested on a single plasma test had a high positive predictive value for subsequent diagnosis and thus strongly associated with clinical endpoints. Therefore, the use of a single simple confirmatory test may limit the effect of biological variability in individual tests and the collection of a second plasma sample, and thus decrease the rate of false-positive diagnosis and increase its specificity [14].
ADA recommendations for diabetes and prediabetes screening in asymptomatic adults include adults aged over 45 years and any overweight adults with at least one risk factor for diabetes. The risk factors include previously tested hemoglobin A1c concentration over 39 mmol/mol, impaired glucose tolerance test (> 7.8 mmol/L), or impaired fasting glucose concentration (> 5.6 mmol/L), first-degree relative with type 2 diabetes, high-risk ethnicity (black, Hispanic, Native American, Alaska Native, Asian, Pacific Islander, or Native Hawaiian), gestational diabetes in women, history of cardiovascular disease, hypertension, high-density lipoprotein cholesterol level of 1.9 mmol/L or less, or triglyceride level of 2.8 mmol/L or more, polycystic ovarian syndrome in women, physical inactivity and other clinical conditions associated with insulin resistance (severe obesity and acanthosis nigricans) [11].
Tinajero and Malik suggested that prediabetes screening should be done earlier (≥ 30 years old) in groups with higher risk of type 2 diabetes and at lower BMI levels (≥ 23 kg/m2). Screening should include the measurement of visceral adiposity (waist circumference) with ethnic-specific cutoff values. Pregnant women should be screened for gestational diabetes early in the second trimester, while high-risk women should be screened earlier [1].
If the fasting glucose concentration, 2-h plasma glucose concentration and/or hemoglobin A1c concentration are within the normal limit, ADA recommended repeated testing at 3-year intervals and annually for individuals with prediabetes [1].
The US Preventive Services Task Force (USPTF) recommended screening for overweight or obese adults aged 40–70 years and adults with at least one of the following risk factors: family history of diabetes, history of gestational diabetes or polycystic ovarian syndrome and high-risk ethnicity. If results are within the normal range, the testing should be repeated at 3-year intervals [21, 22].
Special consideration is needed for ethnic/racial minorities with higher prevalence and complications. Population with African American, American Indians or Alaskan Natives, Asian Americans, Hispanic or Latinos, or Native Hawaiians or Pacific Islanders ethnicity should be considered for screening at a younger age or at a lower BMI cutoff [17].
3.6 Intervention
Management for prediabetic patients may delay or prevent the development of diabetes mellitus. Both ADA and European Society of Cardiology (ESC) recommended a combination of lifestyle modifications and pharmacotherapy for the management of prediabetes [2]. These include optimal glycemic control, lipid-lowering therapy for cardiovascular disease prevention, anti-hypertensive treatment and aspirin therapy for cardiovascular disease prevention if needed [17].
There are currently four approved medications for prediabetes state: Metformin, Pioglitazone, Acarbose and Liraglutide. The American Association of Clinical Endocrinologists (AACE) has also proposed three weight loss therapies: orlistat, lorcaserin and phentermine/topiramate ER; and lastly, the American College of Surgeons Bariatric Surgery Center Network (ACS-BSCN) has also proposed bariatric surgery as a possible intervention for type 2 diabetes and also prediabetes in order to aid body mass loss (Figure 2) [10].
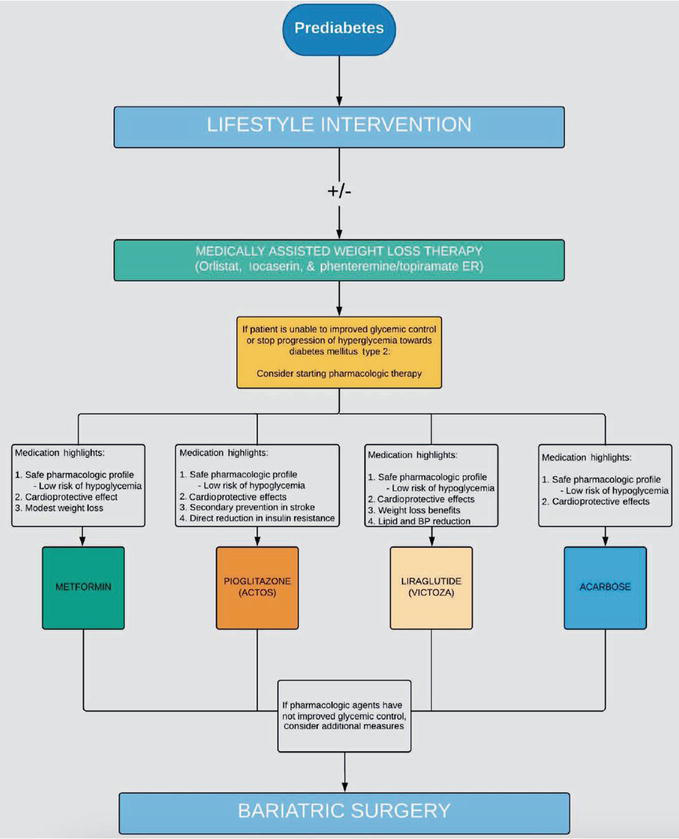
Figure 2.
Management algorithm for people without overt diabetes [
A long-term adherence to lifestyle modification is required in order to ensure the benefits of preventing overt diabetes. Motivational therapy is needed to ensure timely and optimal management of prediabetes and should be personalized based on the patient’s needs, preferences and values. The American Association of Diabetes Educators encouraged the primary care physician to utilize a comprehensive framework for healthy behaviors in the prediabetic population. Common elements in these approaches include digital education program, professional health coaching, tracking and group therapy support [23].
3.7 Reversal of prediabetes to Normal glucose regulation
Several studies on diabetes prevention have reported cases where subjects experienced a restoration of normal glucose regulation. Lifestyle modification with supervision from a physiotherapist was reported to have declines in body weight (2.3–3.7%) and experienced glycemic improvements accompanied by favorable changes in plasma pressure, lipids and hyperinsulinemia and restoration of acute insulin secretory response to glucose [13].
Bariatric surgery has been associated with the reversal or prevention of type 2 diabetes through weight loss, improved insulin sensitivity and alteration in inflammatory cytokines. A previous study showed a ~ 90% reduction in type 2 diabetes incidents during a 15 years period in subjects who underwent bariatric surgery [13].
Prediabetes state has long been associated with micro- and macrovascular complications. A previous study showed that the reversal of prediabetes to normal glucose regulation was associated with lower risk (~56%) of incident diabetes mellitus. The number of times a patient achieved normoglycemic was shown to have a direct association with the risk reduction in the diabetes incidence: 47% if normoglycemia was performed once, 61% if achieved twice and 67% if achieved thrice. This risk reduction was independent of the prior treatment group [13].
The decreased incidence of diabetes mellitus in patients experiencing the reversal to normal glucose regulation had been associated with the corresponding reduction in the prevalence of microvascular disease after a 15-years follow-up. Regression of the prediabetes state into normal glucose regulation was also associated with a reduction in macrovascular risk. The reversion from 2hPG-defined prediabetes to normoglycemia was associated with a reduced risk of cardiovascular disease and mortality [13].
In a previous meta-analysis, the annual relative risk of regression of IGT to normoglycemia was estimated at 33%. This study also mentioned the high variability of the regression based on 2hBG concentrations. In the British Whitehall study, individuals with prediabetes (defined by HbA1c) were less likely to regress to normoglycemia than individuals with isolated IFG or IGT or a combination of both in a 5 years follow-up [14].
4. Conclusion
Prior to the onset of diabetes mellitus, a state known as prediabetic precedes. Similar to diabetes, prediabetes is also associated with macro- and microvascular complications and carries a higher risk for the development of diabetes. Early and effective screening may aid in earlier intervention and result in the delay and even prevention of overt diabetes. Studies have shown that prediabetes individuals may revert to normal glucose regulation with effective interventions and therefore may help reduce the burden of diabetes disease in the general population. Screening for prediabetes, either with fasting glucose concentration, 2-h plasma glucose concentration or hemoglobin A1c concentration, should be done in a high-risk population instead of the general population to maximize the cost-effectiveness of screening tests.
Conflict of interest
There are no conflicts of interest regarding the publication of this article.
References
- 1.
Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes. Endocrinology and Metabolism Clinics of North America. 2021; 50 (3):337-355. DOI: 10.1016/j.ecl.2021.05.013 - 2.
Rett K, Gottwald-Hostalek U. Understanding prediabetes: Definition, prevalence, burden and treatment options for an emerging disease. Current Medical Research and Opinion. 2019; 35 (9):1529-1534. DOI: 10.1080/03007995.2019.1601455 - 3.
Peer N, Balakrishna Y, Durao S. Screening for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2020;(6):5. DOI: 10.1002/14651858.CD005266.pub2 - 4.
Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. The Lancet Diabetes & Endocrinology. 2018; 6 (5):361-369. DOI: 10.1016/S2213-8587(18)30051-2 - 5.
Toledano Y, Knobler H. Not all patients with type 2 diabetes are equal. The American Journal of Medicine. 2021; 134 (6):707-709. DOI: 10.1016/j.amjmed.2021.02.005 - 6.
Ley SH, Schulze MB, Hivert M, Meigs JB, Hu FB. Diabetes in America. 3rd ed. Vol 1. National Library of Medicine’s Bookshelf. Chapter 13 Risk Factors for Type 2 Diabetes. 2017 - 7.
Ismail L, Materwala H, Al Kaabi J. Association of risk factors with type 2 diabetes: A systematic review. Computational and Structural Biotechnology Journal. 2021; 19 :1759-1785. DOI: 10.1016/j.csbj.2021.03.003 - 8.
Laakso M. Biomarkers for type 2 diabetes. Molecular Metabolism. 2019; 27 :S139-S146. DOI: 10.1016/j.molmet.2019.06.016 - 9.
Davarpasand T, Hosseinsabet A. Prediabetes, heart mechanics, and echocardiography: A narrative review. Echocardiography. 2021; 38 (2):304-313. DOI: 10.1111/echo.14929 - 10.
Zand A, Ibrahim K, Patham B. Prediabetes: Why should we care? Methodist DeBakey Cardiovascular Journal. 2018; 14 (4):289. DOI: 10.14797/mdcj-14-4-289 - 11.
Dugan J, Cantillep A, Newberry K, Shubrook J. A call to action on prediabetes. Journal of the American Academy of Physician Assistants. 2018; 31 (10):26-30. DOI: 10.1097/01.JAA.0000545064.33107.8f - 12.
Janet Mary G, Karthiga KA. Prediabetes: Prevalence, screening, risk factors, and interventions: A review. IP Journal of Nutrition, Metabolism and Health Science. 2020; 2 (1):8-13. DOI: 10.18231/j.ijnmhs.2019.002 - 13.
Sallar A, Dagogo-Jack S. Regression from prediabetes to normal glucose regulation: State of the science. Experimental Biology and Medicine. 2020; 245 (10):889-896. DOI: 10.1177/1535370220915644 - 14.
Echouffo-Tcheugui JB, Kengne AP, Ali MK. Issues in defining the burden of prediabetes globally. Current Diabetes Reports. 2018; 18 (11):105. DOI: 10.1007/s11892-018-1089-y - 15.
Mahat RK, Singh N, Arora M, Rathore V. Health risks and interventions in prediabetes: A review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019; 13 (4):2803-2811. DOI: 10.1016/j.dsx.2019.07.041 - 16.
Mathew TK, Zubair M, Tadi P. Blood Glucose Monitoring. 2023 Apr 23. In: StatPearls [Internet]. Treaseure Island (FL): StatPearls Publishing; 2023 Jan. PMID: 32310436 - 17.
Duan D, Kengne AP, Echouffo-Tcheugui JB. Screening for diabetes and prediabetes. Endocrinology and Metabolism Clinics of North America. 2021; 50 (3):369-385. DOI: 10.1016/j.ecl.2021.05.002 - 18.
Gurung P, Zubair M, Jialal I. Plasma Glucose. 2023 Jan 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. PMID: 31082125 - 19.
Matthews DR, Hosker JR, Rudenski AS, Naylor BA, Treacher DF, Turner RC, et al. Homeostasis model assessment: Insulin resistance and fl-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28 (7):412-419. DOI: 10.1007/BF00280883 - 20.
Hrebícek J, Janout V, Malincíková J, Horáková D, Cízek L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. The Journal of Clinical Endocrinology and Metabolism. 2002; 87 (1):144-147. DOI: 10.1210/jcem.87.1.8292 - 21.
Jonas DE, Crotty K, Yun JDY, Middleton JC, Feltner C, Taylor-Phillips S, et al. Screening for prediabetes and type 2 diabetes: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA-Journal of The American Medical Association. 2021; 326 (8):744-760. DOI: 10.1001/jama.2021.10403 - 22.
US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force Recommendation Statement. JAMA-Journal of The American Medical Association. 2021; 326 (8):736-743. DOI: 10.1001/jama.2021.12531 - 23.
Kalra S, Singal A, Kapoor N. Prediabetes: A pragmatic approach to counselling and coaching. Journal of the Pakistan Medical Association. 2022; 72 (4):771-772. DOI: 10.47391/JPMA.22-29