Differentiating rheumatoid arthritis from psoriatic arthritis.
Abstract
Rheumatoid arthritis (RA) and psoriatic arthritis (PsA) are chronic inflammatory illnesses categorized by joint pain and swelling, along with systemic symptoms. The distinction between RA and PsA may be difficult to determine since their clinical presentations and symptoms are so similar. RA and PsA are treated in a palliative manner since they are not curable diseases. Allopathic medicines have serious side effects, and long term-consumption decreases patient quality of life. Hyperacidity, edema, stomach ulcers, gastrointestinal bleeding, perforation, and reduced appetite are some of the most common adverse effects. Curcumin, the primary active component within Curcuma longa (turmeric), has been demonstrated to be helpful in treating RA and PsA, with effectiveness attributed to its mode of activity. This chapter defines the correlation between RA and PsA and reports on the use and mechanism of curcumin in the management of these conditions. According to various literature surveys and evidence, it can be concluded that curcumin is a safe and effective therapeutic option for managing RA and PsA compared to synthetic medications.
Keywords
- rheumatoid arthritis
- psoriasis
- psoriatic arthritis
- curcumin
- anti-inflammatory
- Immunomodulator
1. Introduction
Rheumatoid arthritis (RA) is a severe immune-mediated condition that impacts more women than men and is most common among the elderly. In 2002, the prevalence rate was reported to range from 0.5–1% of the population, with geographical variation [1]. Synovial joint inflammation and deformity, and inflammation of surrounding tissues such as tendons, ligaments, and muscles, characterize this condition. Although RA is associated with an aberrant immune response, the basic causes and pathophysiology of the illness are still unknown. It is said to be induced by aberrant immune system responses as a consequence of numerous hereditary and environmental variables [2]. These immune system alterations might occur several years before patients begin to experience symptoms. RA damages the lining of synovial joints, causing increasing disability, early mortality, and economic hardship. Arthralgia, edema, and erythema, as well as reduction in degree of movement, are all symptoms of symmetrical joint activation [3]. Mild RA is defined as presence of symptoms for less than 6 months, while developed RA is defined as presence of symptoms for longer than 6 months. Joint inflammation and injury that worsens over time can lead to disability and a lower quality of life [4].
Psoriasis is an illness that mostly damages the skin or joints and affects 2–3% of the population. The heart, aorta, and lungs can also be impacted by the condition. The percentage among sufferers with psoriasis who progress to psoriatic arthritis (PsA) remains unclear, with rates varying from 6–42% in various studies. According to one comprehensive study, PsA impacts up to 24% of psoriasis sufferers. Psoriasis often develops 8 to 10 years before PsA. Because both RA and PsA are immune-mediated chronic inflammatory illnesses with similar pathophysiology, they should be treated together to reduce pharmaceutical adverse effects and costs [5].
PsA is a recurrent, inflammatory musculoskeletal condition that is associated with skin psoriasis and is seronegative. It affects men and women equally around the ages of 40 and 50 [6]. Peripheral and axial joints, enthesis, skin, and nails are among the organ system that are impacted. PsA is correlated with comorbidities including osteoporosis, uveitis, subclinical intestinal inflammation, and illness [7].
Identification of PsA has been challenging due to its heterogeneity. However, categorization parameters including CASPAR (ClASsification criteria for Psoriatic ARthritis) [8] as well as various screening methods have simplified the diagnostic process for general practitioners, dermatologists, and rheumatologists. Estimates of prevalence within the general population are quite variable, owing to variances in epidemiological study methods [9]. The estimated frequency of PsA ranges from 0.16 to 0.25% [10], according to updated categorization criteria. PsA is characterized by synovial hyperplasia, immune cell infiltration, and proliferation of both the skin and synovium. Ache, edema, and physical discomfort affect sufferers’ ability to work effectively in everyday activities, leading to a decreased standard of living [11].
Both RA and PsA are severe inflammatory conditions marked by joint discomfort and edema as well as systemic symptoms. Both can cause joint injury as well as loss of function if not detected early. Consequently, early detection is critical for developing treatment methods that will improve therapeutic and radiological results [12].
Clinically, distinguishing between RA and PsA is complicated because their clinical presentations and symptoms are so similar. Both conditions are connected to prevalent varieties of arthritis, including gout or secondary osteoarthritis (OA), and share parallels with other inflammatory disorders [13].
Traditional medicine like curcumin has been used in conjunction with pharmaceuticals to manage RA and PsA. Treatment for these conditions aims to alleviate inflammation and prevent progression of the disease, including irreversible bone loss, preserve joint and muscle function, and reduce disease activation. Nonsteroidal anti-inflammatory drugs (NSAIDs), immunosuppressive agents, slow-acting antirheumatic drugs, immunological and biological agents, and botanicals are among the medications used.
First-line treatment to slow illness progression or decrease discomfort involves the use of biologic disease-modifying antirheumatic medications such as tocilizumab (an interleukin 6 (IL-6), sarilumab (an IL-6 inhibitor), or abatacept (an CD80/86 inhibitor). The mechanism of action of such medicines differs according to the drug used, but the main impact is to reduce inflammatory mediators and induce death in dysfunctional autoimmune cells. Despite their great efficacy, these drugs are hindered by their high costs [14]. NSAIDs are an alternative approach. Although these drugs are helpful exclusively for moderate instances of PsA, they cannot completely reduce symptoms [15].
Turmeric (
2. Epidemiological characteristics of rheumatoid arthritis and psoriatic arthritis
RA and PsA have different epidemiological characteristics [18]. RA affects more than one million people in the United States [19], whereas PsA affects around half a million people [20]. Psoriasis affects roughly 30% of individuals [21]. RA and PsA are prevalent in different regions of the world. RA is common in various populations, with prevalence rates ranging from 0.5 and 1.0%; however, prevalence is significantly greater among Native American Indian communities (5–7%) but lower in China and Japan (0.2–7%) [22, 23, 24]. The ubiquity rate of PsA in the United States and Europe ranges from 0.1 to 0.4%, whereas it is lower in Japan. This diversity of incidence shows that risk of illness is impacted by both environmental and hereditary variables [25, 26].
3. Pathogenesis of rheumatoid arthritis and psoriatic arthritis
Clinically, RA and PsA are both comparable and distinct. This is probably due to underlying genetic variation, with some genes involved in pathogenesis being shared by both diseases and others contributing to each disease’s unique pathogenesis [27]. According to studies, each illness is produced due to integrated and complicated signaling pathways that impact various immune responses that perform distinct roles in disease pathogenesis [28]. It is apparent that innate or adaptive immunological reactions are involved [29]. T cells, B cells, and the coordinated interplay of pro-inflammatory cytokines all play important roles in the pathogenesis of RA. In PsA, activated T lymphocytes and macrophages perform a crucial function [30].
3.1 Rheumatoid arthritis
RA susceptibility is mostly determined by genetic factors, with heritability varying between 50 and 60%. More than 30% of genetic risk is accounted for by the human leukocyte antigen (HLA) locus, while non-HLA genes, such as tumor necrosis factor-alpha (TNF-alpha), have also been identified [31]. The pathophysiology of RA is characterized by a complex interaction between the adaptive and innate immune systems, as well as inflammatory infiltrates in the synovium and synovial fluid. Key contributors to the disease include dendritic cells, mast cells, neutrophils, and macrophages. T cells and B cells generate cytokines, antibodies, and immunological complexes, with the onset of RA attributed to phosphoinositide-3-kinase (PI3K) delta and gamma signaling molecules associated with T cells and B cells, as well as neutrophil and mast cell activity. The production of pro-inflammatory cytokines and chemokines, along with dysregulation of three essential T-helper (TH) cell subtypes—TH1, TH17, and regulatory T (Treg) cells—play crucial roles in the initiation and progression of RA [32].
3.2 Psoriatic arthritis
According to family research, the genes linked with PsA are HLA-CW*0602, IL-23r, and IL-12b [33]. The unavailability of established genetic vulnerability sites may also be associated with reduced incidence and greater variability of PsA [34]. Genetically predisposed people who have a dysfunctional immune response are hypothesized to develop PsA, resulting in immune cell infiltration and cytokine production. Infiltrating cells such as activated T cells and macrophages are assumed to perform a key role in generating inflammatory or inflammatory and degenerative events within joint tissues, along with skin psoriasis [35]. Inflammatory cytokines released via T cells, including IL-1, IL-2, IL-10, interferon (IFN), and tumor necrosis factor (TNF), are abundant in the synovium [36]. Development of PsA is linked to the IL-22 and IL-23/Th17 axis [37]. In matched PsA synovial tissue and skin samples, the expression of IL-17 genes was higher in the skin compared to the synovium, but the overexpression of TNF pathway was equivalent between both sites. The expression of angiogenesis-related genes and IL-6 was elevated in the synovium but not in the skin [38]. According to an assessment of PsA etiology, there may be four clinical phenotypes that are determined by genotype: synovial predominant, entheseal predominant, axial predominant, and mutilans [39].
4. Difference between rheumatoid arthritis and psoriatic arthritis
RA is characterized as an immune-mediated, persistent, inflammatory condition that causes symptoms like synovitis, cartilage degeneration, and bone destruction. PsA is also an autoimmune systemic illness characterized by numerous radiological symptoms [40]. Table 1 lists the features of RA and PsA.
| Features | Rheumatoid arthritis | Psoriatic arthritis |
|---|---|---|
| No. of affected joints | 30–50% with arthritis | Predominant: polyarthritis |
| Contribution of joints | like distal interphalangeal joints is the examle. | Usually, distal interphalangeal joints |
| Enthesitis | Typically, medically diagnosed in 60–80% | Not typical |
| Dactylitis | Present in 30% | Not typical |
| Axial involvement | Axial spondylarthritis phenotypes | Erosive cervical disease |
| Skin, nail disease | 80% in psoriasis and 60% in nail disease | Reduced community threat or background threat |
| Serology | RF and CCP are generally negative | RF or CCP are generally positive |
| Radiographic alterations | Periosteal new bone formation (uncommon especially in early disease) | Erosion or osteopenia |
5. Clinical characteristics of rheumatoid arthritis and psoriatic arthritis
RA categorization guidelines were created by the American College of Rheumatology (ACR) and the European League Against Rheumatism (ELAR) for patient identification or application in the clinical trials [41]. Confirmation of unambiguous, persistent clinical synovitis in at least one joint is the most important clinical feature of RA. The number of afflicted joints, duration of symptoms, and existence of serological indicators or an increased acute-phase reactant round out the criteria for RA. PsA classification criteria are used to classify individuals having inflammatory articular illness for clinical studies in psoriatic arthritis [42]. Psoriasis, psoriatic arthritis, psoriatic nail degradation, and dactylitis are all important clinical features. Neither categorization nor diagnostic criteria should be conflated. In RA, joint involvement is generally symmetric, but joint association in PsA is frequently. Individuals with RA mostly experience polyarthritis (five joints affected), however joint involvement can also be oligoarticular or polyarticular [43].
RA usually affects the wrists, shoulders, elbows, and metacarpophalangeal joints. PsA usually affects the distal interphalangeal joints of the hands and feet, major joints of lower extremities, the axial spine, and sacroiliac joints, as well as the metacarpophalangeal joints. Due to the potential damage to the axial skeleton by PsA, it is classified within the spondylarthritis spectrum rather than as RA. This includes involvement of areas such as the sacroiliac joint and spine [44].
About 35% of individuals with PsA experience enthesis (inflammation of attachment points of tendons or ligaments), but enthesis is rare in RA [45]. In PsA, dactylitis (inflammation of whole digit) is a frequent symptom, occurring in approximately 50% of sufferers. Dactylitis affects only around 5% of RA sufferers [46].
Extra-articular symptoms of RA and PsA include ocular problems [47]. Keratoconjunctivitis sicca is a frequent ocular symptom of RA, affecting about 18% of individuals [48].
Both RA and PsA cause cutaneous symptoms. Rheumatoid nodules, vasculitis skin lesions, and granulomatous dermatoses are the most frequent cutaneous manifestations of RA. In 84% of patient with PsA, skin symptoms manifest before or predate the appearance of joint problems. Approximately 96% of people with PsA either present with or have a history of and even a genetic background of psoriasis [49, 50].
6. Diagnosis of rheumatoid arthritis and psoriatic arthritis
6.1 Rheumatoid arthritis
Two indicators commonly used for diagnosing RA are anti-cyclic citrullinated antibodies (anti-CCP) and rheumatoid factor (RF). Meta-analysis studies suggest that anti-CCP exhibits either identical or greater sensitivity (67% for anti-CCP vs. 69% for RF) and greater specificity (95% for anti-CCP vs. 85% for RF) compared to RF. Moreover, anti-CCP has a stronger predictive value for the onset of erosive illness. It is important to note that while RF and anti-CCP are highly specific, they can also be found in a wide range of other illnesses [51].
In patients with RA, antinuclear antibodies (ANAs) and anti-double-stranded DNA (anti-dsDNA) antibodies are commonly detected. However, it is worth considering that the use of TNF monoclonal antibodies like infliximab for managing RA may elevate the serum concentration of ANA and anti-dsDNA [52].
6.2 Psoriatic arthritis
Diagnosing PsA can be challenging because of the many similarities between it and other rheumatological diseases such as RA, OA, and gout. Furthermore, between 10 and 15% of undiagnosed psoriatic patients in dermatological clinics are undiagnosed, with a delay in diagnosis also connected with poorer disease outcomes [53]. In theory, diagnostic testing for PsA is not available; instead, individuals are diagnosed depending only clinical features. Symptoms are typically diverse, with “domains” such as peripheral arthritis, enthesitis, dactylitis, axial illness, psoriasis, and nail diseases occurring frequently [54].
7. Comorbidities associated with rheumatoid arthritis and psoriatic arthritis
Comorbidities associated with autoimmune inflammatory illnesses include infections, cancer, depression and anxiety, cardiovascular difficulties, non-alcohol fatty liver disease (NAFLD), and obesity [55].
Table 2 lists important comorbidities associated with RA and PsA.
| S.no | Comorbidities | Rheumatoid arthritis | Psoriatic arthritis |
|---|---|---|---|
| 1. | Cardiovascular | RA is associated with an increased risk of cardiovascular illness, with the greatest incidence occurring among the elderly [56]. There has been evidence of a heightened incidence of atrial fibrillation, stroke, and hypertension [57]. Even after accounting for frequent cardiovascular comorbidities or risk factors, systemic inflammation increases the potential of cardiovascular mortality in RA sufferers [58]. | PsA is associated with a greater incidence of myocardial infraction, angina, and hypertension [59]. Level of severity of PsA is a major determinant of cardiovascular morbidity, along with established risk factors for cardiovascular conditions such as diabetes and hyperlipidemia. |
| 2. | Malignancies | Increase in non-Hodgkin’s lymphoma, Hodgkin’s condition, cancer of lungs and skin cancer of nonmelanoma, were reported in a investigation of 20,699 individuals with rheumatoid arthritis between 1977 and 1987, previous studies demonstrate link among rheumatoid arthritis and non-Hodgkin’s lymphoma, Hodgkin’s disease, lung cancer [60]. Other community-based studies found that smoking-related malignancies are 20–50% more likely and nonmelanoma skin cancer is 470% more likely in patients with RA. The majority of cancers are linked to psoriasis and RA not PsA, indicating a disease-specific relationship. Several variables, including patient characteristics [61], illness features, and lifestyle factors, all have an impact [62]. | In a cohort study of PsA, around 10.2% of participants got cancer; however, this rate does not vary from the rate of cancer in the overall community [63]. Malignant neoplasms were shown to be the third leading cause of mortality (17.0%) in patients with PsA [64], after cardiovascular and respiratory system illnesses [64]. |
| 3. | Nonalcoholic fatty liver disease (NAFLD) | NAFLD affects 10–24% of people in the normal community whereas it impacts 57.5–74% of the obese population [65]. According to the analysis of autopsy histologic liver discomfort in RA patients, fatty alterations were seen in 42 of 182 cases (23%) [66]. | PsA was demonstrated to be a determinant of NAFLD in individuals with psoriasis in an Italian study [67] regardless of age, gender, BMI, or obesity. |
Table 2.
Comorbidities associated with rheumatoid arthritis and psoriatic arthritis.
8. Treatment of rheumatoid arthritis and psoriatic arthritis
Treatment methods for RA and PsA may differ due to variations in disease etiology and therapeutic response. Medications that target upstream factors like TNF seem to be beneficial for both PsA and RA. However, medications targeting downstream cytokines show high condition-specific efficacy. For example, targeting IL-6 is highly effective in RA, while targeting IL-17A is effective in PsA but not necessarily in both diseases.
Disease-modifying antirheumatic drugs (DMARDS) have long been utilized for managing rheumatological diseases, but their long-term therapeutic benefits in PsA are primarily based on clinical experience rather than comprehensive trial-based study. PsA can be treated with a number of approaches, including nonpharmacological approaches such as weight loss, smoking cessation, and exercise. NSAIDs and corticosteroid injections are utilized to alleviate symptoms.
8.1 Nonsteroidal anti-inflammatory drugs
Patients benefit from treatment with NSAIDS for alleviation of symptoms, but NSAIDs do not completely resolve or stop joint deterioration or the psoriatic component of PsA [68]. Indeed, no changes in psoriasis area severity index (PASI) score or erythrocyte sedimentation rate (ESR) were identified in controlled investigations [69].
8.2 Conventional synthetic disease-modifying antirheumatic drugs
For the management of RA, methotrexate is the most prescribed conventional synthetic disease-modifying antirheumatic medication (csDMARD). Methotrexate is reported to decrease disease intensity and increase quality of life in individuals with PsA. Leflunomide has proven to be more effective than placebo in treating patients with PsA [70].
8.3 Biologic disease-modifying antirheumatic drugs
8.3.1 TNF-α inhibitors
TNF-α is a proinflammatory cytokine overexpressed in the synovium of inflammatory arthritis patients. The US Food and Drug Administration (FDA) has approved five TNF inhibitors for the treatment of RA and PsA: etanercept, infliximab, adalimumab, golimumab, and certolizumab pegol. TNF inhibition in RA inhibits bone degradation as well as reduces injury via reducing osteoclast production to decrease systemic inflammation. TNF-α inhibitor’s capacity to prevent the production of matrix metalloproteinases and aggrecanases has been proposed as a way to decrease cartilage matrix degradation. TNF-α inhibitors diminish synoviocyte hyperproliferation and T-cell and microphage infiltration in PsA, decreasing synovial thickness. TNF-α inhibitors control angiogenesis and osteoclastogenesis in PsA and decrease synovitis [71].
Inhibitors of IL-17A target various immune cells including T helper (Th) 17 cells, macrophages, mast cells, dendritic cells, natural killer cells, and CD8+ T cells, all of which release IL-17A, a proinflammatory effector cytokine. PsA patients had increased levels of IL-17A in their synovial fluid, which has been linked to pathogenic angiogenesis, osteoclastogenesis, and fibrogenesis in studies. Interactions between IL-17A and synovial-like fibroblast, osteoblast, and osteoclast precursors encouraged persistent inflammation and bone alterations in PsA, which contribute to joint destruction 91. Osteoclastogenesis, upregulated matrix metalloproteinases, and another proinflammatory cytokines can also be induced by TNF-α and IL-17. The existence of IL-17-generating T cells in PsA patients is linked to illness performance markers (such as CRP and ESR) or radiographic degradation. Secukinumab and oxekizumab, both IL-17A inhibitors, have been approved by the FDA for the management of active PsA [72].
8.3.1.1 IL-12 and IL-23 dual inhibitor
IL-12 and IL-23 are two key cytokines associated with the progression of psoriasis and PsA. In response to inflammation, monocytes or macrophages release IL-12, which stimulates Th1 cells, while myeloid dendritic cells produce IL-23, a cytokine that regulates the activity of Th17 cells [73]. IL-12 aids in the differentiation of Th1 cells and the activation of natural killer (NK) cells, while IL-23 is linked to osteoclastogenesis and bone degradation.
Ustekinumab, an inhibitor of the IL-12/23p40 subunit, blocks Th1 and Th17 differentiation and downstream IL-17 production. Phase III clinical studies have shown that ustekinumab significantly reduces the radiographic progression of joint damage and improves clinical symptoms in individuals with active PsA [74].
However, ustekinumab has not been found to be effective in individuals with RA. Similarly, guselkumab, a specific inhibitor of IL-23, has shown ineffectiveness in individuals with RA, although initial results in patients with PsA suggest it may help with joint pain. Further research is needed.
In a phase II study of PsA, risankizumab, a specific inhibitor of IL-23, has shown promising effects [75].
8.3.1.2 IL-6 inhibitors
In acute-phase inflammatory reactions, macrophages and T cells generate IL-6, a proinflammatory cytokine. IL-6, like transforming growth factor and IL-23, helps to keep pathogenic Th17 cells differentiated and produce IL-17. IL-6 levels in the synovium have been found to be elevated in individuals with active RA and PsA [76]. Tocilizumab or sarilumab, two IL-6 inhibitors approved by the FDA for the management of RA, decrease illness activity and radiographic joint damage. For the treatment of RA, further IL-6 inhibitors (such as olokizumab) are being developed [77].
8.3.1.3 T-cell activation inhibitors
Abatacept, a fusion protein that interacts with CD80/CD86 ligands on the surfaces of antigen-presenting cells, works by suppressing T-cell stimulation and reducing levels of proinflammatory cytokines and autoantibodies. The FDA has approved abatacept for the management of active RA, particularly in individuals who have not responded well to TNF inhibitors. Additionally, the FDA has authorized abatacept for the management of active PsA. Findings from a phase III trial have demonstrated the effectiveness of abatacept, regardless of prior exposure to TNF inhibitors. However, it is worth noting that in individuals with both psoriasis and PsA, abatacept did not improve skin symptoms compared to placebo [78].
8.3.1.4 CD20 inhibitors
B cells play a significant role in RA by generating autoantibodies, which are immune proteins that mistakenly target and react with a person’s own tissues or organs. Additionally, B cells produce various cytokines and express the receptor activator of nuclear factor kappa B ligand (RANKL), which promotes the development and activation of osteoclasts. B cells express CD20 molecules, and inhibiting them leads to B cell loss and direct downregulation of RANKL and proinflammatory cytokines.
Rituximab, a CD20 inhibitor, is approved by the FDA for the treatment of RA in individuals who have not responded to TNF inhibitors when used in conjunction with methotrexate. Clinical trials have shown the efficacy of rituximab in treating RA. However, rituximab has not been found to be helpful in individuals with PsA, possibly because they lack circulating autoantibodies [79].
8.4 Targeted synthetic oral small-molecule disease-modifying drugs
8.4.1 Phosphodiesterase-4-inhibitors
Phosphodiesterase-4 (PDE4) is an enzyme that increases cyclic adenosine monophosphate breakdown, upregulating inflammatory responses by increasing levels of cytokines such as TNF-α, IL-12, and IL-23. Apremilast, a PDE4 inhibitor, is approved by the FDA for the management of active PsA. Approval was based on data from phase III human trials that showed apremilast reduced disease activity and improved clinical outcomes. There have been no studies evaluating apremilast’s potential to slow radiographic progression. Apremilast was demonstrated to decrease TNF release from human rheumatoid synovial membrane cells and diminish clinical disease activity in animal models of RA. Furthermore, in a phase II trial of individuals with RA, apremilast was shown to be ineffective when compared to placebo [80].
8.4.1.1 Janus kinase inhibitors
Janus kinases (JAKs) are cytoplasmic tyrosine kinases that control inflammatory cytokine signaling, such as IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, therefore regulating immune responses. Tofacitinib is approved by the FDA for the management of individuals with active RA who have an inadequate response to or cannot tolerate methotrexate. Treatment with tofacitinib was linked to a reduction in active disease signs and symptoms, enhancement of physical as well as wellbeing condition of life, and a reduction in radiographic progress among individuals with RA in clinical trials. Tofacitinib was found to be effective for both PsA and psoriasis in the phase III Oral Psoriatic Arthritis Trial (OPAL). Tofacitinib’s safety profile was similar among individuals with RA and individuals with PsA. The FDA approved tofacitinib for the treatment of PsA in 2017 [80].
9. Curcumin importance for management of rheumatoid arthritis and psoriatic arthritis
Curcumin, an antioxidant, anti-inflammatory, and immune-modulatory agent, is found in turmeric. It has been found to assist with arthritis and joint pain. Given that persistent inflammation is a key feature of arthritis, it is important to note that curcumin has been reported to be beneficial for both inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). Additionally, curcumin may potentially help reduce neuroinflammation associated with Alzheimer’s disease. Recent studies suggest that curcuminoids, when combined with black pepper, may help alleviate the painful joint symptoms associated with RA, PsA, or OA.
PsA is a type of inflammatory arthritis that is similar to RA but with significant differences. PsA results in shiny, red spots on the skin, often known as “plaques.” Psoriatic symptoms are frequently asymmetric, which means that if one wrist hurts, the other may be symptom free. Curcumin can help the immune response by reducing inflammation, which can help to alleviate joint discomfort, increase mobility, and restore functioning in troublesome regions. Curcumin’s anti-inflammatory properties have been proven in several trials, including investigations of back pain, muscular discomfort, and inflammation associated with asthma and allergies.
The antiarthritic effect of curcumin has been investigated in patients with RA. In one study, 45 participants was randomly allocated to one of three groups: treatment with 500 mg of curcumin, treatment with 50 mg of diclofenac sodium, or a combination of the two. The Disease Activity Scores (DAS) of patients across all three groups showed significant statistical improvement. However, the curcumin group showed superior results and had the highest percentage of recovery. According to the American College of Rheumatology (ACR) score, the curcumin group also exhibited the greatest reduction in pain and joint edema. Curcumin has been demonstrated to be safe and effective in treating RA [79]. Figure 1 shows the effect of curcumin on RA and PsA.
Figure 1.
Effect of curcumin on rheumatoid and psoriatic arthritis.
10. Mechanism of curcumin in the management of rheumatoid arthritis and psoriatic arthritis
Curcumin has great potential for managing both RA and PsA via different mechanisms.
10.1 Rheumatoid arthritis
Arthritis produces inflammation and discomfort, yet its root cause is unknown; thus, treatment of its underlying causes is challenging. Current therapies for arthritis focus on alleviating joint pain resulting from inflammation, normal wear and tear, and muscle pain. Steroids, painkillers, and NSAIDs are commonly prescribed to manage arthritis symptoms, reducing inflammation and extreme pain. However, their long-term use is discouraged due to inadequate pain relief, immunological abnormalities, and significant gastrointestinal and cardiovascular side effects. Therefore, there is a need for herbal treatments with anti-inflammatory properties, especially in managing conditions like RA and OA, given the withdrawal of several FDA-approved anti-inflammatory medications. Phytomedicines and chemical compounds derived from plants have attracted global interest for their potential in managing various debilitating diseases [80].
In addition to its use as a cooking spice, curcumin may be utilized as an alternative treatment for arthritis. It has traditionally been used in Chinese and Ayurvedic medicine as an anti-inflammatory therapy.
Curcumin has shown positive immunomodulatory effects by reducing the generation of pro-inflammatory cytokines as well as dysregulated immune cell activities in RA, including TH1, TH17, and regulatory T (Treg), and B cells. Curcumin has been found to reduce the severity of RA complications by inhibiting the production of pro-inflammatory mediators such as nuclear factor-B (NF-B), activator protein-1 (AP-1), and mitogen-activated protein kinases (MAPKs) in immune cells and synovial fibroblast cells. Curcumin also affects the expression of energy-related transcription factors, including signal transducer and activator of transcription, peroxisome proliferator-activated receptor-c, activator protein-1, cAMP responding element binding protein, estrogen response element, and others. Consequently, turmeric as well its constituents are claimed to possess anti-inflammatory, anti-diabetic, and anti-lipidemic properties. In several types of cultured cells and animal experiments, extracts from the roots of
10.2 Psoriatic arthritis
Curcumin inhibits the action of nuclear factor kappa B (NF-kB) and various inflammatory markers, including tumor necrosis factor-alpha (TNF-a) and interleukin-6 (IL-6). Additionally, it can alleviate discomfort associated with immune-mediated inflammatory diseases, aside from its anti-inflammatory properties.
Studies have demonstrated that curcumin reduces inflammation and symptoms associated with autoimmune collagen-induced arthritis (CIA), which is a common animal model used to study RA. These beneficial effects of curcumin may be attributed to its immunomodulatory action via modulation of T lymphocytes.
Combining acupuncture in the style of Chinese medicine with a daily intake of 500 mg turmeric (
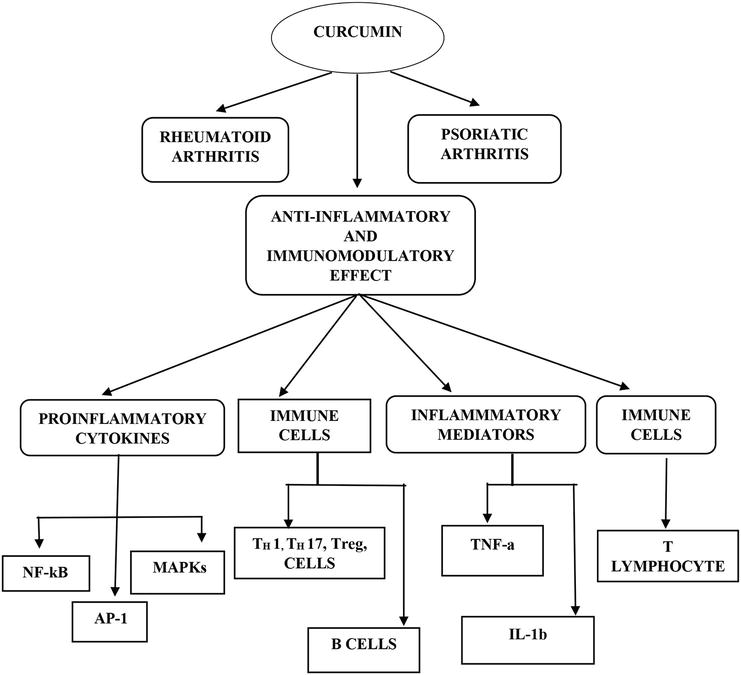
Figure 2.
Diagrammatic representation for mechanism of curcumin in rheumatoid and psoriatic arthritis.
11. Various in vivo and in vitro studies have demonstrated the role of curcumin in the management of rheumatoid and psoriatic arthritis
Table 3 lists some of the studies demonstrating the role of curcumin in managing RA and PsA.
| S.R.NO | Researcher | Work done | References |
|---|---|---|---|
| 1. | Amin et al. | Study of curcumin’s impact on experimentally generated arthritis of the temporomandibular joint. Results show curcumin may be useful as an antioxidant agent for inflammatory and degenerative conditions like arthritis. | [80] |
| 2. | Skyvalidas et al. | Study investigating curcumin’s ability to decrease the production of IFN and IL-17 in psoriasis and PsA patients’ peripheral blood mononuclear cells. Results show that curcumin reduces pro-inflammatory IFN and IL-17 production in vitro in psoriatic illness, suggesting its potential as a dietary immunosuppressant in such individuals. | [81] |
| 3. | Martin et al. | Study of natural medicine therapy in a patient with PsA. Acupuncture, turmeric ( | [82] |
| 4. | Manca et al. | Study of curcumin-loaded hyalurosomes for RA. The ability of these vesicles to downregulate the production of anti-apoptotic proteins IAP1 and IAP2 and stimulate the production of IL-10 while reducing the production of IL-6 and IL-15 and reactive oxygen species was demonstrated in vitro using fibroblast-like synovial cells cultured in synovial fluid. The results suggest the potential use of curcumin-loaded hyalurosomes in controlling the local consequences of RA. | [83] |
| 5. | Wang et al. | Study of curcumin’s therapeutic benefits and pharmacological mechanism in collagen-induced arthritis (CIA) rats. Results show that curcumin has a substantial pharmacological action in lowering the inflammatory response in macrophages and has therapeutic benefits in CIA rats. Its mechanism might be linked to the inhibition of the NF-B signaling pathway and induction of macrophage apoptosis. | [84] |
| 6. | Dewangan et al. | Study of the effectiveness of curcumin-loaded carboxymethyl cellulose acetate butyrate (CMCAB) polymer and the development of well-defined nanoparticles, which improve CUR-CMCAB nanoparticle solubility for RA therapy. It may also be inferred that several phytochemicals, including curcumin, can deliver effective treatments without the negative effects associated with routinely used drugs if properly produced as pharmaceuticals employing specialized carrier systems. | [85] |
| 7. | Jeengar et al. | Study of the capacity of emu oil to penetrate the skin and boost the antiarthritic potential of lipophilic bioactive curcumin, which has weak permeability across biological membranes. In arthritic animals, topical treatment of a curcumin–emu oil combination resulted in significantly lower levels of pro-inflammatory mediators TNF-, IL-1, and IL-6 (p = 0.05, 0.001, and 0.01 1). Topical administration of curcumin with emu oil has the potential to be a noninvasive and effective therapy for inflammatory arthritis. | [86] |
Table 3.
Various in vivo and in vitro studies demonstrating the role of curcumin in the management of rheumatoid and psoriatic arthritis.
12. Conclusion
RA and PsA are both chronic inflammatory, autoimmune conditions that are treated in a palliative manner because they are incurable. The production and use of drugs derived from chemical compounds can have serious negative effects, with long-term usage often decreasing overall quality of life. Curcumin, a compound found in natural vegetation, has garnered attention worldwide for its potential in managing various debilitating diseases. Specifically, it has shown effectiveness in treating RA and PsA. Curcumin interacts with receptors associated with the pathophysiology of these diseases, helping to alleviate symptoms. Arthritis, characterized by persistent inflammation in multiple joints, can be difficult to diagnose due to its chronic nature. Accurate diagnosis is crucial because differences in underlying pathophysiology and medication tolerance can significantly impact treatment outcomes. Curcumin has proven beneficial in effectively treating both diseases without adverse side effects, potentially leading to symptom improvement in both conditions.
Acknowledgments
I acknowledge Invertis University, Bareilly, Pharmacy, for providing the necessary requirement for the accomplishment of this work, I am extremely grateful to our chairmen DR. Umesh Gautam sir for giving me the golden oppurtinity for completion of this article.
Conflict of interest
The author has no conflict of interest, financial or otherwise.
Consent for publication
Not applicable.
References
- 1.
Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Research. 2002; 4 (2):265-272 - 2.
Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: New estimates for a new century. Rheumatology. 2002; 41 (7):793-800 - 3.
van der Linden MP et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis and Rheumatism. 2010; 62 (12):3537-3546 - 4.
Cai Q , Xin Z, Zuo L, Li F, Liu B. Alzheimer's disease and rheumatoid arthritis: A Mendelian randomization study. Frontiers in Neuroscience. 2018; 12 :627 - 5.
Feletar M, Foley P, Brown MA. Developments in psoriasis and psoriatic arthritis. Drug Discovery Today: Disease Mechanisms. 2008; 5 :47-54 - 6.
Chimenti MS, Ballanti E, Perricone C, Cipriani P, Giacomelli R, Perricone R. Immunomodulation in psoriatic arthritis: Focus on cellular and molecular pathways. Autoimmunity. 2013; 12 (5):599-606 - 7.
Sukhov A, Adamopoulos IE, Maverakis E. Interactions of the immune system with skin and bone tissue in psoriatic arthritis: A comprehensive review. Clinical Reviews in Allergy and Immunology. 2016; 51 (1):87-99 - 8.
Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis and Rheumatism. 2006; 54 (8):2665-2673 - 9.
Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of psoriatic arthritis: A systematic review. The Journal of Rheumatology. 2008; 35 (7):1354-1358 - 10.
Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, et al. Epidemiology of psoriatic arthritis in the population of the United States. Journal of the American Academy of Dermatology. 2005; 53 (4):573 - 11.
Prey S, Paul C, Bronsard V, Puzenat E, Gourraud PA, Aractingi S, et al. Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: A systematic review of the literature. Journal of the European Academy of Dermatology and Venereology. 2010; 24 (2):31-35 - 12.
Villeneuve E, Nam JL, Bell MJ, et al. A systematic literature review of strategies promoting early referral and reducing delays in the diagnosis and management of inflammatory arthritis. Annals of the Rheumatic Diseases. 2013; 72 (1):12-22 - 13.
Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheumatic Disease Clinics of North America. 2015; 41 (4):569-579 - 14.
Joshi P, Dhaneshwar SS. An update on disease modifying antirheumatic drugs. Inflammation & Allergy Drug Targets. 2014; 13 (4):249-261 - 15.
Mease PJ, Armstrong AW. Managing patients with psoriatic disease: The diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014; 74 (4):423-441 - 16.
Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clinical and Experimental Pharmacology & Physiology. 2012; 39 (3):283-299 - 17.
Kloesch B, Becker T, Dietersdorfer E, Kiener H, Steiner G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. International Immunopharmacology. 2013; 15 (2):400-405 - 18.
Koeberle A, Werz O. Multi-target approach for natural products in inflammation. Drug Discovery Today. 2014; 19 (12):1871-1882 - 19.
Behrens F, Koehm M, Thaçi D, et al. Anti-citrullinated protein antibodies are linked to erosive disease in an observational study of patients with psoriatic arthritis. Rheumatology. 2016; 55 (10):1791-1795 - 20.
Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis & Rheumatism. 2008; 58 (1):15-25 - 21.
Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. Journal of the American Academy of Dermatology. 2005; 53 (4):553-573 - 22.
Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. Journal of the American Academy of Dermatology. 2013; 69 (5):729-735 - 23.
Shichikawa K, Inoue K, Hirota S, et al. Changes in the incidence and prevalence of rheumatoid arthritis in Kamitonda, Wakayama, Japan, 1965-1996. Annals of the Rheumatic Diseases. 1999; 58 (12):751-756 - 24.
Zeng Q , Huang S, Chen R. 10-year epidemiological study on rheumatic diseases in Shantou area. Zhonghua Nei Ke Za Zhi. 1997; 36 (3):193-197 - 25.
Hunter TM, Boytsov NN, Zhang X, et al. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatology International. 2017; 37 (9):1551-1557 - 26.
Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of psoriatic arthritis: A systematic review. The Journal of Rheumatology. 2008; 35 (7):1354-1358 - 27.
Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Seminars in Cutaneous Medicine and Surgery. 2010; 29 (1):3-9 - 28.
Chen Z, OʼShea JJ. Th17 cells: A new fate for differentiating helper T cells. Immunologic Research. 2008; 41 (2):87-102 - 29.
Fitzgerald O, Winchester R. Psoriatic arthritis: From pathogenesis to therapy. Arthritis Research & Therapy. 2009; 11 (1):214 - 30.
Mohan VK, Ganesan N, Gopalakrishnan R. Association of susceptible genetic markers and autoantibodies in rheumatoid arthritis. Journal of Genetics. 2014; 93 (2):597-605 - 31.
Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: Partners in crime in inflammation in rheumatoid arthritis and beyond? Nature Reviews. Immunology. 2007; 7 (3):191-201 - 32.
Castelino M, Barton A. Genetic susceptibility factors for psoriatic arthritis. Current Opinion in Rheumatology. 2010; 22 (2):152-156 - 33.
O’Rielly DD, Rahman P. Genetics of psoriatic arthritis. Best Practice & Research. Clinical Rheumatology. 2014; 28 (5):673-685 - 34.
Yamamoto T. Psoriatic arthritis: From a dermatological perspective. European Journal of Dermatology. 2011; 21 (5):2011 - 35.
van Kuijk AW, Reinders-Blankert P, Smeets TJ, Dijkmans BA, Tak PP. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Annals of the Rheumatic Diseases 2006; 65 (12): 1551-1557 - 36.
Sabat R, Philipp S, Höflich C, Kreutzer S, Wallace E, Asadullah K, et al. Immunopathogenesis of psoriasis. Experimental Dermatology. 2007; 16 (10):779-798 - 37.
Belasco J, Louie JS, Gulati N, Wei N, Nograles K, Fuentes-Duculan J, et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis & Rhematology. 2015; 67 (4):934-944 - 38.
Fitzgerald O, Haroon M, Giles JT, Winchester R. Concepts of pathogenesis in psoriatic arthritis: Genotype determines clinical phenotype. Arthritis Research & Therapy. 2015; 17 (1):115 - 39.
Firestein GS, Budd RC, Gabriel SE, et al. Kelley and Firestein's Textbook of Rheumatology. 10th ed. Vol. 2(10). Philadelphia, PA: Elsevier; 2017 - 40.
Janssen KMJ, de Smit MJ, Brouwer E, et al. Rheumatoid arthritis-associated autoantibodies in non-rheumatoid arthritis patients with mucosal inflammation: A case–control study. Arthritis Research & Therapy. 2015; 17 (1):174 - 41.
Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis and Rheumatism. 2010; 62 (9):2569-2581 - 42.
Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis & Rhematology. 2015; 67 (1):128-139 - 43.
Helliwell PS, Hetthen J, Sokoll K, et al. Joint symmetry in early and late rheumatoid and psoriatic arthritis: Comparison with a mathematical model. Arthritis & Rheumatism. 2000; 42 (4):865-871 - 44.
Joaquim AF, Appenzeller S. Cervical spine involvement in rheumatoid arthritis—A systematic review. Autoimmunity Reviews. 2014; 13 :1195-1202 - 45.
Schett G, Lories RJ, D’Agostino M-A, et al. Enthesitis: From pathophysiology to treatment. Nature Reviews Rheumatology. 2017; 13 (12):731-741 - 46.
Krüger K, Burmester GR, Wassenberg S, et al. THU0141 A non-interventional clinical study evaluating the use of golimumab in patients with rheumatoid arthritis (RA), psoriatic arthritis (PSA), and ankylosing spondylitis (AS) in a real-life setting in Germany. Annals of the Rheumatic Diseases. 2016; 75 (2):1763 - 47.
Van der Horst-Bruinsma IE, Lems WF, Dijkmans BA. A systematic comparison of rheumatoid arthritis and ankylosing spondylitis. Clinical and Experimental Rheumatology. 2009; 27 (4):43-49 - 48.
Zlatanović G, Veselinović D, Cekić S, et al. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosnian Journal of Basic Medical Sciences. 2010; 10 (4):323-327 - 49.
Shin D, Kim HJ, Kim DS, et al. Clinical features of psoriatic arthritis in Korean patients with psoriasis: A cross-sectional observational study of 196 patients with psoriasis using psoriatic arthritis screening questionnaires. Rheumatology International. 2016; 36 :207-212 - 50.
Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: A population-based study. Arthritis & Rheumatism. 2009; 61 (2):233-239 - 51.
Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Annals of Internal Medicine. 2007; 146 (11):797-808 - 52.
Eriksson C, Engstrand S, Sundqvist K-G, Rantapää-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNFa. Annals of the Rheumatic Diseases. 2005; 64 (3):403-407 - 53.
Villani AP, Rouzaud M, Sevrain M, Barnetche T, Paul C, Richard MA, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2015; 73 (3):242-248 - 54.
Tucker LJ, Coates LC, Helliwell PS. Assessing disease activity in psoriatic arthritis: A literature review. Rheumatology and Therapy. 2019; 6 (1):23-32 - 55.
Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. Journal of the American Heart Association. 2013; 2 (2):e000062 - 56.
Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006; 65 (12):1608-1612 - 57.
Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Svendsen JH, et al. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. British Medical Journal. 2012; 344 :e1257 - 58.
Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis and Rheumatism. 2005; 52 (3):722-732 - 59.
Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Annals of the Rheumatic Diseases. 2009; 68 (7):1131-1135 - 60.
Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. European Journal of Cancer. 1996; 32 (10):1753-1757 - 61.
Chen YJ, Wu CY, Chen TJ, Shen JL, Chu SY, Wang CB, et al. The risk of cancer in patients with psoriasis: A population-based cohort study in Taiwan. Journal of the American Academy of Dermatology. 2011; 65 (1):84-91 - 62.
Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: Nested cohort crossover study. Lancet. 2001; 358 (9287):1042-1045 - 63.
Rohekar S, Tom BD, Hassa A, Schentag CT, Farewell VT, Gladman DD. Prevalence of malignancy in psoriatic arthritis. Arthritis and Rheumatism. 2008; 58 (1):82-87 - 64.
Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: Results from a single outpatient clinic. I. Causes and risk of death. Arthritis and Rheumatism. 1997; 40 (10):1868-1872 - 65.
Angulo P. Nonalcoholic fatty liver disease. The New England Journal of Medicine. 2002; 346 (16):1221-1231 - 66.
Ruderman EM, Crawford JM, Maier A, Liu JJ, Gravallese EM, Weinblatt ME. Histologic liver abnormalities in an autopsy series of patients with rheumatoid arthritis. British Journal of Rheumatology. 1997; 36 (2):210-213 - 67.
Miele L, Vallone S, Cefalo C, La Torre G, Di Stasi C, Vecchio FM, et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. Journal of Hepatology. 2009; 51 (4):778-786 - 68.
Nash P, Clegg DO. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Annals of the Rheumatic Diseases. 2005; 64 (2):74-77 - 69.
Sarzi-Puttini P, Santandrea S, Boccassini L, Panni B, Caruso I. The role of NSAIDs in psoriatic arthritis: Evidence from a controlled study with nimesulide. Clinical and Experimental Rheumatology. 2001; 19 (1 Suppl. 22):17-20 - 70.
Nas K, Karkucak M, Durmus B, et al. Comorbidities in patients with psoriatic arthritis: A comparison with rheumatoid arthritis and psoriasis. International Journal of Rheumatic Diseases. 2015; 18 (8):873-879 - 71.
Cañete JD, Pablos JL, Sanmartí R, et al. Antiangiogenic effects of anti-tumor necrosis factor α therapy with infliximab in psoriatic arthritis. Arthritis and Rheumatism. 2004; 50 (5):1636-1641 - 72.
Ortega C, Fernández-A S, Carrillo JM, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic. Effector cells that secrete Th17-related cytokines. Journal of Leukocyte Biology. 2009; 86 (2):435-443 - 73.
Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Annals of the Rheumatic Diseases. 2017; 76 (1):79-87 - 74.
Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. Journal of Immunology. 2008; 181 (9):5948-5955 - 75.
Kavanaugh A, Puig L, Gottlieb AB, et al. Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: Results from a randomized, placebo-controlled phase III trial. Arthritis Care and Research. 2015; 67 (12):1739-1749 - 76.
Deodhar A, Gottlieb A, Boehncke WH, et al. Efficacy and safety results of guselkumab, an anti-il23 monoclonal antibody, in patients with active psoriatic arthritis over 24 weeks: A phase 2a, randomized, double-blind, placebo-controlled study [abstract]. Annals of the Rheumatic Diseases. 2017; 76 (2):142-143 - 77.
Mease PJ, Kellner H, Morita A. Efficacy and safety results from a phase 2 trial of risankizumab, a selective IL-23p19 inhibitor, in patients with active psoriatic arthritis [abstract]. Arthritis and Rheumatism. 2017; 69 (10): Abstract 2L - 78.
Rooney M, Symons JA, Duff GW. Interleukin 1 beta in synovial fluid is related to local disease activity in rheumatoid arthritis. Rheumatology International. 1990; 10 (5):217-219 - 79.
Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis & Rheumatism. 2006; 54 (8):2665-2673 - 80.
Amina LE, Gamily MEI. Biological impact of curcumin on the healing of tempromandibular joint in experimentally induced arthritis. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology. 2021; 33 :260-266 - 81.
Skyvalidas DN, Mavropoulos A, Tsiogkas S, Dardiotis E, Liaskos C, Mamuris Z, et al. Curcumin mediates attenuation of pro-inflammatory interferon γ and interleukin 17 cytokine responses in psoriatic disease, strengthening its role as a dietary immunosuppressant. Nutrition Research. 2020; 75 :95-108 - 82.
Martin BR. Treatment of psoriatic arthritis with acupuncture, turmeric (Curcuma longa), sarsaparilla (Smilax officinalis) and vitamin D: A case report. Journal of Chiropractic Medicine Martin. 2020; 19 (3):194-200 - 83.
Manca ML, Lattuada D, Valentia D, Marelli O, Corradini C, Fernàndez-Busquets X, et al. Potential therapeutic effect of curcumin loaded hyalurosomes against inflammatory and oxidative processes involved in the pathogenesis of rheumatoid arthritis: The use of fibroblast-like synovial cells cultured in synovial fluid. European Journal of Pharmaceutics and Biopharmaceutics. 2019; 136 :84-92 - 84.
Wang Q , Ye C, Sun S, Li R, Shi X, Wang S, et al. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. International Immunopharmacology. 2019; 72 :292-300 - 85.
Dewangan AK, Perumal Y, Pavurala N, Chopra K, Mazumder S. Preparation, characterization and anti-inflammatory effects of curcumin loaded carboxymethyl cellulose acetate butyrate nanoparticles on adjuvant induced arthritis in rats. Journal of Drug Delivery Science and Technology. 2017; 41 :269-279. DOI: 10.1016/j.jddst.2017.07.022 - 86.
Jeengar MK, Shrivastava S, Chandra Mouli Veeravalli S, VGM N, Sistla R. Amelioration of FCA induced arthritis on topical application of curcumin in combination with emu oil. Nutrition. 2016; 9 :955-964. DOI: 10.1016/j.nut.2016.02.009 - 87.
Coates LC, Helliwell PS. Psoriatic arthritis: State of the art review. Clinical Medicine. 2017; 17 (1):65-70