EWGSOP guidelines for diagnosis of sarcopenia recommend following the pathway: Find cases-assess-confirm-severity (F-A-C-S) [32].
Abstract
Systemic sclerosis (SSc) is a systemic autoimmune connective tissue disease with great clinical and pathogenetic heterogeneity. Although skin is the most visible organ affected, skeletal muscles are affected in up to 96% of SSc patients and this is associated with a worse clinical outcome including increased mortality. Muscle involvement varies from patients experiencing myalgias, fibrosing myopathy to overlaps of SSc and myositis, a condition referred to as scleromyositis. In SSc muscle biopsies, muscular fibrosis, inflammation, microangiopathy and atrophy are observed, which is consistent with most prominent SSc pathophysiologic processes. The damage and fibrosis of the muscle tissue and the reduced ability of the body to build and repair muscle lead to a loss of muscle mass and strength. Studies show that patients with SSc have a higher prevalence of myopenia than the general population, but the exact cause is not yet fully understood. Partially, this phenomenon could be attributed to the disrupted activity of fibro-adipogenic progenitors, driven by alterations in the skeletal muscle microenvironment of SSc patients. These changes are also reflected in shifts in myokine secretion.
Keywords
- skeletal muscle
- autoimmune myositis
- systemic sclerosis
- scleromyositis
- myopathy
- myopenia
- myokines
- fibro-adipogenic progenitors
1. Introduction
Systemic sclerosis (SSc) is an autoimmune connective tissue disease that most commonly occurs between 40 and 60 years of age [1] and affects women more often than men; however, male patients tend to have more severe disease [2].
Patients are clinically divided into limited cutaneous (lcSSc) and diffuse cutaneous (dcSSc) subsets based on the extent of skin involvement. The dcSSc subtype typically has a worse prognosis and more common development of joint contractures, skeletal myopathy, kidney disease, interstitial lung disease and heart involvement, while in lcSSc, digital ischemia, oesophageal disease, pulmonary arterial hypertension and malabsorption occur more commonly. SSc-specific anti-topoisomerase I autoantibodies are more prevalent in dcSSc, while anti-centromere antibodies are more prevalent in lcSSc [3].
While muscle involvement is not the most life-threatening manifestation of SSc, musculoskeletal impairments are one of the main factors for physical disabilities that affect the ability to perform activities of daily living and can have a significant impact on patients’ quality of life [4, 5]. It is important to stress that patients with myopathy also have more severe disease and decreased survival [6, 7, 8, 9].
2. Characteristics of muscle involvement in SSc
Muscle involvement in SSc can present itself in a wide range of pathologies, from indolent subclinical myopathy that manifests as a mild muscle enzyme-level elevation, to aggressive inflammation as in idiopathic inflammatory myopathy. Sometimes it can present itself in a similar manner to other SSc organ involvements in the form of fibrosing myopathy without any existing evidence of inflammation [10].
The estimated prevalence of muscle involvement in SSc ranges widely from 5 to 96%. The variability in data reflects the lack of a formal definition of muscle involvement, which can include muscle weakness, abnormal muscle enzymes, abnormal electromyography (EMG) or abnormal biopsy of skeletal muscle [6]. Risk factors associated with myopathy are male gender, diffuse cutaneous SSc, shorter disease duration and higher modified Rodnan skin score [11].
2.1 Clinical features
Myalgia, muscle weakness and tenderness are being most frequently reported by patients, myalgia by 11 to 56% and muscle weakness by 23 to 60% of SSc patients. Objective muscle weakness detected on physical examination was present in 9 to 83% of cases. Patients generally present with symmetrical proximal upper and lower limb weaknesses. It is suggested that extra muscular involvement, such as skin thickening, interstitial lung disease, pulmonary arterial hypertension and anaemia, can also contribute to exercise limitation in SSc patients [10].
2.2 Laboratory findings
SSc patients with suspected myopathy should be tested for serum levels of muscle enzymes, creatine kinase (CK) and aldolase. Serum CK and aldolase elevation is reported in 6 to 47% and in 11 to 75% of SSc patients, respectively [8]. In the European Scleroderma Trials and Research group (EUSTAR) cohort, a substantial number of patients had muscle weakness, but not all of them had simultaneous CK elevation; 22.8% of patients with lcSSc and 37.1% of patients with dcSSc had muscle weakness, while 4.4 and 11.3% had elevated CK, respectively [12]. CK can also be elevated in myocarditis, where measurement of isoenzymes and serum troponin can be helpful in identifying CK origin [8]. All patients with SSc and suspicion of muscle involvement should also have their thyroid function tested. SSc patients can have specific autoantibodies, which can predict the clinical phenotype, and in patients with SSc-myositis overlap anti-Ku, anti-U1RNP and anti-PM-Scl are most prevalent among autoantibodies [13].
2.3 Electromyography
Laboratory markers can sometimes be unreliable; thus, electromyography (EMG) can be helpful to define the cause of muscle weakness and/or elevated levels of muscle enzymes. EMG was found to be abnormal in 11 to 92% of SSc patients. It is usually altered similar to EMG performed in patients with idiopathic inflammatory myopathies, as myopathic patterns are more frequently demonstrated in proximal than distal muscles [14].
2.4 Magnetic resonance imaging
Magnetic resonance imaging (MRI) can be helpful in determining the aetiology of muscle weakness in SSc patients and can help to identify an appropriate site for muscle biopsy. Muscle oedema is the most commonly reported finding on MRI, and changes such as fatty replacement of muscle tissue on T1 sequences can suggest chronic and irreversible muscle damage [14].
2.5 Muscle biopsy
Muscle biopsy in SSc patients can be helpful if the clinical picture is not clear. The histopathologic features are heterogeneous. According to the European Neuromuscular Centre (ENMC), there are four histopathologic categories of myopathy: polymyositis, dermatomyositis, necrotizing myopathy and non-specific myositis. In one study of 42 patients with SSc and muscle weakness, the most common histopathologic categories were non-specific myositis (35.7%) and necrotizing myopathy (21.4%). This study also showed that higher CK values were associated with inflammation and necrosis [14]. Another study demonstrated that fibrosis (81%) and microangiopathy (92%) are the main histopathologic hallmarks of SSc-related myopathy [15]. Therefore, histopathologic findings allow us to identify patients with predominant features related to inflammation and patients with features suggestive of fibrosis, which may be helpful when considering appropriate treatment.
2.6 Nailfold capillaroscopy
Studies have shown that muscle involvement was observed in 12 to 50% of SSc patients with advanced microvascular damage assessed with nailfold videocapillaroscopy (NVC) [16, 17, 18]. Patients with SSc exhibiting late NVC patterns were found to have decreased whole-body lean and fat mass compared to those with early and active NVC patterns [17]. The decrease in lean mass was more noticeable in the upper limbs, potentially indicating muscle wasting or loss of muscle mass in those areas [17]. It was observed that SSc patients with late NVC pattern had a significantly higher prevalence of low skeletal muscle index (43.75%), while in patients with early (9.1%) and active (12.5%) NVC patterns, the prevalence of a low skeletal muscle index was lower [16].
2.7 Skeletal muscle damage patterns in SSc
Muscle involvement in SSc can present in two major histopathological patterns distinguished by the predominating pathological process in skeletal muscle tissue. In one, fibrosis prevails, while in the other, inflammation is more pronounced (Figure 1).

Figure 1.
Differences between inflammatory and fibrosing patterns of muscle involvement [
2.8 Fibrosing myopathy
SSc patients with muscle weakness and fibrosing myopathy (fibrosis predominance on muscle biopsy) represent a subset of patients that tend to have dcSSc. They have lower values of muscle enzymes (CK, aldolase), more prevalent non-irritable myopathy on EMG and higher mortality rates compared to SSc patients with muscle weakness and a muscle biopsy showing an inflammatory myopathy. The presence of anti-Scl-70 and anti-U3-RNP autoantibodies was detected more frequently in patients with fibrosing myopathy [20]. Patients with fibrosing myopathy show less therapeutic response and thus have a worse prognosis.
2.9 Scleromyositis
SSc and autoimmune myositis (AIM) are classic connective tissue diseases. We usually have specific diagnostic and/or classification criteria for these diseases, but sometimes a patient fulfils criteria for two different diseases. Scleromyositis, or SSc associated with myositis, is one of the best known and recognised overlap syndromes. Scleromyositis patients fulfil the criteria for SSc and AIM, have clinical features of both diseases and most of them also have specific immunological markers [8, 19]. Among myositis-associated autoantibodies, which are generally characteristic for patients with overlap myositis, anti-PM/Scl, anti-Ku, anti-U1RNP and anti-U3RNP are most common in SSc/AIM overlap. In a few patients, anti-RuvBL1/2 and anti-SMN autoantibodies have been reported (Figure 2). Despite the large panel of recognised autoantibodies, about half of SSc/AIM overlap patients are seronegative, meaning no currently known SSc-related autoantibodies can be detected in their serum samples [21]. Subsets of SSc/AIM overlap patients with different autoantibodies differ in the age of disease onset, response to treatment, risk of interstitial lung disease and survival [8].

Figure 2.
Presence of autoantibodies in scleromyositis and survival in SSc and SSc/AIM overlap.
Research studies indicate that muscle weakness, which is typically symmetrical, proximal, bilateral and more pronounced in the upper limbs, was observed in 13 to 100% of patients with scleromyositis. In addition, 21 to 88% of scleromyositis patients reported myalgias [8]. Other typical symptoms of scleromyositis include Raynaud’s phenomenon occurring in approximately 81% of scleromyositis patients [22], and head dropping or camptocormia due to axial muscle weakness, which occurs in approximately one third of patients with SSc/AIM overlap [8].
Due to the systemic nature of the SSc/AIM disease, extramuscular symptoms are common (Figure 3), with approximately 33% of patients suffering from impaired forced vital capacity, 25% from dysphagia and 20% from cardiac involvement [22]. The higher frequency of extramuscular complications is a plausible explanation for the higher mortality among SSc/AIM overlap patients compared to SSc patients with no muscular involvement [8, 23]. The cumulative 5- and 10-year survival from diagnosis for SSc/AIM overlap patients is 82 and 68%, respectively, compared to 93 and 87% for SSc patients with no muscular involvement (Figure 2) [23]. In both, the most common cause of death is pulmonary complications, followed by cardiac complications, scleroderma renal crisis and gastrointestinal involvement [8]. Compared to SSc patients, SSc/AIM overlap patients have a higher risk of interstitial lung disease, one of the leading death-causing complications, and scleroderma renal crisis, which could be explained by the more frequent use of corticosteroids among SSc/AIM patients [8, 24].
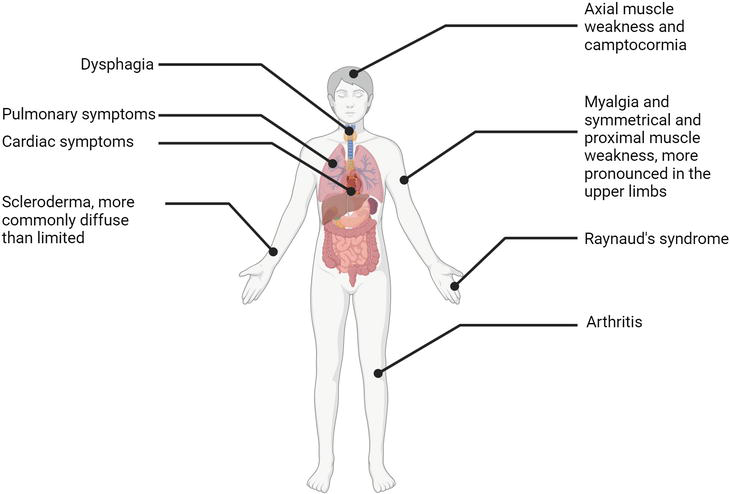
Figure 3.
Symptoms of scleromyositis.
A scoping review of 77 studies that included 559 muscle biopsies from SSc patients with myositis reported upregulation of sarcolemmal major histocompatibility complex class I (MHC-I) proteins (72%), inflammatory infiltrates (57%) that were predominantly T cell and present in endomysium, perimysium and perivascularly, muscle fibre necrosis (56%), myofibre atrophy (48%), regenerative fibres (41%), fibrosis (35%), neurogenic features (23%) and mitochondrial abnormalities (8%) [8, 25]. Vasculopathy and endomysial fibrosis, each present in approximately 33% of SSc/AIM overlap patients, may be the histopathological features suitable to distinguish SSc/AIM overlap from other forms of autoimmune myositis, which is consistent with the fact that vasculopathy and fibrosis are the two main features of SSc pathophysiology, along with immune dysfunction, a common feature with other AIM subgroups [25, 26]. These findings underscore the complex nature of muscle involvement in SSc, resulting from the interplay of vasculopathy, connective tissue and neurogenic changes, immune activation, and other factors.
3. Myopenia and sarcopenia in SSc: Screening and diagnosing
Sarcopenia and myopenia are impairments of musculature that may overlap in features but differ in underlying mechanisms and severity. It has been proposed to define myopenia as the loss of muscle mass in cachexia to distinguish it from sarcopenia, age-related and disease-related muscle wasting, which includes decrease in both muscle quantity and quality (strength and function) [27]. Cachexia is a complex metabolic syndrome characterised by the progressive loss of muscle mass and adipose tissue, often accompanied by systemic inflammation, insulin resistance and increased muscle protein breakdown [28], leading to progressive functional impairment [27]. Although malnutrition can occur in both cachexia and sarcopenia, cachexia is typically associated with a more pronounced and severe degree of malnutrition due to the underlying systemic disease and inflammatory state (Figure 4) [29].

Figure 4.
Development of muscle loss in chronic disease.
Sarcopenia is recognised as a muscle disease with the ICD-10-CM (M62.84) code [30]. It occurs with ageing, inactivity, and/or disease, and increases the risk of falls, disability, and mortality. Over the past decade, many aspects of sarcopenia have been studied, and the definition and diagnostic criteria for sarcopenia have evolved. Originally, sarcopenia was described as an age-related decrease in lean body mass [31]; however, recently the definition and guidelines for the diagnosis of sarcopenia were prepared by the European Working Group on Sarcopenia in Older People (EWGSOP2) (Table 1) [33] requiring decreased muscle quantity or quality must be detected for diagnosis. In particular, the finding of low muscle strength is used as the primary parameter of sarcopenia. Muscle quality, defined as strength and/or power per unit of muscle mass, is an emerging biomarker that relates skeletal muscle structure to function [33, 37, 38, 39]. Muscle quality characteristics include factors such as structural changes in the neuromuscular system with loss of skeletal muscle contractile units, remodelling of fibre types and motor units, decreased muscle regenerative capacity, fat infiltration and mitochondrial dysfunction [40, 41, 42].
| Assessment | SSc specific assessment | |
|---|---|---|
| SARC-F questionnaire consisting of five items for self-reported information from patients regarding symptoms associated with sarcopenia, screening tool to assess the risk of sarcopenia in individuals in various healthcare settings, including community healthcare and clinical environments [33]. | The combination of high diagnostic accuracy and feasibility makes SARC-F with the addition of age and body mass measurements (SARC-F + EBM) appropriate screening tool for routine care of patients with SSc. It can effectively identify individuals with the condition while being practical to use in everyday clinical practice [34]. | |
| Measurements of muscle strength. Low muscle strength could be measured as handgrip strength, as it has been shown to correspond well with strength in other areas of the body (cut-off value of <27 kg for men and < 16 kg women), or isometric torque methods can be used to measure lower limb strength [33]. A suitable measure is the chair stand test, which can be used as a proxy for the strength of the leg muscles (quadriceps) (cut-off value >15 s for five rises) [33]. | In SSc patients with hand disability, lower limb strength can be measured using isometric torque or chair stand methods rather than handgrip strength [33]. | |
| To confirm sarcopenia by detection of low muscle quantity and quality, DXA is advised in clinical practice, and DXA, BIA, CT or MRI in research studies (cut-off value appendicular skeletal muscle mass (ASM)/height2 < 7.0 kg/m2 for men, and < 5.5 kg/m2 for women) [33]. | The skeletal muscle mass expressed as skeletal muscle area (SMA) and skeletal muscle index (SMI) at L1 level on chest CT was demonstrated to be an accurate measure that is useful for detecting myopenia in patients with SSc [35]. In SSc patients with skin involvement (fibrosis, digital ulcers), BIA is unlikely to be suitable for measuring muscle mass as it does not measure muscle mass directly but instead derives an estimate of muscle mass based on whole body electrical conductivity and may underestimate muscle mass in SSc patients [36]. | |
| Severity can be evaluated by performance measures; gait speed (≤0.8 m/s), short physical performance battery (SPPB) (≤8 points score), timed-up-and-go test (TUG) (≥20 s) and 400-m walk tests can be used (non-completion or ≥ 6 min for completion) [33]. |
Table 1.
Impaired muscle function together with fatigue, and disability due to other SSc symptoms (such as skin fibrosis and nutrition issues) and chronic inflammation can lead to myopenia. Overall, the results of the studies show that the prevalence of myopenia in SSc patients varies, with the reported prevalence ranging from 10.7 to 41.9%, depending on the diagnostic criteria and cut-off values used. The majority of SSc patients included in the studies were female, with percentages ranging from 84.4 to 92%. The average age of the patients ranged from 52 to 64 years [17, 32, 43, 44, 45, 46, 47, 48, 49].
Myopenia in SSc patients was associated with factors such as malnutrition [32, 43, 44, 45, 47, 48], low BMI [16, 45, 46, 47], lower fat mass [16, 45, 47], longer disease duration [43, 45, 47, 49], higher mRSS score [43, 44, 47, 48], decline in forced vital capacity [45], capillaroscopy patterns [16, 47] and reduced lung function (DLCO) [16, 45, 47].
Cachectic features are commonly observed in SSc patients with myopenia. The mechanisms underlying muscle wasting in SSc differ from those in other diseases and are primarily related to immunosuppression and malnutrition due to malabsorption syndrome [43]. As the disease progresses, the anabolic resistance caused by the vasculopathy may further accelerate the wasting of muscle and adipose tissue. Inflammation, neurological changes and polypharmacy may contribute to the progression from cachexia to sarcopenia in subsets of SSc patients.
Of the studies conducted with SSc patients, only two specifically address sarcopenia using the diagnostic criteria established by EWGSOP [32], or the AWGS criteria [44]. However, in several studies, the concept and definition of sarcopenia were not appropriate. The diagnosis was primarily based on individual assessment of skeletal muscle mass or muscle strength, without considering other factors contributing to sarcopenia, such as muscle quality [16, 45, 46, 47]. On the other hand, some studies focused on measuring body composition considering muscle mass or fat-free mass in SSc patients [43, 48, 49].
Studies that investigated sarcopenia in SSc patients showed prevalence between 22.5 and 22.7% [33, 37]. Potential predictors of sarcopenia in SSc patients were malnutrition, low body mass index (BMI), inflammation and number of immunosuppressants used. In addition, correlations between sarcopenia and disease subtype dSSc [46, 47] were observed. Sarcopenic SSc patients had a worse capillaroscopy pattern and lower quality of life as measured by the SF-36 survey [32, 47].
In summary, muscle impairment in SSc spans a spectrum ranging from myopenia to sarcopenia, with varying underlying mechanisms and severity. The interplay of immunosuppression, malnutrition, vasculopathy, inflammation and neurological alterations contributes to the muscle wasting and dysfunction observed in SSc patients. Further research is needed to better understand the specific mechanisms and the different subsets of muscle impairment in SSc patients.
4. Importance of non-myogenic cells in muscle pathology of SSs
In the histology of SSc muscles, interstitial and perivascular fibrosis are observed [6, 50]; however, the question remains, why is fibrosis in muscles not as frequent and as extensive as in skin.
Although myofibres are the major cell type in skeletal muscle, other cells are also important for the maintenance of muscle homeostasis. The essential components for the regeneration of muscle tissue are myogenic progenitor cells known as satellite cells. However, estimates in rats suggest that approximately half of all nuclei in skeletal muscles belong to non-muscle cells, including fibroblasts, endothelial cells and pericytes. Among these, fibroblasts are especially prominent [51]. They are associated with the formation of transient fibrosis that occurs following muscle injury, and, where repair is not successful, in the formation of resilient scar tissue, in addition to the gradual accumulation of muscle connective tissue that may occur with ageing (in sarcopenia) [52]. However, they are also implicated in regeneration, as 30 days after injury their number was increased fourfold and they were located preferentially surrounding regenerating muscle fibres. Even more, strong stimulation of myogenesis by fibroblasts was observed in cell culture, pointing towards direct stimulation of satellite cell differentiation and fusion [53]. More recent research found that these cells are fibro-adipogenic progenitors (FAP) that can differentiate into adipocytes or myofibroblasts and thus cause fibrosis and fatty infiltration, the two crucial processes of muscle degeneration and diseases (Figure 5) [55]. The fate of FAPs is strongly dependent on the microenvironment. Myokine IL-15 promotes skeletal muscle regeneration by stimulating the proliferation of FAPs and suppresses fatty infiltration by inhibiting their differentiation into adipocytes [54, 56]. In acute muscle injury, immune cells release IL-4 supporting FAP proliferation, while macrophages induce TNF-α-mediated FAP apoptosis shortly after entering the transient proregenerative phase [57]. Contrary, in chronic muscle injury, such as myositis, the macrophages switch from M1 to M2 and secrete TGF-β1, allowing FAPs to evade apoptosis and continue to secrete ECM components, resulting in fibrosis [57].

Figure 5.
Differentiation of fibro-adipogenic progenitors in skeletal muscle. Based on ref. [
Some of the effects of FAP can be explained by the mediators that they secrete. FAPs are major producers of IL-6, IL-33 and follistatin after acute injury [54].
5. Myokines
Skeletal muscle tissue is not only a component of the locomotor system but also a secretory organ, signalling through direct cell-cell interactions, cell surface molecules and soluble factors [58]. Contracting myofibres are a source of myokines, which exert their functions within the muscle tissue and in other organs such as adipose tissue, brain, liver, bone and pancreas [59]. Myokine signalling positively affects various biological processes, including metabolism, muscle hypertrophy and cognition, as well as tumour growth suppression and inflammation reduction [60]. Myokine molecules are also released from immune cells, possessing additional functions, often of an inflammatory nature, and can therefore also be classified as cytokines (Figure 6). However, further research is needed to identify the exact causes that could explain why, how and in what circumstances a molecule exerts its effects as a myokine or a cytokine.
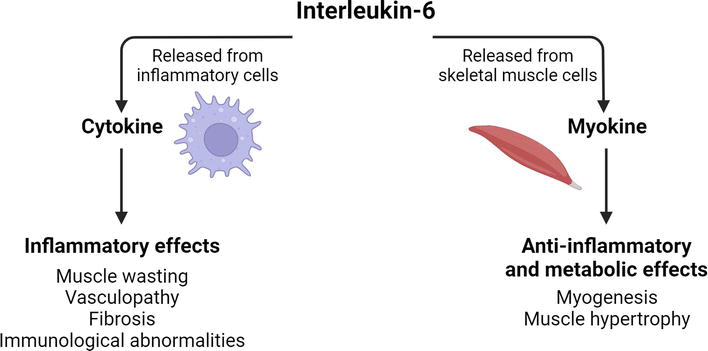
Figure 6.
Duality of IL-6 effects.
Despite the positive effects, exercise is associated with acute inflammation, which is necessary for the repair of exercise-induced damage in the muscle tissue [61]. However, evidence indicates that physical exercise is safe and beneficial, as it does not exacerbate long-term inflammation or the number or degree of joint damage [59]. Long-term engagement in physical activity combined with reduced energy intake decreases the levels of systemic inflammatory markers, suggesting the downregulation of inflammation [59]. Moreover, it suppresses fibrotic processes and promotes circulation and vascular repair [62]. Caution is necessary for patients with interstitial lung disease who are at risk for oxygen desaturation during exercise [63].
The beneficial effects and safety of physical exercise make it an excellent non-pharmaceutical anti-inflammatory therapy for patients with SSc [59]. In SSc, muscle disuse often occurs because of difficulties with performing physical activities due to common disease-related symptoms such as bone and muscle pain, stiffness and immobility. Muscle disuse exacerbates the symptoms, making it increasingly more difficult to break the cycle of physical inactivity [62]. Regular physical activity adjusted to the patient’s abilities can relieve symptoms and improve physical health and psychosocial well-being. Physical activity interventions in patients with SSc have been reported to enhance general health parameters, including exercise tolerance, muscle strength and aerobic capacity, and provide symptom relief, as evidenced by improvement in handgrip strength, microstomia, pain and disability score [62].
5.1 IL-6
Among the factors that trigger IL-6 release from skeletal muscle cells are muscle contraction and reduced availability of metabolic substrates, which is why IL-6 is often referred to as the muscle energy sensor. IL-6 increases serum levels of cortisol, IL-1Ra (interleukin-1 receptor antagonist protein) and IL-10, which exert anti-inflammatory and metabolic effects [59]. As a myokine, IL-6 is essential for satellite cell-dependent myogenesis and overload-induced muscle hypertrophy (Figure 6) [64].
In contrast, when secreted in an inflammatory milieu or when its elevation and activity are prolonged, IL-6 is considered an inflammatory cytokine with the opposite effects on skeletal muscle, causing muscle wasting [65]. Elevated levels of IL-6 are associated with many inflammatory diseases, including SSc. Along with IL-4 and TGFβ, IL-6 is regarded as a fibrogenic cytokine, mediating its profibrotic actions by stimulating collagen production and inhibiting collagenase synthesis [26].
Consistent with its role in SSc-related inflammation and fibrosis, upregulation of IL-6 was shown in the following:
Skin fibroblasts of SSc patients, where IL-6 levels correlate with the degree of collagen production [66],
Serum of SSc patients, where IL-6 levels correlate with the disease severity [26, 66, 67],
Exhaled breath and bronchoalveolar lavage of SSc patients, where IL-6 levels correlate with the extension of lung fibrosis [68].
The pathological role of IL-6 in SSc was demonstrated in experiments on animal SSc models, where IL-6 blockade or gene knockout resulted in the suppression of disease development [69].
IL-6 may have a role in all three features of the early stages of SSc pathogenesis: vasculopathy, immunological abnormalities and fibrosis [26].
In this regard, IL-6 seems to promote SSc pathogenesis; however, the contribution of the myokine portion or better lack of it, enables the dominance of negative effects and is not yet clear.
5.2 IL-15
Interleukin-15 is a proinflammatory cytokine crucial for the growth and survival of NK cells, B and T lymphocytes, eosinophils and mast cells. It is expressed in endothelial cells, vascular smooth muscle cells, fibroblasts, adipocytes, keratinocytes, cardiomyocytes and skeletal muscle cells [71]. There is a rapid increase in circulating levels of IL-15 in response to exercise. As a myokine, IL-15 regulates lipid metabolism and muscle regeneration. Skeletal muscle protects against excessive fat accumulation by releasing IL-15, which inhibits lipid deposition in preadipocytes and accumulation of visceral fat [59, 72].
In SSc, serum levels of IL-15 are elevated and correlate negatively with vital lung capacity, reflecting pulmonary fibrosis [71].
Its role in the transmigration and infiltration of immune cells may contribute to autoimmunity in SSc [71].
Although this correlation suggests a profibrotic effect of IL-15, results from
5.3 SPARC
Secreted Protein Acidic and Rich in Cysteine (SPARC), also known as osteonectin or basement-membrane protein 40 (BM-40), is a calcium-binding, extracellular matrix-associated glycoprotein released into the bloodstream by skeletal muscle in response to aerobic exercise. It mediates interactions between cells and their surrounding matrix by binding to ECM components, cellular receptors and secreted growth factors [74, 75]. It also induces the expression of proteases or their inhibitors and is thus important for the regulation of many processes involved in homeostasis and disease, such as cell proliferation, migration and invasion, tissue remodelling, angiogenesis, immune function, wound healing and fibrosis [74, 75, 76].
In SSc, SPARC appears to be involved in vasculopathy and fibrotic processes. By affecting cytoskeleton reorganisation and cell-ECM interactions, SPARC may induce intracellular gap formation in endothelial cells, leading to alterations in EC barrier function and increased paracellular permeability with perivascular oedema [77]. These are characteristics of SSc vasculopathy, along with the recruitment of inflammatory cells and intravascular platelet activation [78].
SPARC is commonly overexpressed in fibrotic tissue [79]. In SSc, SPARC overexpression was reported in dermal fibroblasts, endothelial cells, pericytes and epidermal keratinocytes [74]. The serum concentration of SPARC is elevated as well, but only in patients with limited SSc [75, 78]. The effects of SPARC on the development of a profibrotic phenotype seem to be associated with TGFβ, a driver of fibrotic processes [75, 79]. SPARC upregulates several ECM- and fibrosis-related genes in SSc dermal fibroblasts, contrary to dermal fibroblasts of healthy individuals, which do not respond to SPARC [75].
The difference between the responsiveness of healthy versus SSc-derived fibroblasts to SPARC is TGFβ-signalling dependent. TGFβ induces SPARC expression and vice versa, forming an autocrine feedback loop [80]. Additionally, SPARC enhances the cellular sensitivity to TGFβ by binding to the TGFβ receptor and altering its conformation, which results in increased expression of ECM- and fibrosis-related genes in cells exposed to both TGFβ and SPARC, compared to TGFβ only [81].
Accordingly,
5.4 LIF
Leukaemia Inhibitory Factor (LIF) is a myokine that acts by binding to its receptor LIFR and a signal transducer gp130, classifying LIF as a cytokine of the IL-6 family [85]. Its name derives from early research showing its ability to inhibit proliferation and induce differentiation of M1 myeloid leukaemia cells [86]. It is now known that LIF is highly pleiotropic, functioning in various processes across several organ systems such as haematopoiesis, embryogenesis, bone formation, skeletal and cardiac muscle and nervous system function [87]. In skeletal muscle, LIF is a contraction-induced myokine; however, no increases plasma levels were observed after exercise, pointing to local effects. LIFR is mainly expressed by satellite cells and LIF induces their proliferation and inhibits their premature differentiation [88]. Due to the angiostatic properties of LIF, loss of LIF function stimulates angiogenesis [89].
Serum levels of LIF are decreased in the early stages of SSc, as is the expression of LIF in SSc skin. Additionally, the expression of LIFR is downregulated in SSc skin in similar dynamics as LIF, while gp130 is downregulated irrespective of the disease stage [89]. Altered LIF function in the early stages of SSc might contribute to impaired angiostasis, leading to vasculopathy; however, it remains to be determined if LIF from muscles contributes to some potential effects in SSc or other tissues/cells contribute the majority.
6. Conclusions
Muscle involvement is a significant burden for patients with SSc and affects most of them. Clinically, muscle involvement is usually manifested by myalgia, muscle weakness and tenderness. Histologically, two main patterns can be identified in the skeletal muscles of SSc patients: fibrosing and inflammatory myopathy. The latter is associated with characteristic autoantibodies: anti-PM/Scl, anti-Ku, anti-U1RNP and anti-U3RNP. A large proportion of SSc patients with muscle involvement fulfil the criteria for both SSc and autoimmune myositis and can therefore be classified into a specific overlap SSc/AIM entity called scleromyositis.
Due to malnutrition, GIT involvement, inflammation, treatments and difficulty with engaging in physical exercise, SSc/AIM overlap patients often suffer from myopenia (muscle wasting), which can later develop into sarcopenia in approximately 20% of patients, which is associated with worse disease outcome and quality of life.
One aspect of muscle involvement in SSc is associated with changes in cellular function and the microenvironment in skeletal muscle tissue. However, essential cell types such as fibro-adipogenic progenitors and their role in SSc are incompletely understood. Their microenvironment depends largely on myokines secreted by contracting myotubes. Research on myokines suggests that some have beneficial and some deleterious effects on skeletal muscle in disease. Although some of the effects are well known, the challenge remains to accurately determine the amounts and effects of myokines released from muscle fibres as opposed to those released by other cell types.
Acknowledgments
This research was funded by the Slovenian Research Agency ARRS, with project J7-3153 and the National Research Program #P3-0314, P3-0043.
The figures were made with BioRender.
Appendices and nomenclature
autoimmune myositis | |
Asian Working Group for Sarcopenia | |
basement-membrane protein 40 | |
body mass index | |
creatinine kinase | |
connective tissue growth factor | |
diffuse cutaneous systemic sclerosis | |
diffusing capacity for carbon monoxid | |
extra-cellular matrix | |
electromyography | |
European Neuro Muscular Centre | |
European Scleroderma Trials and Research group | |
European Working Group on Sarcopenia in Older People | |
fibro-adipogenic progenitors | |
glycoprotein 130 | |
interleukin-10 | |
interleukin-15 | |
interleukin-1 receptor antagonist protein | |
interleukin-4 | |
interleukin-6 | |
limited cutaneous systemic sclerosis | |
leukaemia inhibitory factor | |
leukaemia inhibitory factor receptor | |
major histocompatibility complex class I | |
magnetic resonance imaging | |
modified Rodnan skin score | |
cells natural killer cells | |
nailfold videocapillaroscopy | |
peripheral blood mononuclear cell | |
36-item short form survey | |
secreted protein acidic and rich in cysteine | |
systemic sclerosis | |
transforming growth factor β | |
T-helper cells | |
tumour necrosis factor α |
References
- 1.
Moinzadeh P, Kuhr K, Siegert E, Mueller-Ladner U, Riemekasten G, Gunther C, et al. Older age onset of systemic sclerosis - accelerated disease progression in all disease subsets. Rheumatology (Oxford, England). 2020; 59 (11):3380-3389 - 2.
Peoples C, Medsger TA Jr, Lucas M, Rosario BL, Feghali-Bostwick CA. Gender differences in systemic sclerosis: Relationship to clinical features, serologic status and outcomes. Journal of Scleroderma and Related Disorders. 2016; 1 (2):177-240 - 3.
Hughes M, Herrick AL. Systemic sclerosis. British Journal of Hospital Medicine (London, England). 2019; 80 (9):530-536 - 4.
Lorand V, Czirjak L, Minier T. Musculoskeletal involvement in systemic sclerosis. Presse Médicale. 2014; 43 (10 Pt. 2):e315-e328 - 5.
Martin Calderon L, Chaudhary M, Pope JE. Healthcare utilization and economic burden in systemic sclerosis: A systematic review. Rheumatology (Oxford, England). 2022; 61 (8):3123-3131 - 6.
Walker UA, Clements PJ, Allanore Y, Distler O, Oddis CV, Khanna D, et al. Muscle involvement in systemic sclerosis: Points to consider in clinical trials. Rheumatology (Oxford, England). 2017; 56 (Suppl. 5):v38-v44 - 7.
Zhou M, Jiang L, Nie L, Chen T, Zhang T, Sun W, et al. Myopathy is a risk factor for poor prognosis of patients with systemic sclerosis: A retrospective cohort study. Medicine (Baltimore). 2020; 99 (33):e21734 - 8.
Giannini M, Ellezam B, Leclair V, Lefebvre F, Troyanov Y, Hudson M, et al. Scleromyositis: A distinct novel entity within the systemic sclerosis and autoimmune myositis spectrum. Implications for care and pathogenesis. Frontiers in Immunology. 2022; 13 :974078 - 9.
Jung M, Bonner A, Hudson M, Baron M, Pope JE, Canadian Scleroderma Research G. Myopathy is a poor prognostic feature in systemic sclerosis: Results from the Canadian scleroderma research group (CSRG) cohort. Scandinavian Journal of Rheumatology. 2014; 43 (3):217-220 - 10.
Bratoiu I, Burlui AM, Cardoneanu A, Macovei LA, Richter P, Rusu-Zota G, et al. The involvement of smooth muscle, striated muscle, and the myocardium in scleroderma: A review. International Journal of Molecular Sciences. 2022; 23 (19):12011 - 11.
Paik JJ, Wigley FM, Mejia AF, Hummers LK. Independent association of severity of muscle weakness with disability as measured by the health assessment questionnaire disability index in scleroderma. Arthritis Care & Research (Hoboken). 2016; 68 (11):1695-1703 - 12.
Walker UA, Tyndall A, Czirjak L, Denton C, Farge-Bancel D, Kowal-Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR scleroderma trials and research group database. Annals of the Rheumatic Diseases. 2007; 66 (6):754-763 - 13.
Meyer OC, Fertig N, Lucas M, Somogyi N, Medsger TA. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. The Journal of Rheumatology. 2007; 34 (1):104-109 - 14.
Paik JJ, Wigley FM, Lloyd TE, Corse AM, Casciola-Rosen L, Shah AA, et al. Spectrum of muscle histopathologic findings in forty-two scleroderma patients with weakness. Arthritis Care & Research (Hoboken). 2015; 67 (10):1416-1425 - 15.
Corallo C, Cutolo M, Volpi N, Franci D, Agliano M, Montella A, et al. Histopathological findings in systemic sclerosis-related myopathy: Fibrosis and microangiopathy with lack of cellular inflammation. Therapeutic Advances in Musculoskeletal Disease. 2017; 9 (1):3-10 - 16.
Paolino SGF, Cimmino MA, Casabella A, Pizzorni C, Patanè M, Schenone C, et al. Advanced microvascular damage associated with occurence of sarcopenia in systemic sclerosis patients: Results from a retrospective cohort study. Clinical and Experimental Rheumatology. 2020; 38 :65-72 - 17.
Paolino S, Gotelli E, Goegan F, Casabella A, Ferrari G, Patane M, et al. Body composition and bone status in relation to microvascular damage in systemic sclerosis patients. Journal of Endocrinological Investigation. 2021; 44 (2):255-264 - 18.
Dumitru RB, Goodall AF, Broadbent DA, Del Galdo F, Tan AL, Biglands JD, et al. First pilot study of extracellular volume MRI measurement in peripheral muscle of systemic sclerosis patients suggests diffuse fibrosis. Rheumatology (Oxford, England). 2022; 61 (4):1651-1657 - 19.
Selva-O’Callaghan A, Guillen-Del-Castillo A, Gil-Vila A, Trallero-Araguás E, Matas-García A, Milisenda JC, et al. Systemic sclerosis-associated myopathy: How to treat. Current Treatment Options in Rheumatology. 2023. pp. 1-17 - 20.
Paik JJ, Wigley FM, Shah AA, Corse AM, Casciola-Rosen L, Hummers LK, et al. Association of Fibrosing Myopathy in systemic sclerosis and higher mortality. Arthritis Care & Research (Hoboken). 2017; 69 (11):1764-1770 - 21.
Leclair V, D’Aoust J, Gyger G, Landon-Cardinal O, Meyer A, O’Ferrall E, et al. Autoantibody profiles delineate distinct subsets of scleromyositis. Rheumatology (Oxford, England). 2022; 61 (3):1148-1157 - 22.
Junior JG, Mugii N, Inaoka PT, Sampaio-Barros PD, Shinjo SK. Inflammatory myopathies overlapping with systemic sclerosis: A systematic review. Clinical Rheumatology. 2022; 41 (7):1951-1963 - 23.
Bhansing KJ, van Riel PL, van Engelen BG, Fransen J, Vonk MC. Patients with systemic sclerosis/polymyositis overlap have a worse survival rate than patients without it. The Journal of Rheumatology. 2016; 43 (10):1838-1843 - 24.
Bhansing KJ, Lammens M, Knaapen HK, van Riel PL, van Engelen BG, Vonk MC. Scleroderma-polymyositis overlap syndrome versus idiopathic polymyositis and systemic sclerosis: A descriptive study on clinical features and myopathology. Arthritis Research & Therapy. 2014; 16 (3):R111 - 25.
Lefebvre F, Giannini M, Ellezam B, Leclair V, Troyanov Y, Hoa S, et al. Histopathological features of systemic sclerosis-associated myopathy: A scoping review. Autoimmunity Reviews. 2021; 20 (7):102851 - 26.
Brown M, O’Reilly S. The immunopathogenesis of fibrosis in systemic sclerosis. Clinical and Experimental Immunology. 2019; 195 (3):310-321 - 27.
Fearon K, Evans WJ, Anker SD. Myopenia-a new universal term for muscle wasting. Journal of Cachexia, Sarcopenia and Muscle. 2011; 2 (1):1-3 - 28.
Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: A new definition. Clinical Nutrition. 2008; 27 (6):793-799 - 29.
Berardi E, Madaro L, Lozanoska-Ochser B, Adamo S, Thorrez L, Bouche M, et al. A pound of flesh: What cachexia is and what it is not. Diagnostics (Basel). 2021; 11 (1):116 - 30.
Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. Journal of Cachexia, Sarcopenia and Muscle. 2016; 7 (5):512-514 - 31.
Rosenberg IH. Sarcopenia: Origins and clinical relevance. The Journal of Nutrition. 1997; 127 (Suppl. 5):990S-991S - 32.
Siegert E, March C, Otten L, Makowka A, Preis E, Buttgereit F, et al. Prevalence of sarcopenia in systemic sclerosis: Assessing body composition and functional disability in patients with systemic sclerosis. Nutrition. 2018; 55-56 :51-55 - 33.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age and Ageing. 2019; 48 (1):16-31 - 34.
Hax V, do Espirito Santo RC, Dos Santos LP, Farinon M, de Oliveira MS, Tres GL, et al. Practical screening tools for sarcopenia in patients with systemic sclerosis. PLoS One. 2021; 16 (1):e0245683 - 35.
da Rocha DS, Tessari JA, Mainardi NB, Hax V, Gasparin AA, de Oliveira CAV, et al. Assessment of muscle mass using chest computed tomography-based quantitative and qualitative measurements in patients with systemic sclerosis: A retrospective study with cross-sectional and longitudinal analyses. Seminars in Arthritis and Rheumatism. 2023; 59 :152168 - 36.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gomez J, et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clinical Nutrition. 2004; 23 (6):1430-1453 - 37.
Bauer JM. Muscle function and sarcopenia: Clinical implications of recent research. Journal of the American Medical Directors Association. 2021; 22 (4):725-727 - 38.
Narici M, McPhee J, Conte M, Franchi MV, Mitchell K, Tagliaferri S, et al. Age-related alterations in muscle architecture are a signature of sarcopenia: The ultrasound sarcopenia index. Journal of Cachexia, Sarcopenia and Muscle. 2021; 12 (4):973-982 - 39.
Fragala MS, Fukuda DH, Stout JR, Townsend JR, Emerson NS, Boone CH, et al. Muscle quality index improves with resistance exercise training in older adults. Experimental Gerontology. 2014; 53 :1-6 - 40.
Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, Arosio B. Role of age-related mitochondrial dysfunction in sarcopenia. International Journal of Molecular Sciences. 2020; 21 (15):5236 - 41.
Hepple RT. When motor unit expansion in ageing muscle fails, atrophy ensues. The Journal of Physiology. 2018; 596 (9):1545-1546 - 42.
Li CW, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. Journal of Cachexia, Sarcopenia and Muscle. 2022; 13 (2):781-794 - 43.
Rosato E, Gigante A, Pellicano C, Villa A, Iannazzo F, Alunni Fegatelli D, et al. Symptoms related to gastrointestinal tract involvement and low muscularity in systemic sclerosis. Clinical Rheumatology. 2022; 41 (6):1687-1696 - 44.
Sangaroon A, Foocharoen C, Theerakulpisut D, Srichompoo K, Mahakkanukrauh A, Suwannaroj S, et al. Prevalence and clinical association of sarcopenia among Thai patients with systemic sclerosis. Scientific Reports. 2022; 12 (1):18198 - 45.
Caimmi C, Caramaschi P, Venturini A, Bertoldo E, Vantaggiato E, Viapiana O, et al. Malnutrition and sarcopenia in a large cohort of patients with systemic sclerosis. Clinical Rheumatology. 2018; 37 (4):987-997 - 46.
Allen TS, Southwood CR, Doerfler BM, Hirano I, Sheean PM. The impact of medical nutrition therapy for patients with advanced systemic sclerosis (MNT PASS). Journal of the Academy of Nutrition and Dietetics. 2014; 114 (9):A44 - 47.
Corallo C, Fioravanti A, Tenti S, Pecetti G, Nuti R, Giordano N. Sarcopenia in systemic sclerosis: The impact of nutritional, clinical, and laboratory features. Rheumatology International. 2019; 39 (10):1767-1775 - 48.
Sari A, Esme M, Aycicek GS, Armagan B, Kilic L, Ertenli AI, et al. Evaluating skeletal muscle mass with ultrasound in patients with systemic sclerosis. Nutrition. 2021; 84 :110999 - 49.
Marighela TF, Genaro PS, Pinheiro MM, Szejnfeld VL, Kayser C. Risk factors for body composition abnormalities in systemic sclerosis. Clinical Rheumatology. 2013; 32 (7):1037-1044 - 50.
Medsger TA Jr, Rodnan GP, Moossy J, Vester JW. Skeletal muscle involvement in progressive systemic sclerosis (scleroderma). Arthritis and Rheumatism. 1968; 11 (4):554-568 - 51.
Schmalbruch H, Hellhammer U. The number of nuclei in adult rat muscles with special reference to satellite cells. The Anatomical Record. 1977; 189 (2):169-175 - 52.
Mendias CL. Fibroblasts take the Centre stage in human skeletal muscle regeneration. The Journal of Physiology. 2017; 595 (15):5005 - 53.
Mackey AL, Magnan M, Chazaud B, Kjaer M. Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. The Journal of Physiology. 2017; 595 (15):5115-5127 - 54.
Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro-Adipogenic progenitors cross-talk in skeletal muscle: The social network. Frontiers in Physiology. 2019; 10 :1074 - 55.
Kang X, Yang MY, Shi YX, Xie MM, Zhu M, Zheng XL, et al. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Communication and Signaling: CCS. 2018; 16 (1):42 - 56.
Chen W, You W, Valencak TG, Shan T. Bidirectional roles of skeletal muscle fibro-adipogenic progenitors in homeostasis and disease. Ageing Research Reviews. 2022; 80 :101682 - 57.
Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nature Medicine. 2015; 21 (7):786-794 - 58.
Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. eBioMedicine. 2019; 49 :381-388 - 59.
Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nature Reviews Rheumatology. 2015; 11 (2):86-97 - 60.
Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: The emerging roles of Myokines. Endocrine Reviews. 2020; 41 (4):594-609 - 61.
Cerqueira E, Marinho DA, Neiva HP, Lourenco O. Inflammatory effects of high and moderate intensity exercise-a systematic review. Frontiers in Physiology. 2019; 10 :1550 - 62.
Pettersson H, Alexanderson H, Poole JL, Varga J, Regardt M, Russell AM, et al. Exercise as a multi-modal disease-modifying medicine in systemic sclerosis: An introduction by the global fellowship on rehabilitation and exercise in systemic sclerosis (G-FoRSS). Best Practice & Research. Clinical Rheumatology. 2021; 35 (3):101695 - 63.
Someya F, Mugii N, Hasegawa M, Yahata T, Nakagawa T. Predictors of exercise-induced oxygen desaturation in systemic sclerosis patients with interstitial lung disease. Respiratory Care. 2014; 59 (1):75-80 - 64.
Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? The FEBS Journal. 2013; 280 (17):4131-4148 - 65.
Moresi V, Adamo S, Berghella L. The JAK/STAT pathway in skeletal muscle pathophysiology. Frontiers in Physiology. 2019; 10 :500 - 66.
Muangchan C, Pope JE. Interleukin 6 in systemic sclerosis and potential implications for targeted therapy. The Journal of Rheumatology. 2012; 39 (6):1120-1124 - 67.
Muangchant C, Pope JE. The significance of interleukin-6 and C-reactive protein in systemic sclerosis: A systematic literature review. Clinical and Experimental Rheumatology. 2013; 31 (2 Suppl. 76):122-134 - 68.
Baraut J, Michel L, Verrecchia F, Farge D. Relationship between cytokine profiles and clinical outcomes in patients with systemic sclerosis. Autoimmunity Reviews. 2010; 10 (2):65-73 - 69.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology. 2014; 6 (10):a016295 - 70.
Barnes TC, Spiller DG, Anderson ME, Edwards SW, Moots RJ. Endothelial activation and apoptosis mediated by neutrophil-dependent interleukin 6 trans-signalling: A novel target for systemic sclerosis? Annals of the Rheumatic Diseases. 2011; 70 (2):366-372 - 71.
Wuttge DM, Wildt M, Geborek P, Wollheim FA, Scheja A, Akesson A. Serum IL-15 in patients with early systemic sclerosis: A potential novel marker of lung disease. Arthritis Research & Therapy. 2007; 9 (5):R85 - 72.
Nadeau L, Aguer C. Interleukin-15 as a myokine: Mechanistic insight into its effect on skeletal muscle metabolism. Applied Physiology, Nutrition, and Metabolism. 2019; 44 (3):229-238 - 73.
Wuttge DM, Wildt M, Scheja A, Westergren-Thorsson G. Interleukin-15 attenuates transforming growth factor-beta1-induced myofibroblast differentiation in human fetal lung fibroblasts. European Cytokine Network. 2010; 21 (3):165-176 - 74.
Davies CA, Jeziorska M, Freemont AJ, Herrick AL. Expression of osteonectin and matrix Gla protein in scleroderma patients with and without calcinosis. Rheumatology (Oxford, England). 2006; 45 (11):1349-1355 - 75.
Carvalheiro T, Malvar Fernandez B, Ottria A, Giovannone B, Marut W, Reedquist KA, et al. Extracellular SPARC cooperates with TGF-beta signalling to induce pro-fibrotic activation of systemic sclerosis patient dermal fibroblasts. Rheumatology (Oxford, England). 2020; 59 (9):2258-2263 - 76.
Zhou X, Tan FK, Reveille JD, Wallis D, Milewicz DM, Ahn C, et al. Association of novel polymorphisms with the expression of SPARC in normal fibroblasts and with susceptibility to scleroderma. Arthritis and Rheumatism. 2002; 46 (11):2990-2999 - 77.
Young BA, Wang P, Goldblum SE. The counteradhesive protein SPARC regulates an endothelial paracellular pathway through protein tyrosine phosphorylation. Biochemical and Biophysical Research Communications. 1998; 251 (1):320-327 - 78.
Macko RFGA, Young BA, Lowitt MH, White B, Wigley FM, Goldblum SE. Increased circulating concentrations of the counteradhesive proteins SPARC and thrombospondin-1 in systemic sclerosis (scleroderma). Relationship to platelet and endothelial cell activation. The Journal of Rheumatology. 2002; 29 (12):2565-2570 - 79.
Ding W, Pu W, Jiang S, Ma Y, Liu Q , Wu W, et al. Evaluation of the antifibrotic potency by knocking down SPARC, CCR2 and SMAD3. eBioMedicine. 2018; 38 :238-247 - 80.
Scavelli K, Chatterjee A, Rhee DJ. Secreted protein acidic and rich in cysteine in ocular tissue. Journal of Ocular Pharmacology and Therapeutics. 2015; 31 (7):396-405 - 81.
Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, et al. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. Journal of Cellular Biochemistry. 2004; 91 (5):915-925 - 82.
Zhou X, Tan FK, Guo X, Arnett FC. Attenuation of collagen production with small interfering RNA of SPARC in cultured fibroblasts from the skin of patients with scleroderma. Arthritis and Rheumatism. 2006; 54 (8):2626-2631 - 83.
Strandjord TP, Madtes DK, Weiss DJ, Sage EH. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. The American Journal of Physiology. 1999; 277 (3):L628-L635 - 84.
Wang JC, Lai S, Guo X, Zhang X, de Crombrugghe B, Sonnylal S, et al. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Research & Therapy. 2010; 12 (2):R60 - 85.
Jorgensen MM, de la Puente P. Leukemia inhibitory factor: An important cytokine in pathologies and cancer. Biomolecules. 2022; 12 (2):217 - 86.
Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, et al. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). The EMBO Journal. 1987; 6 (13):3995-4002 - 87.
Metcalf D. The unsolved enigmas of leukemia inhibitory factor. Stem Cells. 2003; 21 (1):5-14 - 88.
Broholm C, Pedersen BK. Leukaemia inhibitory factor–An exercise-induced myokine. Exercise Immunology Review. 2010; 16 :77-85 - 89.
Taniguchi T, Miyagawa T, Tamaki Z, Nakamura K, Yamashita T, Saigusa R, et al. A possible implication of reduced levels of LIF, LIFR, and gp130 in vasculopathy related to systemic sclerosis. Archives of Dermatological Research. 2017; 309 (10):833-842