Abstract
Molecular autopsy makes it possible to identify the genetic alteration responsible for an inherited arrhythmogenic disease, main suspected cause of sudden death in cases that remain unexplained after a complete medico-legal autopsy. By using next-generation sequencing technology, a massive genetic study can be carried out that identifies a rare variant classified as potentially pathogenic in up to 25% of sudden death cases in the young population. To carry out a post-mortem genetic study, it is necessary to have samples in suitable conservation conditions. Our chapter focuses on the type of samples that are used today in massively parallel genetic analyses.
Keywords
- samples
- molecular autopsy
- genetics
- tissue
- paraffin
- blood
1. Introduction
Currently, in nearly 5% of all cases after comprehensive forensic autopsy no definite cause of death is found, allowing for the definition of “negative autopsy” [1]. When autopsy fails to find the cause of decease, sudden death can be called “unexplained” (SUD) [2]. In SUD cases, especially in those younger than 35 years, inherited arrhythmogenic syndrome (IAS), are frequent cause of death and thus, sudden cardiac death (SCD) should always be suspected [3]. All IAS are of genetic origin and therefore, family members can be carriers of pathogenic genetic alterations, and in consequence, be at risk for SUD. The first manifestation of any of IAS may be a lethal arrhythmogenic episode, highlighting early identification of genetic carriers and allowing the adoption of preventive personalized therapeutic measures [4]. In 2001, a post-mortem genetic analysis or post-mortem molecular analysis (also called “molecular autopsy”) was firstly proposed [5] as a fundamental tool in order to unravel the genetic origin of an IAS as cause of SUD [2]. To date, molecular autopsy has become a complement to autopsies process in the current forensic area. Molecular autopsy has been shown to be a reliable diagnostic tool during a comprehensive forensic investigation of SUDs and may have important implications for the first-degree relatives of the victim leading to further analyses to predict and prevent the risk of life-threatening events [6].
2. Genetic analysis
First genetic approach in molecular autopsy was Sanger technology which has played a significant role allowing the identification of the first genes associated with IAS related to SCD. Although, the Sanger sequencing technique has played an important role in the history of molecular genetics and has been very useful in the study of SCD for many years, at present, its use in clinical practice has been reduced [7]. For years, Sanger sequencing was the gold standard for investigating SUD cases until it was replaced by second-generation high-throughput techniques, called Next generation sequencing (NGS) [8]. This is due to the fact that Sanger technology only allows the study of a limited number of genes and at a high cost, compared to massive next-generation sequencing techniques. However, it remains the gold standard technique for variant confirmation, especially for small deletions and insertions. Nowadays available NGS technologies allow a rapid and cost-effective genetic analysis of numerous genes (even whole exome -WES- and genome -WGS- sequencing). NGS has enabled the identification of more than 2400 new disease-associated genes and more than 150 new genetic diseases [9].
Finally, third-generation sequencing technologies, also known as single-molecule sequencing, allow for the direct sequencing of single DNA molecules without the need for amplification or fragmentation. Although this technology promises to improve the range of detection of causal variants in a wide range of pathologies, since it has some advantages such as the possibility of studying structural variants and repetitive elements, its implementation in clinical practice has not yet materialized. It is partly due to certain limitations such as the high cost compared to NGS, the need for a more complex bioinformatics analysis, and perhaps the most important limitation in our field, the fact that it requires fresh material to obtain ultralong DNA of high molecular weight, which can be a great challenge in post-mortem analysis [10, 11].
3. NGS applicability to SUD
The routinary genetic study carried out using NGS technology in SUD consist of analyzing the main genes currently associated with IAS, either by amplification and sequencing of gene panels, or by performing WES and subsequent filtering of the genes of interest [12, 13, 14]. The number of genes analyzed increase as progress is made in the field of IAS [15]. In young population, molecular autopsy using NGS reveals a definite pathogenic genetic alteration responsible of an IAS in near 20% of cases [16, 17, 18, 19, 20, 21, 22]. It is important to remark that genetic alterations identified in IAS are genetic defects in ion channels expressed in heart as well as in brain, therefore being the main cause of sudden death episodes during epilepsy (Sudden Unexpected Death in Epilepsy, SUDEP) [23, 24, 25].
After molecular autopsy with a positive genetic diagnosis, due to other family members could harbor the same genetic variant and, thus at risk of IAS, a clinical translation of genetic results should be performed [4]. In such cases, first-degree relatives of a SUD victim should undergo a multidisciplinary evaluation including clinical examination and genetic analysis [6, 26]. To date, main challenge in clinical translation of genetic data is the interpretation of large part of variants identified, remaining of unknown significance (VUS). Firstly, this is due to the stricter classification provided by the American College of Medical Genetics (ACMG) [27]. Moreover, either variants are found in genes with no definite association with any of IAS or available data does not allow a deleterious role of a variant to be assigned. Despite this fact, current clinical guidelines recommend molecular autopsy in SUD cases when the victim is young (< 50 years of age) and/or the circumstances of death and/or the family history support an IAS as the most plausible cause of SUD [4, 6, 28, 29].
4. Samples
Collection of samples for molecular autopsy is a crucial step in the forensic analysis of SUD and is recommended by several guidelines [6, 30]. Suitable sampling in terms of site and of timing is crucial also because of the risk of low-template DNA, i.e., inadequate quality and/or quantity of extracted DNA. Indeed, in IAS, up to 40% of samples are not collected adequately for post-mortem genetic study preventing an appropriate analysis [31]. In an exploratory study, fresh blood and frozen blood were reported as the most common types of post mortem samples. In addition, fresh blood and frozen blood had the highest number of successful DNA extractions, but blood spot cards, frozen liver, and frozen heart tissue were also reported to have successful DNA extractions [32]. Recently, a consensus focused on post-mortem study of SCD cases was published, recommending blood as optimal sample for molecular autopsy despite other kind of samples can be also used if appropriate collection and storage, such as fresh/frozen tissues or formalin-fixed and paraffin-embedded (FFPE) tissues [14].
4.1 Blood
This is the easier approach to obtain and storage a post-mortem sample in order to perform a molecular autopsy. The sample will be preferably extracted from the subclavian vasculature by puncture prior to opening the thoracic cavity. If not possible, the thoracic and abdominal cavities will be opened, removing the visceral blockage and puncturing the right atrium afterwards for blood collection [33]. It is recommended to collect 3–5 ml of peripheral or intracardiac blood less than 48 hours post-mortem in Ethylene Diamine Tetra Acetic acid (EDTA) tubes (Figures 1 and 2A). If sample is collected more than 48 hours post-mortem, the degradation of DNA increases progressively despite conservation of body at cold temperature. This degradation may impede a proper DNA extraction and NGS analysis. EDTA tubs should be also store at cold temperature (4–8°C) but if DNA will be extracted during first 48 hours after collection, tubes can be retained at room temperature (no more than 20°C) [34]. If DNA extraction will be programmed more than 2 days after collection, it is highly recommended store tubes at 4°C (maximum 2–4 weeks) [34]. More than one month after extraction, DNA may be progressively degraded, so freezing at a minimum of −20 to −80°C is recommended [14, 35]. However, it is important to note that freezing the EDTA tube should be avoided as much as possible, as the freezing and thawing process damages the DNA structure. In this situation, and in order to preserve DNA integrity, thawing process should be performed progressively (−20 to 4–8°C for at least 1–2 days, and then to room temperature). DNA extraction should be performed at routine room temperature in laboratories (around 15°C). This is the optimal protocol to avoid a rapid DNA degradation, which prevents a proper NGS analysis.
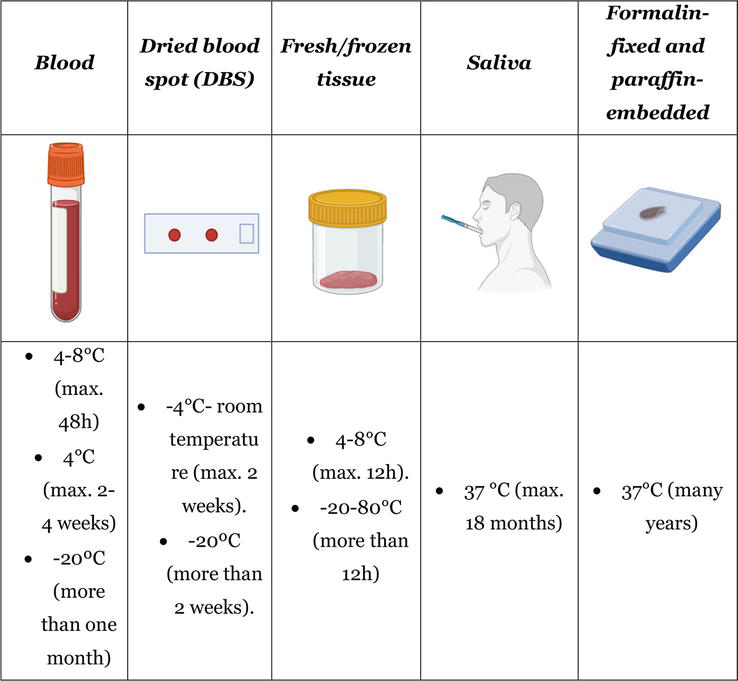
Figure 1.
Samples used in molecular autopsy analysis and their conservation methods.
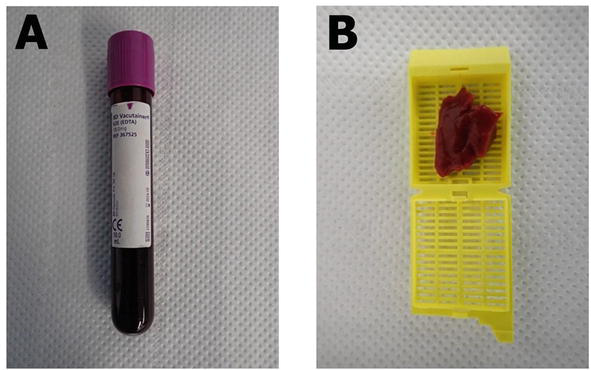
Figure 2.
Real samples mostly used in post-mortem NGS analysis. A. Blood sample. B. Fresh tissue sample.
4.2 Dried blood spot (DBS)
Most NGS analyses are performed using EDTA-anticoagulated peripheral blood, because of the high quality of DNA extraction obtained from such samples (Figure 1). However, collecting, transporting and storing blood in some circumstances can represent a challenge. An alternative may be the use of Dried blood spots (DBS), since NGS techniques require only short fragments of DNA for sequencing, which can be obtained with this collection technique [14, 35, 36]. DBS is an inexpensive method that is easy to handle in all conditions and does not require a trained professional for collection [37]. DBS can be storage at ambient temperature for many months and even years but the quality of DNA extracted could be reduced progressively [38]. Although recent studies support a sequencing yield with DNA from DBS samples, similar to that obtained with DNA samples from peripheral blood, both in the detection of single nucleotide variants, insertions and deletions, as well as copy number variants and mitochondrial heteroplasmy, such studies are limited and their throughput in NGS techniques applied to postmortem analysis of SUD has not been confirmed [39, 40, 41].
4.3 Fresh/frozen tissue
Fresh tissue samples from cadaver should be collected for post-mortem genetic testing (Figures 1 and 2B). The 2020 APHRS/HRS expert consensus statement recommends that samples of heart (especially if SCD is suspected) and at least one spleen/liver/skeletal muscles sample should always be saved [35]. About 5 g of tissue is optimal to perform a quick DNA extraction (no more than 6–12 hours after extraction and retained at 4–8°C) in order to avoid DNA degradation. Focused on SUD and suspected IAS, tissue should be of heart (optimal from ventricular myocardium). If not analyzed in this time period after extraction, the fresh samples should be stored at freezing or ultra-freezing temperatures (−20 to −80°C) [35]. In this situation, defrosting should be carried out progressively (−80 to −20°C, then to 4–8°C for at least 1–2 days, and finally to room temperature) before DNA extraction to avoid degradation, as mentioned for frozen blood. DNA extraction should be performed at routine room temperature in laboratories (around 15°C).
4.4 Saliva
DNA extraction from saliva for high-quality genetic analysis is widely used for living people and many devices are available for the sample collection (Figure 1) [42]. It is a widely used sample in the genetic study of many pathologies given the multiple advantages it presents in terms of sample collection, transport and storage. Saliva can be stored at room temperature for up to 18 months without compromising its quality for genetic analysis [43]. Although in forensic genetics buccal swabs are indicated for identification in recent corpses, sampling cadaveric saliva is usually not recommended, since it is technically challenging and can be biased by post-mortem changes. Other samples with the same or lower extraction complexity may yield better quality DNA samples.
4.5 Formalin-fixed and paraffin-embedded (FFPE) tissues
Currently, FFPE tissue samples are processed and stored, as part of routine forensic protocol in order to unravel any tissular alteration (Figure 1) [44]. In heart FFPE tissue, several alterations such as inflammatory cell infiltration, myocyte apoptosis or any other alteration may clarify the cause of death. Focused on IAS, tissular alterations may confirm the suspected diagnosis. Sometimes, macroscopic study does not reveal any alteration but FFPE analysis identify alterations such as disarray or fibro-fatty infiltration in myocardium, hallmarks of cardiomyopathies [45]. It is possible to extract DNA from FFPE stored for more than 25 years for molecular analysis [46]. These samples represent a suboptimal material for molecular autopsy as storage in formalin has been shown to damage DNA, which is then variable in both quantity and quality [44, 47].
In order to use FFPE for NGS analysis, protocols of tissue fixation and paraffin embedding usually damages the DNA integrity preventing an adequate NGS study from being performed according the recommended protocols [48]. Tissue samples that were formalin-fixed did not have high rates of successful DNA extraction, which is consistent with evidence found in a past study that showed formalin-fixed samples are unreliable for post mortem genetic testing in cases of sudden unexplained death [32]. DNA from FFPE have been considered error prone and unreliable in comprehensive surveillance of SUD-associated genes. Given these shortcomings, the standard autopsy for SUD should include archiving EDTA-preserved blood or frozen tissue to facilitate post mortem genetic testing [49].
However, several studies have been carried out obtaining proper DNA from FFPE heart tissue in IAS but with a wide range of technical variables preventing a standardized use of FFPE for a comprehensive NGS analysis [50, 51]. In order to solve this limitation, in last years, adapting protocols and special kits focused on DNA extraction from FFPE samples has been developed, helping to use this kind of samples, especially in old cases if no other sample available.
5. Ethics considerations
Although in some countries it is not necessary to have a specific and express consent to perform a genetic analysis on the sample of a deceased person, current legislation must be taken into account before performing a molecular autopsy. When identifying a genetic alteration responsible for the pathology or if the results of the studies are of interest to other members of the deceased’s family, it is important to take into account ethical concepts to preserve the privacy and protection of the family members’ data, as well as the impact on the health of the individuals who may be at risk. In the case of minors or individuals with intellectual disabilities, the parents or guardians/legal representatives are the ones who must make the decision for them. Communication of the results of genetic testing and autopsy to the family should ideally be performed by a multidisciplinary team composed of cardiologists and genetic counselors specialized in cardiovascular genetics at a medical center, in the context of genetic counseling [14].
6. Conclusions
Finding the cause of the death in SUD cases should be considered a public health priority since, especially in young population, these events are mainly due to IAS. The current standard techniques for postmortem molecular analysis are those focused on panels of known SUD-associated genes, both because it allows the analysis of a large number of genes at low cost, and because it requires a small amount of DNA obtained from a wide variety of samples for analysis. Sampling is a crucial step in molecular autopsy to avoid the risk of low-template DNA and thus to maximize DNA yield. Post-mortem blood and fresh or frozen highly vascularized tissues are optimal sources of DNA, while the recourse to FFPE tissues should be reserved when other strategies are not feasible, since the risk of low-template DNA.
Acknowledgement/funding
This work was supported by Obra Social “La Caixa Foundation” (LCF/PR/GN19/50320002). Co-funded by Instituto de Salud Carlos III (FIS PI21/00094). CIBERCV is an initiative of the ISCIII, Spanish Ministry of Economy and Competitiveness. Funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Conflict of interest
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.
Lawler W. The negative coroner’s necropsy: A personal approach and consideration of difficulties. Journal of Clinical Pathology. 1990; 43 (12):977-980 - 2.
Basso C, Aguilera B, Banner J, Cohle S, d’Amati G, de Gouveia RH, et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Archiv. 2017; 471 (6):691-705 - 3.
Oliva A, Flores J, Merigioli S, LeDuc L, Benito B, Partemi S, et al. Autopsy investigation and Bayesian approach to coronary artery disease in victims of motor-vehicle accidents. Atherosclerosis. 2011; 218 (1):28-32 - 4.
Wilde AAM, Semsarian C, Marquez MF, Sepehri Shamloo A, Ackerman MJ, Ashley EA, et al. European heart rhythm association (EHRA)/heart rhythm society (HRS)/Asia pacific heart rhythm society (APHRS)/Latin American heart rhythm society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Heart Rhythm. 2022 Jul; 19 (7):e1-e60. - 5.
Ackerman MJ, Tester DJ, Driscoll DJ. Molecular autopsy of sudden unexplained death in the young. The American Journal of Forensic Medicine and Pathology. 2001; 22 (2):105-111 - 6.
Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. European Heart Journal. 2022; 43 (40):3997-4126 - 7.
Heather JM, Chain B. The sequence of sequencers: The history of sequencing DNA. Genomics. 2016; 107 (1):1-8 - 8.
Castiglione V, Modena M, Aimo A, Chiti E, Botto N, Vittorini S, et al. Molecular autopsy of sudden cardiac death in the genomics era. Diagnostics (Basel). 2021 Jul 30; 11 (8):1378 - 9.
Rabbani B, Nakaoka H, Akhondzadeh S, Tekin M, Mahdieh N. Next generation sequencing: Implications in personalized medicine and pharmacogenomics. Molecular BioSystems. 2016; 12 (6):1818-1830 - 10.
Xiao T, Zhou W. The third generation sequencing: The advanced approach to genetic diseases. Translational Pediatrics. 2020; 9 (2):163-173 - 11.
van Dijk EL, Jaszczyszyn Y, Naquin D, Thermes C. The third revolution in sequencing technology. Trends in Genetics. 2018; 34 (9):666-681 - 12.
Alcalde M, Nogue-Navarro L, Tiron C, Fernandez-Falgueras A, Iglesias A, Simon A, et al. Rare variants in genes encoding structural myocyte contribute to a thickened ventricular septum in sudden death population without ventricular alterations. Forensic Science International Genetics. 2022; 58 :102688 - 13.
Coll M, Fernandez-Falgueras A, Tiron C, Iglesias A, Buxo M, Simon A, et al. Post-mortem toxicology analysis in a young sudden cardiac death cohort. Forensic Science International Genetics. 2022; 59 :102723 - 14.
Kelly KL, Lin PT, Basso C, Bois M, Buja LM, Cohle SD, et al. Sudden cardiac death in the young: A consensus statement on recommended practices for cardiac examination by the pathologist from the Society for Cardiovascular Pathology. Cardiovascular Pathology. 2023 Mar-Apr; 63 :107497 - 15.
Martinez-Barrios E, Grassi S, Brion M, Toro R, Cesar S, Cruzalegui J, et al. Molecular autopsy: Twenty years of post-mortem diagnosis in sudden cardiac death. Frontiers in Medicine. 2023; 10 :1118585 - 16.
Campuzano O, Sanchez-Molero O, Allegue C, Coll M, Mademont-Soler I, Selga E, et al. Post-mortem genetic analysis in juvenile cases of sudden cardiac death. Forensic Science International. 2014; 245C :30-37 - 17.
Chugh SS, Senashova O, Watts A, Tran PT, Zhou Z, Gong Q, et al. Postmortem molecular screening in unexplained sudden death. Journal of the American College of Cardiology. 2004; 43 (9):1625-1629 - 18.
Di Paolo M, Luchini D, Bloise R, Priori SG. Postmortem molecular analysis in victims of sudden unexplained death. The American Journal of Forensic Medicine and Pathology. 2004; 25 (2):182-184 - 19.
Skinner JR, Crawford J, Smith W, Aitken A, Heaven D, Evans CA, et al. Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm. 2011; 8 (3):412-419 - 20.
Winkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Hansen SH, et al. Sudden unexpected death in infancy in Denmark. Scandinavian Cardiovascular Journal: SCJ. 2011; 45 (1):14-20 - 21.
Tester DJ, Ackerman MJ. The molecular autopsy: Should the evaluation continue after the funeral? Pediatric Cardiology. 2012; 33 (3):461-470 - 22.
Isbister JC, Nowak N, Butters A, Yeates L, Gray B, Sy RW, et al. “Concealed cardiomyopathy” as a cause of previously unexplained sudden cardiac arrest. International Journal of Cardiology. 2021; 324 :96-101 - 23.
Bagnall RD, Crompton DE, Petrovski S, Lam L, Cutmore C, Garry SI, et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Annals of Neurology. 2016; 79 (4):522-534 - 24.
Chahal CAA, Salloum MN, Alahdab F, Gottwald JA, Tester DJ, Anwer LA, et al. Systematic review of the genetics of sudden unexpected death in epilepsy: Potential overlap with sudden cardiac death and arrhythmia-related genes. Journal of the American Heart Association. 2020; 9 (1):e012264 - 25.
Chahal CAA, Tester DJ, Fayyaz AU, Jaliparthy K, Khan NA, Lu D, et al. Confirmation of cause of death via comprehensive autopsy and whole exome molecular sequencing in people with epilepsy and sudden unexpected death. Journal of the American Heart Association. 2021; 10 (23):e021170 - 26.
Hansen BL, Jacobsen EM, Kjerrumgaard A, Tfelt-Hansen J, Winkel BG, Bundgaard H, et al. Diagnostic yield in victims of sudden cardiac death and their relatives. Europace. 2020; 22 (6):964-971 - 27.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of medical genetics and genomics and the Association for molecular pathology. Genetics in Medicine. 2015; 17 (5):405-424 - 28.
Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: This document was developed as a partnership between the heart rhythm society (HRS) and the European heart rhythm association (EHRA). Europace. 2011; 13 (8):1077-1109 - 29.
Priori SG, Blomstrom-Lundqvist C. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. European Heart Journal. 2015; 36 (41):2757-2759 - 30.
Stiles MK, Fauchier L, Morillo CA, Wilkoff BL. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2020; 17 (1):e220-e228 - 31.
Williams N, Manderski E, Stewart S, Bao R, Tang Y. Lessons learned from testing cardiac channelopathy and cardiomyopathy genes in individuals who died suddenly: A two-year prospective study in a large medical examiner’s office with an in-house molecular genetics laboratory and genetic counseling services. Journal of Genetic Counseling. 2020; 29 (2):293-302 - 32.
Liu G, MacLeod H, Webster G, McNally EM, O’Neill SM, Dellefave-Castillo L. Genetic counselors’ approach to postmortem genetic testing after sudden death: An exploratory study. Academic Forensic Pathology. 2018; 8 (3):738-751 - 33.
Singh D, Tiwari RC, Kumar A, Bhute AR, Meshram RP, Dikshit M, et al. A comprehensive review of pathological examination in forensic medicine: Past, present, and future. Cureus. 2022; 14 (3):e22740 - 34.
Sanchez O, Campuzano O, Fernandez-Falgueras A, Sarquella-Brugada G, Cesar S, Mademont I, et al. Natural and undetermined sudden death: Value of post-mortem genetic investigation. PLoS One. 2016; 11 (12):e0167358 - 35.
Stiles MK, Wilde AAM, Abrams DJ, Ackerman MJ, Albert CM, Behr ER, et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm. 2021; 18 (1):e1-e50 - 36.
Boemer F, Fasquelle C, d’Otreppe S, Josse C, Dideberg V, Segers K, et al. A next-generation newborn screening pilot study: NGS on dried blood spots detects causal mutations in patients with inherited metabolic diseases. Scientific Reports. 2017; 7 (1):17641 - 37.
Martial LC, Aarnoutse RE, Schreuder MF, Henriet SS, Bruggemann RJ, Joore MA. Cost evaluation of dried blood spot home sampling as compared to conventional sampling for therapeutic drug monitoring in children. PLoS One. 2016; 11 (12):e0167433 - 38.
Gruner N, Stambouli O, Ross RS. Dried blood spots--preparing and processing for use in immunoassays and in molecular techniques. Journal of Visualized Experiments. 2015 Mar 13; 97 :52619 - 39.
Agrawal P, Katragadda S, Hariharan AK, Raghavendrachar VG, Agarwal A, Dayalu R, et al. Validation of whole genome sequencing from dried blood spots. BMC Medical Genomics. 2021; 14 (1):110 - 40.
Anderson C, Fry RC, Hartwell H, Kleeberger C, Sandler DP, Nichols HB. Measurement of mitochondrial DNA copy number in dried blood spots: A pilot study. Mitochondrion. 2021; 56 :35-39 - 41.
Hendrix MM, Cuthbert CD, Cordovado SK. Assessing the performance of dried-blood-spot DNA extraction methods in next generation sequencing. International Journal of Neonatal Screening. 2020 Jun; 6 (2):36 - 42.
Goode MR, Cheong SY, Li N, Ray WC, Bartlett CW. Collection and extraction of saliva DNA for next generation sequencing. Journal of Visualized Experiments. 2014 Aug 27; 90 :51697 - 43.
Anthonappa RP, King NM, Rabie AB. Evaluation of the long-term storage stability of saliva as a source of human DNA. Clinical Oral Investigations. 2013; 17 (7):1719-1725 - 44.
Reid KM, Maistry S, Ramesar R, Heathfield LJ. A review of the optimisation of the use of formalin fixed paraffin embedded tissue for molecular analysis in a forensic post-mortem setting. Forensic Science International. 2017; 280 :181-187 - 45.
Mathieson W, Thomas GA. Why formalin-fixed, paraffin-embedded biospecimens must be used in genomic medicine: An evidence-based review and conclusion. The Journal of Histochemistry and Cytochemistry. 2020; 68 (8):543-552 - 46.
Lin J, Kennedy SH, Svarovsky T, Rogers J, Kemnitz JW, Xu A, et al. High-quality genomic DNA extraction from formalin-fixed and paraffin-embedded samples deparaffinized using mineral oil. Analytical Biochemistry. 2009; 395 (2):265-267 - 47.
Tester DJ, Ackerman MJ. The role of molecular autopsy in unexplained sudden cardiac death. Current Opinion in Cardiology. 2006; 21 (3):166-172 - 48.
Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. The American Journal of Pathology. 2002; 161 (6):1961-1971 - 49.
Carturan E, Tester DJ, Brost BC, Basso C, Thiene G, Ackerman MJ. Postmortem genetic testing for conventional autopsy-negative sudden unexplained death: An evaluation of different DNA extraction protocols and the feasibility of mutational analysis from archival paraffin-embedded heart tissue. American Journal of Clinical Pathology. 2008; 129 (3):391-397 - 50.
Baudhuin LM, Leduc C, Train LJ, Avula R, Kluge ML, Kotzer KE, et al. Technical advances for the clinical genomic evaluation of sudden cardiac death: Verification of next-generation sequencing panels for hereditary cardiovascular conditions using formalin-fixed paraffin-embedded tissues and dried blood spots. Circulation. Cardiovascular Genetics. 2017 Dec; 10 (6):e001844 - 51.
Lin Y, Gryazeva T, Wang D, Zhou B, Um SY, Eng LS, et al. Using postmortem formalin fixed paraffin-embedded tissues for molecular testing of sudden cardiac death: A cautionary tale of utility and limitations. Forensic Science International. 2020; 308 :110177
Notes
- †Both equally contributed as co-first authors
- Both equally contributed as co-senior authors.