Lethal concentration 50 (LC50) of the total methanolic extract of
Abstract
The termiticidal activity of methanolic extracts of Senna occidentalis and Tithonia diversifolia leaves was determined on the crop pest termite, Ancistrotermes cavithorax. Both extracts were toxic to termite workers by contact and inhalation. The T. diversifolia extract (LC50 of 29, 76 mg/l) was more toxic than the S. occidentalis extract (LC50 of 84.90 mg/l). The two extracts are not repellent but anti-palatable. To recommend insecticidal plants in the culture medium requires the knowledge of their harmfulness to the environment and mammals. This and the toxicity of its two extracts were determined on female rats Rattus norvegicus. The LD 50 of these two extracts exceeded 5000 mg/kg of body mass. The extracts did not cause any renal or hepatic damage after 14 days. The use of these insecticidal plant extracts can therefore be recommended to farmers.
Keywords
- toxicity
- termites
- mammals
- Tithonia diversifolia
- Senna occidentalis
1. Introduction
Termites are one of the greatest scourges in tropical agriculture and agroforestry [1, 2]. In Côte d’Ivoire, West Africa, they regularly attack and destroy food and industrial crops [3, 4, 5, 6]. To control termite attacks and damage, several methods are used by producers. Among these techniques is the use of chemical inputs [7]. Excessive use of chemical inputs can adversely affect living organisms and their environment [8] with very high repair costs. In sub-Saharan Africa, the potential cost of treating pesticide-related illnesses between 2005 and 2020 is about US$90 billion [9]. These problems with synthetic pesticides have led to the search for new termiticidal substances from plants that are environmentally and human health friendly [5, 10, 11]. Pesticidal plants are toxic to pests and generally less harmful to beneficial insects and the environment [12]. The present study set out to evaluate the insecticidal properties of two common plants in West Africa,
2. Materials and methods
2.1 Plant materials
Fresh leaves of
2.2 Animal material
The pesticide tests were conducted on adult workers of the termite
The acute toxicity tests and the histopathology study were conducted on
2.3 Preparation of extracts
Extracts were prepared according to the method described by Kaushik and Vir [13]. Thus, 30 g of powder from the leaves of each plant was mixed in 1 l of 70% methanol. The resulting mixture was stirred for 48 h at room temperature (25°C) using a magnetic stirrer type IKAMAG RCT (Staufen, Germany). The mixture was filtered three times on Whatman paper (No. 4) and then using cotton. The collected solution was subjected to rotary evaporation with a rotavapor BUCHI R-100/32 to obtain the methanolic extract. This solution was then dried under vacuum.
2.4 Biological tests
2.4.1 Toxicity by contact
The test was carried out in Petri dishes 90 mm in diameter containing 3.5 g of soil moistened with 1 ml of distilled water [14]. Using a micropipette, 10, 20, 50, and 100 μl doses of each extract were deposited and mixed with the soil. After depositing, the dishes are air-dried for 1 h. Fifty adult workers of
LD50 is calculated on the basis of 24-h mortality.
2.4.2 Inhalation toxicity
The inhalation test was performed in a large Plexiglass® box of 180 × 20 × 70 mm height according to the method of Tahiri et al. [14]. Into each large box was introduced a Petri dish containing 3.5 g of soil previously moistened with 1 ml of distilled water. Fifty
2.4.3 Barrier soil test
The barrier soil test was conducted according to the method of Tahiri [11]. Nine plastic boxes (chambers) (diameter 5 × 3.5 cm high), closed with plastic covers, were used per arena. One introduction chamber containing sieved nest soil, four substrate chambers containing nest soil, and four food chambers, each containing two pieces of Whatman no. 4 paper of 4 cm2 were used.
The chambers were connected to each other and to the introduction chamber located in the center of the arena by transparent tubes (7 cm length) (LABELL Ch/Fr), length 49 inches. In each trial, only one substrate chamber per arena was treated as barrier soil (50 g of sand moistened with 7.5 ml of the product) to prevent termites from reaching the food source. The other untreated substrate chambers contained 50 g of sand moistened with 7.5 ml of distilled water.
Three hundred
After 10 days of continuous exposure to the product, surviving workers are counted in all arenas, and their total weight is calculated. The tunneling task and food consumption of workers were compared between treated and untreated insects.
2.4.4 Acute toxicity in female rats
The experiment was conducted according to the European OECD guideline 423 [15]. Nine female rats were divided into three batches of three rats (one control and two treated batch) and used for the experiments. The animals were deprived of food overnight. Prior to the experiment, the animals were provided free access to water. Control animals received ml/100 g body weight of distilled water. Due to the low toxicity of
2.4.4.1 Relative mass of vital organs
At the end of the 14-day test, each animal was weighed and then sacrificed. The kidneys, liver, and heart were removed and weighed. Relative masses were calculated according to the following formula:
2.4.4.2 Hematologic and biochemical examinations
At the time of animal sacrifice, volumes of blood were collected in EDTA tubes and dry tubes for hematologic and biochemical analysis, respectively. The hematologic analysis was performed using a Sysmex XS-500i automated analyzer. The blood count consisted of determining the number of white blood cells (WBC), red blood cells (RBC), and blood platelets (PLT), the hematocrit (HCT) and hemoglobin (HGB), the mean blood volume (MCV), the mean corpuscular hemoglobin (MCH) and the mean corpuscular hemoglobin concentration (MCHC).
Biochemical analysis of blood was performed after centrifugation at 3000 rpm for 4 min. An aliquot of serum was collected in Eppendorf tubes. Urea, creatinine, blood glucose, cholesterol, alanine aminotransferase (ASAT), and aspartate aminotransferase (ALAT) were determined using a HITACHI 704 R (Japan) automated analyzer.
2.4.4.3 Histopathology of the liver, heart, and kidney
During the sacrifice, the liver, heart, and kidney of treated and control rats were removed, rinsed with 9‰ NaCl solution, and then fixed in 10% formalin. Flaps of each organ were arranged in cassettes and successively bathed in 80°, 90°, 96°, and 96° ethanol for dehydration. They were then cleared n toluene I and II for 1, 2, and 2 h and then cleared in a step for 2 h in the same reagent. The cassettes were removed from toluene, drained, and impregnated, respectively, in two liquid kerosene baths (I and II) for 2 and 3 h in an oven at 50°C. The cassettes were then cured in the open air and then in the freezer. Sections of 5 μm were made with a Leica RM 2125 RTS® microtome and then stained with hematoxylin and eosin (H&E). The sections were observed with an Olympus CKX41 microscope (Germany) connected to a computer equipped with Videomet software. Pictures of the different histological sections were taken.
2.5 Statistical analysis
The results were expressed as mean plus or minus standard deviation, compared by the ANOVA test and separated by the Newman–Keuls test at the 5% threshold. The statistical software used was Statistica 7.0.
3. Results
3.1 Toxic effect by contact of the extracts
The results of the contact toxicity test indicate that the methanolic extract of
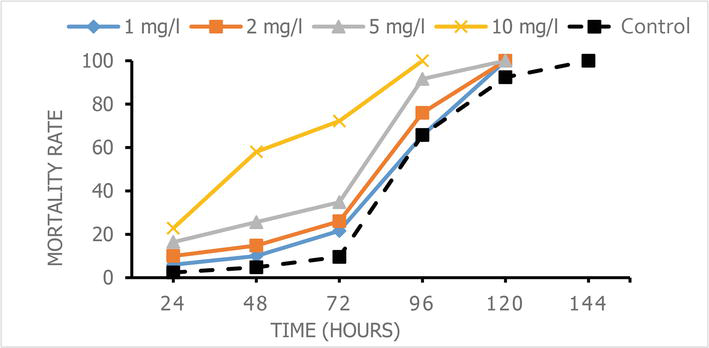
Figure 1.
Variation in mortality of
Percentage mortality as a function of time for the contact toxicity test of the methanolic extract of
The mortality rate of termites increased proportionally with the concentration and time of exposure. Fifty percent (50%) of the mortality of
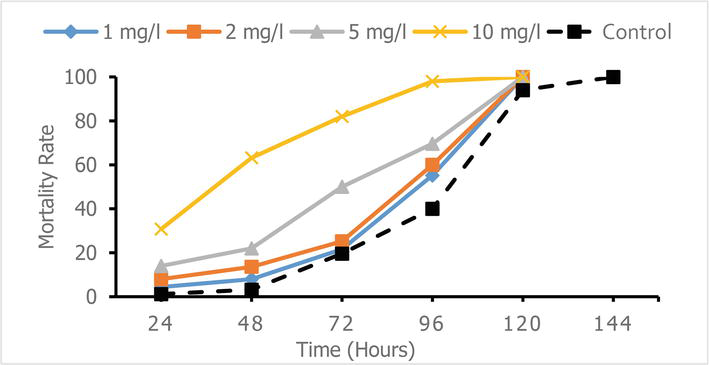
Figure 2.
Variation in mortality of
3.2 Lethal concentrations of extracts
The Probit analysis performed on the basis of 24-h mortality, showed that the amount of the methanolic extract of
| Products | LC50 (mg/l) | Boundary below 95% (mg/l) | Borne above 95% (mg/l) |
|---|---|---|---|
| 29.76 | 19.87 | 55.90 | |
| 84.90 | 37.57 | 426.22 |
Table 1.
3.3 Inhalation toxicity of extracts
After 24 h of inhalation, the mortality rates of 95.99% and 91.66% obtained with the methanolic extract of
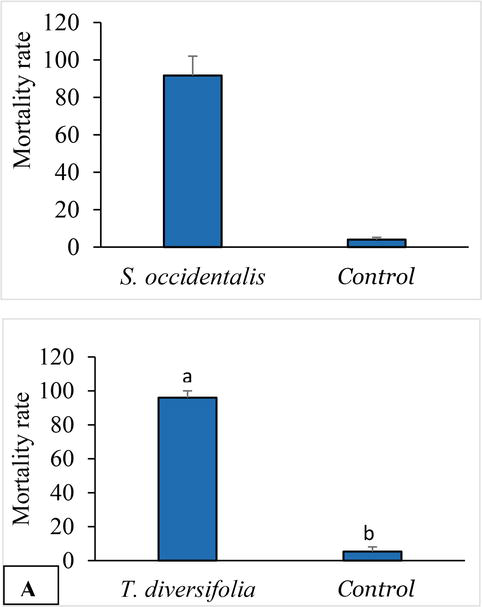
Figure 3.
Distribution of mortality of
3.4 Toxic effect by barrier soil of extracts
In the test and control arenas, after 10 days of continuous exposure of 300 workers to moistened soil, the mortality rates of 88.89 ± 3.37% and 85.33 ± 9.61%, respectively, in the tests with
| Soil moistening | Average mortality (%) ± standard deviation |
|---|---|
| Extract of | 85.33 ± 9.61 a |
| Extract of | 88.89 ± 3.37 a |
| Distilled water | 26.0 ± 1.85 c |
Table 2.
Average mortality rate of
Values followed by the same letters in the same column are not significantly different at the 5% threshold (Kruskal–Wallis). N = 300 workers/test; paper area = 2 × 4 cm2/room.
| Soil moistening | Cumulative consumption (mm2) ± standard deviation |
|---|---|
| Extract of | 08.04 ± 1.82 a |
| Extract of | 06.09 ± 1.5 a |
| Distilled water | 175.92 ± 8.46 c |
Table 3.
Cumulative consumption of
Values followed by the same letters in the same column are not significantly different at the 5% threshold (Kruskal–Wallis). N = 300 workers/test; paper area = 2 × 4 cm2/room.
3.5 Mortality and symptoms in female rats
Single-dose oral administration of 2000 mg/kg body weight of methanolic extract of
| Rats | Doses (mg/kg) | Number of dead rats | Symptoms |
|---|---|---|---|
| Lot 1 (Control, n = 3) | 0 | 0 | — |
| Lot 2 (EMSO, n = 3) | 2000 | 0 | — |
| Lot 3 (EMTD, n = 3) | 2000 | 0 | — |
Table 4.
Acute toxicity to female rats of methanolic extracts
EMSO, Methanolic extract of
3.6 Effects of extracts on relative organ mass
There was no significant change in the relative mass of the kidneys, liver, and heart of treated rats compared with the relative mass of the organs of control rats (ANOVA, P > 0.05) (Table 5).
| Parameters | Lot 1 control | Lot 2 | Lot 2 |
|---|---|---|---|
| Kidneys | 0.256 ± 0.005 a | 0.268 ± 0.006 a | 0.256 ± 0.005 a |
| Livers | 3620 ± 0.300 a | 3920 ± 0.360 a | 3780 ± 0.270 a |
| Heart | 0.351 ± 0.013 a | 0.335 ± 0.003 a | 0.326 ± 0.001 a |
Table 5.
Effects of total methanolic extract of
Values assigned to the letter a in the same row do not differ significantly (P > 0.05).
3.7 Effects of extracts on biochemical and hematologic parameters
The urea level of rats treated with methanolic extract of
| Parameters | Control (distilled water) | ||
|---|---|---|---|
| Urea (mmol/l) | 0.37 ± 0.026 | 0.37 ± 0.015 | 0.27 ± 0.023* |
| Creatinine (mmol/l) | 3.66 ± 0.33 | 3.33 ± 0.33 | 3,.66 ± 0.33 |
| Blood glucose (mmol/l) | 0.97 ± 0.033 | 1.03 ± 0.039* | 0.96 ± 0.045 |
| Cholesterol (g/l) | 0.58 ± 0.017 | 0.62 ± 0.014 | 0.56 ± 0.015 |
| ASAT (U/I) | 161.5 ± 1964 | 134.7 ± 1.76* | 131.3 ± 1.85* |
| ALAT (U/I) | 40.33 ± 0.88 | 38.33 ± 1.20 | 36.33 ± 0.88 |
Table 6.
Effects of total methanolic extract of
ALAT, alanine aminotransferase; ASAT, Aspartate aminotransferase. In the Table, on each line *: significant difference at P < 0.05; Absence of asterisk (*) on values indicates no significant difference p > 0.05, ANOVA, Newman–Keuls test.
| Parameters | Control (distilled water) | ||
|---|---|---|---|
| WBC (103/μl) | 19.36 ± 1.16 | 20.08 ± 0.86 | 21.30 ± 1.35 |
| RBC (106/μl) | 8100 ± 0.38 | 7120 ± 0.27 | 7515 ± 0.255 |
| HGB (g/dl) | 12.93 ± 0.26 | 12.20 ± 0.52 | 12.90 ± 0.7 |
| HCT (%) | 38.90 ± 0.83 | 37.70 ± 0.77 | 38.10 ± 0.9 |
| MCV (fL) | 51.50 ± 0.81 | 51.53 ± 0.58 | 51.60 ± 0.7 |
| MCH (pg) | 17.20 ± 0.23 | 17.13 ± 0.12 | 17.05 |
| MCHC (g/dl) | 31.53 ± 0.37 | 32.71 ± 0.32 | 33.00 ± 0.2 |
| PLT (103/μl) | 723.7 ± 17.07 | 710.3 ± 16.29 | 705.5 ± 14.5 |
| NEUT (%) | 21.45 ± 0.65 | 21.45 ± 0.88 | 21.12 ± 1.34 |
Table 7.
Effects of methanolic extract of
WBC, white blood cells; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; MCV, mean blood volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, blood platelets; NEUT, neutrophil. The absence of an asterisk (*) on the values indicates that there is no significant difference p > 0.05, ANOVA, Newman–Keuls test.
3.8 Effects of methanolic extracts on histological parameters
The histopathological study performed on the liver, kidney, and heart revealed no structural abnormalities (inflammation, hepatic cell necrosis, and apoptosis) in the rats treated with different doses of total methanolic extracts of
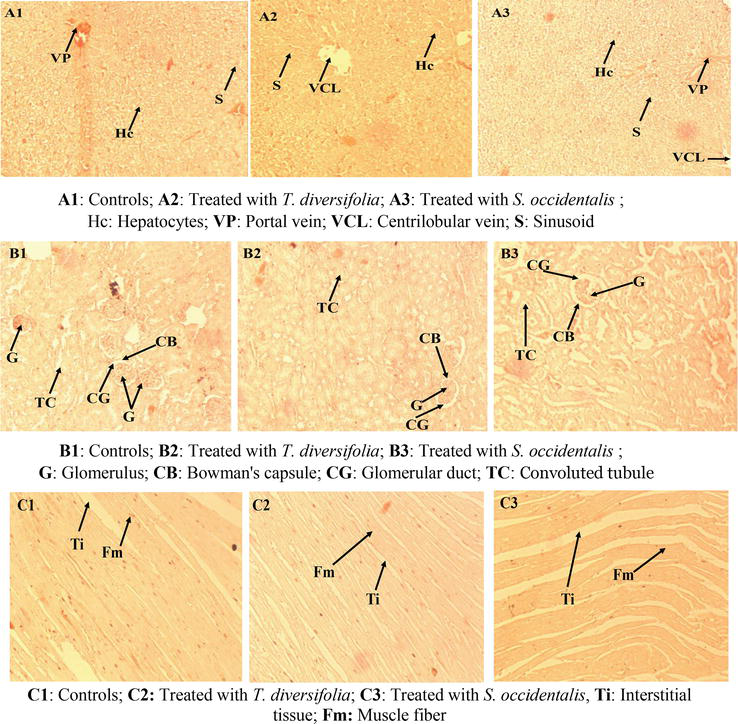
Figure 4.
Histological sections of rat liver (A), kidney (B), and heart (C). Staining: Hematoxylin and eosin, G × 100.
4. Discussion
In the laboratory, methanolic extracts of the leaves of
The mortality rate of
The LC50 values confirmed that the methanolic extract of
The mortality of termites recorded during the experiment shows that the biological efficacy of the methanolic extract of
The extracts also seemed to act by inhalation. Indeed, inhalation with the methanolic extract of
For the barrier soil tests, the sensitivity of the
Although termites have penetrated the barrier soil (treated soil), food in the food chamber is not consumed. In nearby untreated food chambers, termite consumption is affected and is much lower than in controls. Zhu et al. [23] and Tahiri [11] showed that other botanical insecticides tested on
The acute in vivo toxicity study of the two plant extracts, 14 days after oral administration of methanolic extracts of
The results obtained in this study corroborate those obtained by other authors on the same plants. Thus, it was shown that the total methanolic extract of
Relative organ weights showed no significant difference from the control at the end of 14 days of treatment. This result suggested that the single dose of 2000 mg/kg body weight of methanolic extracts of
Analysis of biochemical parameters showed that the creatine level of the rats did not change significantly (P ˃ 0.5) compared to the control at the end of 14 days of treatment at the dose of 2000 mg/kg body weight. Only urea level decreased significantly (P ˂ 0.5) in rats treated with methanolic extracts of
ALAT activity also showed no significant change (P ˃ 0.05) with the administration of the methanolic extracts of
Macroscopic and microscopic examinations of the liver, kidney, and heart of animals treated with different doses of methanolic extracts of
5. Conclusion
This toxicity study showed that methanolic extracts of
References
- 1.
Watt AD, Stork NE, Mc Beath C, Lawson GL. Impact of forest management on insect abundance and damage in a lowland tropical forest in southern Cameroon. Journal of Applied Ecology. 1997; 34 :985-998 - 2.
Wood TG. The agricultural importance of termites in the tropic. Agricultural Zoology Reviews. 1996; 7 :117-155 - 3.
Akpesse AAM, Yao NGR, Coulibaly T, Diby YKS, Kouassi KP, Koua KH. Attacks and damage of termites (Insecta : Isoptera) in cocoa plantations ( Theobroma Cacao L.) of M'Brimbo S.A.B station (South Côte D’Ivoire). International Journal of Advanced Research. 2019;7 (1):438-445 - 4.
Coulibaly T, Akpesse AAM, Yapi A, Zirihi GN, Kouassi KP. Dégâts des termites dans les pépinières de manguiers du nord de la Côte d’Ivoire (Korhogo) et essai de lutte par utilisation d’extraits aqueux de plantes. Journal of Animal &Plant Sciences. 2014; 22 (3):3454-3468 - 5.
Diby YKS, Tahiri YA, Akpesse AAM, Tra Bi CS, Kouassi KP. Évaluation de l’effet insecticide de l’extrait aqueux de Tithonia diversifolia (Hemsl.) gray (Asteracee) sur les termites en culture du riz (NERICA 1) au centre de la Côte d’Ivoire. Journal of Animal & Plant Sciences. 2015;25 (3):3966-3976 - 6.
Tahiri A, Mangué J. Stratégie d’attaque des jeunes plants d’hévéa ( Hevea Brasilliensis Muell.) par les termites et effet comparé de deux insecticides utilisés pour leur protection en basse Côte d’Ivoire. Science & Nature. 2007;4 (1):45-55 - 7.
Cowie RH, Wood S. Damage to crops, forestry and rangeland by fungus-growing termites (Termitidae : Macrotermitinae) in Ethiopia. Sociobiology. 1989; 15 (2):139-153 - 8.
Rahimi R, Abdollahi M. A review on the mechanisms involvedin hyperglycemia induced by orgaophosphorus pesticides. Pesticide Biochemistry and Physiology. 2007; 88 :21-115 - 9.
Anjorwalla P, Belmain S, Sola P, Jamnadass R, Stevenson PC. Guide des plantes pesticides. Nairobi, Kenya: World Agroforestry Centre (ICRAF); 2016. p. 74 - 10.
Siapo YM, Tahiri A, Diby YKS, Ano EJ. Evaluation insecticidal potential of methanolic extracts of Senna occidentalis Link (1829) andTithonia diversifolia (Hemsl) A Gray (1883) on thetermiteAncistrotermes . European Journal of Biotechnology and Bioscience. 2018;6 (4):50-54 - 11.
Tahiri A. Toxicité du macérât de Carica papaya L. contreCoptotermes formosanus Shiraki (Isoptera : Rhinotermitidae). Afrique Science. 2012;8 (3):93-101 - 12.
Mkenda P, Mwanauta R, Stevenson PC, Ndakidemi P, Mtei K, Belmain SR. Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. Public Library of Science. 2015; 10 (11):e0143530 - 13.
Kaushik N, Vir S. Variations in fatty acid and composition of Neem seeds collected from the Rajasthan State of India. Biochemical Society Transactions. 2000; 28 :880-882 - 14.
Tahiri A, Amissa AA, Adjé AF, Amusant N. Effet pesticide et screening des extraits d’ Azadirachta indica (A.) Juss. Sur le termiteMacrotermes bellicosus Rambur. Bois et Forêts Tropicales. 2011;310 (4):79-88 - 15.
Bounihi A. Criblage phytochimique, Étude Toxicologique et Valorisation Pharmacologique de Melissa officinalis et de Mentha rotundifolia (Lamiacées) [Thèse de Doctorat en Pharmacie]. Rabat (Maroc): Université Mohammed V; 2015. p. 199 - 16.
Silva Mirtes GB, Aragao TP, Vasconcelos CFB, Ferreira PA, Andrade BA, Costa IMA, et al. Acute and subacute toxicity of Cassia occidentalis L. stem and leaf in Wistar rats. Journal of Ethnopharmacology. 2011; 136 (2):341-346 - 17.
Elufioyer TO, Alatise OI, Fakoya FA, Agbedahunsi JM, Houghton PJ. Toxicity studies of Tithonia diversifolia A. Gray (Asteraceae) in rats. Journal of Ethnopharmacology. 2009;122 (2):410-415 - 18.
Tahiri A, Amissa AA, Assi M. Toxicité et mode d’action des extraits de Carica papaya L. (Caricaceae) surMacrotermes bellicosus Rambur (Isoptera; Macrotermitinae). Cahiers Agricultures. 2010;19 (4):267-272 - 19.
Diby YKS, Tahiri A, Adja NA, Danho M, Akpesse AAM, Kouassi KP. Repellent and toxic of macerât of Tithonia diversifolia (Hemsl.) gray (Asteraceae) onAncistrotermes sp. at the laboratory. International Journal of Multidisciplinary Research and Development. 2018;5 (12):143-147 - 20.
Maistrello L, Henderson G, Laine RA. Efficacy of vetiver oil and nootkatone as soil barriers against Formosan subterranean termite (Isoptera: Rhinotermitidae). Journal of Economic Entomology. 2001; 94 :1532-1537 - 21.
Zhu W, Chan EK, Li J, Hemmerich P, Tan EM. Transcription activating property of autoantigen SG2NA and modulating effect of WD-40 repeats. Experimental Cell Research. 2001; 269 (2):312-321 - 22.
Blaske VU, Hertel H. Repellent and toxic effects of plants extracts on subterranean termites (Isoptera: Rhinotermitidae). Journal of Economic Entomology. 2001; 94 (5):1200-1208 - 23.
Zhu BCR, Henderson G, Adams RP, Mao L, Yu Y, Lain RA. Repellency of vetiver oils from different biogenetic and geographical origins against Formosan subterranean termites (Isoptera: Rhinotermitidae). Sociobiology. 2003;42 :623-638 - 24.
Irie-N’guessan AG, Kablan BJ, Kouakou-Siransy NG, Leblais V, Champy P. Evaluation de la toxicité de cinq plantes antiasthmatiques de la médecine traditionnelle ivoirienne. International Journal of Biological and Chemical Sciences. 2011; 5 (3):1316-1319 - 25.
Ejelonu OC, Elekofehintia OO, Adanlaw IG. Tithonia diversifolia saponin blood lipid interaction and its influence on immune system of normal Wistar rats. Biomedicine & Pharmacotherapy. 2017;87 :589-595 - 26.
Gowda S, Desai PB, Kulkami SS, Hull VV, Math AAK, Vernekar SN. Markers of renal function tests. North American Journal of Medicine & Science. 2010; 2010 :2170-2173 - 27.
Goddard C, Warnes T. Raised liver enzymes in asymptomatic patients: Investigation and outcome. Digestive Disease. 1992; 10 :218-226 - 28.
Kebieche M. Activité biochimique des extraits flavonoïdiques de la plante Ranunculus repens L.: effet sur le diabète expérimental et l’hépatotoxicité induite par l’Epirubicine [Thèse de Doctorat]. Algerie: Université Mentouri Constantine; 2009. p. 144 - 29.
Sudha A, Sumathi K, Manikandaselvi S, Prabhu NM, Srinivasan P. Anti-hepatotoxic activity of crude flavonoid fraction of Lippia nodiflora L. on ethanol induced liver injury in rats. Asian Journal of Animal Sciences. 2013; 7 (1):1-13