Abstract
A generalized damage to the microcirculation (microvasculopathy) is a cardinal feature of systemic sclerosis and its first manifestation is Raynaud’s phenomenon. Early detection of microvasculopathy enables to establish the right diagnosis at the very early stage of the disease and to identify those patients with the greater risk of internal organ involvement or developmental digital tip ulcers. Dynamic methods help to monitor the response to treatment that influences on the vasomotoric functions of the microcirculation. The gold standard for the assessment of microvascular involvement constitutes nailfold capillaroscopy, which can be performed using stereomicroscopy, videocapillaroscopy, or dermoscopy. Other non-invasive diagnostic methods include sidestream dark field imaging, optical coherence tomography, laser Doppler and laser-related methods, and thermography.
Keywords
- microvasculopathy
- nailfold capillaroscopy
- dermoscopy
- sidestream dark field imaging laser methods
- optical coherence tomography
- doppler methods
- thermography
- systemic sclerosis
1. Introduction
A generalized damage to the microcirculation (microvasculopathy) is a cardinal feature of systemic sclerosis (SSc) and its first manifestation is Raynaud’s phenomenon (RP) [1]. The gold standard for the assessment of microvascular involvement constitutes nailfold capillaroscopy (NFC), but attempts to evaluate the microcirculation in nailfolds date back to 1912. Warren Lombard from the Physiological Institute of the University of Würzburg found improved visibility of dermal papillae and the superficial blood vessels after application of glycerine and transparent oil on the skin [2]. This proposal to use immersion oils for visual exploration of skin capillaries allowed for later development of nailfold capillaroscopic assessment in patients with scleroderma by Brown and O’Leary with stereomicroscope [3]. Intense works of Maricq’s and LeRoy brought the renaissance on the use of NFC in the diagnostic management of systemic sclerosis [4, 5, 6].
2. Nailfold capillaroscopy (NFC)
NFC is the first step at the diagnostic management in patients with RP to distinguish primary from secondary symptom. Abnormalities seen in NFC in patients with RP which coexist with positivity to specific for SSc antinuclear antibodies help to identify those subjects at the very early stage of the disease [7]. SSc is the only systemic connective tissue disease (CTD), which includes capillaroscopic abnormalities in classification criteria established by the European League Against Rheumatism and American College of Rheumatology [8]. The significance of NFC for other CTDs is lower and neither classification criteria for systemic lupus erythematosus [9] nor ones for idiopathic inflammatory myopathies [10] require evaluation of nailfolds. NFC does not only help to establish the diagnosis of SSc, but it also enables to follow-up a progression of microvasculopathy [11]. Capillaroscopic patterns proposed more than twenty years ago by Cutolo et al. (Figure 1) have been still the most valuable grading tool for the damage to the microcirculation [12].
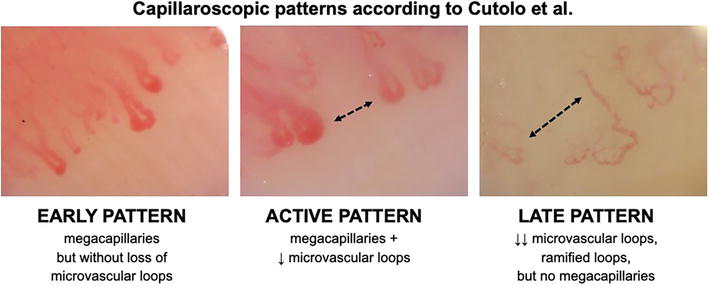
Figure 1.
Classification of microvasculopathy to capillaroscopic patterns.
Although microvasculopathy in SSc is generalized, the damage to the microcirculation is preferentially evaluated in nailfolds of fingers. Skin capillaries are parallel to the epidermis in nailfolds, while in other areas of the body they run perpendicularly (Figure 2). This parallel arrangement allows the observation of the capillaries along instead of only their top-like features in the majority of body surface [13, 14]. One may notice there is an easy development of multiple telangiectasia in patients with SSc due to a ramification of microvessels, which run parallel to the epidermis. Their shape and arrangement are mostly randomized and less predictable than in nailfolds, as well as these telangiectasias may overlap with physiologically already developed ones [15]. Overall, the standardization for the assessment of microvasculopathy in patients with Raynaud’s phenomenon and systemic sclerosis was developed by the European Scleroderma Trials and Research group only in fingers [1].
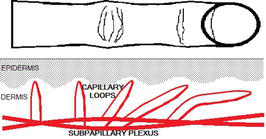
Figure 2.
Capillary loops in the papillary dermis run mostly perpendicularly but in nailfolds they are distributed parallelly to the epidermis.
Initially enlarged capillary loops and then megacapillaries, cap hemorrhages, bushy, and ramified vascular loops, together with disarrangement of vascularity and avascular areas, are typically seen in nailfolds of patients with SSc (Figure 3) [12]. The microcirculation in nailfolds can be evaluated with a use of stereomicroscope, USB microscope (including a dedicated videocapillaroscope), as well as handheld dermoscope.
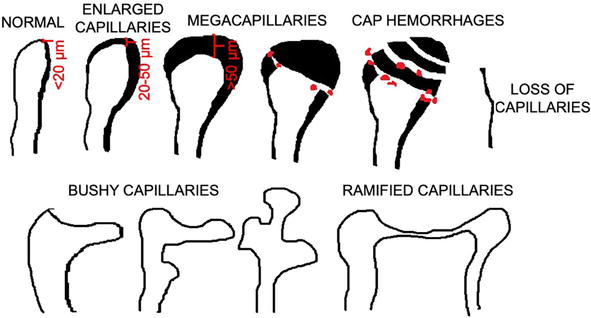
Figure 3.
Different forms of capillary loops seen typically in patients with systemic sclerosis with the possibility of measuring diameter of capillary loop at the apical part of the vascular loop.
Stereomicroscopy (Figure 4) helped to develop NFC technique yet by Brown and O’Leary [3] or later by Maricq and LeRoy [4, 5, 6]. It shows capillaries at 10–40 magnification, which allows for widefield evaluation of capillary loops in the entire nailfold; however, stereomicroscopy shares same limitations. Firstly, it is non-portable technique, which requires an additional lighting with a fiber-optic illuminator. The distance between the optical system of the stereomicroscope and the nailfold may require additional fixation of the finger to capture sharp pictures of capillaries. Space restriction between the objective and the stage may limit the use of stereomicroscopy in case of flexural contracture of fingers developing in some of SSc patients.
Figure 4.
NFC with the use of stereomicroscopy.
Development of USB microscopy has enabled to perform NFC at the bed of patient even in case of advanced deformation of fingers. Dedicated videocapillaroscopy systems (Figure 5) may show vascular loops with 50–600 magnification and they are equipped with integrated LED diodes that illuminate the nailfold. High-power magnification restricts, however, the assessment of the nailfold to only selected region at a time in a contrast to stereomicroscopy, which allows for a quick global evaluation of the microcirculation in the nailfold due to the widefield of vision. Direct contact with the finger shortens the distance between the optical axis and the nailfold, which reduces the blur of pictures due to the movements of fingers. Capillaries are mostly sharply visible on pictures, which enable for their further qualitative and quantitative assessment with the use of dedicated software systems [16, 17].

Figure 5.
NFC with use of nailfold videocapillaroscopy.
3. Dermoscopy
Dermoscopy is an imagining technique primarily used by dermatologists for evaluation of pigmental lesions, but it was shown to be sufficient for early assessment of microvessels. A handheld device reveals vascular structures at the nailfold with a standard 10-fold magnification (Figures 6–8), which may help to distinguish normal from abnormal microcirculation at the first consultation of a patient with RP. Identification of abnormalities of microvessels usually requires referring the patient to a full NFC at greater magnification for detailed analysis of nailfolds. Attachment of dermoscope to a smartphone may enhance the magnification increasing the zoom up to 20–30× (Figures 9–11), therefore allowing for more precise evaluation of the microcirculation and its classification to capillaroscopic patterns [18]. One should remember not to lean the dermoscope firmly on the nailfold to avoid squeezing the vessels and the appearance of pseudo-avascularization (Figure 11).
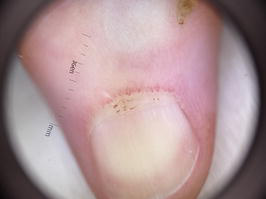
Figure 6.
NFC with the use of dermoscopy (10× zoom).
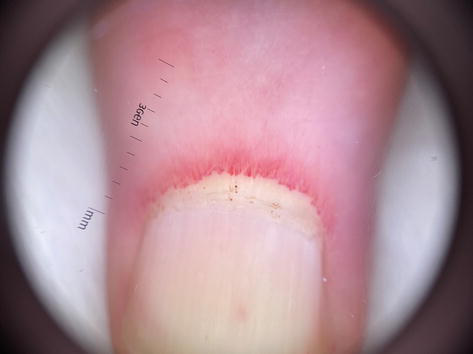
Figure 7.
Megacapillaries seen under dermoscopy in nailfolds (10× zoom).
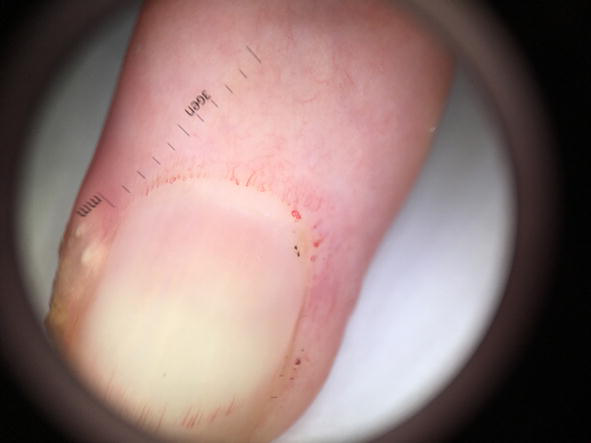
Figure 8.
Megacapillaries and microaneurysma seen under dermoscopy in nailfolds (10× zoom).
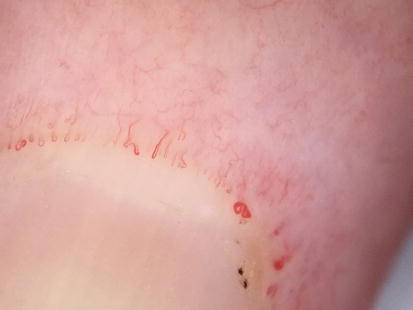
Figure 9.
Megacapillaries, microaneurysma and ramified capillaries seen under dermoscopy in nailfolds (20× zoom enhanced by smartphone).
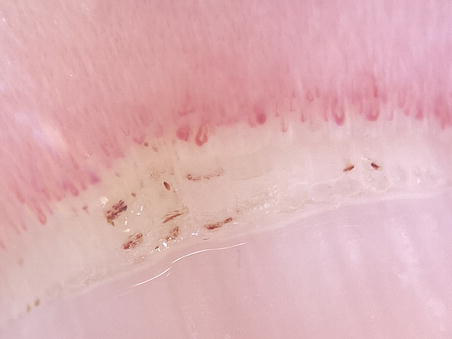
Figure 10.
NFC with the use of dermoscopy after attachment to smartphone (20× zoom).

Figure 11.
Pseudo-avascularization due to the squeezing of microvessels by dermoscope (20× zoom enhanced by smartphone).
4. Sidestream dark field imaging
The generalized microvasculopathy contributes to the involvement of other than cutaneous vascular bed. There was proposed evaluation of the microcirculation in oral cavity by sidestream dark field imaging, which is an automatic real-time microcirculation analysis tool. Evaluation of the capillary bed in the sublingual region showed previously significantly lower capillary density in SSc patients than in healthy controls [19, 20]. There were found positive correlations between NFC and sublingual measurement of perfusion and capillary density [20].
5. Optical coherence tomography
The involvement of the microcirculation in the eye was investigated using optical coherence tomography angiography (OCTA). It showed significantly reduced both retinal and choroidal perfusions in SSc patients when compared to controls [21, 22]. The use of optical coherence tomography of the skin showed earlier to be a valuable assessment tool potent to obtain virtual skin biopsy. A use of infrared light enables to produce images of micron resolution. OCT A-scans were previously referred to skin biopsies obtained in SSc patients from fingers (dorsal and volar aspect), hand (dorsal aspect), and forearm (dorsal and volar aspect). These images revealed blurred dermal-epidermal junction and the loss of capillaries in the skin of SSc patients [23]. A flow in the cutaneous microcirculation can be better explored with newly developed dynamic OCT (D-OCT). It recently demonstrated the reduced blood flow in nailfolds according to capillaroscopic patterns, but only a moderate correlation was seen between microvascular flow density measured by D-OCT and the number of capillaries calculated in NFC. One should notice that D-OCT analyzes the blood perfusion in a portion of the skin (3D space), whereas NFC counts capillaries only in the distal raw of the nailfold (2D surface) [24].
6. Laser doppler and laser related methods
A measurement of a shift in light beam backscattered by moving erythrocytes is the basis of laser Doppler techniques (Figure 12). Laser Doppler flowmetry (LDF) shows fluctuations in a blood flow in about 1 mm3 of tissue based on a single-point technique measurement [25]. This method is mostly used for recording of perfusion in fingers during the attack of RP and the change of the blood perfusion after pharmacological interventions. Device head remains, however, attached to the point on the skin and any cutaneous irregularities may impair the read of underlying perfusion. Additionally, movements of fingers may produce artifacts in the record of the blood flow [26]. Combining of several single measurements over the surface of finger enables to measure perfusion across the finger. Laser Doppler perfusion imaging (LDPI) uses a moveable mirror that directs the laser beam on the different measurement allowing for raster scanning or line scanning of a surface. Obtained images are composed of multiple pixels and displayed on the screen [25]. This imaging modality helps to analyze change in the perfusion of entire finger when evaluation of the risk for the development of digital tip ulcers is needed. Technique that does not require time-consuming scanning is laser speckle contrast analysis (LASCA), because it shows continuously perfusion in the hand. Laser Doppler methods and LASCA may seem different imaging techniques which developed separately, but a mathematical analysis shows that the two approaches are, in fact, identical. Illumination of a tissue leads herein to irregular backscattering of the laser light, which creates dark and light interference pattern (speckle pattern) [27]. CCD camera with a fixed exposure time records these changes in the speckle pattern as a motion. The speckle pattern is stationary when illuminated object remains static but moving red blood cells in a tissue produces blurring over time. The image is more blurred when there are more moving erythrocytes and the contrast between speckles decreases. Lack of movements means to a loss of blurring and therefore the contrast between speckles is large [25].
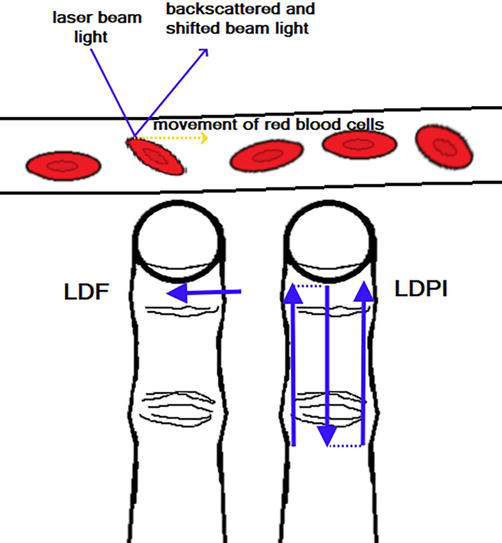
Figure 12.
Laser Doppler methods with a detection of backscattered laser beam light, which is shifted due to the movement of red blood cells. This can be laser Doppler flowmetry (LDF) with single point measurement or laser Doppler perfusion imaging (LDPI) with line or raster scanning.
7. Infrared thermography
Imaging method that enables for indirect evaluation of the microvasculopathy is thermography. Skin surface emits infrared radiation (IR), which energy can be collected by lenses and converted to an electric signal, amplified and shown on a screen of thermal imaging camera. A colorful map of temperature distribution is created over the body surface and one may determine maximal, minimal and average temperature in a region of interest. These measurements can be done using both industrial and mobile thermal imaging cameras attached to smartphone [28].
A change in a local blood perfusion influences on IR and temperature, but it also depends on a body area and underlying inflammation [28, 29, 30]. One should remember to ensure right environmental conditions (e.g., room temperature 19–21 C, fixed humidity) and adaptation of patients not to disturb the measurements of IR. When there is the measurement of temperature distribution in hands, the patient should be asked to avoid smoking, coffee and alcohol drinking, hand washing, and moisturizing before testing not to force a change in IR emission [30, 31]. The recording should be performed perpendicularly to the dorsal aspect of hands (from the distance of 0.4–0.6 m), which are isolated from the background, for example, by the cork plate or gauze (Figure 13). To investigate nailfolds of finger II-V both hands should be positioned flat. The measure of temperature in nailfolds of thumbs seems to be preferentially performed in the fist, when thumbs lay on a cork plate or gauze and fingers II–V remain covered below (Figure 14). Regions of interests can be determined over the nailfolds, but measurements may include a gradient of temperatures between the center of the metacarpus and the nailfolds of separate fingers [32, 33].
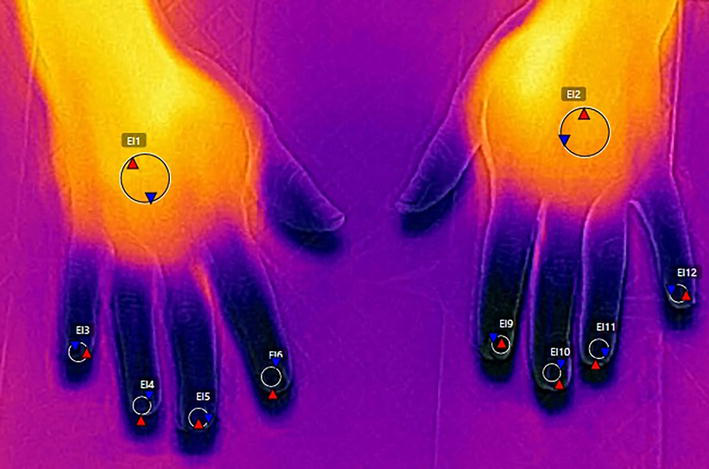
Figure 13.
Thermography of finger II–V performed with the use of FLIR T420 camera.
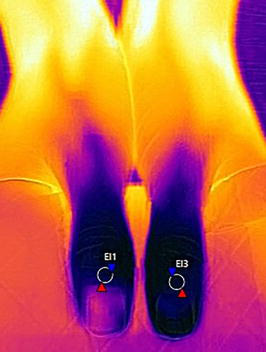
Figure 14.
Thermography of thumbs performed with the use of FLIR T420 camera.
Although there was found only moderate correlation between temperature measurements in nailfolds and the density of capillaries, thermography showed dependence of IR emission on capillaroscopic patterns in patients with SSc. One should remember that thermography measures IR emission from the portion of the tissue (3D space), whereas capillaroscopy quantifies density of vascular loops on a short distance (2D space) [32]. Thermography recently demonstrated differences in the control of temperature in hands between SSc patients with limited and diffuse cutaneous involvement [33]. Finally, thermography seems to be promising for the identification of fingers at then greater risk for the development of digital tip ulcers [32].
References
- 1.
Smith V, Herrick AL, Ingegnoli F, Damjanov N, De Angelis R, Denton CP, et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmunity Reviews. 2020; 19 (3):102458 - 2.
Lombard WP. The blood pressur in the arterioles, capillaries, and small veins of the human skin. The American Journal of Physiology. 1912; 29 (3):335-362 - 3.
Brown GE, O’Leary PA. Skin capillaries in scleroderma. Archives of Internal Medicine (Chicago, Ill). 1925; 36 (1):73-88 - 4.
Maricq HR, LeRoy EC. Capillary blood flow in scleroderma. Bibliotheca Anatomica. 1973; 11 :352-358 - 5.
Maricq HR, LeRoy EC. Patterns of finger capillary abnormalities in connective tissue disease by “wide-field” microscopy. Arthritis and Rheumatism. 1973; 16 (5):619-628 - 6.
Maricq HR, Downey JA, LeRoy EC. Standstill of nailfold capillary blood flow during cooling in scleroderma and Raynaud’s syndrome. Blood Vessels. 1976; 13 (6):338-349 - 7.
Minier T, Guiducci S, Bellando-Randone S, Bruni C, Lepri G, Czirják L, et al. Preliminary analysis of the very early diagnosis of systemic sclerosis (VEDOSS) EUSTAR multicentre study: Evidence for puffy fingers as a pivotal sign for suspicion of systemic sclerosis. Annals of the Rheumatic Diseases. 2014; 73 (12):2087-2093 - 8.
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Annals of the Rheumatic Diseases. 2013; 72 (11):1747-1755 - 9.
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2019; 78 (9):1151-1159 - 10.
Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Annals of the Rheumatic Diseases. 2017; 76 (12):1955-1964 - 11.
Smith V, Riccieri V, Pizzorni C, et al. Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. The Journal of Rheumatology. 2013; 40 :2023-2028 - 12.
Cutolo M, Sulli A, Pizzorni C, Accardo S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. The Journal of Rheumatology. 2000; 27 :155-160 - 13.
Li N, Zang H, Sun H, Jiao X, Wang K, Liu TCT, et al. A Noninvasive accurate measurement of blood glucose levels with raman spectroscopy of blood in microvessels. Molecules. 2019; 24 (8):1500 - 14.
Fedorovich AA, Drapkina OM, Pronko KN, Sinopalnikov VI, Zemskov VM. Telemonitoring of capillary blood flow in the human skin: New opportunities and prospects. Clinics and Practice. 2018; 15 (2):561-567 - 15.
Hurabielle C, Avouac J, Lepri G, de Risi T, Kahan A, Allanore Y. Skin telangiectasia and the identification of a subset of systemic sclerosis patients with severe vascular disease. Arthritis Care & Research (Hoboken). 2016; 68 (7):1021-1027 - 16.
Sekiyama JY, Camargo CZ, Eduardo LC, Kayser AC. Reliability of widefield nailfold capillaroscopy and videocapillaroscopy in the assessment of patients with raynaud’s phenomenon. Arthritis Care & Research (Hoboken). 2013; 65 (11):1853-1861 - 17.
Grassi W, Rossella De Angelis R. Capillaroscopy: Questions and answers. Clinical Rheumatology. 2007; 26 (12):2009 - 18.
Miziołek B, Pieczyrak R, Polak K, Frątczak A, Jedlecka A, Grosicka A, et al. Role of short courses on nailfold capillaroscopy in obtaining abilities for the identification of microvasculopathy in patients with Raynaud’s phenomenon. Skin Research and Technology. 2023; 29 (1):e13223 - 19.
Sha M, Griffin M, Denton CP, Butler PE. Sidestream Dark Field (SDF) imaging of oral microcirculation in the assessment of systemic sclerosis. Microvascular Research. 2019; 126 :103890 - 20.
Miranda S, Armengol G, Le Besnerais M, Lévesque H, Benhamou Y. New insights into systemic sclerosis related microcirculatory dysfunction by assessment of sublingual microcirculation and vascular glycocalyx layer. Results from a preliminary study. Microvascular Research. 2015; 99 :72-77 - 21.
Ranjbar M, Rothe M, Klapa S, Lange T, Prasuhn M, Grisanti S, et al. Evaluation of choroidal substructure perfusion in patients affected by systemic sclerosis: An optical coherence tomography angiography study. Scandinavian Journal of Rheumatology. 2020; 49 (2):141-145 - 22.
Rommel F, Prangel D, Prasuhn M, Grisanti S, Ranjbar M. Correlation of retinal and choroidal microvascular impairment in systemic sclerosis. Orphanet Journal of Rare Diseases. 2021; 16 (1):27 - 23.
Abignano G, Aydin SZ, Castillo-Gallego C, Liakouli V, Woods D, Meekings A, et al. Virtual skin biopsy by optical coherence tomography: The first quantitative imaging biomarker for scleroderma. Annals of the Rheumatic Diseases. 2013; 72 (11):1845-1851 - 24.
Abignano G, Green L, Eng S, Emery P, Del Galdo F. Nailfold microvascular imaging by dynamic optical coherence tomography in systemic sclerosis: A case-controlled pilot study. The Journal of Investigative Dermatology. 2022; 142 (4):1050-1057 - 25.
Eriksson S, Nilsson J, Sturesson C. Non-invasive imaging of microcirculation: A technology review. Medical Devices (Auckland). 2014; 7 :445-452 - 26.
Herrick AL, Dinsdale G, Murray A. New perspectives in the imaging of Raynaud’s phenomenon. European Journal of Rheumatology. 2020; 7 (Suppl 3):S212-S221 - 27.
Briers JD. Laser doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiological Measurement. 2001; 22 :R35-R66 - 28.
Lahiri BB, Bagavathiappan S, Jayakumar T, Philip J. Medical applications of infrared thermography: A review. Infrared Physics & Technology. 2012; 55 (4):221-235 - 29.
Ludwig N, Formenti D, Gargano M, Alberti G. Skin temperature evaluation by infrared thermography: Comparison of image analysis methods. Infrared Physics & Technology. 2014; 62 :1-6 - 30.
Lis-Święty A, Miziołek B, Ranosz-Janicka I, Bierzyńska-Macyszyn G, Brzezińska-Wcisło L. Thermal imaging and dermoscopy for detecting inflammation in frontal fibrosing alopecia. Journal of Cosmetic Dermatology. 2018; 17 (2):268-273 - 31.
Szczepanek M, Frątczak A, Polak K, Lis-Święty A. Narrow-band reflectance spectrophotometry and infrared thermography for assessment of skin lesions in localized scleroderma. Journal of the European Academy of Dermatology and Venereology. 2022; 36 (12):2451-2458 - 32.
Miziołek B, Lis-Święty A, Skrzypek-Salamon A, Brzezińska-Wcisło L. Correlation between the infrared thermogram and microvascular abnormalities of the nailfold in patients with systemic sclerosis. Postepy Dermatologii I Alergologii. 2021; 38 (2):115-122 - 33.
Miziołek B, Lis-Święty A, Kucharz E, Pieczyrak R, Polak K, Szczepanek M, et al. Clinical assessment of patients with systemic sclerosis: Is there a place for thermography? Archives of Dermatological Research. 2023; 315 (3):387-393