1. Introduction
Autoimmune diseases, significant challenges to global health, result from immune responses targeting self-antigens [1]. Understanding these diseases requires exploring the underlying molecular mechanisms, offering crucial perspectives on targeted therapy development.
2. Balancing immunity: tolerance and defense
The immune system aims to maintain cellular and tissue integrity, reject foreign entities, and tolerate self-antigens [2, 3]. This delicate balance relies on distinguishing nonself (pathogens) and modified self-components from unmodified self-antigens, which are tolerated.
3. Key features of innate and adaptive immunity
Innate and adaptive immunity are crucial for maintaining immune system integrity. Innate immunity acts as a first line of immune defense, while adaptive immunity provides immunological memory. This feature might similarly apply to innate immunity, albeit in a distinctive manner without clonal distribution. In this regard, it’s important to mention that we are presently exploring the concept of trained immunity.
4. Orchestrating tolerance: comprehensive insights into T-cell regulation and research in therapeutic potentials
T-cells under the orchestration of various regulatory mechanisms are pivotal in maintaining immunological tolerance [4]. This orchestration extends from the central tolerance mechanisms within the thymus that eliminate autoreactive T-cells [5] to peripheral tolerance mechanisms that prevent T-cell responses to self-antigens in peripheral tissues.
In the periphery, mature lymphocytes navigate through a sophisticated process of encountering self-antigens, leading to intrinsic anergy, apoptosis, or regulatory control by regulatory T-cells (Tregs) [6, 7, 8]. This complex ballet of peripheral tolerance is essential in curbing T-cell responses to self-antigens.
The fate of naive T-cells is intricately influenced by the balance between antigen (Signal 1) and co-stimulation (Signal 2). Co-stimulation mediated by immune checkpoints, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), plays a crucial role in regulating T-cell activation [9]. Anergy in T-cells encountering self-antigens emerges from inadequate co-stimulation, involving aberrant T-cell receptor (TCR) signaling and heightened co-inhibitory signals [10, 11].
The orchestration of immune balance extends to the interplay between activating receptors (TCR complex, CD28) and inhibitory receptors (CTLA-4, PD-1). CTLA-4 halts T-cell activation by removing B7 (CD80/CD86) ligands from antigen-presenting cells (APCs), while PD-1 inhibits T-cell activation through downstream signaling [12, 13, 14]. Recognition of self-antigens induces apoptosis in T-cells through multiple mechanisms [15, 16].
This comprehensive understanding of T-cell regulation provides insights into the intricate mechanisms that maintain immunological equilibrium.
One such approach involves unleashing the immune arsenal by targeting inhibitory receptors like CTLA-4 and PD-1 in therapies. While this amplifies antitumor immune responses, it may also pose the risk of triggering autoimmune reactions. Ongoing research delves into the exploration of other inhibitory receptors as potential targets for checkpoint blockade therapy. Another avenue involves tapping into Treg cells, which play a vital role in immune balance. Treg cells suppress harmful lymphocytes through different mechanisms, including cytokine production (IL-10, TGF-β) and expression of inhibitory molecules, such as CTLA-4. Treg cell therapy is gaining traction for addressing autoimmune diseases, graft-versus-host reactions, and graft rejection. Additionally, ongoing trials are investigating the potential of IL-2 in regulating immune reactions and highlighting the therapeutic applications of Treg cells [17].
5. B-cell tolerance
Balancing B-cell tolerance is a multifaceted process crucial for immune homeostasis. During B-cell development, negative selection eliminates cells with high self-antigen affinity, preventing the production of autoantibodies. Positive selection evaluates receptor functionality with a moderate self-antigen response, while excessive self-reactivity triggers receptor reformatting, known as receptor editing, involving gene rearrangements in the immunoglobulin M (IgM) light chain loci [18].
Self-reactive B-cell tolerance mechanisms encompass various strategies, including receptor editing, deletion, anergy, and competition for growth factors. Anergy renders B-cells functionally incapacitated, particularly when recognizing soluble proteins with low avidity in the bone marrow or specific microenvironments [19].
Similar to T-cells, B-cells also undergo two types of tolerance mechanisms–central and peripheral. Central tolerance of B-cells occurs during development in the bone marrow and involves receptor editing or negative selection, targeting B-cells with high-affinity receptors for prevalent autoantigens [20]. In peripheral lymphoid tissues, mature B-cells may undergo anergy, becoming unresponsive to autoantigens independently of T-cell assistance [21]. While essential for thymus-independent self-antigens such as polysaccharides and lipids, these anergic B-cells exit lymphoid follicles, but their survival may be compromised without essential stimuli.
Loss of B-cell anergy is a pivotal aspect of the intricate B-cell tolerance mechanism. In the periphery, 20% of B-cells express self-reactive receptors, restrained by inhibitory signals that are swiftly reversed upon dissociation from self-antigens. The loss of B-cell anergy precedes selected autoimmune disorders, highlighting its potential contributions to pathogenic B-cells in autoimmunity [22]. This delicate balance is further influenced by the regulatory roles of Tregs and regulatory B cells (Bregs), contributing to immune equilibrium by curbing excessive inflammatory responses [23, 24].
6. Microbiome and fetal antigen tolerance
6.1 Microbial harmony: essential roles in immune tolerance
Commensal microbes in the gut, respiratory tract, and skin perform vital functions. Mature lymphocytes recognize microbes without triggering immune responses, aided by some mechanisms like the regulation exerted by IL-10-producing Treg cells. Intestinal dendritic cells (DCs) contribute to food antigen tolerance [25, 26, 27].
6.2 Treg cells in pregnancy
6.2.1 Orchestrating fetal antigen tolerance
Tolerance to fetal antigens during pregnancy avoids immune responses against paternal antigens. Peripheral transcription factor forkhead box protein 3 (FoxP3, also known as scurfin) specific to paternal antigens play a crucial role in immune suppression, modulating various mechanisms for fetal tolerance. Treg cells peak during trophoblast invasion, decreasing during labor, highlighting their dynamic role throughout pregnancy [28, 29].
6.2.2 Mechanisms of fetal antigen tolerance
Treg cells influence cytokines and immunological signals, excluding inflammatory cells from the uterus, and establishing an immunosuppressive placental microenvironment. Disruptions in these mechanisms may lead to immune complications during pregnancy [30, 31].
7. Autoimmunity: genetic and environmental influences
Autoimmunity emerges as a consequence of the immune system’s aberrant overactivation targeting its own unaltered components. This phenomenon involves a combination of genetic predisposition, epigenetic changes, and various environmental influences, such as infections, ultraviolet (UV) radiation, medications, vaccination, and sex hormones. These factors, as depicted in Figure 1, interact intricately to impact disease susceptibility, prompting activation of self-reactive T-cells and B-cells. The orchestration of T-cells, B-cells, APCs, antibodies, inflammatory cells, and cytokines intricately contributes to the complexity of the autoimmune process [1].
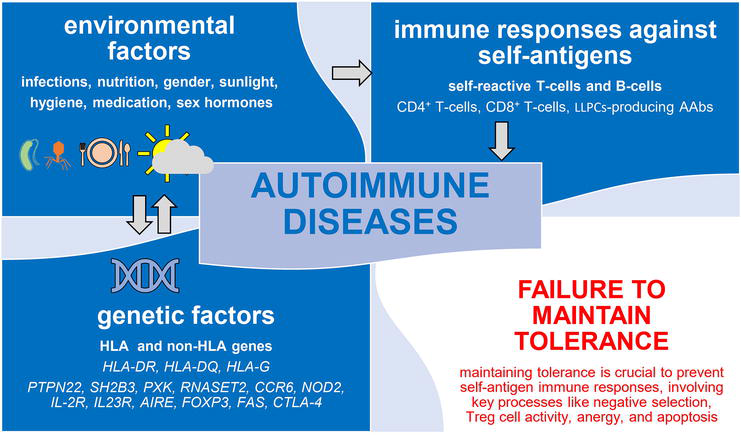
Figure 1.
Interplay of genetic and environmental factors in autoimmune diseases. This figure provides a succinct visual overview of the complex interrelationships driving autoimmune diseases. Genetic factors include variants of the HLA and non-HLA genes. Environmental factors encompass mainly infections, nutrition, gender, low sunlight (ultraviolet rays) exposure, hygiene, medications, and sex hormones. Immune responses against self-antigens involve CD4+ T-cells, CD8+ T-cells, and B-cells, as well as long-lived “memory” plasma cells producing autoantibodies. The failure to maintain tolerance, governed by key mechanisms such as negative selection, Treg cells, anergy, and apoptosis, is pivotal in understanding autoimmune disease development. These intricate interactions contribute to the development of autoimmune diseases. AIRE: Autoimmune regulator, CCR6: C-C motif chemokine receptor 6, CTLA-4: Cytotoxic T-lymphocyte antigen 4, FAS: FAS cell surface death receptor (also known as tumor necrosis factor [TNF] receptor superfamily member cluster of differentiation 95 [CD95] or apoptosis antigen 1 [APO-1 or APT]), FOXP3: Transcription factor forkhead box protein 3, HLA: Human leukocyte antigen, IL23R: Interleukin-23 receptor, IL-2R: Interleukin-2 receptor, LLPCs: Long-lived plasma cells, NOD2: Nucleotide-binding oligomerization domain-containing protein 2, PTPN22: Protein tyrosine phosphatase non-receptor type 22, RNASET2: Ribonuclease T2, SH2B3: SH2B adaptor protein 3 (also referred to as lymphocyte adapter protein [Lnk]), PXK: Phox (PX) domain-containing serine/threonine kinase.
7.1 Genetic factors
7.1.1 Role of MHC alleles
Human leukocyte antigen (HLA) alleles, particularly class II (HLA-DR and HLA-DQ), modulate immune responses involving autoreactive CD4+ T-cells. Specific HLA alleles increase autoimmune disease risk, with their exact role remaining elusive. Non-classical HLA-G gene, especially soluble HLA-G (sHLA-G), is crucial for immune tolerance at the maternal-fetal interface [32, 33, 34, 35, 36].
7.1.2 Non-HLA genes
A myriad of non-HLA genes actively contribute to the complex landscape of autoimmune diseases, introducing polymorphisms that disrupt self-tolerance or set off abnormal lymphocyte activation. Noteworthy genes implicated in various autoimmune conditions include protein tyrosine phosphatase non-receptor type 22 (
7.2 Environmental factors
7.2.1 Infections and type I interferons
In the intricate interplay between infections and the immune system, a dual role emerges. On one hand, infections disrupt peripheral T-cell tolerance, setting the stage for autoimmune responses. Notably, viral infections prompt the production of Type I interferons, a key player in the initiation of autoimmune diseases. The phenomenon of molecular mimicry adds another layer, wherein infections generate antigens resembling self-antigens, contributing significantly to the breakdown of immune tolerance [42, 43, 44, 45]. Additionally, infections and tissue damage introduce chemical alterations to peripheral tissue antigens, releasing self-antigens. This interaction with autoreactive cells becomes a pivotal factor in the path toward autoimmune diseases, with the cumulative impact of childhood infections potentially serving as the ignition for autoimmunity [46].
7.2.2 Influence of gender, sunlight, and hygiene on autoimmunity
Gender and sunlight exposure, affecting vitamin D3 levels, have been linked to the prevalence and progression of autoimmune diseases [47]. The “hygiene hypothesis,” which posits that exposure to certain infections may protect against autoimmunity, further underscores the impact of environmental factors on autoimmune disease development [48, 49].
8. Autoimmune diseases
8.1 Complexity of autoimmune diseases
Exceeding 130, autoimmune diseases vary in severity, falling into organ-specific and systemic categories [1]. Complexity arises from genetic and phenotypic diversity, with a notable delay in symptom manifestation and diagnostic phenotype development. Autoantibodies aid diagnosis and prognosis, yet the coexistence of multiple disorders complicates management.
8.2 Autoimmune challenges: temporal insights
Understanding autoimmune diseases reveals shared processes with inherent complexities. Challenges include defining early events recognizable only after diagnostic phenotype development. Recent studies on autoantibody development suggest a temporal separation between the onset of an autoimmune response and clinical symptoms.
8.3 Phases of autoimmune disease development
Examining autoimmune disease development unveils four phases: susceptibility, initiation, propagation, and regulation/resolution. The susceptibility phase (Phase I) explores genetic complexities, such as Mendelian patterns in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) and immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. The initiation phase (Phase II) focuses on adaptive immune responses, emphasizing immunodominance and antigen processing. The propagation phase (Phase III) amplifies the autoimmune process, shedding light on adjuvant properties and innate immune receptors [50].
9. Conclusions
Autoimmune diseases, numbering over a hundred, present significant global health challenges due to their diverse manifestations. The breakdown of immunological tolerance in both central and peripheral mechanisms is crucial for maintaining balance, and disruptions can lead to misguided immune responses. Processes such as negative selection, Treg cell activity, as well as other immune regulatory cells, anergy, and apoptosis are integral to immunological tolerance. Genetic factors, encompassing HLA and non-HLA elements, along with environmental triggers, play pivotal roles in disease initiation. Ongoing research aims to advance diagnosis and treatment, recognizing distinct phases in disease development for potential intervention. Exploring natural regulatory mechanisms provides promising avenues for therapeutic development, acknowledging the active role of target tissues and emphasizing a comprehensive understanding. Future investigations focus on genetic and epigenetic factors, interactions between innate and adaptive immunity, contributions of Treg cells, and the involvement of target tissues in the ongoing amplification process.
References
- 1.
Aribi M. Introductory chapter: Immune system dysfunction and autoimmune diseases. In: Aribi M, editor. Immunopathogenesis Immune-based Therapy for Selected Autoimmune Disorders. London, UK, Rijeka: IntechOpen; 2017. DOI: 10.5772/67671 - 2.
Dembic Z. Immune system protects integrity of tissues. Molecular Immunology. 2000; 37 :563-569. DOI: 10.1016/s0161-5890(00)00084-5 - 3.
Dembic Z. Response to Cohn: The immune system rejects the harmful, protects the useful and neglects the rest of microorganisms. Scandinavian Journal of Immunology. 2004; 60 :3-5; discussion 6-8. DOI: 10.1111/j.0300-9475.2004.01451.x - 4.
Gol-Ara M, Jadidi-Niaragh F, Sadria R, Azizi G, Mirshafiey A. The role of different subsets of regulatory T cells in immunopathogenesis of rheumatoid arthritis. Arthritis. 2012; 2012 :805875. DOI: 10.1155/2012/805875 - 5.
Xing Y, Hogquist KA. T-cell tolerance: Central and peripheral. Cold Spring Harbor Perspectives in Biology. 2012; 4 :a006957. DOI: 10.1101/cshperspect.a006957 - 6.
Greenwood R, Frelinger J. Mechanisms of unresponsiveness: T- and B-cell mediated mechanisms of anergy. Advances in Experimental Medicine and Biology. 2001; 489 :109-117. DOI: 10.1007/978-1-4615-1277-6_10 - 7.
Gurung P, Kucaba TA, Ferguson TA, Griffith TS. Activation-induced CD154 expression abrogates tolerance induced by apoptotic cells. Journal of immunology Baltimore Md. 2009; 1950 (183):6114-6123. DOI: 10.4049/jimmunol.0901676 - 8.
Cheru N, Hafler DA, Sumida TS. Regulatory T cells in peripheral tissue tolerance and diseases. Frontiers in Immunology. 2023; 14 :1154575. DOI: 10.3389/fimmu.2023.1154575 - 9.
Dai J, Fang P, Saredy J, Xi H, Ramon C, Yang W, et al. Metabolism-associated danger signal-induced immune response and reverse immune checkpoint-activated CD40+ monocyte differentiation. Journal of Hematology & Oncology. 2017; 10 :141. DOI: 10.1186/s13045-017-0504-1 - 10.
Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunological Reviews. 2011; 241 :180-205. DOI: 10.1111/j.1600-065X.2011.01011.x - 11.
Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Current Opinion in Immunology. 2013; 25 :214-221. DOI: 10.1016/j.coi.2012.12.003 - 12.
Walker LSK. Treg and CTLA-4: Two intertwining pathways to immune tolerance. Journal of Autoimmunity. 2013; 45 :49-57. DOI: 10.1016/j.jaut.2013.06.006 - 13.
Intlekofer AM, Thompson CB. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. Journal of Leukocyte Biology. 2013; 94 :25-39. DOI: 10.1189/jlb.1212621 - 14.
Bardhan K, Anagnostou T, Boussiotis VA. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Frontiers in Immunology. 2016; 7 :550. DOI: 10.3389/fimmu.2016.00550 - 15.
Murali AK, Mehrotra S. Apoptosis - an Ubiquitous T cell Immunomodulator. Journal of Clinical and Cellular Immunology. 2011; S3 :2. DOI: 10.4172/2155-9899.S3-002 - 16.
Hughes P, Bouillet P, Strasser A. Role of Bim and other Bcl-2 family members in autoimmune and degenerative diseases. Current Directions in Autoimmunity. 2006; 9 :74-94. DOI: 10.1159/000090773 - 17.
van der Vliet HJJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clinical & Developmental Immunology. 2007; 2007 :89017. DOI: 10.1155/2007/89017 - 18.
Aribi M. Immunogenetic aspect of B-cell antigen receptor diversity generation. In: Aribi M, editor. Normal and Malignant B-Cell. London, UK: IntechOpen; 2020. DOI: 10.5772/intechopen.90637 - 19.
Ferry H, Leung JCH, Lewis G, Nijnik A, Silver K, Lambe T, et al. B-cell tolerance. Transplantation. 2006; 81 :308-315. DOI: 10.1097/01.tp.0000203830.79357.39 - 20.
Wang Y, Liu J, Burrows PD, Wang J-Y. B cell development and maturation. Advances in Experimental Medicine and Biology. 2020; 1254 :1-22. DOI: 10.1007/978-981-15-3532-1_1 - 21.
Zoghi S, Masoumi F, Rezaei N. The immune system. In: Clinical Immunology. London, UK: Elsevier; 2023. pp. 1-46. DOI: 10.1016/B978-0-12-818006-8.00005-0 - 22.
Getahun A. Role of inhibitory signaling in peripheral B cell tolerance. Immunological Reviews. 2022; 307 :27-42. DOI: 10.1111/imr.13070 - 23.
Abo-Helo N, Vadasz Z, Kessel A, Toubi E. Regulatory B cells. Harefuah. 2016; 155 :50-53, 66 - 24.
Mauri C, Bosma A. Immune regulatory function of B cells. Annual Review of Immunology. 2012; 30 :221-241. DOI: 10.1146/annurev-immunol-020711-074934 - 25.
Canny GO, McCormick BA. Bacteria in the intestine, helpful residents or enemies from within? Infection and Immunity. 2008; 76 :3360-3373. DOI: 10.1128/IAI.00187-08 - 26.
Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity. 2018; 49 :1004-1019. DOI: 10.1016/j.immuni.2018.12.001 - 27.
Stagg AJ. Intestinal dendritic cells in health and gut inflammation. Frontiers in Immunology. 2018; 9 :2883. DOI: 10.3389/fimmu.2018.02883 - 28.
Than NG, Hahn S, Rossi SW, Szekeres-Bartho J. Editorial: Fetal-maternal immune interactions in pregnancy. Frontiers in Immunology. 2019; 10 :2729. DOI: 10.3389/fimmu.2019.02729 - 29.
Huang N, Chi H, Qiao J. Role of regulatory T cells in regulating fetal-maternal immune tolerance in healthy pregnancies and reproductive diseases. Frontiers in Immunology. 2020; 11 :1023. DOI: 10.3389/fimmu.2020.01023 - 30.
Nevers T, Kalkunte S, Sharma S. Uterine regulatory T cells, IL-10 and hypertension. American Journal of Reproductive Immunology. 2011; 66 Suppl. 1 (Suppl. 1):88-92. DOI: 10.1111/j.1600-0897.2011.01040.x - 31.
Jørgensen N, Persson G, Hviid TVF. The Tolerogenic function of regulatory T cells in pregnancy and cancer. Frontiers in Immunology. 2019; 10 :911. DOI: 10.3389/fimmu.2019.00911 - 32.
Castelli EC, Ramalho J, Porto IOP, Lima THA, Felício LP, Sabbagh A, et al. Insights into HLA-G genetics provided by worldwide haplotype diversity. Frontiers in Immunology. 2014; 5 :476. DOI: 10.3389/fimmu.2014.00476 - 33.
Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA-G: At the Interface of maternal-fetal tolerance. Trends in Immunology. 2017; 38 :272-286. DOI: 10.1016/j.it.2017.01.009 - 34.
Tantengco OAG, Richardson L, Lee A, Kammala A, Silva DMC, Shahin H, et al. Histocompatibility antigen, class I, G (HLA-G)‘s role during pregnancy and parturition: A systematic review of the literature. Life Basel Switz. 2021; 11 :1061. DOI: 10.3390/life11101061 - 35.
Rouas-Freiss N, Paul P, Dausset J, Carosella ED. HLA-G promotes immune tolerance. Journal of Biological Regulators and Homeostatic Agents. 2000; 14 :93-98 - 36.
Martín-Villa JM, Vaquero-Yuste C, Molina-Alejandre M, Juarez I, Suárez-Trujillo F, López-Nares A, et al. HLA-G: Too much or too little? Role in cancer and autoimmune disease. Frontiers in Immunology. 2022; 13 :796054. DOI: 10.3389/fimmu.2022.796054 - 37.
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001; 411 :603-606. DOI: 10.1038/35079114 - 38.
Chen L, Cao S-Q , Lin Z-M, He S-J, Zuo J-P. NOD-like receptors in autoimmune diseases. Acta Pharmacologica Sinica. 2021; 42 :1742-1756. DOI: 10.1038/s41401-020-00603-2 - 39.
Harris F, Berdugo YA, Tree T. IL-2-based approaches to Treg enhancement. Clinical and Experimental Immunology. 2023; 211 :149-163. DOI: 10.1093/cei/uxac105 - 40.
Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nature Reviews. Immunology. 2014; 14 :585-600. DOI: 10.1038/nri3707 - 41.
Abbas AK, Lichtman AH, Pillai S. Basic Immunology: Functions and Disorders of the Immune System. 6th ed. Philadelphia, PA: Elsevier; 2020 - 42.
Ehl S, Hombach J, Aichele P, Rülicke T, Odermatt B, Hengartner H, et al. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. The Journal of Experimental Medicine. 1998; 187 :763-774. DOI: 10.1084/jem.187.5.763 - 43.
Enouz S, Carrié L, Merkler D, Bevan MJ, Zehn D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. The Journal of Experimental Medicine. 2012; 209 :1769-1779. DOI: 10.1084/jem.20120905 - 44.
Herbeuval J-P. Editorial: Type 1 interferon in pathologies, autoimmune diseases, and chronic viral infections: Understanding the fascinating biologic role of type 1 interferons. Frontiers in Immunology. 2023; 14 :1239086. DOI: 10.3389/fimmu.2023.1239086 - 45.
Damian RT. Molecular mimicry: Antigen sharing by parasite and host and its consequences. The American Naturalist. 1964; 98 :129-149. DOI: 10.2307/2459352 - 46.
Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nature Immunology. 2017; 18 :716-724. DOI: 10.1038/ni.3731 - 47.
Aribi M, Mennechet FJD, Touil-Boukoffa C. Editorial: The role of vitamin D as an immunomodulator. Frontiers in Immunology. 2023; 14 :1186635. DOI: 10.3389/fimmu.2023.1186635 - 48.
Angum F, Khan T, Kaler J, Siddiqui L, Hussain A. The prevalence of autoimmune disorders in women: A narrative review. Cureus. 2020; 12 :e8094. DOI: 10.7759/cureus.8094 - 49.
Lahita RG. Sex and gender influence on immunity and autoimmunity. Frontiers in Immunology. 2023; 14 :1142723. DOI: 10.3389/fimmu.2023.1142723 - 50.
Antiochos B, Rosen A. Mechanisms of autoimmunity. In: Rich RR, Shearer WT, Schroeder H, Frew AJ, editors. Clinical Immunology: Principles and Practice. Amsterdam, Netherlands: Elsevier; 2019. pp. 677-684. DOI: 10.5772/67671